Long Shelf-Life Ready-to-Eat Plant-Based Whole Hard-Boiled Eggs: Low Allergenic and Regular Formulas
Abstract
1. Introduction
2. Materials and Methods
2.1. Ingredients and Formulations for the Plant-Based Egg
2.2. Development of Low Allergenic and Regular Formulations
2.3. Study of Preservation Methods for Shelf-Life Extension
2.4. Shelf Life Determination
2.5. Microbial Analysis
2.6. Lipid Oxidation Test
2.7. Firmness Measurement
2.8. Sensory Evaluation and Consumer Acceptance Testing
2.9. Statistical Analysis
3. Results
3.1. Physical and Color Properties of Plant-Based Whole Hard-Boiled Eggs Formulated with Different Proteins
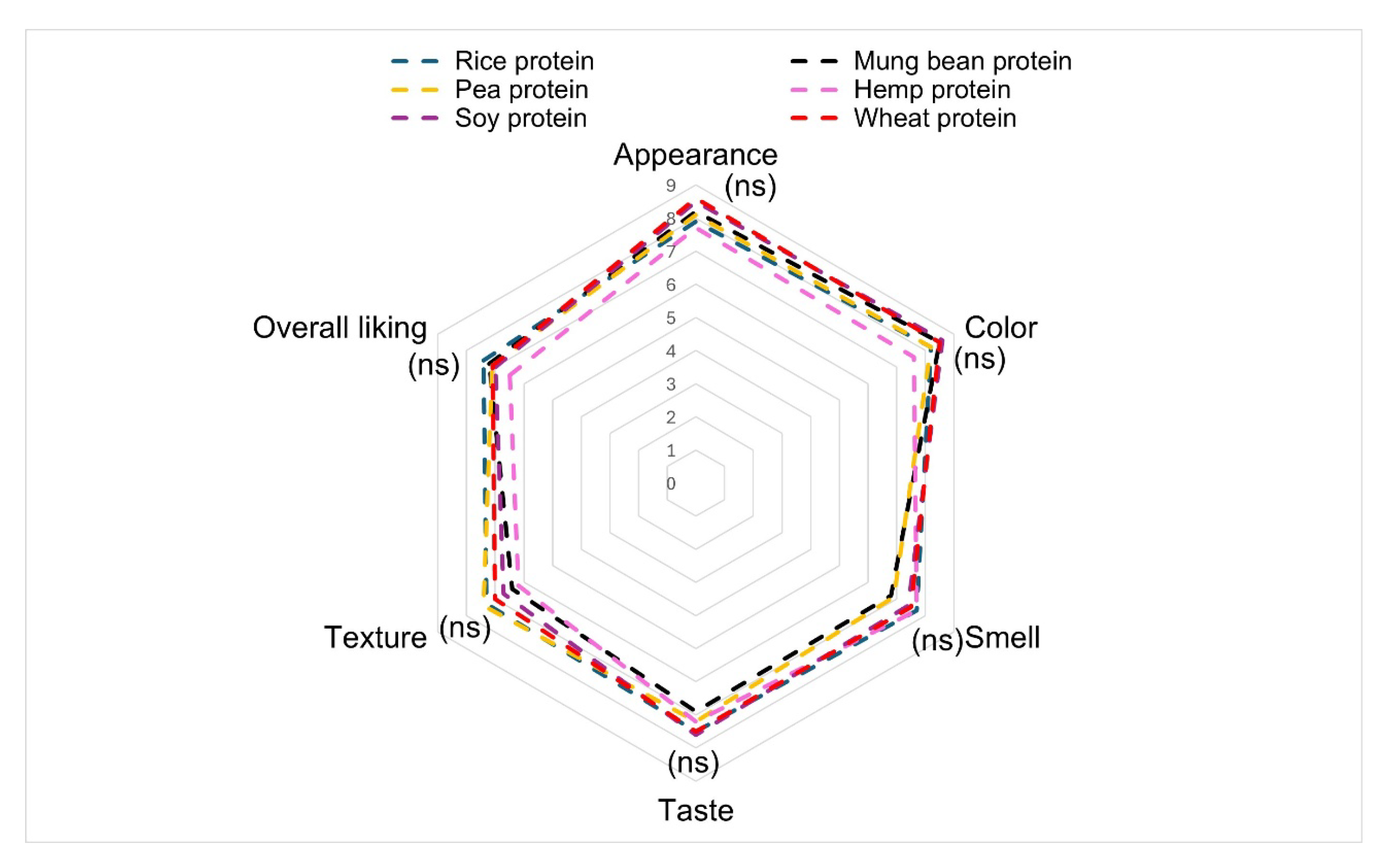
3.2. Sensory Test Results
3.3. Protein and Oil Contents
3.4. Preservation of Plant-Based Whole Hard-Boiled Eggs
3.4.1. Thermal Preservation: Optimization of Pasteurization Conditions
3.4.2. Gamma Irradiation Preservation: Optimization of Sterilization Conditions
3.5. The Shelf-Life Estimation
3.5.1. Shelf Life Estimation: Thermal Pasteurization:
3.5.2. Shelf Life Estimation: Gamma Ray Sterilization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, X.; Zou, P.-R.; Hu, F.; Zhu, W.; Wei, Z.-J. Updates on Plant-Based Protein Products as an Alternative to Animal Protein: Technology, Properties, and Their Health Benefits. Molecules 2023, 28, 4016. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F.; Gagaoua, M. Vegan Egg: A Future-Proof Food Ingredient? Foods 2022, 11, 161. [Google Scholar] [CrossRef]
- Alcorta, A.; Porta, A.; Tárrega, A.; Alvarez, M.D.; Vaquero, M.P. Foods for Plant-Based Diets: Challenges and Innovations. Foods 2021, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Gains, N.; Thi, X.-N.H. Do Plant-Based Food Products Communicate Sustainability? A Case Study of Brand Meanings in Thailand and Their Relationship to Sustainable Diets. NIDA Case Res. J. 2023, 15, 1. [Google Scholar] [CrossRef]
