Widely Targeted Metabolomics Reveals the Quality Characteristics of a New Tea Cultivar, ‘Baiyun 0495’
Abstract
1. Introduction
2. Materials and Methods
2.1. Tea Samples and Chemicals
2.2. Sensory Evaluation and Quantitative Descriptive Analysis (QDA)
2.3. Sample Preparation and Extraction
2.4. UPLC and ESL-Q TRAPMS/MS Conditions
- Column: An Agilent SB-C18 column with a particle size of 1.8 µm, an inner diameter of 2.1 mm, and a length of 100 mm was utilized.
- Mobile Phase: Solvent A was pure water containing 0.1% formic acid, while solvent B was acetonitrile with 0.1% formic acid.
- Gradient Program: The analysis started with an initial mobile-phase composition of 95% A and 5% B. Over a period of 9 min, a linear gradient was applied to reach a composition of 5% A and 95% B. This 5% A and 95% B composition was maintained for 1 min. Subsequently, within 1.1 min, the composition was adjusted back to 95% A and 5.0% B, which was then held for 2.9 min.
- Flow Rate: The flow velocity of the mobile phase was set at 0.35 mL per minute.
- Column Temperature: The column oven was maintained at 40 °C.
- Injection Volume: A volume of 2 μL of the sample was injected into the system.
2.5. Statistical Analysis
3. Results and Discussion
3.1. Sensory Evaluation Results of White Tea
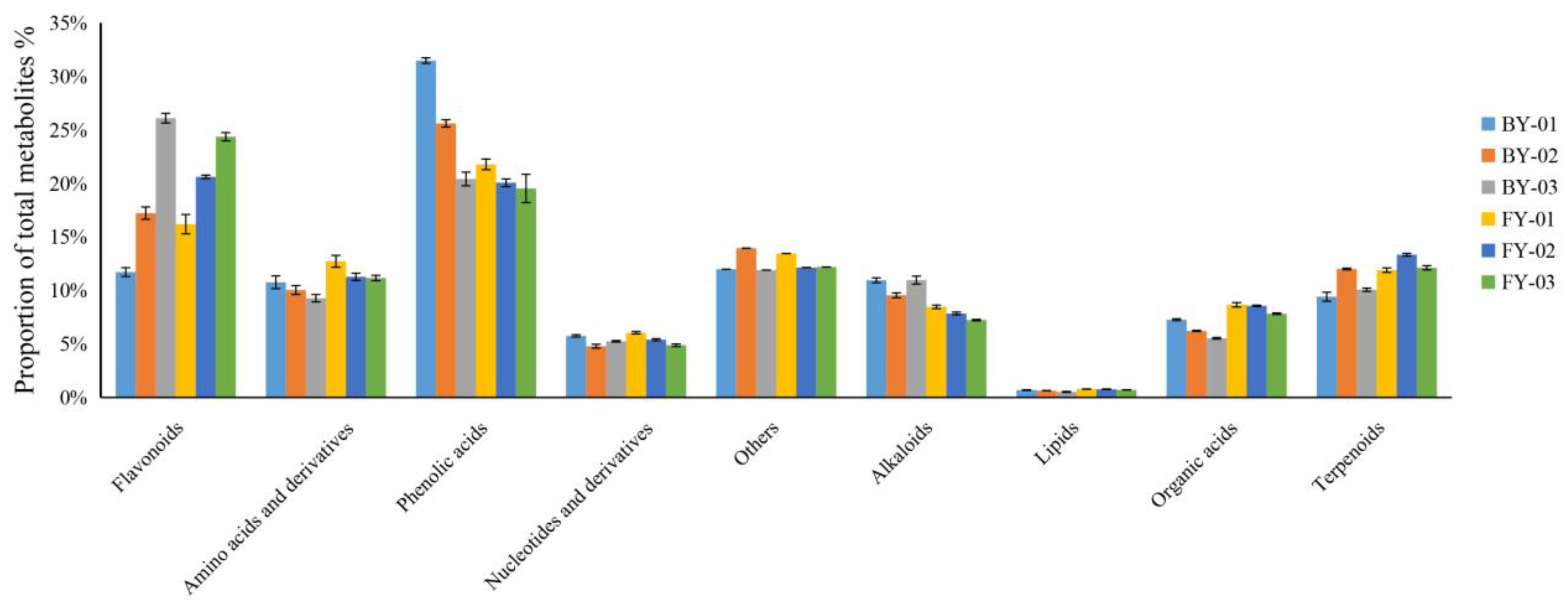
3.2. Widely Targeted Metabolomics Analysis of Baiyun 0492 and Fuyun 6 White Tea
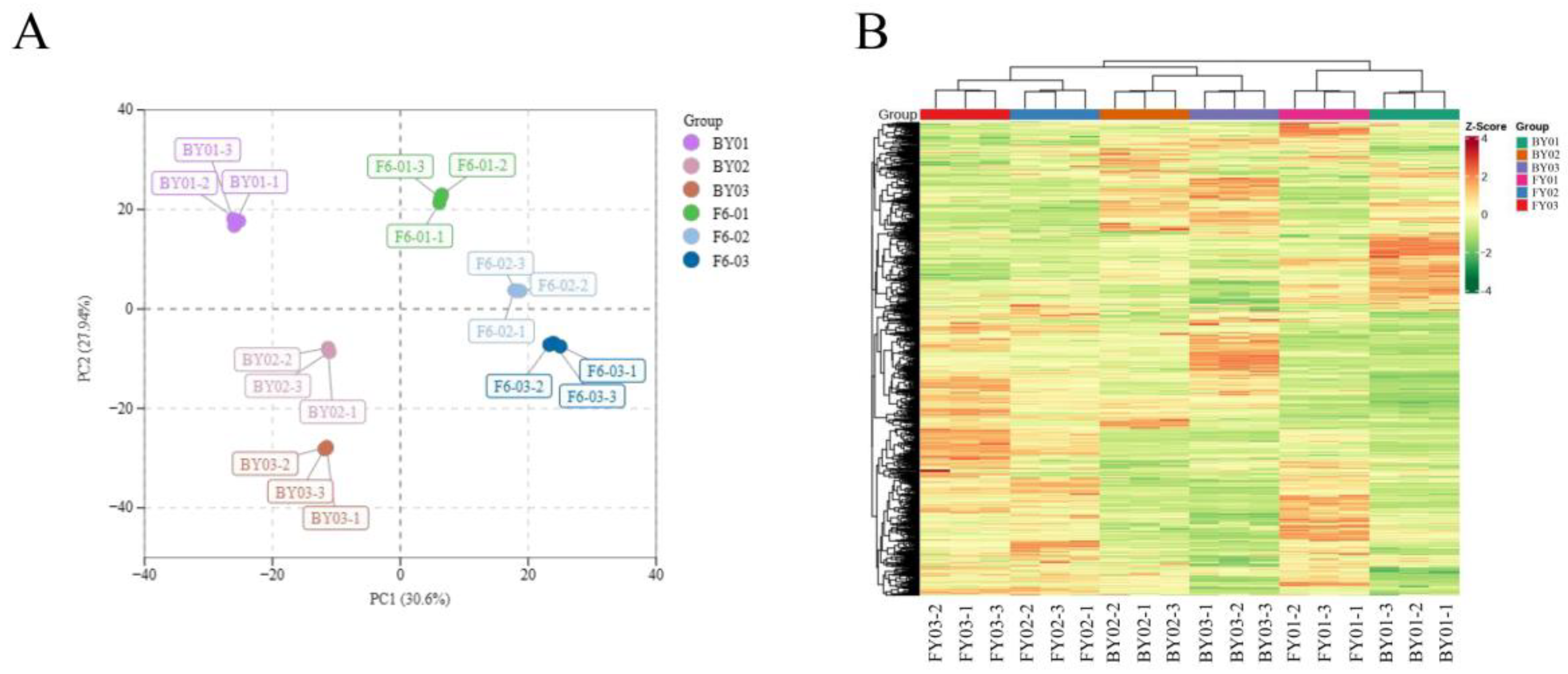
3.3. PCA and OPLS-DA Between Baiyun 0492 White Tea and Fuyun 6 White Tea
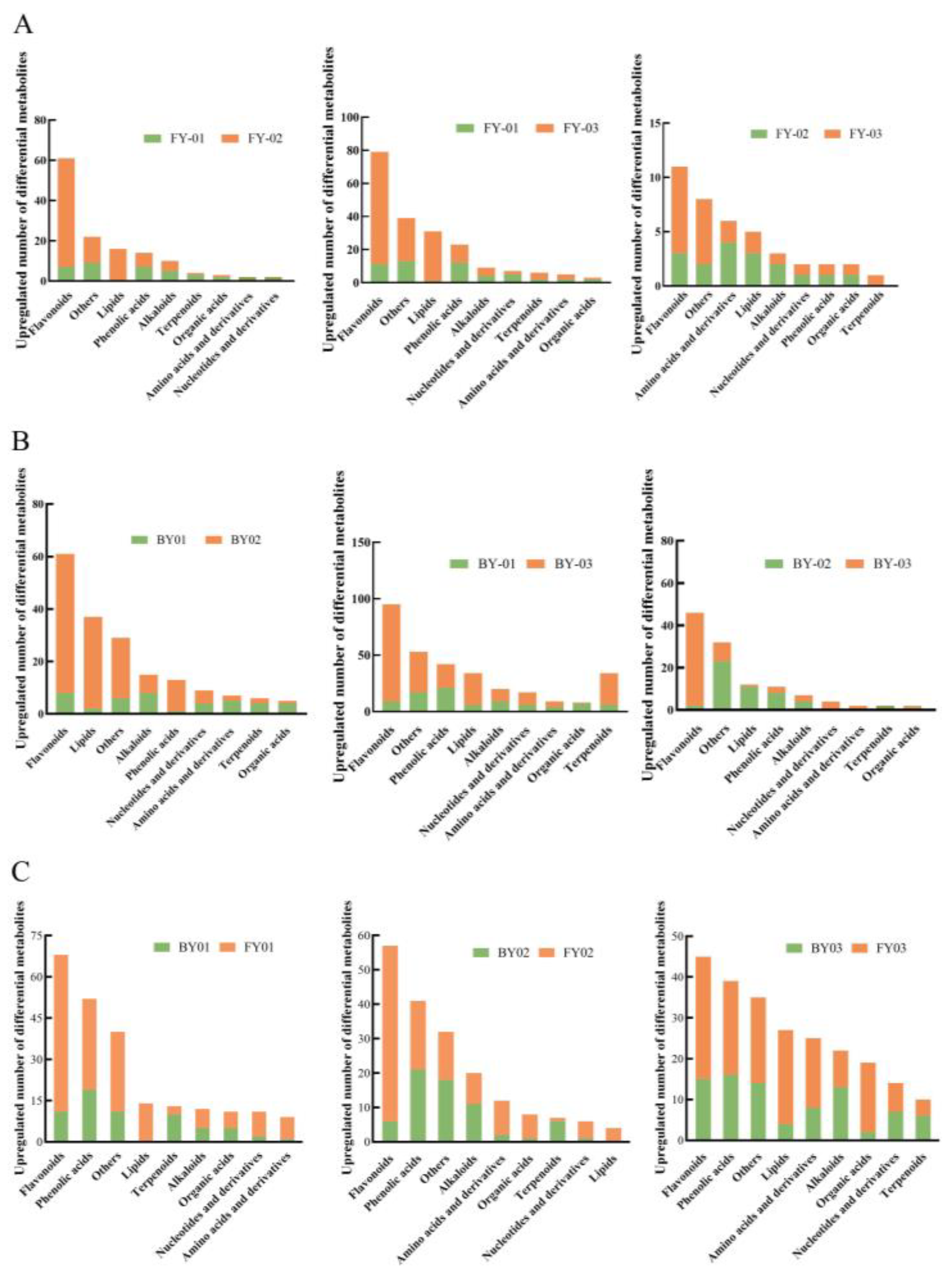
3.4. Analysis of Different Metabolites of Different Varieties of White Tea
3.4.1. Screening of Differentially Abundant Metabolites
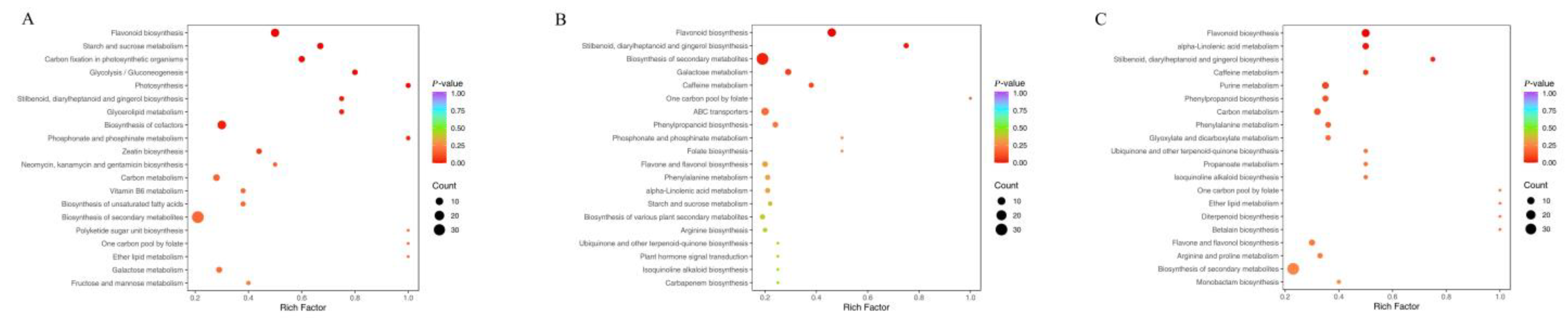
3.4.2. Functional Annotation and Enrichment Analysis of Differentially Abundant KEGG Metabolites in Various White Tea Cultivars
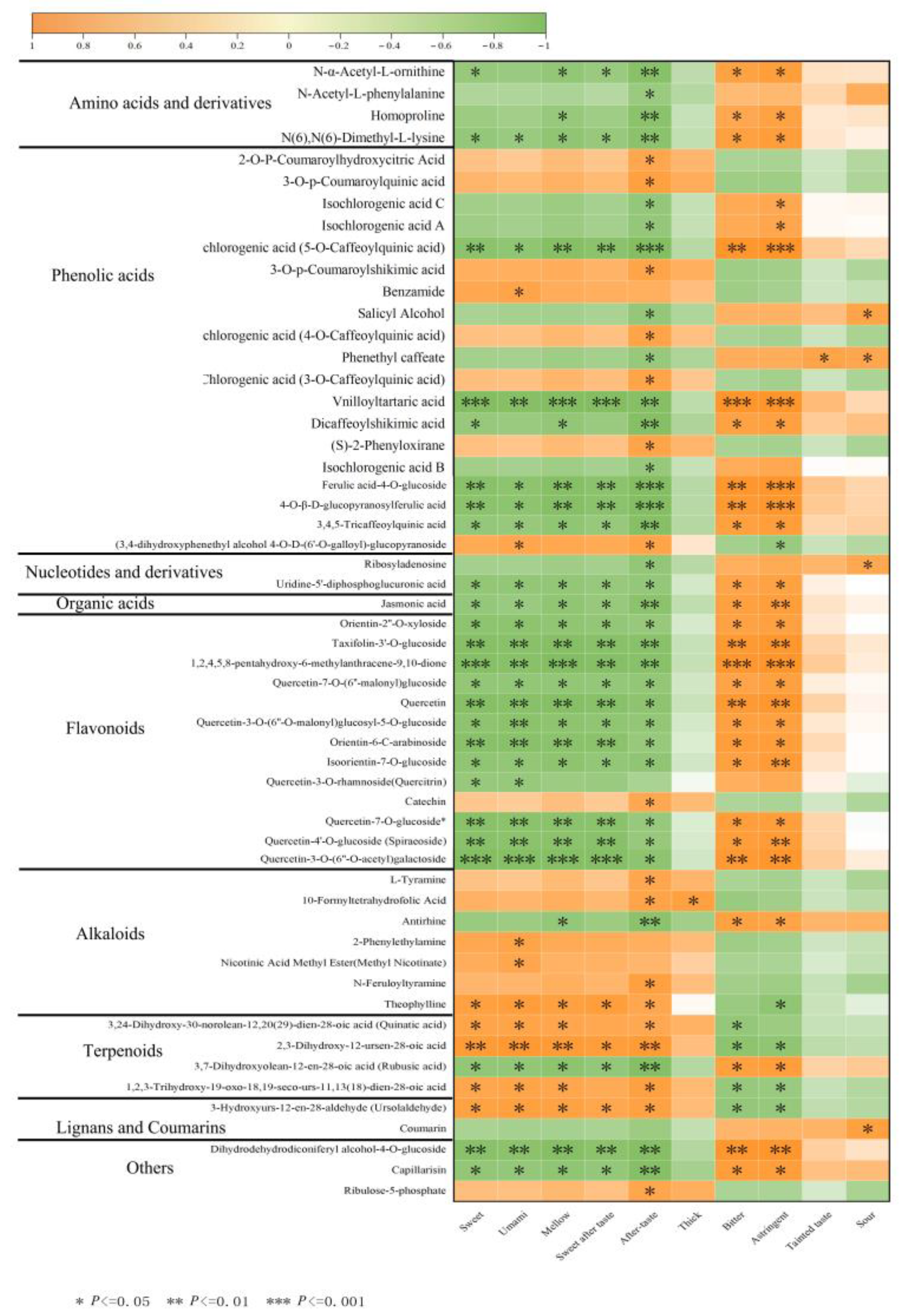
3.5. Correlation Analysis of Differentially Abundant Metabolites and Quality of Baiyun 0492 White Tea
