Concentration and Potential Non-Carcinogenic and Carcinogenic Health Risk Assessment of Metals in Locally Grown Vegetables
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Sample Processing
2.3. Quantification of Heavy Metals
2.4. Quality Control and Quality Assurance
2.5. Health Risk Assessment of Vegetable Consumption
2.5.1. Estimated Daily Intake (EDI)
2.5.2. Non-Carcinogenic and Carcinogenic Risk Assessment
3. Results and Discussion
3.1. Metal Concentrations of Vegetables
| n | Na | Mg | K | Ca | Fe | Zn | Mn | Cu | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Potato | 8 | Mean | 44.51 | 1138 | 23,880 | 32.73 | 13.36 | 11.73 | 5.388 | 5.463 |
| SD | 33.22 | 311.4 | 5577 | 8.880 | 4.539 | 2.851 | 1.389 | 1.285 | ||
| Onion | 7 | Mean | 129.6 | 1067 | 18,311 | 321.7 | 23.67 | 12.50 | 9.046 | 2.854 |
| SD | 48.21 | 90.66 | 1983 | 50.28 | 6.289 | 3.278 | 1.960 | 1.047 | ||
| Tomato | 7 | Mean | 273.5 | 2425 | 44,547 | 338.8 | 45.40 | 14.38 | 20.29 | 8.928 |
| SD | 127.9 | 382.8 | 13,050 | 152.4 | 12.45 | 4.116 | 5.035 | 2.906 | ||
| Sugar Beet | 7 | Mean | 6434 | 2118 | 34,222 | 211.9 | 25.61 | 13.04 | 26.05 | 5.224 |
| SD | 1869 | 385.5 | 12,806 | 98.74 | 20.28 | 5.821 | 10.84 | 2.495 | ||
| Green Chili | 7 | Mean | 56.58 | 1737 | 25,561 | 225.0 | 44.49 | 11.50 | 15.19 | 5.162 |
| SD | 39.27 | 100.6 | 4101 | 57.80 | 9.534 | 4.279 | 3.355 | 1.198 | ||
| Dill | 5 | Mean | 252.9 | 2728 | 20,363 | 1619.9 | 71.78 | 38.38 | 87.78 | 6.313 |
| SD | 104.4 | 769.2 | 5691 | 440.0 | 23.67 | 8.728 | 22.53 | 1.477 | ||
| Corn | 7 | Mean | 4.357 | 1384 | 10,795 | 9.531 | 17.59 | 17.39 | 8.441 | 1.592 |
| SD | 4.294 | 571.9 | 4069 | 7.051 | 6.724 | 6.480 | 4.551 | 1.167 | ||
| Spinach | 5 | Mean | 4348 | 13,517 | 69,105 | 1683.9 | 199.3 | 78.76 | 216.6 | 3.140 |
| SD | 3279 | 3426 | 22,705 | 351.8 | 41.21 | 8.618 | 37.73 | 0.856 | ||
| White Eggplant | 5 | Mean | 59.40 | 2738 | 39,199 | 305.0 | 27.98 | 15.34 | 15.46 | 8.313 |
| SD | 24.65 | 368.3 | 9585 | 61.25 | 4.872 | 2.021 | 1.605 | 1.472 | ||
| Kale | 5 | Mean | 1071 | 2939 | 29,971 | 3092 | 73.17 | 16.64 | 45.24 | 1.838 |
| SD | 658.7 | 692.7 | 5908 | 420.5 | 12.26 | 3.024 | 13.23 | 0.439 | ||
| Green Bean | 7 | Mean | 15.75 | 2240 | 23,911 | 427.9 | 58.89 | 20.93 | 16.07 | 6.199 |
| SD | 5.789 | 116.0 | 5305 | 112.2 | 8.548 | 3.499 | 8.479 | 1.776 | ||
| Capsicum | 6 | Mean | 84.96 | 1431 | 22,557 | 184.2 | 49.08 | 11.51 | 13.04 | 4.435 |
| SD | 43.53 | 179.5 | 5699 | 44.12 | 10.92 | 2.831 | 3.546 | 1.240 | ||
| Cucumber | 6 | Mean | 243.8 | 2695 | 33,742 | 465.7 | 37.28 | 15.23 | 10.70 | 4.706 |
| SD | 63.09 | 376.5 | 9934 | 116.7 | 7.678 | 3.209 | 2.305 | 0.855 |
3.2. Metal Concentration Comparison Among Different Vegetable Groups
3.3. Health Risk Assessment of Metals via Food Consumption
3.3.1. Estimated Daily Intakes (EDIs)
3.3.2. Non-Carcinogenic and Carcinogenic Health Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chauhan, G.; Chauhan, U.K. Human health risk assessment of heavy metals via dietary intake of vegetables grown in wastewater irrigated area of Rewa, India. Int. J. Sci. Res. Publ. 2014, 4, 1–9. [Google Scholar]
- Zhao, D.; Wang, P.; Zhao, F.J. Toxic metals and metalloids in food: Current status, health risks, and mitigation strategies. Curr. Environ. Health Rep. 2024, 11, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Noli, F.; Tsamos, P. Concentration of heavy metals and trace elements in soils, waters and vegetables and assessment of health risk in the vicinity of a lignite-fired power plant. Sci. Total Environ. 2016, 563, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, M.R.; Ugarte, O.M.; Lima, L.H.V.; Silva, J.R.; da Silva, F.B.V.; da Silva Lins, S.A.; do Nascimento, C.W.A. Risk assessment of heavy metals in soils and edible parts of vegetables grown on sites contaminated by an abandoned steel plant in Havana. Environ. Geochem. Health 2022, 44, 43–56. [Google Scholar] [CrossRef]
- Antoniadis, V.; Golia, E.E.; Liu, Y.T.; Wang, S.L.; Shaheen, S.M.; Rinklebe, J. Soil and maize contamination by trace elements and associated health risk assessment in the industrial area of Volos, Greece. Environ. Int. 2019, 124, 79–88. [Google Scholar] [CrossRef]
- Aslam, M.A.; Abbas, M.S.; Mustaqeem, M.; Bashir, M.; Shabbir, A.; Saeed, M.T.; Irfan, R.M. Comprehensive assessment of heavy metal contamination in soil-plant systems and health risks from wastewater-irrigated vegetables. Colloids Surf. C Environ. Asp. 2024, 2, 100044. [Google Scholar] [CrossRef]
- Sharma, A.; Nagpal, A.K. Contamination of vegetables with heavy metals across the globe: Hampering food security goal. J. Food Sci. Technol. 2020, 57, 391–403. [Google Scholar] [CrossRef]
- Dias, J.S. Nutritional quality and health benefits of vegetables: A review. Food Nutr. Sci. 2012, 3, 1354–1374. [Google Scholar] [CrossRef]
- Alegbe, P.J.; Appiah-Brempong, M.; Awuah, E. Heavy metal contamination in vegetables and associated health risks. Sci. Afr. 2025, 27, e02603. [Google Scholar] [CrossRef]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef]
- Bambhaneeya, S.M.; Garaniya, N.H.; Surve, V.H.; Deshmukh, S.P. Assessment of heavy metal contamination and accumulation in soil and leafy vegetables collected from industrial belt in Bharuch district, Gujarat. Vegetos 2025, 38, 103–110. [Google Scholar] [CrossRef]