- Dang, T.D.; Peters, R.L.; Koplin, J.J.; Dharmage, S.C.; Gurrin, L.C.; Ponsonby, A.L.; Martino, D.J.; Neeland, M.; Tang, M.L.K.; Allen, K.J. Egg Allergen Specific IgE Diversity Predicts Resolution of Egg Allergy in the Population Cohort HealthNuts. Allergy 2019, 74, 318–326. [Google Scholar] [CrossRef]
- Gomes, A.; Sobral, P.J.D.A. Plant Protein-Based Delivery Systems: An Emerging Approach for Increasing the Efficacy of Lipophilic Bioactive Compounds. Molecules 2021, 27, 60. [Google Scholar] [CrossRef]
- Lei, Y.; Ma, L.; Ouyang, H.; Peng, W.; Xu, F.; Wang, P.; Jin, L.; Li, S. Influence of Soy Protein Isolate on the Gel Properties of Walnut Protein Isolate-κ-Carrageenan Treated with NaCl. J. Future Foods 2023, 3, 364–373. [Google Scholar] [CrossRef]
- Lu, Z.; Lee, P.-R.; Yang, H. Chickpea Flour and Soy Protein Isolate Interacted with κ-Carrageenan via Electrostatic Interactions to Form Egg Omelets Analogue. Food Hydrocoll. 2022, 130, 107691. [Google Scholar] [CrossRef]
- Lu, Z.; Lee, P.-R.; Yang, H. Synergistic Adsorption of Surface-Active Components at the Air-Liquid Interface Improves Foaming Properties of Plant-Based Egg Analogues. Food Hydrocoll. 2023, 137, 108414. [Google Scholar] [CrossRef]
- Hu, X.; Meng, Z. Flourless Plant-Based Egg Analogue Based on Protein and Curdlan: Thermogel Behavior Regulation and Foam Stabilization Analysis. Food Hydrocoll. 2024, 156, 110346. [Google Scholar] [CrossRef]
- Grochowicz, J.; Fabisiak, A.; Ekielski, A. Importance of Physical and Functional Properties of Foods Targeted to Seniors. J. Future Foods 2021, 1, 146–155. [Google Scholar] [CrossRef]
- WATTPoultry.com. Plant-Based, Hard-Boiled, RTE Eggs to Hit the Market. 2024. Available online: https://www.wattagnet.com/egg/article/15534095/plant-based-hard-boiled-rte-eggs-to-hit-the-market (accessed on 12 February 2025).