3.5.1. Correlation Analysis of Different Metabolites and Quality of BY01 and FY01
3.5.2. Correlation Analysis of Different Metabolites and Quality of BY02 and FY02
3.5.3. Correlation Analysis of Different Metabolites and Quality of BY03 and FY03
3.5.4. Correlation Analysis of Shared Metabolites and Quality Traits Between Baiyun 0492 and Fuyun 6
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, F.-Y.; Huang, C.-S.; Tong, Y.-L.; Guo, H.-W.; Zhou, S.-J.; Ye, J.-H.; Gong, S.-Y. Widely targeted metabolomics analysis of white peony teas with different storage time and association with sensory attributes. Food Chem. 2021, 362, 130257. [Google Scholar] [CrossRef]
- Sanlier, N.; Atik, İ.; Atik, A. A minireview of effects of white tea consumption on diseases. Trends Food Sci. Technol. 2018, 82, 82–88. [Google Scholar] [CrossRef]
- Yang, C.; Hu, Z.; Lu, M.; Li, P.; Tan, J.; Chen, M.; Lv, H.; Zhu, Y.; Zhang, Y.; Guo, L. Application of metabolomics profiling in the analysis of metabolites and taste quality in different subtypes of white tea. Food Res. Int. 2018, 106, 909–919. [Google Scholar] [CrossRef]
- Ma, C.; Ma, B.; Wang, J.; Wang, Z.; Chen, X.; Zhou, B.; Li, X. Geographical origin identification of Chinese white teas, and their differences in tastes, chemical compositions and antioxidant activities among three production regions. Food Chem. X 2022, 16, 100504. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.-M.; Ding, D.; Song, Y.-S.; Zhang, Z.-Z.; Luo, X.; Wan, X.-C. Chemical constituents analysis of white tea of different qualities and different storage times. Eur. Food Res. Technol. 2016, 242, 2093–2104. [Google Scholar] [CrossRef]
- Tan, J.; Engelhardt, U.H.; Lin, Z.; Kaiser, N.; Maiwald, B. Flavonoids, phenolic acids, alkaloids and theanine in different types of authentic Chinese white tea samples. J. Food Compos. Anal. 2017, 57, 8–15. [Google Scholar] [CrossRef]
- GB/T 23,776-2018; Method for Sensory Evaluation of Tea. Standards Press of China: Beijing, China, 2018.