- Radwan, M.A.; Salama, A.K. Market basket survey for some heavy metals in Egyptian fruits and vegetables. Food Chem. Toxicol. 2006, 44, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Gupta, A.K.; Bhatt, K.; Pandey, K.; Rai, U.N.; Singh, K.P. Distribution of metals in the edible plants grown at Jajmau, Kanpur (India) receiving treated tannery wastewater: Relation with physico-chemical properties of the soil. Environ. Monit. Assess. 2006, 115, 1–22. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Arowolo, T.A.; Bamgbose, O. Cadmium, copper and nickel levels in vegetables from industrial and residential areas of Lagos City, Nigeria. Food Chem. Toxicol. 2003, 41, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Li, Y.; Sun, Q.; Zhang, H. Trace elements in soils and selected agricultural plants in the Tongling mining area of China. Int. J. Environ. Res. Public Health 2018, 15, 202. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Dogan, I.; Ozyigit, I.I.; Demir, G. Influence of aluminum on mineral nutrient uptake and accumulation in Urtica pilulifera L. J. Plant Nutr. 2014, 37, 469–481. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Nepovimova, E.; Kuca, K.; Valko, M. Heavy metals: Toxicity and human health effects. Arch. Toxicol. 2025, 99, 153–209. [Google Scholar] [CrossRef]
- Foulkes, M.E.; Sadee, B.A.; Hill, S.J. Arsenic speciation and its DNA fractionation in the rice plant Oryza sativa. J. Anal. At. Spectrom. 2020, 35, 1989–2001. [Google Scholar] [CrossRef]
- Rusin, M.; Domagalska, J.; Rogala, D.; Razzaghi, M.; Szymala, I. Concentration of cadmium and lead in vegetables and fruits. Sci. Rep. 2021, 11, 11913. [Google Scholar] [CrossRef]
- Mawari, G.; Kumar, N.; Sarkar, S.; Daga, M.K.; Singh, M.M.; Joshi, T.K.; Khan, N.A. Heavy metal accumulation in fruits and vegetables and human health risk assessment: Findings from Maharashtra, India. Environ. Health Insights 2022, 16, 11786302221119151. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Lu, G.; Fan, B.; Xiang, W.; Bao, Z. Bioaccumulation and risk assessment of heavy metals in soil-crop systems in Liujiang karst area, Southwestern China. Environ. Sci. Pollut. Res. 2021, 28, 9657–9669. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, Y.; Sahito, Z.A.; Liu, C.; Li, Z.; Yu, C.; Feng, Y.; Guo, W. Intraspecific variation in tomato: Impact on production quality and cadmium phytoremediation efficiency in intercropping systems with hyperaccumulating plant. Ecotoxicol. Environ. Saf. 2024, 282, 116715. [Google Scholar] [CrossRef] [PubMed]
- Bani, A.; Echevarria, G.; Zhang, X.; Benizri, E.; Laubie, B.; Morel, J.L.; Simonnot, M.O. The effect of plant density in nickel-phytomining field experiments with Alyssum murale in Albania. Aust. J. Bot. 2015, 63, 72–77. [Google Scholar] [CrossRef]
- Radulescu, C.; Stihi, C.; Popescu, I.V.; Dulama, I.D.; Chelarescu, E.D.; Chilian, A. Heavy metal accumulation and translocation in different parts of Brassica oleracea L. Rom. J. Phys. 2013, 58, 1337–1354. [Google Scholar]
- Nowar, A.; Islam, M.H.; Islam, S.; Jubayer, A.; Nayan, M.M. A systematic review on heavy metals contamination in Bangladeshi vegetables and their associated health risks. Front. Environ. Sci. 2024, 12, 1425286. [Google Scholar] [CrossRef]
- Kasemodel, M.C.; Sakamoto, I.K.; Varesche, M.B.A.; Rodrigues, V.G.S. Potentially toxic metal contamination and microbial community analysis in an abandoned Pb and Zn mining waste deposit. Sci. Total Environ. 2019, 675, 367–379. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Zhao, Z.; Cai, Y. Heavy metal pollution in reservoirs in the hilly area of southern China: Distribution, source apportionment and health risk assessment. Sci. Total Environ. 2018, 634, 158–169. [Google Scholar] [CrossRef]
- Silva, F.B.V.; Do Nascimento, C.W.A.; Araújo, P.R.M.; da Silva, F.L.; Lima, L.H.V. Soil contamination by metals with high ecological risk in urban and rural areas. Int. J. Environ. Sci. Technol. 2017, 14, 553–562. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, D.; Ren, F.; Huang, L. Spatiotemporal variation of soil heavy metals in China: The pollution status and risk assessment. Sci. Total Environ. 2023, 871, 161768. [Google Scholar] [CrossRef]
- Boahen, E. Heavy metal contamination in urban roadside vegetables: Origins, exposure pathways, and health implications. Discov. Environ. 2024, 2, 145. [Google Scholar] [CrossRef]
- Sidhu, G.P.S.; Bali, A.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Insights into the tolerance and phytoremediation potential of Coronopus didymus L.(Sm) grown under zinc stress. Chemosphere 2020, 244, 125350. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.M.; Kwon, E.E.; Biswas, J.K.; Tack, F.M.; Ok, Y.S.; Rinklebe, J. Arsenic, chromium, molybdenum, and selenium: Geochemical fractions and potential mobilization in riverine soil profiles originating from Germany and Egypt. Chemosphere 2017, 180, 553–563. [Google Scholar] [CrossRef] [PubMed]
- White, K.B.; Liber, K. Early chemical and toxicological risk characterization of inorganic constituents in surface water from the Canadian oil sands first large-scale end pit lake. Chemosphere 2018, 211, 745–757. [Google Scholar] [CrossRef]
- Saleem, M.; Iqbal, J.; Shah, M.H. Seasonal variations, risk assessment and multivariate analysis of trace metals in the freshwater reservoirs of Pakistan. Chemosphere 2019, 216, 715–724. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, S.; Sun, Q.; Wadood, S.A.; Guo, B. Source identification and spatial distribution of arsenic and heavy metals in agricultural soil around Hunan industrial estate by positive matrix factorization model, principle components analysis and geo statistical analysis. Ecotoxicol. Environ. Saf. 2018, 159, 354–362. [Google Scholar] [CrossRef]