- Puangwerakul, Y.; Soithongsuk, S. Innovation for Plant-Based Foods: Allergen-Free Vegan Meat and Egg Products from Rice Processing By-Products. J. Curr. Sci. Technol. 2022, 12, 2. [Google Scholar]
- Puangwerakul, Y.; Soithongsuk, S.; Sajjabut, S.; Pewlong, W. Effect of Electron Beam Irradiation Doses on Quality and Shelf Life Extension of Non-Allergenic Ready-to-Eat Plant-Based Meat and Egg. J. Curr. Sci. Technol. 2024, 14, 1. [Google Scholar] [CrossRef]
- Lee, S.-Y. IgE Mediated Food Allergy in Korean Children: Focused on Plant Food Allergy. Asia Pac. Allergy 2013, 3, 15–22. [Google Scholar] [CrossRef]
- Leech, S.C.; Ewan, P.W.; Skypala, I.J.; Brathwaite, N.; Erlewyn-Lajeunesse, M.; Heath, S.; Ball, H.; James, P.; Murphy, K.; Clark, A.T. BSACI 2021 Guideline for the Management of Egg Allergy. Clin. Exp. Allergy 2021, 51, 1262–1278. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Y.; Fan, M.; Qian, H.; Wang, L. Recent Advances in Mung Bean Protein: From Structure, Function to Application. Int. J. Biol. Macromol. 2024, 273, 133210. [Google Scholar] [CrossRef]
- Gouda, M.; Bekhit, A. Allergenicity Risks Associated with Novel Proteins and Rapid Methods of Detection. In Novel Proteins: Food Allergy Safety Assessment; CRC Press: Boca Raton, FL, USA, 2022; pp. 379–406. [Google Scholar] [CrossRef]
- Mamone, G.; Picariello, G.; Ramondo, A.; Nicolai, M.A.; Ferranti, P. Production, Digestibility and Allergenicity of Hemp (Cannabis sativa L.) Protein Isolates. Food Res. Int. 2019, 115, 562–571. [Google Scholar] [CrossRef]
- Wiederstein, M.; Baumgartner, S.; Lauter, K. Soybean (Glycine max) Allergens—A Review on an Outstanding Plant Food with Allergenic Potential. ACS Food Sci. Technol. 2023, 3, 363–378. [Google Scholar] [CrossRef]
- Abi-Melhem, R.; Hassoun, Y. Is Pea Our Hidden Allergen? An American Pediatric Case Series. J. Allergy Clin. Immunol. Glob. 2023, 2, 100090. [Google Scholar] [CrossRef]
- Goel, S.; Singh, M.; Grewal, S.; Razzaq, A.; Wani, S.H. Wheat Proteins: A Valuable Resource to Improve Nutritional Value of Bread. Front. Sustain. Food Syst. 2021, 5, 769681. [Google Scholar] [CrossRef]
- Khule, G.D.; Ranvare, A.R.; Singh, A.; Suresh, B.C. Texture Profile Analysis: A Comprehensive Insight into Food Texture Evaluation. J. Dyn. Control. 2024, 8, 30–45. [Google Scholar] [CrossRef]
- Loikaeo, T. Quality Characteristics of Healthy Bread Produced from Germinated Brown Rice, Germinated Mung Bean, and Germinated White Kidney Bean. J. Curr. Sci. Technol. 2024, 14, 1. [Google Scholar] [CrossRef]
