Bio-Packaging Based on Pectin/Tragacanth Gum with Added Extracts of Cherry Waste from the Wine Industry as a New Generation of Active Films for the Food Industry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extracts Preparation
2.3. Preparation of Films
2.4. Evaluation of the Bioactive Potential of Extracts and Films
2.5. Total Polyphenols Content
2.6. Antioxidant Activity
2.7. FTIR Study
2.8. Barrier Properties of Active Eco-Packaging Materials
2.9. Migration Test
2.10. Mechanical Properties
2.11. Opacity Research
2.12. Images Analysis
2.13. Statistical Analysis
2.13.1. Spearman Rank Correlation
2.13.2. Cluster Analysis
3. Results and Discussion
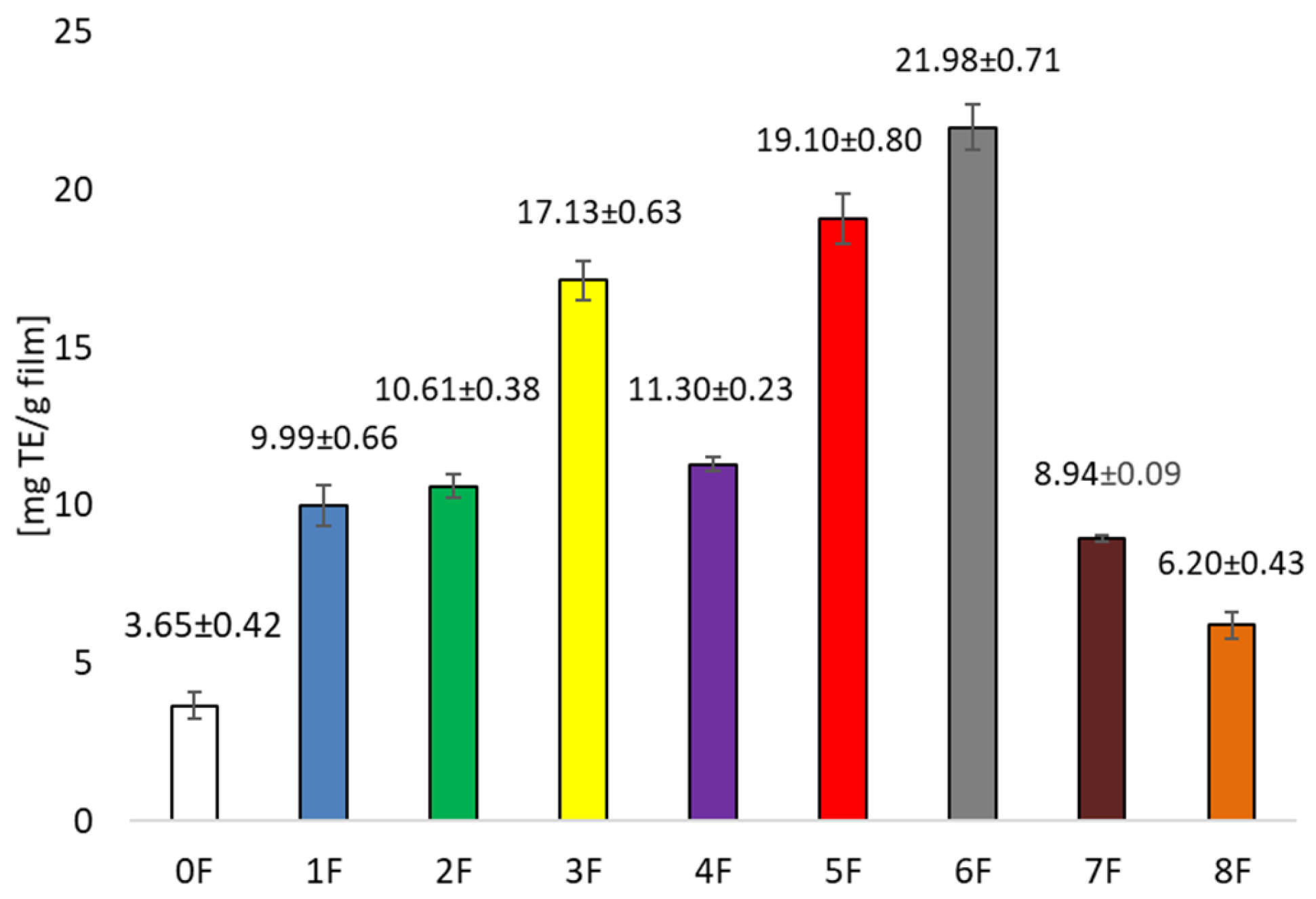
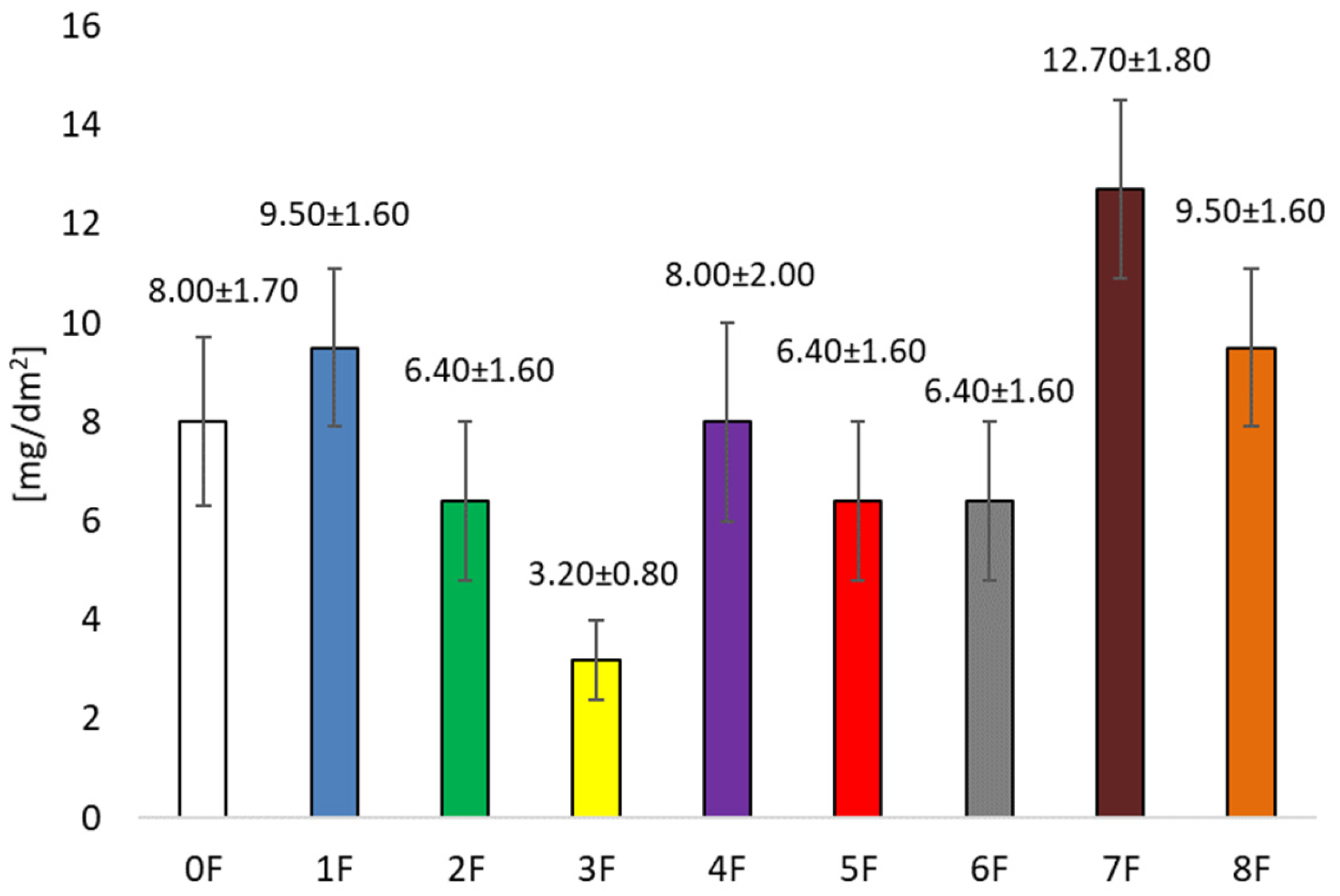
3.1. Testing Antioxidant Activity and Total Polyphenols in Pomace Extracts After Cherry Wine Production and in Prepared Films