- Sadee, B.A.; Ali, R.J. Determination of heavy metals in edible vegetables and a human health risk assessment. Environ. Nanotechnol. Monit. Manag. 2023, 19, 100761. [Google Scholar]
- Singh, A.; Sharma, R.K.; Agrawal, M.; Marshall, F.M. Risk assessment of heavy metal toxicity through contaminated vegetables from waste water irrigated area of Varanasi, India. Trop. Ecol. 2010, 51, 375–387. [Google Scholar]
- Shahriar, S.M.S.; Hossain, M.S.; Dipti, S.; Salam, S.M.A. Heavy metal contamination in soil and vegetables: A review with health risk assessments. J. Sci. Eng. Pap. 2024, 1, 40–48. [Google Scholar] [CrossRef]
- González-Muñoz, M.J.; Peña, A.; Meseguer, I. Monitoring heavy metal contents in food and hair in a sample of young Spanish subjects. Food Chem. Toxicol. 2008, 46, 3048–3052. [Google Scholar] [CrossRef] [PubMed]
- Lupolt, S.N.; Santo, R.E.; Kim, B.F.; Green, C.; Codling, E.; Rule, A.M.; Chen, R.; Scheckel, K.G.; Strauss, M.; Cocke, A.; et al. The safe urban harvests study: A community-driven cross-sectional assessment of metals in soil, irrigation water, and produce from urban farms and gardens in Baltimore, Maryland. Environ. Health Perspect. 2021, 129, 117004. [Google Scholar] [CrossRef]
- Chinnannan, K.; Somagattu, P.; Yammanuru, H.; Reddy, U.K.; Nimmakayala, P. Health risk assessment of heavy metals in soil and vegetables from major agricultural sites of Ohio and West Virginia. Biocatal. Agric. Biotechnol. 2024, 57, 103108. [Google Scholar] [CrossRef]
- Burke, M.W.; Rundquist, B.C.; Zheng, H. Detection of Shelterbelt Density Change Using Historic APFO and NAIP Aerial Imagery. Remote. Sens. 2019, 11, 218. [Google Scholar] [CrossRef]
- Saleem, M.; Pierce, D.; Wang, Y.; Sens, D.A.; Somji, S.; Garrett, S.H. Heavy metal (oid)s contamination and potential ecological risk assessment in agricultural soils. J. Xenobiot. 2024, 14, 634–650. [Google Scholar] [CrossRef]
- Abbasi, A.M.; Iqbal, J.; Khan, M.A.; Shah, M.H. Health risk assessment and multivariate apportionment of trace metals in wild leafy vegetables from Lesser Himalayas, Pakistan. Ecotoxicol. Environ. Saf. 2013, 92, 237–244. [Google Scholar] [CrossRef]
- Sharafi, K.; Mansouri, B.; Omer, A.K.; Bashardoust, P.; Ebrahimzadeh, G.; Sharifi, S.; Massahi, T.; Soleimani, H. Investigation of health risk assessment and the effect of various irrigation water on the accumulation of toxic metals in the most widely consumed vegetables in Iran. Sci. Rep. 2022, 12, 20806. [Google Scholar] [CrossRef]
- Hu, J.; Wu, F.; Wu, S.; Cao, Z.; Lin, X.; Wong, M.H. Bioaccessibility, dietary exposure and human risk assessment of heavy metals from market vegetables in Hong Kong revealed with an in vitro gastrointestinal model. Chemosphere 2013, 91, 455–461. [Google Scholar] [CrossRef]
- Voica, C.; Nechita, C.; Iordache, A.M.; Roba, C.; Zgavarogea, R.; Ionete, R.E. ICP-MS assessment of essential and toxic trace elements in foodstuffs with different geographic origins available in Romanian supermarkets. Molecules 2021, 26, 7081. [Google Scholar] [CrossRef]
- Islam, M.N.; Das, B.K.; Huque, M.E. Risk assessment for Bangladesh is due to arsenic exposure from consumption of vegetables grown with natural arsenic contaminated groundwater. Indian J. Sci. Technol. 2018, 11. [Google Scholar] [CrossRef]
- Shamsollahi, H.R.; Alimohammadi, M.; Momeni, S.; Naddafi, K.; Nabizadeh, R.; Khorasgani, F.C.; Masinaei, M.; Yousefi, M. Assessment of the health risk induced by accumulated heavy metals from anaerobic digestion of biological sludge of the lettuce. Biol. Trace Elem. Res. 2019, 188, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Adefa, T.; Tefera, M. Heavy metal accumulation and health risk assessment in Moringa oleifera from Awi zone, Ethiopia. Chem. Afr. 2020, 3, 1073–1079. [Google Scholar] [CrossRef]
- Zakaria, Z.; Zulkafflee, N.S.; Mohd Redzuan, N.A.; Selamat, J.; Ismail, M.R.; Praveena, S.M.; Tóth, G.; Abdull Razis, A.F. Understanding potential heavy metal contamination, absorption, translocation and accumulation in rice and human health risks. Plants 2021, 10, 1070. [Google Scholar] [CrossRef] [PubMed]
- Kimmons, J.; Gillespie, C.; Seymour, J.; Serdula, M.; Blanck, H.M. Fruit and vegetable intake among adolescents and adults in the United States: Percentage meeting individualized recommendations. Medscape J. Med. 2009, 11, 26. [Google Scholar]
- Hadayat, N.; De Oliveira, L.M.; Da Silva, E.; Han, L.; Hussain, M.; Liu, X.; Ma, L.Q. Assessment of trace metals in five most-consumed vegetables in the US: Conventional vs. organic. Environ. Pollut. 2018, 243, 292–300. [Google Scholar] [CrossRef]
- Bayissa, L.D.; Gebeyehu, H.R. Vegetables contamination by heavy metals and associated health risk to the population in Koka area of central Ethiopia. PLoS ONE 2021, 16, 0254236. [Google Scholar] [CrossRef]
- Lučić, M.; Miletić, A.; Savić, A.; Lević, S.; Ignjatović, I.S.; Onjia, A. Dietary intake and health risk assessment of essential and toxic elements in pepper (Capsicum annuum). J. Food Compos. Anal. 2022, 111, 104598. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxic Substances Portal. U.S. Department of Health and Human Services. Available online: https://wwwn.cdc.gov/TSP/index.aspx (accessed on 9 June 2025).