- Cao, Q.-Q.; Wang, F.; Wang, J.-Q.; Chen, J.-X.; Yin, J.-F.; Li, L.; Meng, F.-K.; Cheng, Y.; Xu, Y.-Q. Effects of brewing water on the sensory attributes and physicochemical properties of tea infusions. Food Chem. 2021, 364, 130235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, Q.-Q.; Granato, D.; Xu, Y.-Q.; Ho, C.-T. Association between chemistry and taste of tea: A review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Scharbert, S.; Holzmann, N.; Hofmann, T. Identification of the astringent taste compounds in black tea infusions by combining instrumental analysis and human bioresponse. J. Agric. Food Chem. 2004, 52, 3498–3508. [Google Scholar] [CrossRef]
- Matsui, T.; Ebuchi, S.; Fujise, T.; Abesundara, K.J.; Doi, S.; Yamada, H.; Matsumoto, K. Strong antihyperglycemic effects of water-soluble fraction of Brazilian propolis and its bioactive constituent, 3, 4, 5-tri-O-caffeoylquinic acid. Biol. Pharm. Bull. 2004, 27, 1797–1803. [Google Scholar] [CrossRef]
- Lee, C.S.; Lee, S.A.; Kim, Y.J.; Seo, S.J.; Lee, M.W. 3, 4, 5-Tricaffeoylquinic acid inhibits tumor necrosis factor-α-stimulated production of inflammatory mediators in keratinocytes via suppression of Akt-and NF-κB-pathways. Int. Immunopharmacol. 2011, 11, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Davies, J.; Doldán, N.G.; Arao, S.; Ferdousi, F.; Szele, F.G.; Isoda, H. 3, 4, 5-Tricaffeoylquinic acid induces adult neurogenesis and improves deficit of learning and memory in aging model senescence-accelerated prone 8 mice. Aging 2019, 11, 401. [Google Scholar] [CrossRef]
- Ou, S.; Kwok, K.C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Tang, H. Effects of ferulic acid and isoferulic acid on proliferation of HepG2 cells and its cytochrome P450 enzymes. Chin. Tradit. Herb. Drugs 2014, 24, 1726–1730. [Google Scholar]
- Li, C.; Zong, B.; Guo, H.; Luo, Z.; He, P.; Gong, S.; Fan, F. Discrimination of white teas produced from fresh leaves with different maturity by near-infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117697. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, A.; Bandyopadhyay, S.K. Seasonal variation of L-Theanine content in tea: A study on Darjeeling Black Tea. Int. J. Sci. Res. Sci. Eng. Technol. 2016, 2, 522–524. [Google Scholar]
- Turkmen, N.; Velioglu, Y.S. Determination of alkaloids and phenolic compounds in black tea processed by two different methods in different plucking seasons. J. Sci. Food Agric. 2007, 87, 1408–1416. [Google Scholar] [CrossRef]
- Yu, P.; Huang, H.; Zhao, X.; Zhong, N.; Zheng, H.; Gong, Y. Distinct variation in taste quality of Congou black tea during a single spring season. Food Sci. Nutr. 2020, 8, 1848–1856. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, Y.; Ma, L.; Yi, X.; Ruan, J. Metabolomic analysis using ultra-performance liquid chromatography-quadrupole-time of flight mass spectrometry (UPLC-Q-TOF MS) uncovers the effects of light intensity and temperature under shading treatments on the metabolites in tea. PLoS ONE 2014, 9, e112572. [Google Scholar] [CrossRef] [PubMed]
- Guadagni, D.G.; Maier, V.P.; Turnbaugh, J.G. Effect of some citrus juice constituents on taste thresholds for limonin and naringin bitterness. J. Sci. Food Agric. 1973, 24, 1277–1288. [Google Scholar] [CrossRef]
- Xu, G.; Liu, D.; Chen, J.; Ye, X.; Ma, Y.; Shi, J. Juice components and antioxidant capacity of citrus varieties cultivated in China. Food Chem. 2008, 106, 545–551. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Arts, I.C.W. Flavonols, flavones and flavanols–nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1081–1093. [Google Scholar] [CrossRef]