- Puangwerakul, Y.; Chaisakdanukul, C.; Soithongsuk, S.; Sajjaut, S.; Pewlong, W. Comparative Effects of HPP and Irradiation on Plant-Based Whole Hard-Boiled Eggs. J. Curr. Sci. Technol. 2024, 14, 3. [Google Scholar] [CrossRef]
- Limsitthichaikoon, S.; Kuljanabhagavad, T.; Vutthipong, A.; Panidthananon, W.; Thongphasuk, P. Consequences of Gamma Irradiation on Triphala’s Phytochemical Compositions, Microbial Burden and Antioxidant Properties. J. Curr. Sci. Technol. 2024, 14, 2. [Google Scholar] [CrossRef]
- Manzocco, L.; Calligaris, S.; Nicoli, M.C. Methods for Food Shelf Life Determination and Prediction. In Oxidation in Foods and Beverages and Antioxidant Applications; Decker, E.A., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2010; pp. 196–222. [Google Scholar] [CrossRef]
- Haouet, M.N.; Tommasino, M.; Mercuri, M.L.; Benedetti, F.; Bella, S.D.; Framboas, M.; Pelli, S.; Altissimi, M.S. Experimental Accelerated Shelf Life Determination of a Ready-to-Eat Processed Food. Ital. J. Food Saf. 2019, 7, 6919. [Google Scholar] [CrossRef]
- Abeyrathne, N.; Nam, K.; Ahn, D.U. Analytical Methods for Lipid Oxidation and Antioxidant Capacity in Food Systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef]
- Lawless, H.; Heymann, H. Sensory Evaluation of Food: Principles and Practices, 2nd ed.; Springer: Ithaca, NY, USA, 2010; Chapter 1. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Wang, X. Effects of Bioactive Compounds and Pharmacological Activities in Medicinal Fruits and Vegetables by Thermal Processing. J. Future Foods 2023, 3, 252–262. [Google Scholar] [CrossRef]
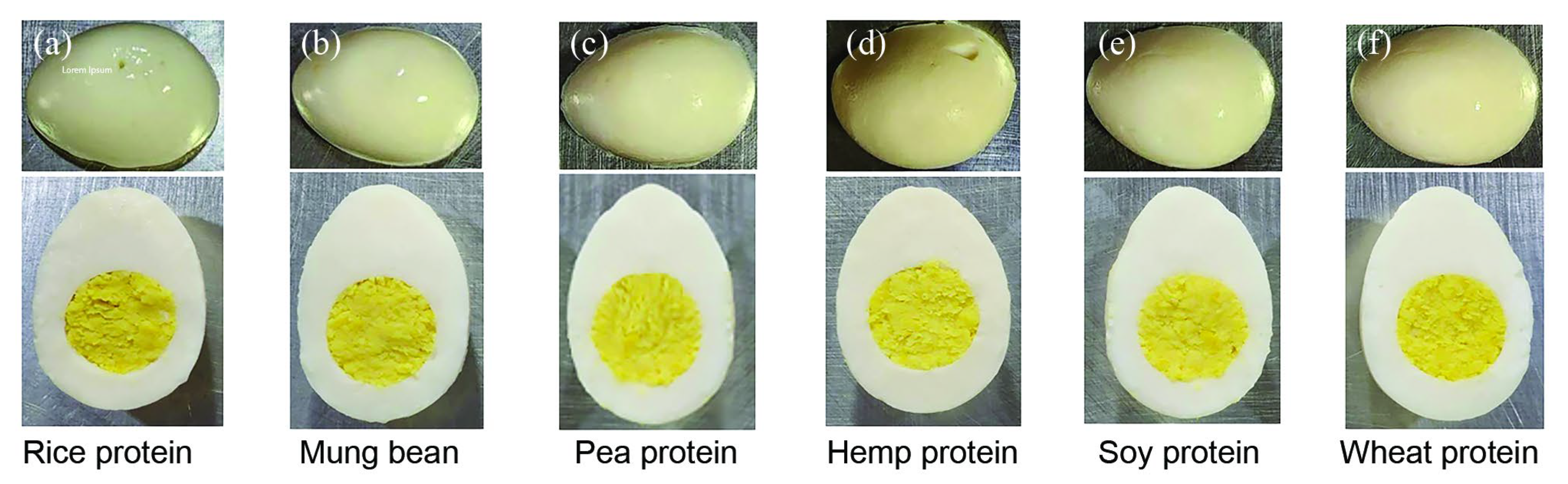


| Properties | Rice Protein | Ratio of Rice Protein: Mung Bean Protein | ||||
|---|---|---|---|---|---|---|
| Mung Bean Protein 0:100 | Pea Protein 50:50 | Hemp Protein 70:30 | Soy Protein 0:100 | Wheat Protein 0:100 | ||
| Physical properties | ||||||
| Texture firmness (mm) | 139.8 ± 1.6 bc | 143 ± 1.5 bc | 144.0 ± 1.0 b | 145.0 ± 3.5 b | 159.0 ± 2.0 a | 125.4 ± 3.0 c |
| L* | 73.0 ± 0.5 a | 75.7 ± 0.3 a | 71.6 ± 1.3 a | 68.2 ± 1.5 b | 82.1 ± 1.5 a | 71.6 ± 1.6 a |
| a* | 10.8 ± 1.5 a | 9.1 ± 1.5 a | 11.0 ± 0.1 a | 8.2 ± 0.9 a | 9.1 ± 1.0 a | 11.0 ± 1.4 a |
| b* | 11.7 ± 1.5 c | 11.5 ± 1.5 c | 11.6 ± 0.5 c | 22.4 ± 2.2 ab | 15.6 ± 1.2 b | 29.2 ± 1.0 a |
| Sensory Test | Rice Protein | Ratio of Rice Protein: Mung Bean Protein | ||||
|---|---|---|---|---|---|---|
| Mung Bean Protein 0:100 | Pea Protein 50:50 | Hemp Protein 70:30 | Soy Protein 0:100 | Wheat Protein 0:100 | ||
| appearance | 7.9 ± 0.6 a | 8.2 ± 0.4 a | 8.1 ± 0.2 a | 7.7 ± 0.2 a | 8.5 ± 0.2 a | 8.6 ± 0.4 a |
| color | 8.2 ± 0.3 a | 8.5 ± 0.2 a | 8.2 ± 0.4 a | 7.6 ± 0.3 a | 8.6 ± 0.4 a | 8.5 ± 0.3 a |