- United States Environmental Protection Agency (USEPA). Integrated Risk Information System (IRIS). Available online: https://www.epa.gov/iris (accessed on 9 June 2025).
- Antoine, J.M.; Fung, L.A.H.; Grant, C.N. Assessment of the potential health risks associated with the aluminium, arsenic, cadmium and lead content in selected fruits and vegetables grown in Jamaica. Toxicol. Rep. 2017, 4, 181–187. [Google Scholar] [CrossRef]
- Ashraf, I.; Ahmad, F.; Sharif, A.; Altaf, A.R.; Teng, H. Heavy metals assessment in water, soil, vegetables and their associated health risks via consumption of vegetables, District Kasur, Pakistan. SN Appl. Sci. 2021, 3, 552. [Google Scholar] [CrossRef]
- Kamunda, C.; Mathuthu, M.; Madhuku, M. Health risk assessment of heavy metals in soils from witwatersrand gold mining basin, South Africa. Int. J. Environ. Res Public Health 2016, 13, 663. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Hadei, M.; Sharafi, K. Human health risk assessment by Monte Carlo simulation method for heavy metals of commonly consumed cereals in Iran-Uncertainty and sensitivity analysis. J. Food Compos. Anal. 2021, 96, 103697. [Google Scholar] [CrossRef]
- Kusin, F.M.; Azani, N.N.M.; Hasan, S.N.M.S.; Sulong, N.A. Distribution of heavy metals and metalloid in surface sediments of heavily-mined area for bauxite ore in Pengerang, Malaysia and associated risk assessment. Catena 2018, 165, 454–464. [Google Scholar] [CrossRef]
- Demissie, S.; Mekonen, S.; Awoke, T.; Teshome, B.; Mengistie, B. Examining carcinogenic and noncarcinogenic health risks related to arsenic exposure in Ethiopia: A longitudinal study. Toxicol. Rep. 2024, 12, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Pajević, S.; Arsenov, D.; Nikolić, N.; Borišev, M.; Orčić, D.; Župunski, M.; Mimica-Dukić, N. Heavy metal accumulation in vegetable species and health risk assessment in Serbia. Environ. Monit. Assess. 2018, 190, 459. [Google Scholar] [CrossRef]
- Ahmed, S.; Mahdi, M.M.; Nurnabi, M.; Alam, M.Z.; Choudhury, T.R. Health risk assessment for heavy metal accumulation in leafy vegetables grown on tannery effluent contaminated soil. Toxicol. Rep. 2022, 9, 346–355. [Google Scholar] [CrossRef]
- Alexander, P.D.; Alloway, B.J.; Dourado, A.M. Genotypic variations in the accumulation of Cd, Cu, Pb and Zn exhibited by six commonly grown vegetables. Environ. Pollut. 2006, 144, 736–745. [Google Scholar] [CrossRef]
- Finster, M.E.; Gray, K.A.; Binns, H.J. Lead levels of edibles grown in contaminated residential soils: A field survey. Sci. Total Environ. 2004, 320, 245–257. [Google Scholar] [CrossRef]
- Säumel, I.; Kotsyuk, I.; Hölscher, M.; Lenkereit, C.; Weber, F.; Kowarik, I. How healthy is urban horticulture in high traffic areas? Trace metal concentrations in vegetable crops from plantings within inner city neighbourhoods in Berlin, Germany. Environ. Pollut. 2012, 165, 124–132. [Google Scholar] [CrossRef]
- Douay, F.; Pelfrêne, A.; Planque, J.; Fourrier, H.; Richard, A.; Roussel, H.; Girondelot, B. Assessment of potential health risk for inhabitants living near a former lead smelter. Part 1: Metal concentrations in soils, agricultural crops, and homegrown vegetables. Environ. Monit. Assess. 2013, 185, 3665–3680. [Google Scholar] [CrossRef]
- Mapanda, F.; Mangwayana, E.; Nyamangara, J.; Giller, K. The effect of long-term irrigation using wastewater on heavy metal contents of soils under vegetables in Harare, Zimbabwe. Agric. Ecosyst. Environ. 2005, 107, 151–165. [Google Scholar] [CrossRef]
- Chowdhury, A.I.; Shill, L.C.; Raihan, M.M.; Rashid, R.; Bhuiyan, M.N.H.; Reza, S.; Alam, M.R. Human health risk assessment of heavy metals in vegetables of Bangladesh. Sci. Rep. 2024, 14, 15616. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.L.; Liu, C.P.; Wang, Y.; Liu, X.; Li, F.B.; Zhang, C.; Li, X.D. Heavy metal contamination in soils and vegetables near an e-waste processing site, south China. J. Hazard. Mater. 2011, 186, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Yu, H.Y.; Chen, J.J.; Li, F.B.; Zhang, H.H.; Liu, C.P. Accumulation of heavy metals in leaf vegetables from agricultural soils and associated potential health risks in the Pearl River Delta, South China. Environ. Monit. Assess. 2014, 186, 1547–1560. [Google Scholar] [CrossRef] [PubMed]
- Sheet, N.N.F. Joint Report of Food Planning and Nutrition Unit (FMPU) of the Ministry of Food of Government of Bangladesh and Food and Agricultural Organization of the United Nation (FAO); National Food Policy Plan of Action and Country Investment Plan; Government of the People’s Republic of Bangladesh: Dhaka, Bangladesh, 2011; pp. 1–2.