- Lee, V.S.; Dou, J.; Chen, R.J.; Lin, R.-S.; Lee, M.-R.; Tzen, J.T. Massive accumulation of gallic acid and unique occurrence of myricetin, quercetin, and kaempferol in preparing old oolong tea. J. Agric. Food Chem. 2008, 56, 7950–7956. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Lv, H.; Zhang, X.; Chen, Z.; Shi, J.; Lu, M.; Lin, Z. Identification of regioisomers of methylated kaempferol and quercetin by ultra high performance liquid chromatography quadrupole time-of-flight (UHPLC–QTOF) tandem mass spectrometry combined with diagnostic fragmentation pattern analysis. Anal. Chim. Acta 2013, 795, 15–24. [Google Scholar] [CrossRef]
- Pastoriza, S.; Mesías, M.; Cabrera, C.; Rufián-Henares, J. Healthy properties of green and white teas: An update. Food Funct. 2017, 8, 2650–2662. [Google Scholar]
- Deng, X.; Shang, H.; Chen, J.; Wu, J.; Wang, T.; Wang, Y.; Zhu, C.; Sun, W. Metabolomics combined with proteomics provide a novel interpretation of the changes in flavonoid glycosides during white tea processing. Foods 2022, 11, 1226. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, S.; Li, Y.; Liu, M.; Ni, K.; Yi, X.; Shi, Y.; Ma, L.; Willmitzer, L.; Ruan, J. Characterization of three different classes of non-fermented teas using untargeted metabolomics. Food Res. Int. 2019, 121, 697–704. [Google Scholar] [CrossRef]
- Purpura, M.; Jäger, R.; Falk, M. An assessment of mutagenicity, genotoxicity, acute-, subacute and subchronic oral toxicity of paraxanthine (1, 7-dimethylxanthine). Food Chem. Toxicol. 2021, 158, 112579. [Google Scholar] [CrossRef]
- Scharbert, S.; Hofmann, T. Molecular definition of black tea taste by means of quantitative studies, taste reconstitution, and omission experiments. J. Agric. Food Chem. 2005, 53, 5377–5384. [Google Scholar] [CrossRef]
- Zhu, M.; Li, N.; Zhou, F.; Ouyang, J.; Lu, D.-M.; Xu, W.; Li, J.; Lin, H.-Y.; Zhang, Z.; Xiao, J.-B. Microbial bioconversion of the chemical components in dark tea. Food Chem. 2020, 312, 126043. [Google Scholar] [CrossRef]
- Yuasa, M.; Koe, M.; Maeda, A.; Eguchi, A.; Abe, H.; Tominaga, M. Characterization of flavor component in Japanese instant soup stocks ‘dashi’. Int. J. Gastron. Food Sci. 2017, 9, 55–61. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Yue, C.-N.; Tong, H.-R. Analysis of taste characteristics identification of key chemical components of fifteen Chinese yellow tea samples, J. Food Sci. Technol. 2021, 58, 1378–1388. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, R.; Shi, W.; Li, L.; Liu, H.; Chen, Z.; Wu, L. Metabolism and pharmacological activities of the natural health-benefiting compound diosmin. Food Funct. 2020, 11, 8472–8492. [Google Scholar] [CrossRef]
- Xu, K.; Tian, C.; Zhou, C.; Zhu, C.; Weng, J.; Sun, Y.; Lin, Y.; Lai, Z.; Guo, Y. Non-targeted metabolomics analysis revealed the characteristic non-volatile and volatile metabolites in the Rougui Wuyi rock tea (Camellia sinensis) from different culturing regions. Foods 2022, 11, 1694. [Google Scholar] [CrossRef] [PubMed]
- Wüpper, S.; Lüersen, K.; Rimbach, G. Chemical composition, bioactivity and safety aspects of Kuding tea—From beverage to herbal extract. Nutrients 2020, 12, 2796. [Google Scholar] [CrossRef]
- Fan, F.-Y.; Zhou, S.-J.; Qian, H.; Zong, B.-Z.; Huang, C.-S.; Zhu, R.-L.; Guo, H.-W.; Gong, S.-Y. Effect of Yellowing duration on the chemical profile of yellow tea and the associations with sensory traits. Molecules 2022, 27, 940. [Google Scholar] [CrossRef] [PubMed]
- Pękal, A.; Biesaga, M.; Pyrzynska, K. Trace metals and flavonoids in different types of tea. Food Sci. Biotechnol. 2013, 22, 925–930. [Google Scholar] [CrossRef]
- Wang, H.; Hua, J.; Yu, Q.; Li, J.; Wang, J.; Deng, Y.; Yuan, H.; Jiang, Y. Widely targeted metabolomic analysis reveals dynamic changes in non-volatile and volatile metabolites during green tea processing. Food Chem. 2021, 363, 130131. [Google Scholar] [CrossRef]