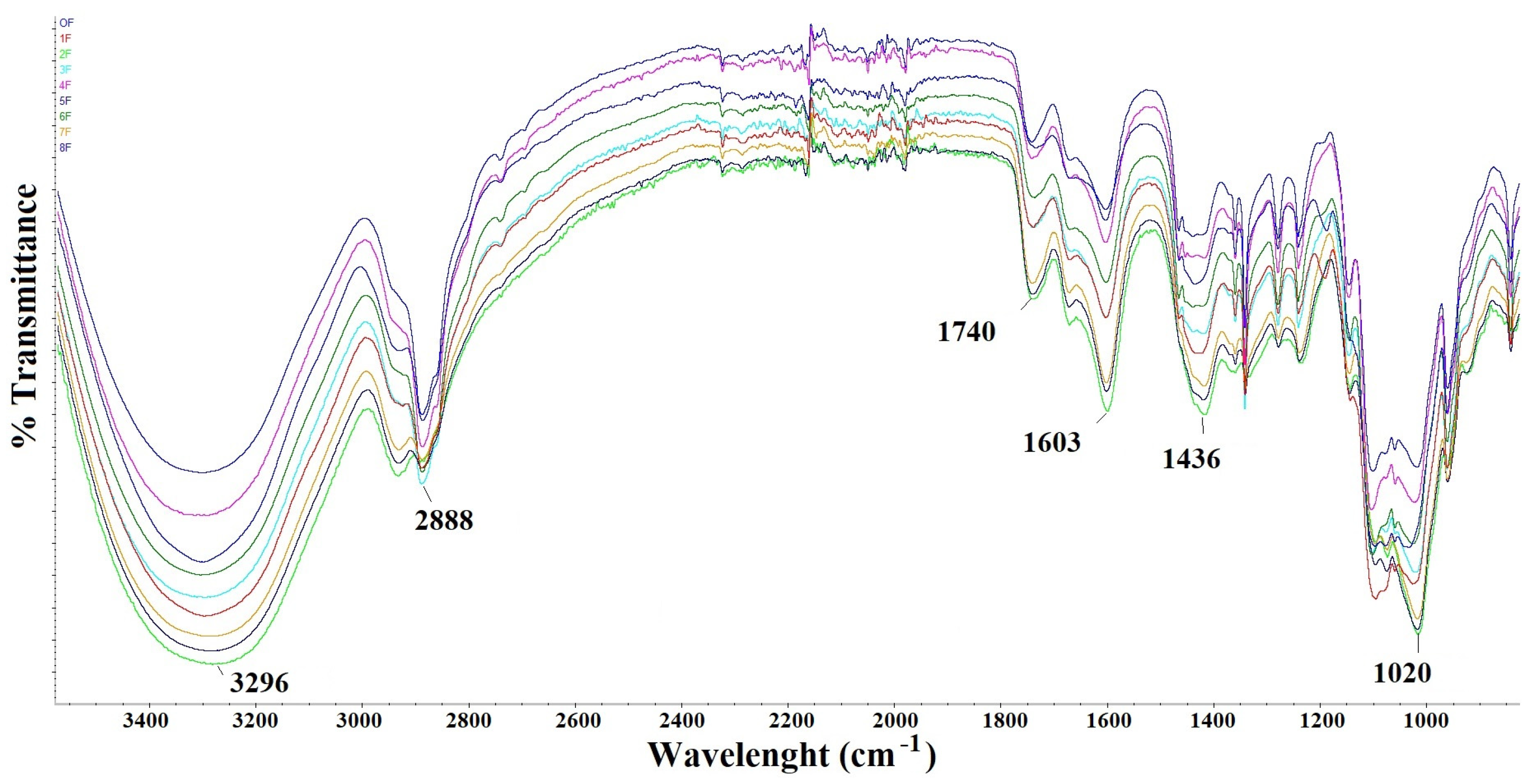
3.2. FTIR Study of Eco-Friendly Packaging Materials
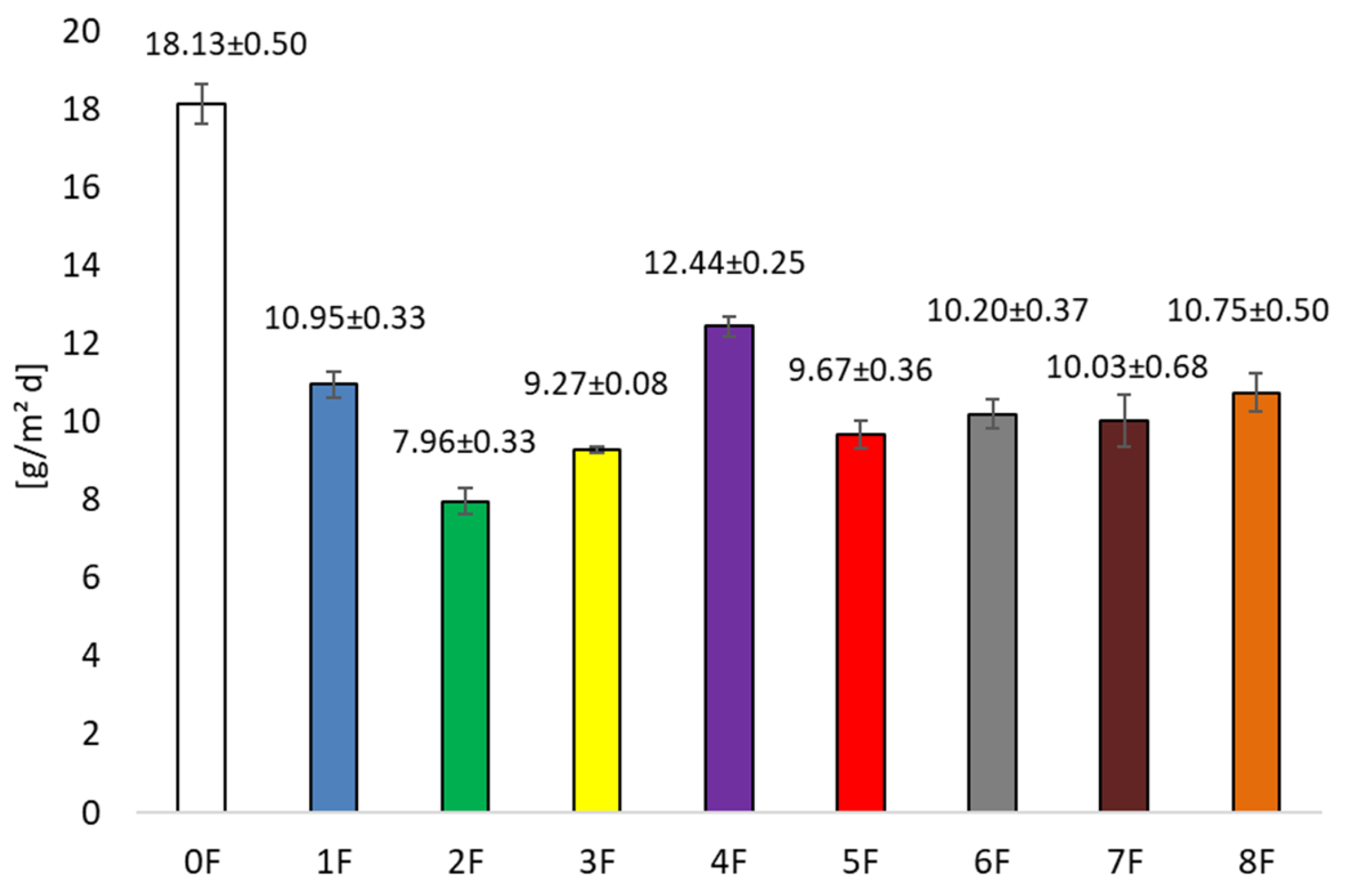
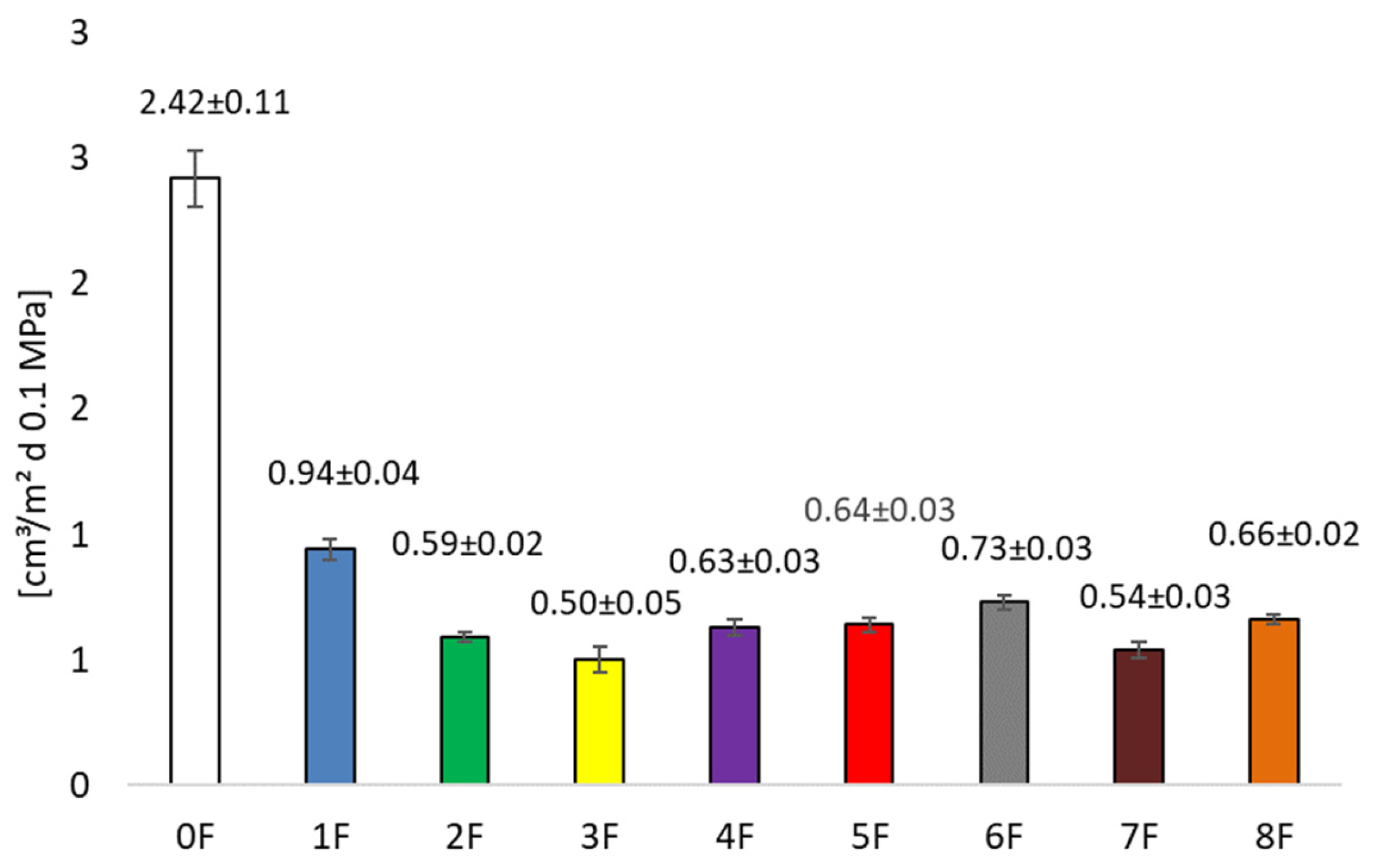
3.3. Barrier Research of Eco-Friendly Packaging Materials
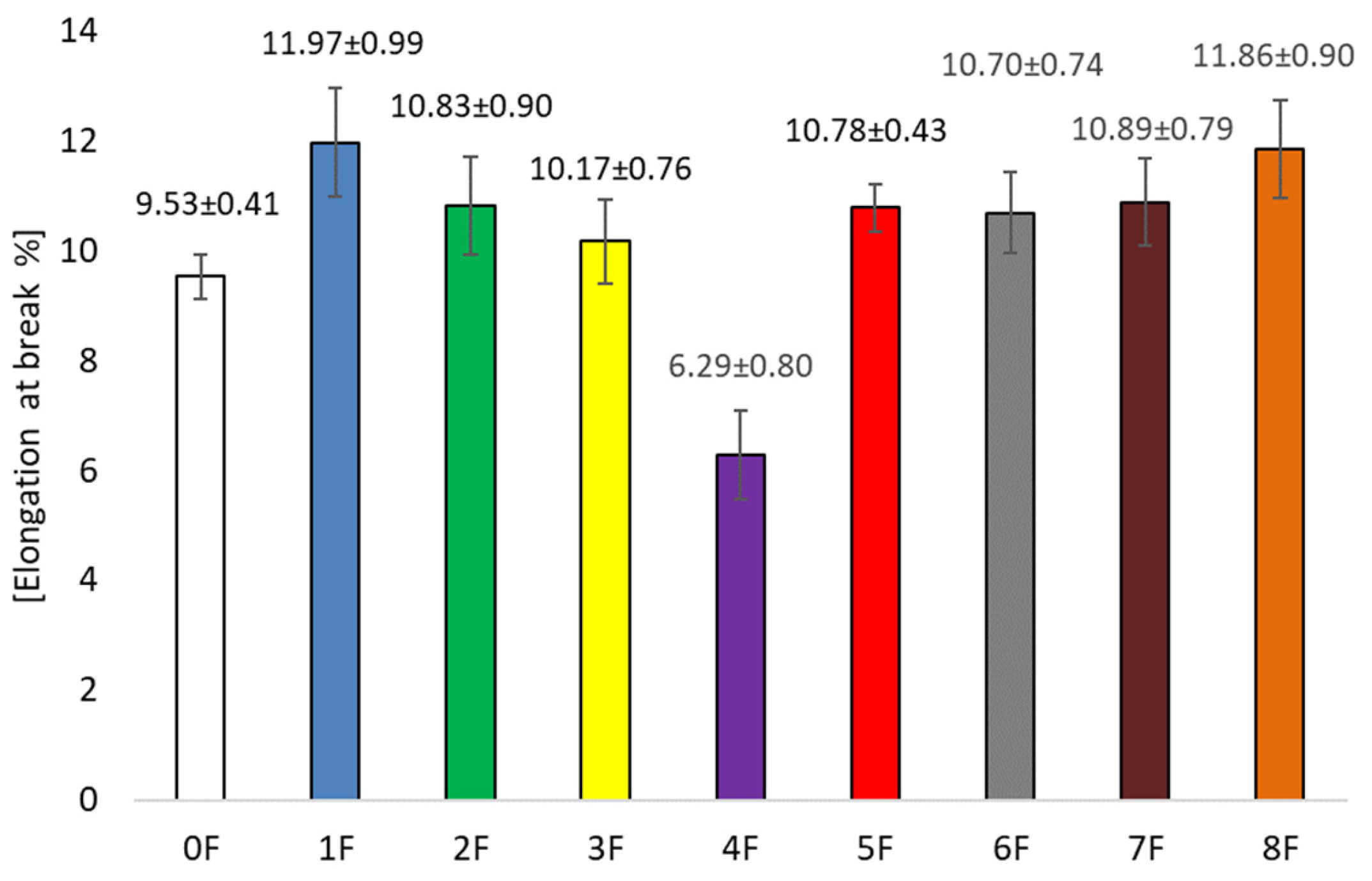
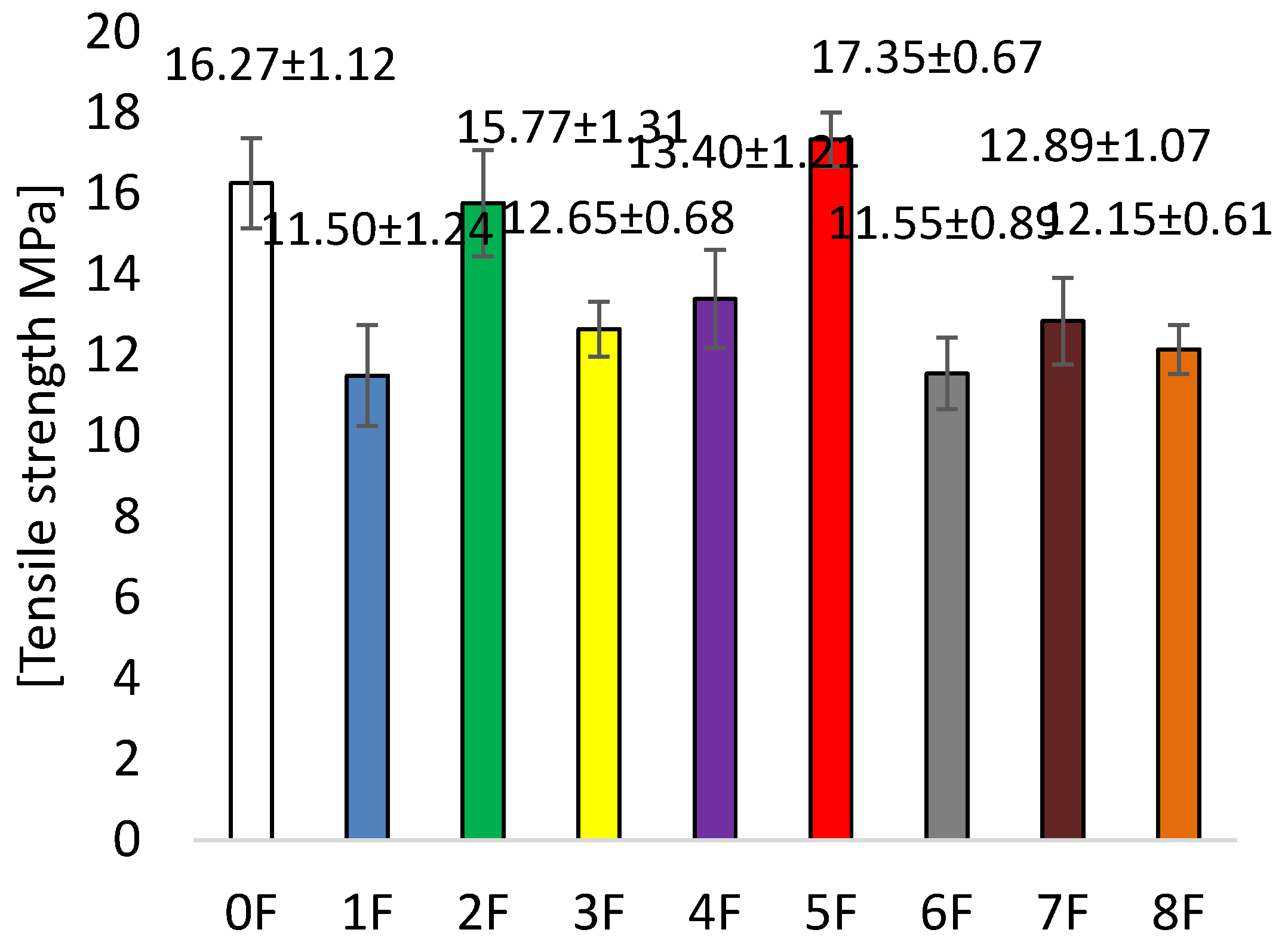
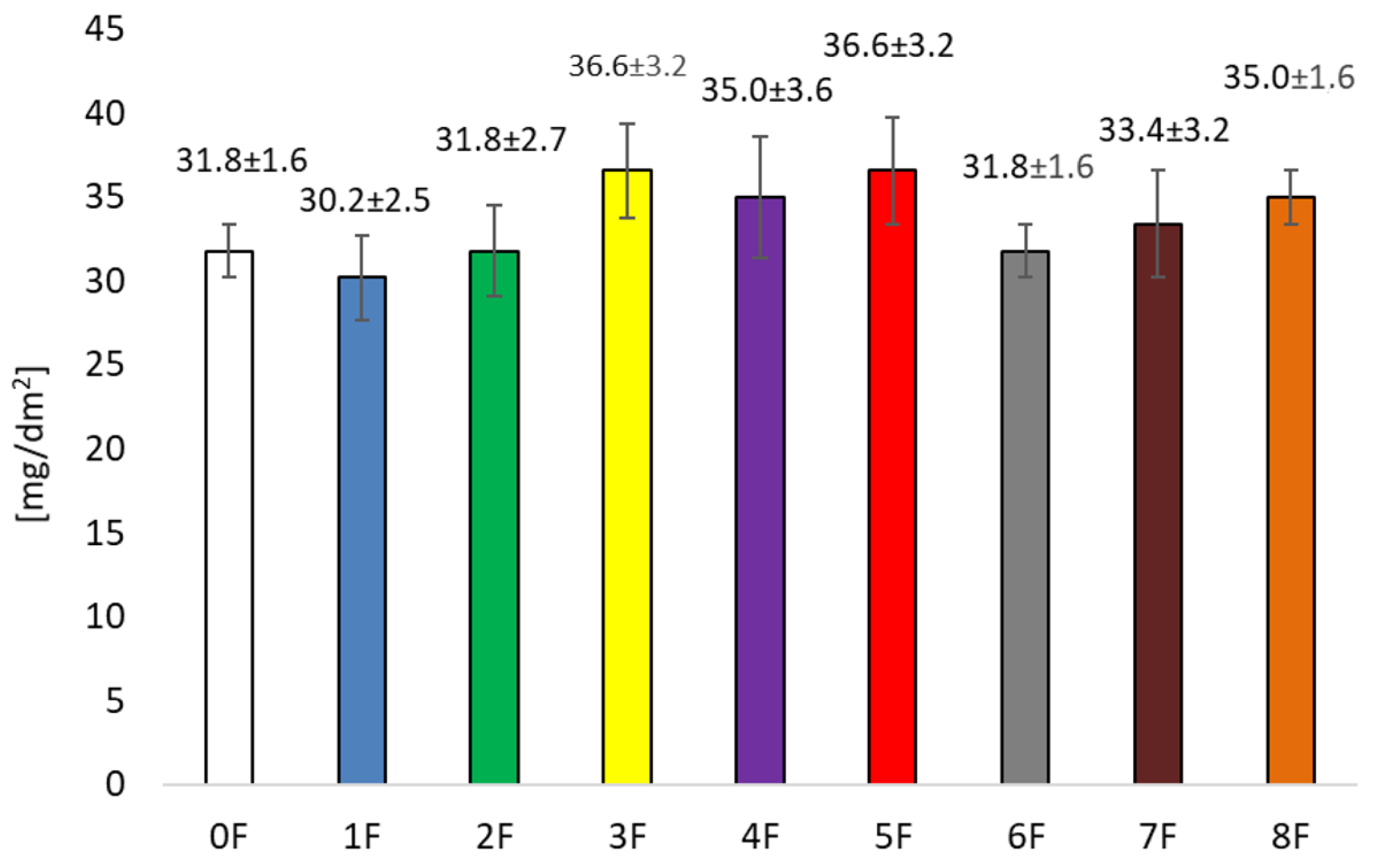
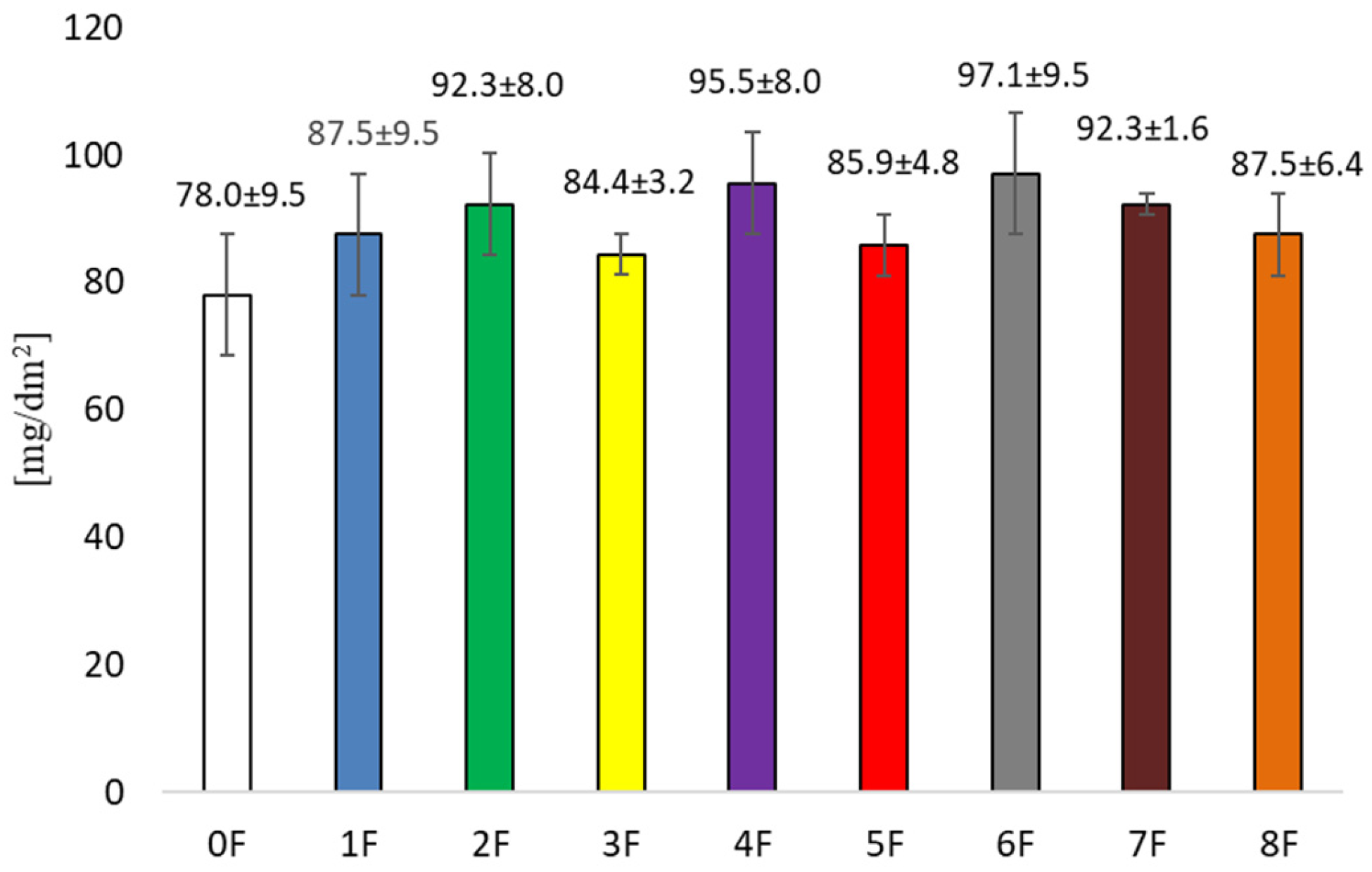
3.4. Mechanical Tests of Eco-Friendly Packaging Materials
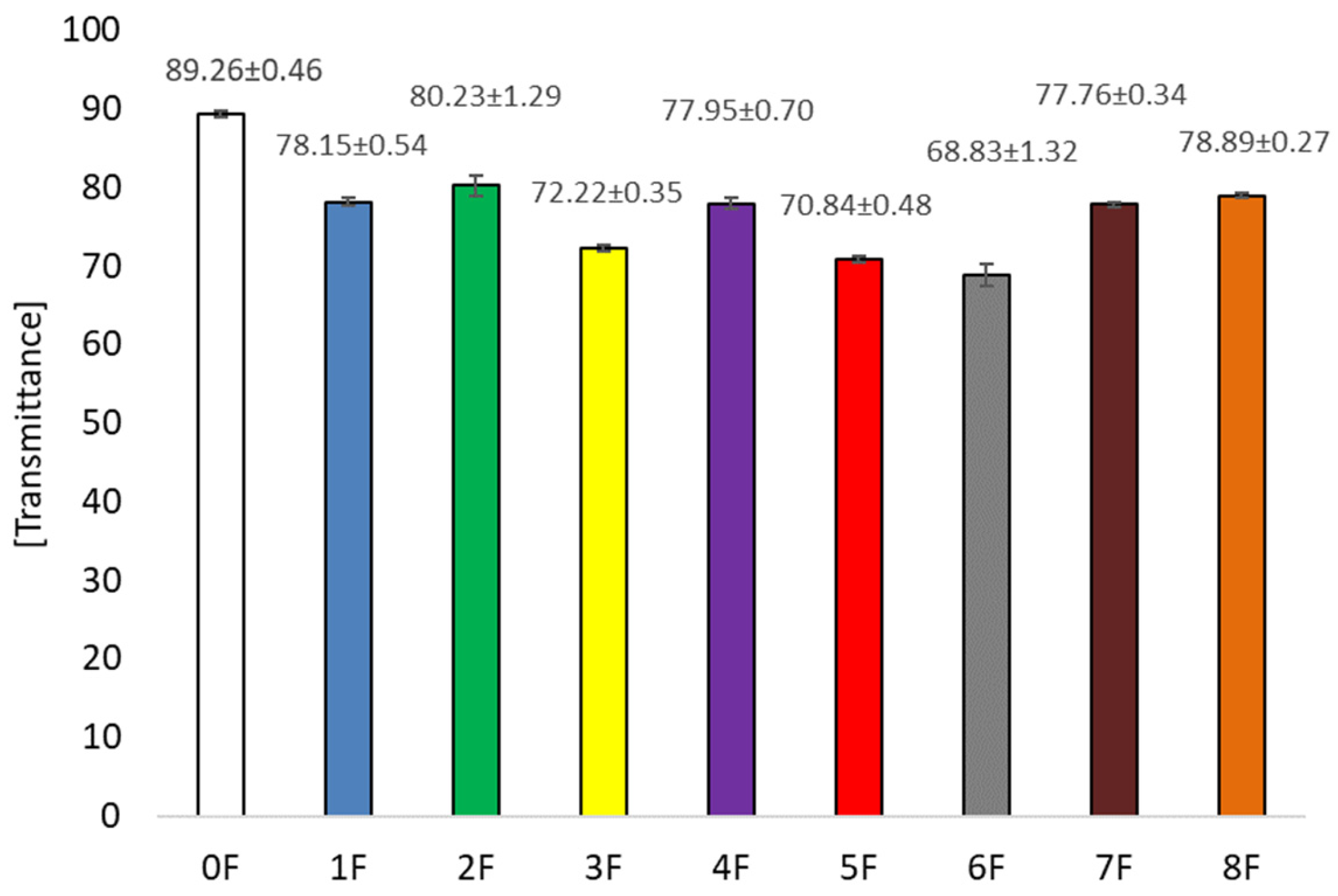
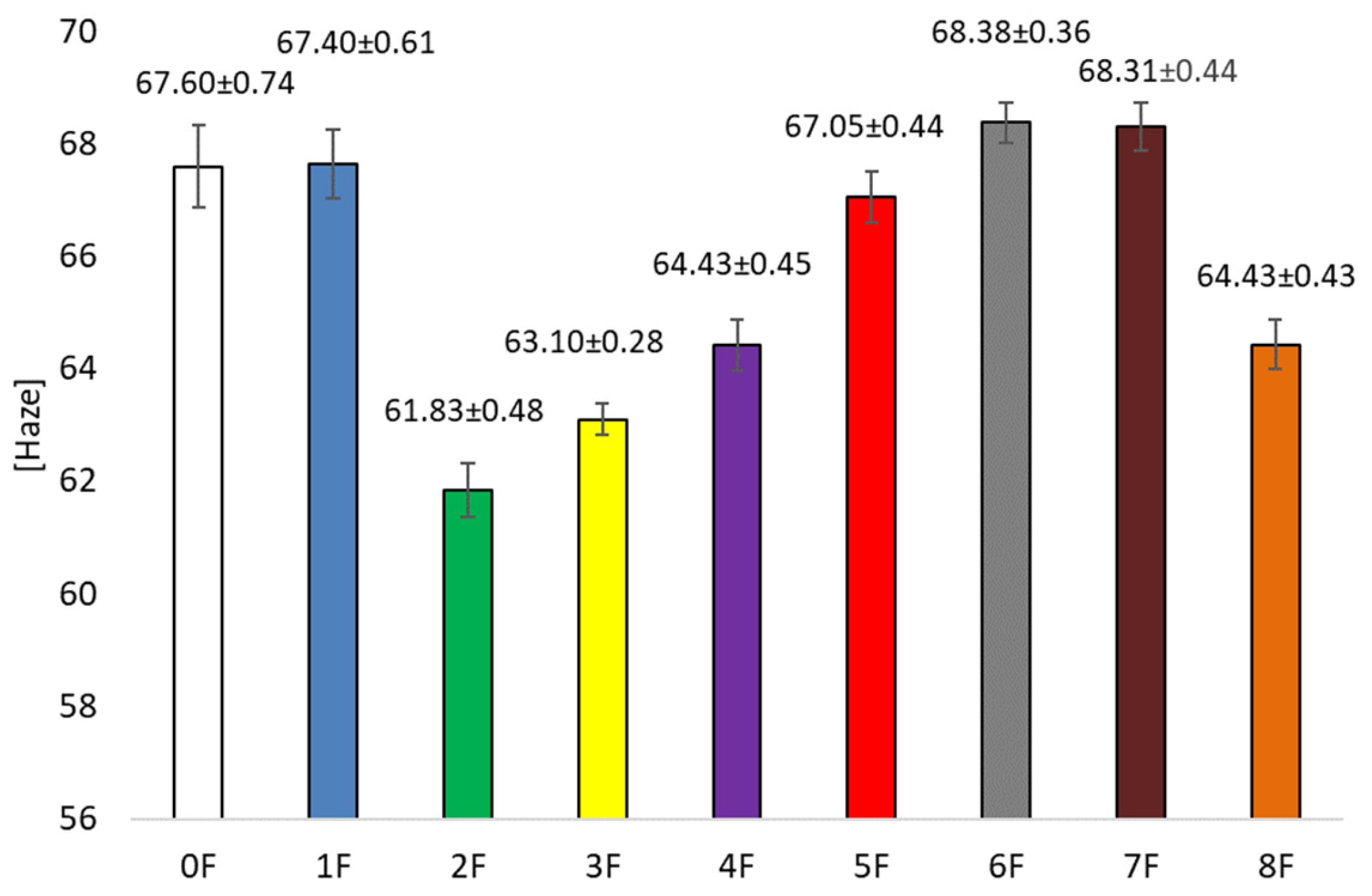
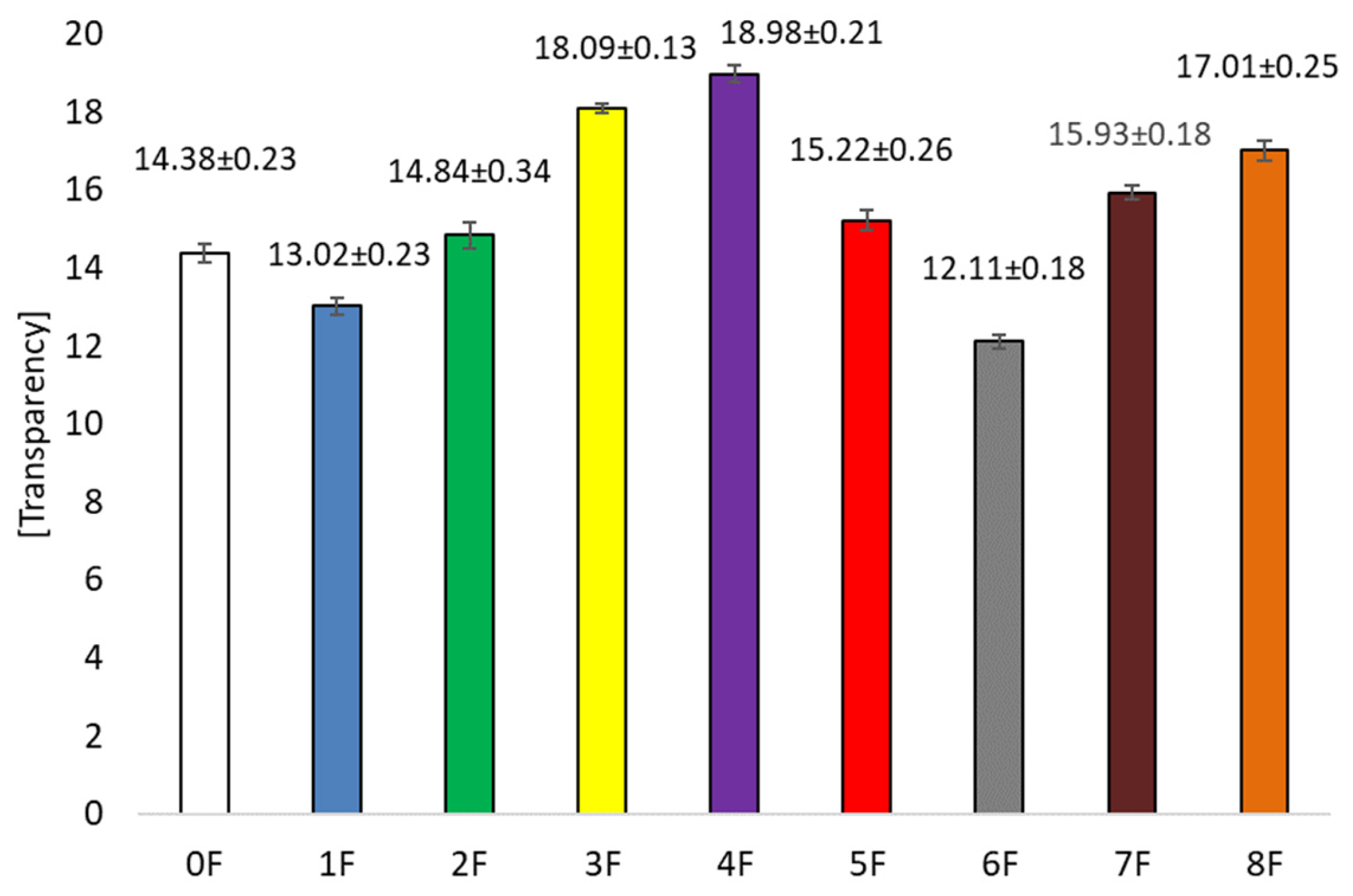
3.5. Optical Tests of Eco-Friendly Packaging Materials
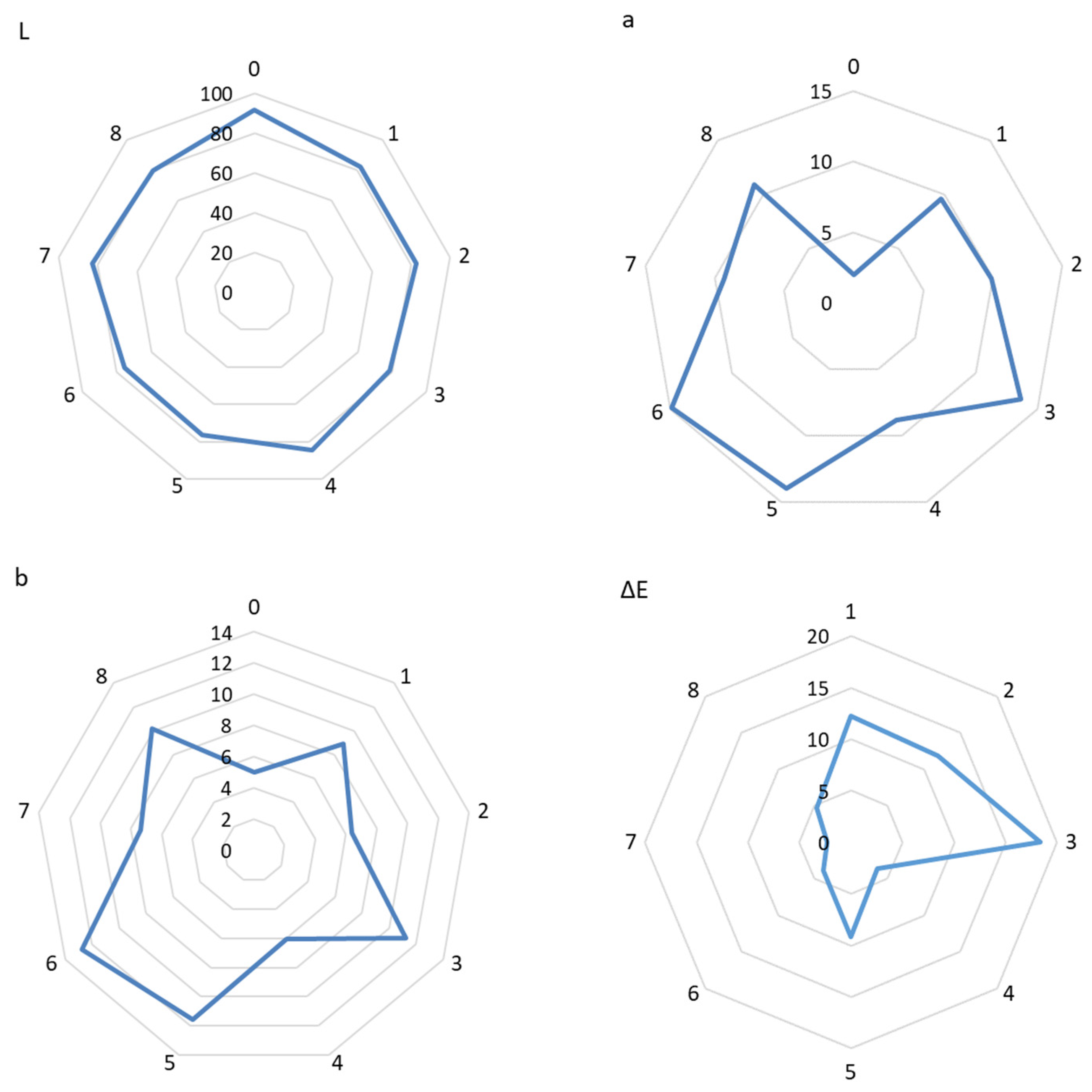
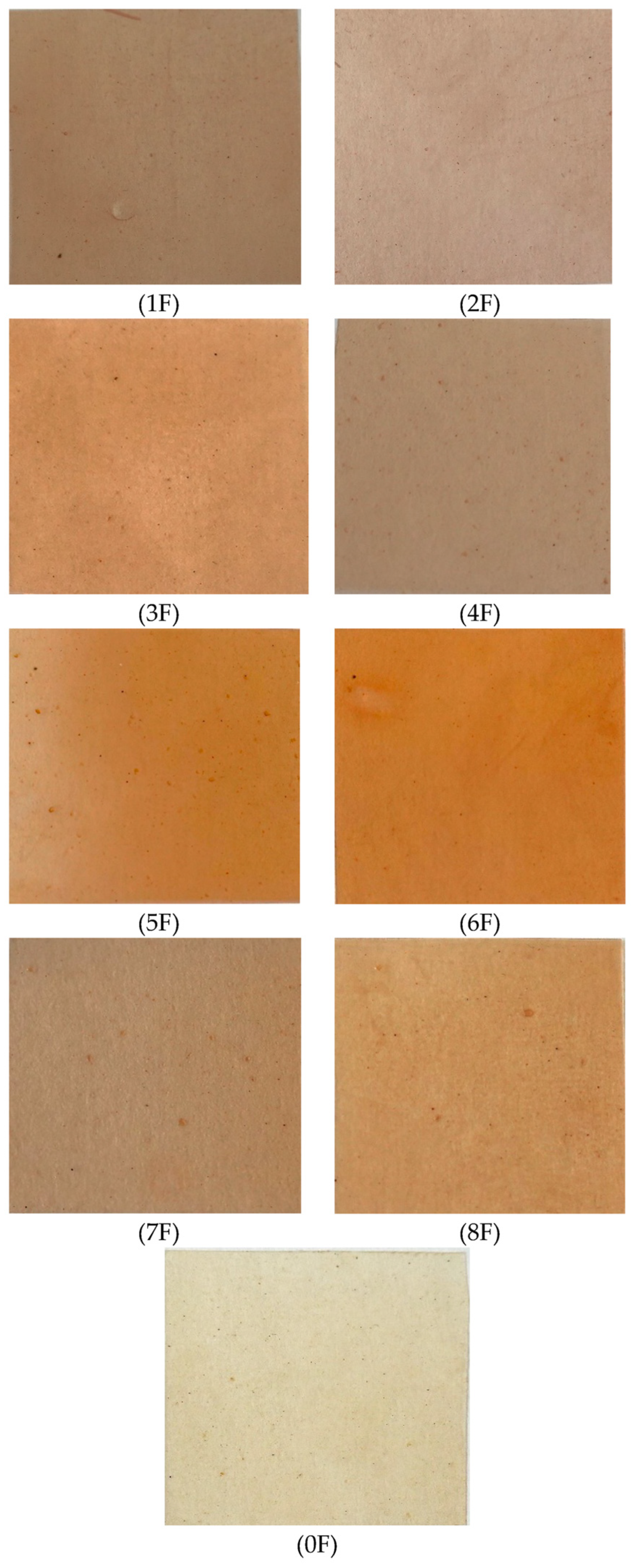
3.6. Color Testing of Eco-Friendly Packaging Materials
3.7. Studies on the Migration of Eco-Friendly Packaging Materials
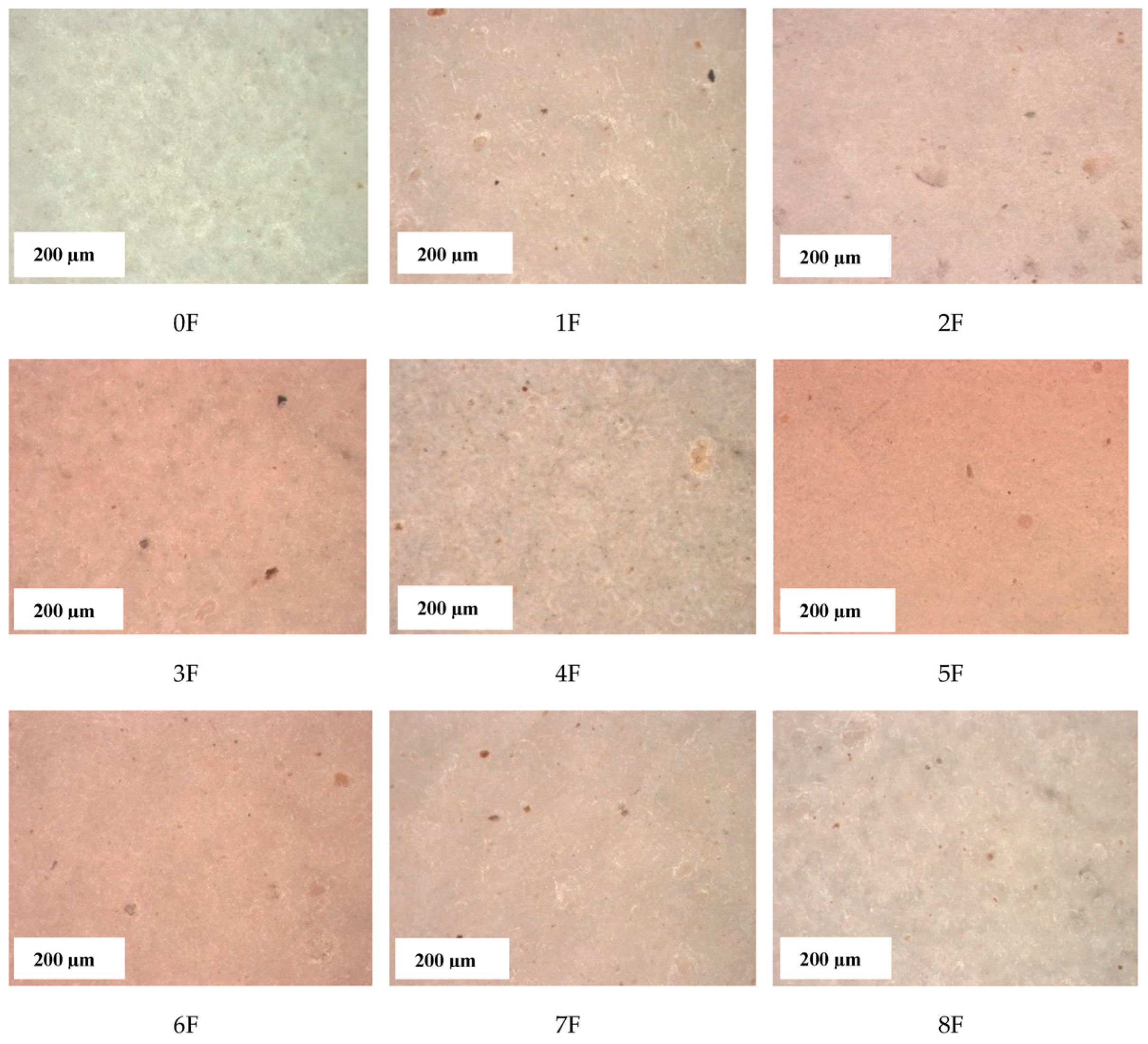