- Khan, S.; Reid, B.J.; Li, G.; Zhu, Y.G. Application of biochar to soil reduces cancer risk via rice consumption: A case study in Miaoqian village, Longyan, China. Environ. Int. 2014, 68, 154–161. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agrawal, M.; Marshall, F. Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol. Environ. Saf. 2007, 66, 258–266. [Google Scholar] [CrossRef]
- Zhong, T.; Xue, D.; Zhao, L.; Zhang, X. Concentration of heavy metals in vegetables and potential health risk assessment in China. Environ. Geochem. Health 2018, 40, 313–322. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, W.T.; Zhou, X.; Liu, L.; Gu, J.F.; Wang, W.L.; Zou, J.L.; Tian, T.; Peng, P.Q.; Liao, B.H. Accumulation of heavy metals in vegetable species planted in contaminated soils and the health risk assessment. Int. J. Environ. Res. Public Health 2016, 13, 289. [Google Scholar] [CrossRef]
- Cherfi, A.; Cherfi, M.; Maache-Rezzoug, Z.; Rezzoug, S.A. Risk assessment of heavy metals via consumption of vegetables collected from different supermarkets in La Rochelle, France. Environ. Monit. Assess. 2016, 188, 136. [Google Scholar] [CrossRef]
- Razzak, A.; Mahjabin, T.; Khan, M.R.M.; Hossain, M.; Sadia, U.; Zzaman, W. Effect of cooking methods on the nutritional quality of selected vegetables at Sylhet City. Heliyon 2023, 9, e21709. [Google Scholar] [CrossRef]
- Lee, J.G.; Hwang, J.Y.; Lee, H.E.; Choi, J.D.; Kang, G.J. Comparative analysis of lead content during food processing. Food Sci. Biotechnol. 2020, 29, 1063–1069. [Google Scholar] [CrossRef]
- Prashanth, L.; Kattapagari, K.K.; Chitturi, R.T.; Baddam, V.R.R.; Prasad, L.K. A review on role of essential trace elements in health and disease. J. Dr. YSR Univ. Health Sci. 2015, 4, 75–85. [Google Scholar]
- Esposito, M.; De Roma, A.; Sansone, D.; Capozzo, D.; Iaccarino, D.; di Nocera, F.; Gallo, P. Non-essential toxic element (Cd, As, Hg and Pb) levels in muscle, liver and kidney of loggerhead sea turtles (Caretta caretta) stranded along the southwestern coasts of Tyrrhenian sea. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 231, 108725. [Google Scholar] [CrossRef] [PubMed]
- Adam Branson, 2023, China Releases the Standard for Maximum Levels of Contaminants in Foods. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=China%20Releases%20the%20Standard%20for%20Maximum%20Levels%20of%20Contaminants%20in%20Foods_Beijing_China%20-%20People%27s%20Republic%20of_CH2023-0040 (accessed on 12 March 2025).
- FAO/WHO, 2023, General Standard for Contaminants and Toxins in Food and Feed, CXS 193-1995, Codex Alimentariu, International Food Standards. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/fr/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B193-1995%252FCXS_193e.pdf (accessed on 12 March 2025).
- McBride, M.B.; Shayler, H.A.; Spliethoff, H.M.; Mitchell, R.G.; Marquez-Bravo, L.G.; Ferenz, G.S.; Russell-Anelli, J.M.; Casey, L.; Bachman, S. Concentrations of lead, cadmium and barium in urban garden-grown vegetables: The impact of soil variables. Environ. Pollut. 2014, 194, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.E.; Lauri, D.C.; Silveira, P.C.L. Assessment of daily intake of trace elements due to consumption of foodstuffs by adult inhabitants of Rio de Janeiro city. Sci Total Environ. 2004, 327, 69–79. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Habibullah-Al-Mamun, M.; Raknuzzaman, M.; Ali, M.M.; Eaton, D.W. Health risk assessment due to heavy metal exposure from commonly consumed fish and vegetables. Environ. Syst. Decis. 2016, 36, 253–265. [Google Scholar] [CrossRef]
- USEPA. In Risk Assessment Guidance for Superfund: Volume 3—Process for Conducting Probabilistic Risk Assessment Chapter l, Part A; USEPA: Washington, IX, USA, 2001.