- Zhao, M.; Su, X.Q.; Nian, B.; Chen, L.J.; Zhang, D.L.; Duan, S.M.; Wang, L.Y.; Shi, X.Y.; Jiang, B.; Jiang, W.W.; et al. Integrated Meta-omics Approaches to Understand the Microbiome of Spontaneous Fermentation of Traditional Chinese Pu-erh Tea. mSystems 2019, 4, 00680–19. [Google Scholar] [CrossRef]
- He, Q.; Yang, K.; Wu, X.; Zhang, C.; He, C.; Xiao, P. Phenolic compounds, antioxidant activity and sensory evaluation of sea buckthorn (Hippophae rhamnoides L.) leaf tea. Food Sci. Nutr. 2023, 11, 1212–1222. [Google Scholar] [CrossRef]
- Deng, X.; He, S.; Han, Y.; Chen, Y. Metabolites profiling reveals the dynamic changes of non-volatiles in Pu-erh during Ganpu tea processing. Food Chem. X 2023, 19, 100774. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-R.; Yukio, K.; Kim, K.M.; Lee, C.-H. Tastes and structures of bitter peptide, asparagine-alanine-leucine-proline-glutamate, and its synthetic analogues. J. Agric. Food Chem. 2008, 56, 5852–5858. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jin, Y.; Jiang, R.; Xu, Y.; Zhang, Y.; Luo, Y.; Huang, J.; Wang, K.; Liu, Z. Dynamic changes in the metabolite profile and taste characteristics of Fu brick tea during the manufacturing process. Food Chem. 2021, 344, 128576. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Liu, M.; Shi, Y.; De Vos, R.C.; Ruan, J. Stimulated biosynthesis of delphinidin-related anthocyanins in tea shoots reducing the quality of green tea in summer. J. Sci. Food Agric. 2020, 100, 1505–1514. [Google Scholar] [CrossRef]
- Narukawa, M.; Kimata, H.; Noga, C.; Watanabe, T. Taste characterisation of green tea catechins. Int. J. Food Sci. Technol. 2010, 45, 1579–1585. [Google Scholar] [CrossRef]
- Yu, P.; Yeo, A.S.-L.; Low, M.-Y.; Zhou, W. Identifying key non-volatile compounds in ready-to-drink green tea and their impact on taste profile. Food Chem. 2014, 155, 9–16. [Google Scholar] [CrossRef]
- Zhu, K.; Zeng, X.; Tan, F.; Li, W.; Li, C.; Song, Y.; Zhao, X. Effect of insect tea on D-galactose-induced oxidation in mice and its mechanisms. Food Sci. Nutr. 2019, 7, 4105–4115. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xu, X.; Zou, X. Biotransformation of caffeine in oolong tea by Paecilomyces gunnii. Int. Biodeterior. Biodegrad. 2016, 114, 141–144. [Google Scholar] [CrossRef]
- Bonvehi, J.S.; Coll, F.V. Evaluation of bitterness and astringency of polyphenolic compounds in cocoa powder. Food Chem. 1997, 60, 365–370. [Google Scholar] [CrossRef]
- Zhou, C.-Z.; Chen, Z.; Li, X.-Z.; Lan, C.; Xie, S.-Y.; Chen, G.-W.; Zhang, H.; Lai, Z.-X.; Lin, Y.-L.; Guo, Y.-Q. Transcriptome and phytochemical analyses reveal the roles of characteristic metabolites in the taste formation of white tea during the withering process. J. Integr. Agric. 2022, 21, 862–877. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Y.; Peng, A.; Cui, J.; Zhao, M.; Pan, Y.; Zhang, M.; Tian, K.; Schwab, W.; Song, C. Single-cell transcriptome atlas reveals developmental trajectories and a novel metabolic pathway of catechin esters in tea leaves. Plant Biotechnol. J. 2022, 20, 2089–2106. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Yang, C.; Zhang, X.; Wang, Y.; Li, Z.; Chen, Y.; Liu, Z.; Zhu, M.; Xiao, Y. Effects of solid-state fermentation with Bacillus subtilis LK-1 on the volatile profile catechins composition antioxidant activity of dark teas. Food Chem. X 2023, 19, 100811. [Google Scholar] [CrossRef] [PubMed]

| Attribute | Definition | Reference |
|---|---|---|
| Sweet | Intensity of sweet taste | Sucrose solution (0.15 mg/mL = 1; 4 mg/mL = 10) |
| Bitter | Intensity of bitter taste | Caffeine solution (0.05 mg/mL = 1; 0.8 mg/mL = 10) |
| Umami | Intensity of umami taste | Glutamic acid solution (0.04 mg/mL = 1; 0.32 mg/mL = 10) |
| Sour | Intensity of sour taste | Citric acid solution (0.15 mg/mL = 1; 0.8 mg/mL = 10) |
| Astringent | Intensity of astringent taste | Alum solution (0.1 mg/mL = 1; 0.24 mg/mL = 10) |