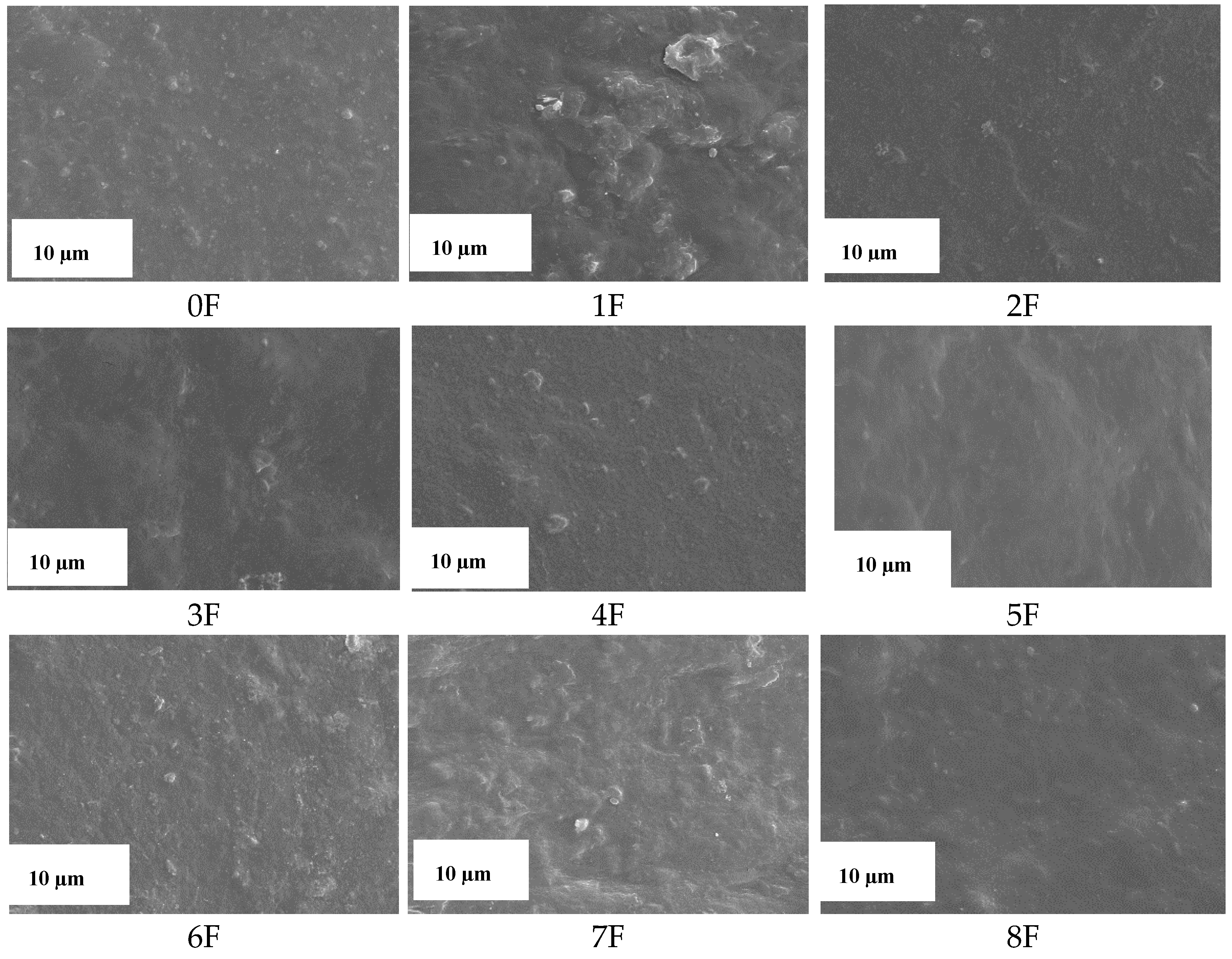
3.8. Images of Films of Eco-Friendly Packaging Materials
3.9. Statistical Analysis of the Results Obtained
3.9.1. Spearman’s Rank Correlation
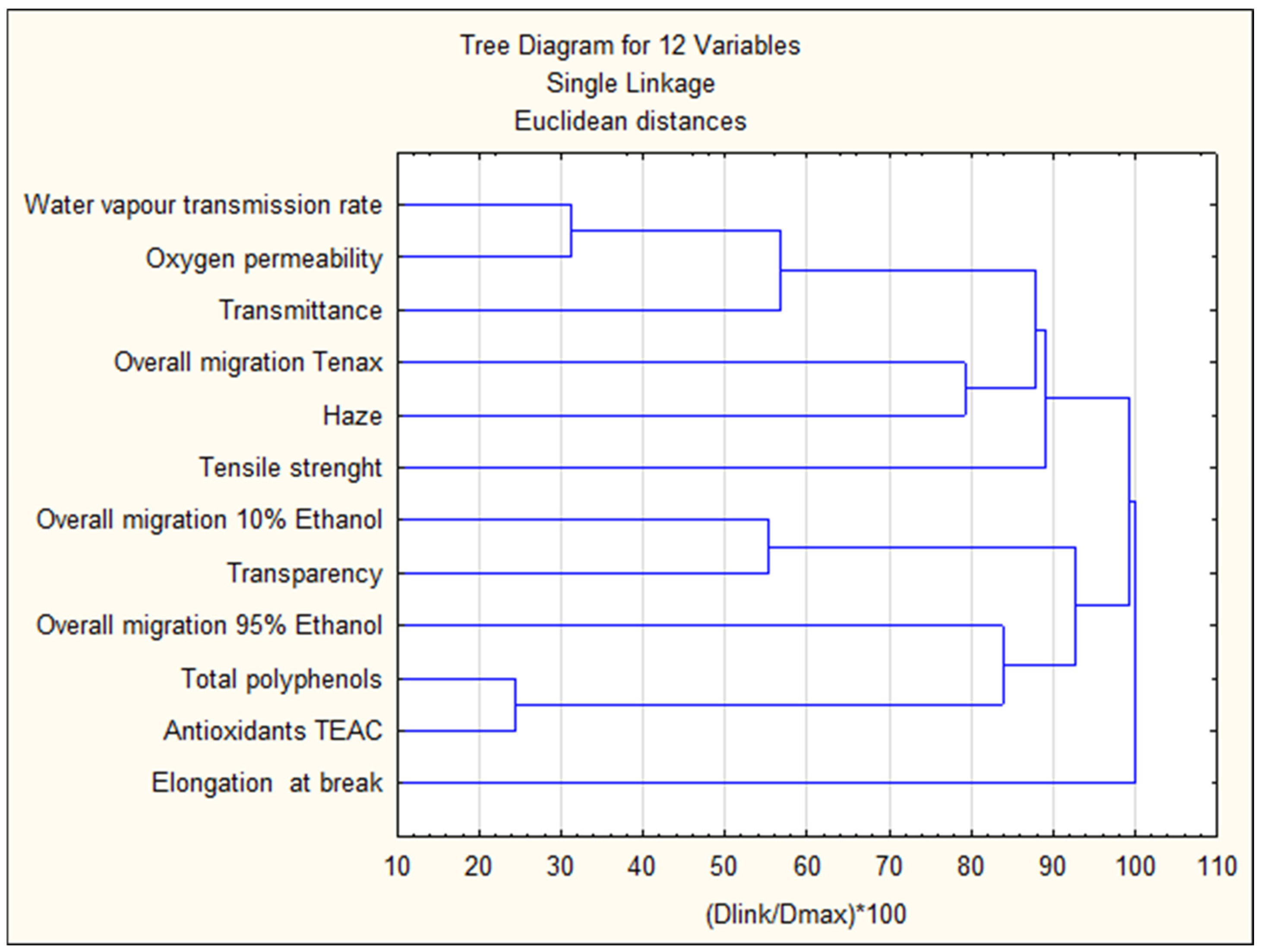
3.9.2. Cluster Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dobrucka, R.; Pawlik, M.; Szymański, M. Green Packaging Films with Antioxidant Activity Based on Pectin and Camellia sinensis Leaf Extract. Molecules 2024, 29, 4699. [Google Scholar] [CrossRef] [PubMed]
- Jakrawatana, N.; Ngammuangtueng, P.; Vorayos, N.; Gheewala, S.H. Replacing single-use plastics with biomaterial packaging in Thailand and impacts on the water-energy-climate Nexus. Sustain. Prod. Consum. 2023, 39, 506–520. [Google Scholar] [CrossRef]