- Zeng, F.; Wei, W.; Li, M.; Huang, R.; Yang, F.; Duan, Y. Heavy metal contamination in rice-producing soils of Hunan province, China and potential health risks. Int. J. Environ. Res. Public Health 2015, 12, 15584–15593. [Google Scholar] [CrossRef]
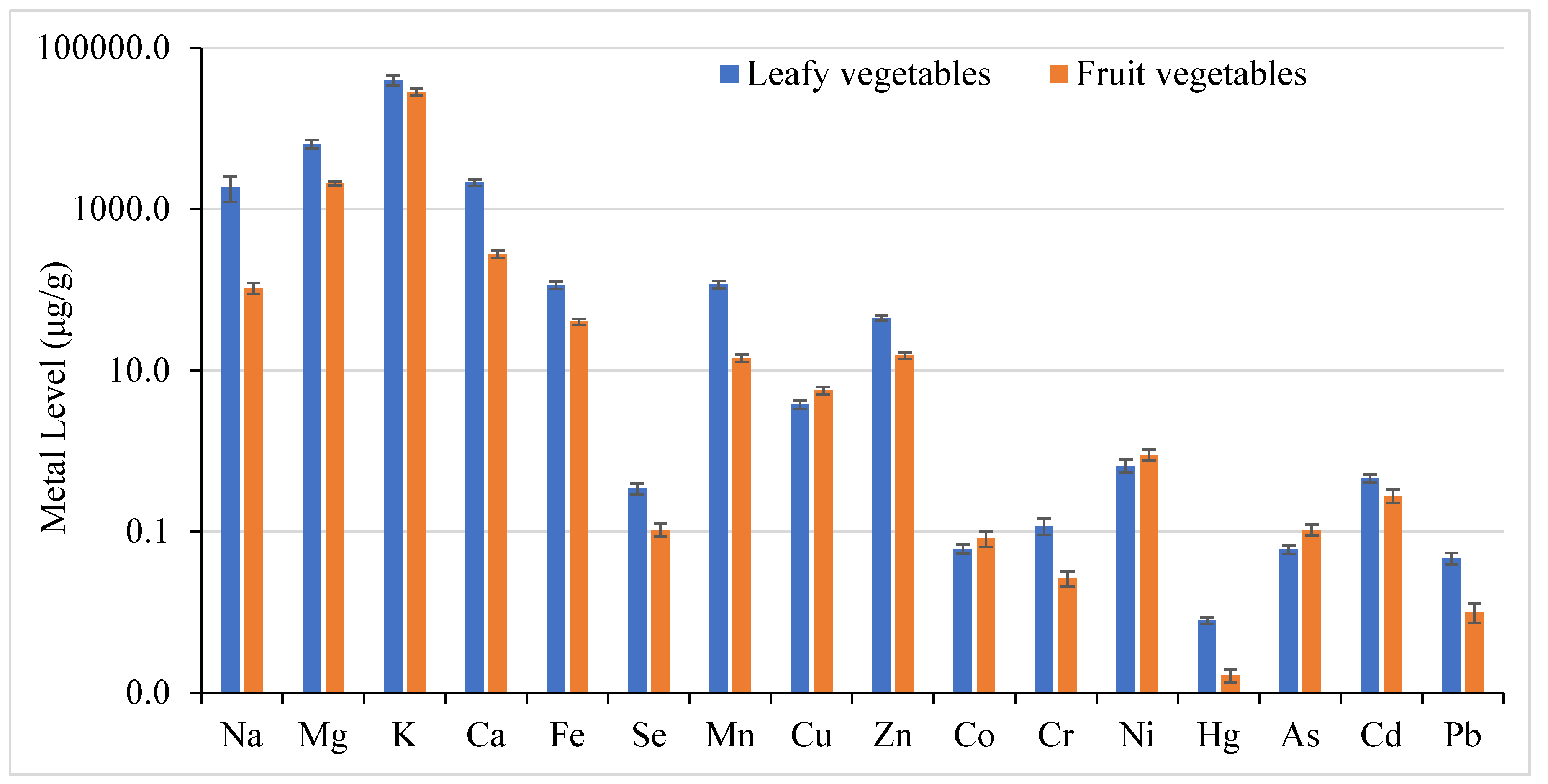

| Se | Co | Cr | Ni | Hg | As | Cd | Pb | ||
|---|---|---|---|---|---|---|---|---|---|
| Potato | Mean | 0.056 | 0.047 | 0.014 | 0.535 | 0.0017 | 0.010 | 0.218 | 0.009 |
| SD | 0.036 | 0.024 | 0.005 | 0.206 | 0.0009 | 0.002 | 0.087 | 0.003 | |
| Onion | Mean | 0.312 | 0.015 | 0.032 | 0.400 | 0.0010 | 0.058 | 0.080 | 0.014 |
| SD | 0.361 | 0.005 | 0.021 | 0.245 | 0.0002 | 0.055 | 0.033 | 0.007 | |
| Tomato | Mean | 0.065 | 0.070 | 0.030 | 0.484 | 0.0029 | 0.022 | 0.369 | 0.007 |
| SD | 0.046 | 0.031 | 0.020 | 0.223 | 0.0017 | 0.011 | 0.101 | 0.003 | |
| Sugar Beet | Mean | 0.082 | 0.073 | 0.042 | 0.349 | 0.0009 | 0.090 | 0.245 | 0.018 |
| SD | 0.078 | 0.048 | 0.043 | 0.128 | 0.0002 | 0.087 | 0.153 | 0.016 | |
| Green Chili | Mean | 0.098 | 0.218 | 0.033 | 0.975 | 0.0008 | 0.048 | 0.657 | 0.007 |
| SD | 0.062 | 0.135 | 0.025 | 0.284 | 0.0001 | 0.025 | 0.442 | 0.003 | |
| Dill | Mean | 0.481 | 0.030 | 0.061 | 1.270 | 0.0047 | 0.079 | 0.286 | 0.040 |
| SD | 0.172 | 0.011 | 0.019 | 0.685 | 0.0002 | 0.024 | 0.075 | 0.035 | |
| Corn | Mean | 0.075 | 0.020 | 0.019 | 0.366 | 0.0012 | 0.013 | 0.006 | 0.007 |
| SD | 0.045 | 0.014 | 0.009 | 0.172 | 0.0004 | 0.009 | 0.004 | 0.000 | |
| Spinach | Mean | 0.225 | 0.125 | 0.179 | 0.342 | 0.0113 | 0.064 | 0.985 | 0.081 |
| SD | 0.055 | 0.029 | 0.063 | 0.070 | 0.0035 | 0.016 | 0.223 | 0.013 | |
| White Eggplant | Mean | 0.070 | 0.034 | 0.021 | 0.922 | 0.0009 | 0.190 | 0.453 | 0.010 |
| SD | 0.013 | 0.007 | 0.013 | 0.264 | 0.0002 | 0.075 | 0.061 | 0.013 | |
| Kale | Mean | 0.327 | 0.028 | 0.114 | 0.365 | 0.0077 | 0.039 | 0.105 | 0.021 |
| SD | 0.120 | 0.006 | 0.088 | 0.058 | 0.0009 | 0.010 | 0.021 | 0.003 | |
| Green Bean | Mean | 0.116 | 0.067 | 0.024 | 2.139 | 0.0020 | 0.009 | 0.014 | 0.009 |
| SD | 0.055 | 0.026 | 0.008 | 0.774 | 0.0013 | 0.001 | 0.025 | 0.004 | |
| Capsicum | Mean | 0.109 | 0.100 | 0.028 | 0.712 | 0.0017 | 0.027 | 0.411 | 0.008 |
| SD | 0.036 | 0.090 | 0.011 | 0.333 | 0.0001 | 0.023 | 0.304 | 0.007 | |
| Cucumber | Mean | 0.208 | 0.068 | 0.034 | 0.713 | 0.0023 | 0.436 | 0.045 | 0.021 |
| SD | 0.083 | 0.022 | 0.013 | 0.274 | 0.0013 | 0.145 | 0.025 | 0.013 |
| Se | Mn | Cu | Zn | Co | Hg | Cr | Ni | As | Cd | Pb | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Potato | 2.7 × 10−4 | 2.6 × 10−2 | 2.7 × 10−2 | 5.7 × 10−2 | 2.3 × 10−4 | 8.5 × 10−6 | 6.8 × 10−5 | 2.6 × 10−3 | 4.9 × 10−5 | 1.1 × 10−3 | 4.4 × 10−5 |
| Onion | 1.5 × 10−3 | 4.4 × 10−2 | 1.4 × 10−2 | 6.1 × 10−2 | 7.4 × 10−5 | 4.8 × 10−6 | 1.5 × 10−4 | 1.9 × 10−3 | 2.8 × 10−4 | 3.9 × 10−4 | 6.8 × 10−5 |