| smell | 7.7 ± 0.8 a | 6.8 ± 0.4 a | 6.9 ± 0.4 a | 7.7 ± 1.2 a | 7.4 ± 0.7 a | 7.5 ± 0.5 a |
| taste | 7.5 ± 0.4 a | 6.9 ± 0.6 a | 7.2 ± 0.3 a | 7.2 ± 0.3 a | 7.6 ± 0.3 a | 7.5 ± 0.2 a |
| texture | 7.3 ± 0.4 a | 6.4 ± 0.5 bc | 7.4 ± 1.2 a | 6.2 ± 0.5 c | 6.7 ± 0.2 b | 7.0 ± 0.5 ab |
| Overall liking | 7.4 ± 0.2 a | 7.2 ± 0.3 a | 7.1 ± 1.3 a | 6.5 ± 0.3 a | 7.0 ± 0.6 a | 7.1 ± 0.3 a |
| Contents | |||||||
|---|---|---|---|---|---|---|---|
| Rice Protein | Mung Bean | Pea Protein | Hemp Protein | Soy Protein | Wheat Protein | Limitation | |
| Total protein (g/egg) | 7.38 ± 1.34 a | 7.08 ± 0.94 a | 7.23 ± 1.15 a | 6.77 ± 0.93 a | 7.35 ± 1.22 a | 7.08 ± 1.24 a | 6–7 g/egg |
| Oil content (g/egg) | 1.92 ± 0.44 a | 1.29 ± 0.35 b | 1.98 ± 0.36 a | 0.36 ± 0.13 d | 0.36 ± 0.11 d | 0.72 ± 0.10 c | <1.5 g/egg |
| Quality | Before Heating | Temperature (°C) | Criteria | |||||
|---|---|---|---|---|---|---|---|---|
| 75 | 80 | 85 | ||||||
| Physical | 15 min | 20 min | 15 min | 20 min | 15 min | 20 min | ||
| Firmness(mm) | 143.0 ± 1.5 | 143.0 ± 1.0 a | 143.9 ± 1.3 a | 144.1 ± 1.1 a | 144.6 ± 1.0 a | 144.9 ± 1.7 a | 145.0 ± 1.8 a | Small different from before heating |
| Color L* | 75.7 ± 0.3 | 75.7 ± 3.8 a | 75.6 ± 3.6 a | 74.5 ± 3.7 a | 74.7 ± 3.5 a | 74.5 ± 5.4 a | 74.0 ± 5.6 a | |
| a* | 9.1 ± 1.5 | 9.2 ± 1.6 a | 9.3 ± 1.4 a | 9.4 ± 0.9 a | 9.5 ± 0.4 a | 9.6 ± 0.5 a | 9.7 ± 0.8 a | |
| b* | 11.5 ± 1.5 | 11.5 ± 1.3 a | 11.8 ± 1.4 a | 12.0 ± 1.8 a | 12.8 ± 1.2 a | 13.2 ± 1.1 a | 13.6 ± 1.3 a | |
| Sensory | ||||||||
| Appearance | 8.2 ± 0.2 a | 8.0 ± 0.0 a | 8.0 ± 0.0 a | 8.0 ± 0.0 a | 8.0 ± 0.0 a | 8.0 ± 0.0 a | >6 (9 Scale) | |
| Color | 8.0 ± 0.0 a | 8.0 ± 0.0 a | 8.0 ± 0.0 a | 8.0 ± 0.0 a | 8.0 ± 0.0 a | 8.0 ± 0.0 a | >6 (9 Scale) | |
| Smell | 7.4 ± 0.5 a | 7.3 ± 0.8 a | 7.2 ± 0.6 a | 7.1 ± 0.7 a | 7.2 ± 0.6 a | 7.2 ± 0.6 a | >6 (9 Scale) | |
| Taste | 7.0 ± 0.5 a | 6.9 ± 0.6 a | 7.0 ± 0.0 a | 6.7 ± 0.6 a | 6.9 ± 0.6 a | 6.6 ± 0.5 a | >6 (9 Scale) | |
| Texture | 7.0 ± 0.0 a | 6.9 ± 0.1 a | 6.5 ± 0.2 b | 6.4 ± 0.2 b | 6.1 ± 0.1 bc | 6.0 ± 0.0 c | >6 (9 Scale) | |
| Overall liking | 7.0 ± 0.5 a | 6.9 ± 0.6 a | 7.0 ± 0.0 a | 6.9 ± 0.5 a | 7.0 ± 0.0 a | 6.8 ± 0.4 a | >6 (9 Scale) | |
| Microorganism (CFU/g) | ||||||||
| Total aerobic bacteria | 0 | 0 | 0 | 0 | 0 | 0 | Not detected | |
| Yeast & Mold | 0 | 0 | 0 | 0 | 0 | 0 | Not detected | |
| E. coil | 0 | 0 | 0 | 0 | 0 | 0 | Not detected | |
| Clostridium perfringens | 0 | 0 | 0 | 0 | 0 | 0 | <100 CFU/g | |
| Staphylococcus aureus | 0 | 0 | 0 | 0 | 0 | 0 | Not found in 0.1 g | |
| Salmonella spp. | 0 | 0 | 0 | 0 | 0 | 0 | Not found in 25 g | |