- Melchor-Martínez, E.M.; Macías-Garbett, R.; Alvarado-Ramírez, L.; Araújo, R.G.; Sosa-Hernández, J.E.; Ramírez-Gamboa, D.; Parra-Saldívar, R. Towards a circular economy of plastics: An evaluation of the systematic transition to a new generation of bioplastics. Polymers 2022, 14, 1203. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.A.; Sanguansri, L.; Fox, E.M.; Cobiac, L.; Cole, M.B. Recovery of wasted fruit and vegetables for improving sustainable diets. Trends Food Sci. Technol. 2020, 95, 75–85. [Google Scholar] [CrossRef]
- Food Wastage Footprint (Project). Food Wastage Footprint Full-Cost Accounting; Food & Agriculture Organization of the UN (FAO): Rome, Italy, 2014. [Google Scholar]
- Szymański, M.; Długaszewska, J.; Pawlik, M.; Dobrucka, R. Development of Innovative Environmental Safety: Bioactives Against Pathogenic Bacteria Red Pectin Films from Hibiscus sabdariffa Flos Extract for Circular Economy. Coatings 2024, 14, 1500. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; Da Silva, M.A.; Dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Yong, H.; Wang, Z.; Huang, J.; Liu, J. Preparation, characterization and application of antioxidant packaging films based on chitosan-epicatechin gallate conjugates with different substitution degrees. Int. J. Biol. Macromol. 2024, 260, 129568. [Google Scholar] [CrossRef]
- Piasecka, I.; Brzezińska, R.; Kalisz, S.; Wiktor, A.; Górska, A. Response Surface Methodology for Optimization of Ultrasound-Assisted Antioxidants Extraction from Blackberry, Chokeberry and Raspberry Pomaces. Plants 2024, 13, 1120. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Abdul Aziz, A.H.; Che Yunus, M.A.; Veza, I.; Harny, I.; Tirta, A. Waste to Wealth of Apple Pomace Valorization by Past and Current Extraction Processes: A Review. Sustainability 2023, 15, 830. [Google Scholar] [CrossRef]