| Tomato | 3.2 × 10−4 | 9.9 × 10−2 | 4.3 × 10−2 | 7.0 × 10−2 | 3.4 × 10−4 | 1.4 × 10−5 | 1.4 × 10−4 | 2.4 × 10−3 | 1.1 × 10−4 | 1.8 × 10−3 | 3.6 × 10−5 |
| Sugar Beet | 4.0 × 10−4 | 1.3 × 10−1 | 2.5 × 10−2 | 6.3 × 10−2 | 3.6 × 10−4 | 4.4 × 10−6 | 2.1 × 10−4 | 1.7 × 10−3 | 4.4 × 10−4 | 1.2 × 10−3 | 9.0 × 10−5 |
| Green Chili | 4.7 × 10−4 | 7.4 × 10−2 | 2.5 × 10−2 | 5.6 × 10−2 | 1.1 × 10−3 | 3.7 × 10−6 | 1.6 × 10−4 | 4.7 × 10−3 | 2.3 × 10−4 | 3.2 × 10−3 | 3.5 × 10−5 |
| Dill | 2.3 × 10−3 | 4.3 × 10−1 | 3.1 × 10−2 | 1.9 × 10−1 | 1.5 × 10−4 | 2.3 × 10−5 | 3.0 × 10−4 | 6.2 × 10−3 | 3.8 × 10−4 | 1.4 × 10−3 | 1.9 × 10−4 |
| Corn | 3.7 × 10−4 | 4.1 × 10−2 | 7.7 × 10−3 | 8.4 × 10−2 | 9.8 × 10−5 | 5.7 × 10−6 | 9.1 × 10−5 | 1.8 × 10−3 | 6.1 × 10−5 | 3.1 × 10−5 | 3.5 × 10−5 |
| Spinach | 1.1 × 10−3 | 1.1 × 100 | 1.5 × 10−2 | 3.8 × 10−1 | 6.1 × 10−4 | 5.5 × 10−5 | 8.7 × 10−4 | 1.7 × 10−3 | 3.1 × 10−4 | 4.8 × 10−3 | 3.9 × 10−4 |
| White Eggplant | 3.4 × 10−4 | 7.5 × 10−2 | 4.0 × 10−2 | 7.5 × 10−2 | 1.6 × 10−4 | 4.2 × 10−6 | 1.0 × 10−4 | 4.5 × 10−3 | 9.2 × 10−4 | 2.2 × 10−3 | 5.0 × 10−5 |
| Kale | 1.6 × 10−3 | 2.2 × 10−1 | 8.9 × 10−3 | 8.1 × 10−2 | 1.4 × 10−4 | 3.7 × 10−5 | 5.6 × 10−4 | 1.8 × 10−3 | 1.9 × 10−4 | 5.1 × 10−4 | 1.0 × 10−4 |
| Green Bean | 5.6 × 10−4 | 7.8 × 10−2 | 3.0 × 10−2 | 1.0 × 10−1 | 3.2 × 10−4 | 9.5 × 10−6 | 1.1 × 10−4 | 1.0 × 10−2 | 4.1 × 10−5 | 6.9 × 10−5 | 4.5 × 10−5 |
| Capsicum | 5.3 × 10−4 | 6.3 × 10−2 | 2.2 × 10−2 | 5.6 × 10−2 | 4.9 × 10−4 | 8.0 × 10−6 | 1.3 × 10−4 | 3.5 × 10−3 | 1.3 × 10−4 | 2.0 × 10−3 | 3.8 × 10−5 |
| Cucumber | 1.0 × 10−3 | 5.2 × 10−2 | 2.3 × 10−2 | 7.4 × 10−2 | 3.3 × 10−4 | 1.1 × 10−5 | 1.7 × 10−4 | 3.5 × 10−3 | 2.1 × 10−3 | 2.2 × 10−4 | 1.0 × 10−4 |
| Min | 2.7 × 10−4 | 2.6 × 10−2 | 7.7 × 10−3 | 5.6 × 10−2 | 7.4 × 10−5 | 3.7 × 10−6 | 6.8 × 10−5 | 1.7 × 10−3 | 4.1 × 10−5 | 3.1 × 10−5 | 3.5 × 10−5 |
| Max | 2.3 × 10−3 | 1.1 × 100 | 4.3 × 10−2 | 3.8 × 10−1 | 1.1 × 10−3 | 5.5 × 10−5 | 8.7 × 10−4 | 1.0 × 10−2 | 2.1 × 10−3 | 4.8 × 10−3 | 3.9 × 10−4 |
| MTDI * | - | 2.5–3 | 60–65 | 5.0 × 10−2 | 4.0 × 10−2 | 0.035–0.2 | 0.1–0.3 | 1.3 × 10−1 | 0.02–0.07 | 2.1 × 10−1 |
| Se | Mn | Cu | Zn | Co | Hg | Cr | Ni | As | Cd | Pb | HI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potato | 0.05 | 0.19 | 0.66 | 0.19 | 0.77 | 0.08 | 0.02 | 0.13 | 0.16 | 1.06 | 0.01 | 3.33 |
| Onion | 0.30 | 0.31 | 0.35 | 0.20 | 0.25 | 0.05 | 0.05 | 0.10 | 0.95 | 0.39 | 0.02 | 2.96 |
| Tomato | 0.06 | 0.70 | 1.08 | 0.23 | 1.14 | 0.14 | 0.05 | 0.12 | 0.35 | 1.79 | 0.01 | 5.69 |
| Sugar Beet | 0.08 | 0.90 | 0.63 | 0.21 | 1.18 | 0.04 | 0.07 | 0.08 | 1.46 | 1.19 | 0.03 | 5.88 |
| Green Chili | 0.09 | 0.53 | 0.63 | 0.19 | 3.54 | 0.04 | 0.05 | 0.24 | 0.77 | 3.19 | 0.01 | 9.27 |
| Dill | 0.47 | 3.05 | 0.77 | 0.62 | 0.48 | 0.23 | 0.10 | 0.31 | 1.28 | 1.39 | 0.05 | 8.74 |