| Bacillus cereus | 0 | 0 | 0 | 0 | 0 | 0 | <100 CFU/g | |
| Quality | Temperature (°C) | Criteria | |||||
|---|---|---|---|---|---|---|---|
| 75 | 80 | 85 | |||||
| Physical | 15 min | 20 min | 15 min | 20 min | 15 min | 20 min | |
| Firmness(mm) | 127.2 ± 2.2 d | 133.1 ± 1.1 c | 134.1 ± 2.4 c | 138.2 ± 5.0 bc | 143.1 ± 1.7 b | 153.5 ± 1.5 a | 125.4 ± 3.0 |
| Color L* | 73.5 ± 1.3 a | 73.0 ± 1.0 a | 72.5 ± 1.4 a | 73.2 ± 1.0 a | 72.1 ± 2.5 a | 72.0 ± 2.0 a | 71.6 ± 1.6 |
| a* | 10.8 ± 1.7 a | 11.0 ± 1.5 a | 11.2 ± 1.1 a | 11.0 ± 0.4 a | 11.9 ± 1.2 a | 11.1 ± 1.5 a | 11.0 ± 1.4 |
| b* | 28.1 ± 1.4 a | 27.8 ± 1.2 a | 28.8 ± 1.6 a | 28.8 ± 1.5 a | 29.3 ± 1.4 a | 29.2 ± 1.2 a | 29.2 ± 1.0 |
| Sensory | |||||||
| Appearance | 8.0 ± 0.0 a | 8.2 ± 0.4 a | 8.0 ± 0.0 a | 8.0 ± 0.0 a | 8.0 ± 0.0 a | 8.0 ± 0.0 a | >6 (9 Scale) |
| Color | 8.0 ± 0.0 a | 8.0 ± 0.0 a | 8.0 ± 0.0 a | 8.0 ± 0.0 a | 8.0 ± 0.0 a | 8.0 ± 0.0 a | >6 (9 Scale) |
| Smell | 7.0 ± 0.0 a | 7.3 ± 0.8 a | 7.2 ± 0.6 a | 7.1 ± 0.7 a | 7.2 ± 0.6 a | 7.2 ± 0.6 a | >6 (9 Scale) |
| Taste | 7.0 ± 0.0 a | 6.9 ± 0.6 a | 7.0 ± 0.0 a | 6.7 ± 0.6 a | 6.9 ± 0.6 a | 6.6 ± 0.5 a | >6 (9 Scale) |
| Texture | 7.0 ± 0.0 ab | 6.7 ± 0.5 a | 6.4 ± 0.2 ab | 6.1 ± 0.1 b | 6.1 ± 0.1 b | 6.0 ± 0.0 b | >6 (9 Scale) |
| Overall liking | 7.0 ± 0.0 a | 7.0 ± 0.0 a | 6.9 ± 0.5 a | 6.9 ± 0.5 a | 7.0 ± 0.0 a | 6.8 ± 0.4 a | >6 (9 Scale) |
| Microorganism (CFU/g) | |||||||
| Total aerobic bacteria | 0 | 0 | 0 | 0 | 0 | 0 | Not detected |
| Yeast & Mold | 0 | 0 | 0 | 0 | 0 | 0 | Not detected |
| E. coil | 0 | 0 | 0 | 0 | 0 | 0 | Not detected |
| Clostridium perfringens | 0 | 0 | 0 | 0 | 0 | 0 | <100 CFU/g |
| Staphylococcus aureus | 0 | 0 | 0 | 0 | 0 | 0 | Not found in 0.1 g |
| Salmonella spp. | 0 | 0 | 0 | 0 | 0 | 0 | Not found in 25 g |
| Bacillus cereus | 0 | 0 | 0 | 0 | 0 | 0 | <100 CFU/g |
| Quality | Level (KGy) | Criteria | |||
|---|---|---|---|---|---|
| 0 | 2 | 3.5 | 5 | ||
| Physical | |||||
| Firmness (mm) | 143.0 ± 1.5 a | 142.0 ± 2.0 a | 144.7 ± 1.7 a | 145.0 ± 1.5 a | 143.0 ± 1.5 |
| Color L* | 75.7 ± 3.8 a | 75.5 ± 2.5 a | 75.0 ± 1.6 a | 72.0 ± 2.0 a | 75.7 ± 0.3 |
| a* | 9.2 ± 1.6 a | 9.2 ± 1.8 a | 9.2 ± 1.0 a | 9.3 ± 1.5 a | 9.1 ± 1.5 |
| b* | 12.0 ± 2.3 a | 12.6 ± 2.1 a | 12.3 ± 2.5 a | 12.8 ± 2.4 a | 11.5 ± 1.5 |
| Sensory | |||||
| Appearance | 8.0 ± 0.0 a | 6.3 ± 0.5 b | 6.2 ± 0.4 b | 6.4 ± 0.5 b | >6 (9 Scale) |
| Color | 8.0 ± 0.0 a | 6.5 ± 0.5 b | 6.5 ± 0.5 b | 6.4 ± 0.5 b | >6 (9 Scale) |
| Smell | 8.0 ± 0.0 a | 6.5 ± 0.5 b | 6.5 ± 0.5 b | 5.4 ± 0.7 c | >6 (9 Scale) |
| Taste | 8.0 ± 0.5 a | 6.9 ± 0.3 b | 6.9 ± 0.6 b | 5.3 ± 0.5 c | >6 (9 Scale) |