| Sweet aftertaste | After drinking tea, the root of the tongue and throat have a sweet feeling, and have a feeling of moisture. | Liquorice solution |
| Mellow | Shade is moderate, soft palate. | Mixed solution of pectin and sucrose |
| Aftertaste | The taste felt in the throat of the mouth after drinking tea | |
| Thick | The contents are rich and sticky. | |
| Tainted taste | It seems to be colloidal suspension or to contain impurities |
| Compounds | Amount | BY01 | BY02 | BY03 | F6-01 | F6-02 | F6-03 |
|---|---|---|---|---|---|---|---|
| Flavonoids | 178 | 176 (Isoorientin-7-O-glucoside) (Quercetin-3-O-(6”-O-malonyl) glucosyl-5-O-glucoside) | 176 (Isoorientin-7-O-glucoside) (Vitexin-7-O-(6”-feruloyl) glucoside-4’-O-glucoside) | 176(Vitexin-7-O-(6”-feruloyl) glucoside-4’-O-glucoside) (Kaempferol-3-O-[2-O-(trans-p-coumaroyl)-3-O-α-D-glucopyranosyl]-α-D-glucopyranoside) | 176(Luteolin-7-O-rutinoside) (Brassicin) | 178 | 178 |
| Amino acids and derivatives | 119 | 119 | 119 | 119 | 119 | 119 | 119 |
| Phenolic acids | 191 | 189 (3,4,5-Tricaffeoylquinic acid) (3-(3-Hydroxyphenyl)-propionic acid) | 189(3,4,5-Tricaffeoylquinic acid) (3-(3-Hydroxyphenyl)-propionic acid) | 190 (3,4,5-Tricaffeoylquinic acid) | 190 ((S)-2-Phenyloxirane) | 190 (3,6-Di-O-caffeoyl glucose) | 190 ((S)-2-Phenyloxirane) |
| Nucleotides and derivatives | 60 | 60 | 59(5-Methyluridine) | 59 (5-Methyluridine) | 60 | 60 | 59(Hypoxanthine) |
| Quinones | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
| Lignans and Coumarins | 31 | 31 | 31 | 31 | 31 | 31 | 31 |
| Others | 119 | 117 (Vitamin K2) (Trans-dehydrorosinone) | 119 | 119 | 117(Vitamin K2)) (Trans-dehydrorosinone) | 118(D-Fructose) | 119 |
| Tannins | 27 | 27 | 27 | 27 | 27 | 27 | 27 |
| Alkaloids | 90 | 90 | 89 (Dihydrocaffeoylputrescine) | 89 (Dihydrocaffeoylputrescine) | 87(Cinnamoyltyramine) (N-Feruloyltyramine) (N-(4-hydroxyphenethyl) cinnamamide) | 89 (N-Feruloyltyramine) | 87 (Cinnamoyltyramine)(N-Feruloyltyramine) (N-(4-hydroxyphenethyl)cinnamamide) |
| Terpenoids | 28 | 27(Pimaric acid) | 28 | 27 (Pimaric acid) | 27(8-Epiloganic acid) | 26 (2,3-Dihydroxy-12-ursen-28-oic acid) (Quinatic acid) | 26 (2,3-Dihydroxy-12-ursen-28-oic acid) (Quinatic acid) |
| Organic acids | 90 | 90 | 90 | 90 | 90 | 90 | 90 |
| Lipids | 136 | 134 (LysoPE 16:3) (10,16-Dihydroxypalmitic acid) | 136 | 136 | 136 | 136 | 136 |
| Total | 1083 | 1074 | 1078 | 1077 | 1074 | 1077 | 1076 |
| Sample | R2x | R2y | Q2 |
|---|---|---|---|
| FY01 vs. FY02 | 0.616 | 1 | 0.972 |
| FY01 vs. FY03 | 0.650 | 1 | 0.976 |
| FY02 vs. FY03 | 0.525 | 1 | 0.939 |
| BY01 vs. BY02 | 0.763 | 1 | 0.983 |
| BY01 vs. BY03 | 0.718 | 1 | 0.987 |
| BY02 vs. BY03 | 0.617 | 0.999 | 0.972 |
| BY01 vs. FY01 | 0.683 | 1 | 0.982 |
| BY02 vs. FY02 | 0.647 | 0.999 | 0.976 |
| BY03 vs. FY03 | 0.692 | 0.999 | 0.984 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Liang, Z.; Zhang, L.; Chen, Z.; Zhao, Y.; Chen, Q.; Ye, N.; Yang, R. Widely Targeted Metabolomics Reveals the Quality Characteristics of a New Tea Cultivar, ‘Baiyun 0495’. Foods 2025, 14, 2206. https://doi.org/10.3390/foods14132206
Yu Y, Liang Z, Zhang L, Chen Z, Zhao Y, Chen Q, Ye N, Yang R. Widely Targeted Metabolomics Reveals the Quality Characteristics of a New Tea Cultivar, ‘Baiyun 0495’. Foods. 2025; 14(13):2206. https://doi.org/10.3390/foods14132206
Chicago/Turabian StyleYu, Ying, Zijun Liang, Lei Zhang, Zhizhi Chen, Yixuan Zhao, Qiang Chen, Naixing Ye, and Ruxing Yang. 2025. "Widely Targeted Metabolomics Reveals the Quality Characteristics of a New Tea Cultivar, ‘Baiyun 0495’" Foods 14, no. 13: 2206. https://doi.org/10.3390/foods14132206
APA StyleYu, Y., Liang, Z., Zhang, L., Chen, Z., Zhao, Y., Chen, Q., Ye, N., & Yang, R. (2025). Widely Targeted Metabolomics Reveals the Quality Characteristics of a New Tea Cultivar, ‘Baiyun 0495’. Foods, 14(13), 2206. https://doi.org/10.3390/foods14132206