| Corn | 0.07 | 0.29 | 0.19 | 0.28 | 0.33 | 0.06 | 0.03 | 0.09 | 0.20 | 0.03 | 0.01 | 1.59 |
| Spinach | 0.22 | 7.51 | 0.38 | 1.28 | 2.03 | 0.55 | 0.29 | 0.08 | 1.04 | 4.79 | 0.11 | 18.3 |
| White Eggplant | 0.07 | 0.54 | 1.01 | 0.25 | 0.55 | 0.04 | 0.03 | 0.22 | 3.07 | 2.20 | 0.01 | 8.00 |
| Kale | 0.32 | 1.57 | 0.22 | 0.27 | 0.45 | 0.37 | 0.19 | 0.09 | 0.63 | 0.51 | 0.03 | 4.65 |
| Green Bean | 0.11 | 0.56 | 0.75 | 0.34 | 1.08 | 0.10 | 0.04 | 0.52 | 0.14 | 0.07 | 0.01 | 3.71 |
| Capsicum | 0.11 | 0.45 | 0.54 | 0.19 | 1.62 | 0.08 | 0.04 | 0.17 | 0.43 | 1.99 | 0.01 | 5.64 |
| Cucumber | 0.20 | 0.37 | 0.57 | 0.25 | 1.11 | 0.11 | 0.06 | 0.17 | 7.05 | 0.22 | 0.03 | 10.1 |
| Cr | As | Pb | Cd | Ni | |
|---|---|---|---|---|---|
| Potato | 3.4 × 10−5 | 7.3 × 10−5 | 3.7 × 10−7 | 4.0 × 10−4 | 4.4 × 10−3 |
| Onion | 7.7 × 10−5 | 4.3 × 10−4 | 5.8 × 10−7 | 1.5 × 10−4 | 3.3 × 10−3 |
| Tomato | 7.2 × 10−5 | 1.6 × 10−4 | 3.0 × 10−7 | 6.8 × 10−4 | 4.0 × 10−3 |
| Sugar Beet | 1.0 × 10−4 | 6.6 × 10−4 | 7.6 × 10−7 | 4.5 × 10−4 | 2.9 × 10−3 |
| Green Chili | 8.1 × 10−5 | 3.5 × 10−4 | 3.0 × 10−7 | 1.2 × 10−3 | 8.0 × 10−3 |
| Dill | 1.5 × 10−4 | 5.8 × 10−4 | 1.6 × 10−6 | 5.3 × 10−4 | 1.0 × 10−2 |
| Corn | 4.5 × 10−5 | 9.2 × 10−5 | 2.9 × 10−7 | 1.2 × 10−5 | 3.0 × 10−3 |
| Spinach | 4.3 × 10−4 | 4.7 × 10−4 | 3.3 × 10−6 | 1.8 × 10−3 | 2.8 × 10−3 |
| White Eggplant | 5.0 × 10−5 | 1.4 × 10−3 | 4.3 × 10−7 | 8.4 × 10−4 | 7.6 × 10−3 |
| Kale | 2.8 × 10−4 | 2.8 × 10−4 | 8.7 × 10−7 | 1.9 × 10−4 | 3.0 × 10−3 |
| Green Bean | 5.7 × 10−5 | 6.2 × 10−5 | 3.8 × 10−7 | 2.6 × 10−5 | 1.8 × 10−2 |
| Capsicum | 6.7 × 10−5 | 1.9 × 10−4 | 3.2 × 10−7 | 7.6 × 10−4 | 5.9 × 10−3 |
| Cucumber | 8.3 × 10−5 | 3.2 × 10−3 | 8.8 × 10−7 | 8.4 × 10−5 | 5.9 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, M.; Wang, Y.; Pierce, D.; Sens, D.A.; Somji, S.; Garrett, S.H. Concentration and Potential Non-Carcinogenic and Carcinogenic Health Risk Assessment of Metals in Locally Grown Vegetables. Foods 2025, 14, 2264. https://doi.org/10.3390/foods14132264
Saleem M, Wang Y, Pierce D, Sens DA, Somji S, Garrett SH. Concentration and Potential Non-Carcinogenic and Carcinogenic Health Risk Assessment of Metals in Locally Grown Vegetables. Foods. 2025; 14(13):2264. https://doi.org/10.3390/foods14132264
Chicago/Turabian StyleSaleem, Muhammad, Yuqiang Wang, David Pierce, Donald A. Sens, Seema Somji, and Scott H. Garrett. 2025. "Concentration and Potential Non-Carcinogenic and Carcinogenic Health Risk Assessment of Metals in Locally Grown Vegetables" Foods 14, no. 13: 2264. https://doi.org/10.3390/foods14132264
APA StyleSaleem, M., Wang, Y., Pierce, D., Sens, D. A., Somji, S., & Garrett, S. H. (2025). Concentration and Potential Non-Carcinogenic and Carcinogenic Health Risk Assessment of Metals in Locally Grown Vegetables. Foods, 14(13), 2264. https://doi.org/10.3390/foods14132264