| Texture | 8.0 ± 0.0 a | 7.0 ± 0.0 b | 6.8 ± 0.4 b | 5.2 ± 0.6 c | >6 (9 Scale) |
| Overall liking | 8.5 ± 0.5 a | 6.6 ± 0.5 b | 6.7 ± 0.4 b | 5.3 ± 0.6 c | >6 (9 Scale) |
| Microorganism (CFU/g) | |||||
| Total aerobic bacteria | 1.23 × 104 ± 0.36 a | 2.8 × 102 ± 0.31 b | 0 | 0 | Not detected |
| Yeast & Mold | 106 ± 8.7 a | 10 ± 2.6 b | 0 | 0 | Not detected |
| E. coil | 0 | 0 | 0 | 0 | Not detected |
| Clostridium perfringens | 4.3 ± 2.5 a | 0 b | 0 | 0 | <100 CFU/g |
| Staphylococcus aureus | 0 | 0 | 0 | 0 | Not found in 0.1 g |
| Salmonella spp. | 0 | 0 | 0 | 0 | Not found in 25 g |
| Bacillus cereus | 0 | 0 | 0 | 0 | <100 CFU/g |
| Quality | Level (KGy) | Criteria | |||
|---|---|---|---|---|---|
| 0 | 2 | 3.5 | 5 | ||
| Physical | |||||
| Firmness (mm) | 125.1 ± 1.8 a | 126.8 ± 0.8 a | 127.1 ± 0.6 a | 128.3 ± 1.1 a | 125.4 ± 3.0 |
| Color L* | 72.5 ± 0.6 a | 72.0 ± 0.8 a | 71.8 ± 0.5 a | 71.5 ± 0.6 a | 71.6 ± 1.6 |
| a* | 11.8 ± 1.0 a | 11.8 ± 0.8 a | 12.0 ± 0.7 a | 12.3 ± 0.5 a | 11.0 ± 1.4 |
| b* | 29.6 ± 0.5 a | 29.8 ± 0.4 a | 30.1 ± 0.7 a | 30.0 ± 1.1 a | 29.2 ± 1.0 |
| Sensory | |||||
| Appearance | 8.0 ± 0.0 a | 6.5 ± 0.5 b | 6.8 ± 0.4 b | 6.3 ± 0.4 b | >6 (9 Scale) |
| Color | 8.0 ± 0.0 a | 6.5 ± 0.5 b | 6.1 ± 07 b | 6.4 ± 0.5 b | >6 (9 Scale) |
| Smell | 7.0 ± 0.0 a | 6.4 ± 0.5 b | 6.0 ± 0.6 b | 5.4 ± 0.5 c | >6 (9 Scale) |
| Taste | 7.0 ± 0.0 a | 6.3 ± 0.4 b | 6.2 ± 0.8 b | 5.3 ± 0.5 c | >6 (9 Scale) |
| Texture | 7.0 ± 0.0 a | 6.8 ± 0.4 b | 6.2 ± 0.4 b | 5.2 ± 0.4 c | >6 (9 Scale) |
| Overall liking | 7.0 ± 0.0 a | 6.5 ± 0.5 b | 6.3 ± 0.4 b | 5.3 ± 0.5 c | >6 (9 Scale) |
| Microorganism (CFU/g) | |||||
| Total aerobic bacteria | 1.36 × 104 ± 4.0 a | 3.3 × 102 ± 0.40 b | 0 c | 0 c | Not detected |
| Yeast & Mold | 139 ± 6.6 a | 11.0 ± 3.1 b | 0 c | 0 c | Not detected |
| E. coil | 0 a | 0 a | 0 a | 0 a | Not detected |
| Clostridium perfringens | 6.3 ± 1.5 a | 2.3 ± 2.0 b | 0 b | 0 b | <100 CFU/g |
| Staphylococcus aureus | 0 a | 0 a | 0 a | 0 a | Not found in 0.1 g |
| Salmonella spp. | 0 a | 0 a | 0 a | 0 a | Not found in 25 g |
| Bacillus cereus | 0 a | 0 a | 0 a | 0 a | <100 CFU/g |
| Day | Temperature 45 °C | Temperature 55 °C | ||
|---|---|---|---|---|
| Total Microbial Count (CFU/g) | TBA (mmol/kg) | Total Microbial Count (CFU/g) | TBA (mmol/kg) | |
| 0 | 0 b | 28.15 ± 0.50 c | 0 a | 28.15 ± 0.50 c |
| 7 | 0 b | 28.10 ± 0.28 c | 0 a | 29.84 ± 0.50 c |
| 14 | 0 b | 28.17 ± 0.30 c | 0 a | 30.02 ± 0.50 c |
| 21 | 0 b | 28.42 ± 0.20 c | 0 a | 30.12 ± 0.36 c |
| 28 | 0 b | 28.86 ± 0.25 c | 0 a | 32.28 ± 0.20 b |
| 35 | 0 b | 29.00 ± 0.50 bc | 0 a | 32.46 ± 0.24 b |
| 42 | 0 b | 29.03 ± 0.55 bc | 0 a | 32.77 ± 0.25 b |
| 49 | 0 b | 29.46 ± 0.20 bc | 0 a | 35.18 ± 0.45 a |
| 56 | 0 b | 30.11 ± 0.20 bc | 0 a | 35.26 ± 0.40 a |
| 63 | 0 b | 30.42 ± 0.25 b | 0 a | 36.20 ± 0.55 a |
| 70 | 0 b | 30.76 ± 0.10 b | 0 a | 36.46 ± 0.20 a |
| 77 | 0 b | 30.82 ± 0.15 b | 0 a | 36.68 ± 0.46 a |
| 84 | 0.66 ± 0.58 a | 32.47 ± 0.10 a | 0 a | 36.92 ± 0.50 a |
| 91 | 1.66 ± 0.58 a | 32.90 ± 0.15 a | 0 a | 37.10 ± 0.26 a |
| Day | Temperature 40 °C | Temperature 50 °C | ||
|---|---|---|---|---|
| Total Microbial Count (CFU/g) | TBA (mmol/kg) | Total Microbial Count (CFU/g) | TBA (mmol/kg) | |
| 0 | 0 b | 35.02 ± 5.50 ᵈ | 0 a | 35.02 ± 5.50 c |
| 9 | 0 b | 35.04 ± 5.20 ᵈ | 0 a | 36.10 ± 5.00 c |
| 18 | 0 b | 35.56 ± 5.30 ᵈ | 0 a | 35.35 ± 5.50c |
| 27 | 0 b | 34.82 ± 5.20 ᵈ | 0 a | 43.66 ± 5.20 b |
| 36 | 0 b | 36.04 ± 5.50 ᵈ | 0 a | 42.74 ± 5.50 b |
| 45 | 0 b | 38.42 ± 5.50 cᵈ | 0 a | 42.65 ± 5.40 b |
| 54 | 0 b | 39.10 ± 5.60 cᵈ | 0 a | 64.06 ± 5.50 a |
| 63 | 0 b | 41.54 ± 5.20 cᵈ | 0 a | 64.52 ± 5.30 a |
| 72 | 0 b | 45.27 ± 5.60 c | 0 a | 65.85 ± 5.20 a |
| 81 | 0 b | 46.45 ± 5.30 c | 0 a | 65.30 ± 5.60 a |
| 90 | 0 b | 46.05 ± 5.10 c | 0 a | 65.20 ± 5.20 a |
| 99 | 0 b | 53.62 ± 5.00 bc | 0 a | 66.65 ± 5.60 a |
| 108 | 0 b | 53.07 ± 5.20 bc | 0 a | 66.32 ± 5.50 a |
| 117 | 6.6 ± 2.0 a | 67.12 ± 5.00 b | 0 a | 66.50 ± 5.50 a |
| 126 | 21.0 ± 3.6 ᵃ | 78.06 ± 5.50 ᵃ | 0 ᵃ | 66.65 ± 5.00 ᵃ |
| Store temperature °C | Prediction Shelf Life (days) | |
|---|---|---|
| Thermal Pasteurization | Gamma Ray Sterilization | |
| 5 | 715 | |
| 10 | 547 | |
| 15 | 419 | |
| 20 | 320 | 545 |
| 25 | 245 | 371 |
| 30 | 188 | 253 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongwailikhit, K.; Soithongsuk, S.; Puangwerakul, Y. Long Shelf-Life Ready-to-Eat Plant-Based Whole Hard-Boiled Eggs: Low Allergenic and Regular Formulas. Foods 2025, 14, 2220. https://doi.org/10.3390/foods14132220
Wongwailikhit K, Soithongsuk S, Puangwerakul Y. Long Shelf-Life Ready-to-Eat Plant-Based Whole Hard-Boiled Eggs: Low Allergenic and Regular Formulas. Foods. 2025; 14(13):2220. https://doi.org/10.3390/foods14132220
Chicago/Turabian StyleWongwailikhit, Kanda, Suvimol Soithongsuk, and Yupakanit Puangwerakul. 2025. "Long Shelf-Life Ready-to-Eat Plant-Based Whole Hard-Boiled Eggs: Low Allergenic and Regular Formulas" Foods 14, no. 13: 2220. https://doi.org/10.3390/foods14132220
APA StyleWongwailikhit, K., Soithongsuk, S., & Puangwerakul, Y. (2025). Long Shelf-Life Ready-to-Eat Plant-Based Whole Hard-Boiled Eggs: Low Allergenic and Regular Formulas. Foods, 14(13), 2220. https://doi.org/10.3390/foods14132220