- Castellanos-Gallo, L.; Ballinas-Casarrubias, L.; Espinoza-Hicks, J.C.; Hernández-Ochoa, L.R.; Muñoz-Castellanos, L.N.; Zermeño-Ortega, M.R.; Borrego-Loya, A.; Salas, E. Grape Pomace Valorization by Extraction of Phenolic Polymeric Pigments: A Review. Processes 2022, 10, 469. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape Pomace as a Sustainable Source of Bioactive Compounds: Extraction, Characterization, and Biotechnological Applications of Phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; Brevik, E.C.; Bayoumi, Y.; Shalaby, T.A.; El-Mahrouk, M.E.; Taha, N.; Elbasiouny, H.; Elbehiry, F.; Amer, M.; Abdalla, N.; et al. An Overview of Agro-Waste Management in Light of the Water-Energy-WasteNexus. Sustainability 2022, 14, 15717. [Google Scholar] [CrossRef]
- Venkidasamy, B.; Samynathan, R.; Ramasamy, P.; Kumar, M.P.S.; Thiruvengadam, M.; Khayrullin, M.; Shariati, M.A.; Nile, A.S.; Nile, S.H. Unveiling novel applications of fruit pomace for sustainable production of value-added products and health benefits: A review. Food Biosci. 2024, 61, 104533. [Google Scholar] [CrossRef]
- Sarker, A.; Matak, K.; Jaczynski, J. Effect of transglutaminase concentration on the properties of soy protein isolate-pectin composite edible films. Food Packag. Shelf Life 2025, 47, 101418. [Google Scholar] [CrossRef]
- Min, B.; Bae, I.Y.; Lee, H.G.; Yoo, S.H.; Lee, S. Utilization of pectin-enriched materials from apple pomace as a fat replacer in a model food system. Biores. Technol. 2010, 101, 5414–5418. [Google Scholar] [CrossRef]
- Dobrucka, R.; Długaszewska, J.; Pawlik, M.; Szymański, M. Innovative active bio-based food packaging material with Cannabis sativa L. seeds extract as an agent to reduce food waste. Colloids Surf. B-Biointerfaces 2025, 245, 114313. [Google Scholar] [CrossRef]
- Lasik-Kurdyś, M.; Gumienna, M.; Górna, B.; Adzahan, N.M. Influence of Green Tea Added to Cherry Wine on Phenolic Content, Antioxidant Activity and Alpha-Glucosidase Inhibition during an In Vitro Gastrointestinal Digestion. Foods 2022, 11, 3298. [Google Scholar] [CrossRef]
- Singelton, V.L.; Rossi, J.A. Colorymetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Gumienna, M.; Lasik, M.; Czarnecki, Z. Bioconversion of grape and chokeberry wine polyphenols during simulated gastrointestinal in vitro digestion. Int J Food Sci Nutr. 2011, 62, 226–233. [Google Scholar] [CrossRef]
- Re, R.; Pellegirini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- DIN 55474:2015; Auxiliary Means of Packaging-Desiccants in Bag-Application, Calculation of the Required Number of Desiccant Units. Available online: https://www.dinmedia.de/de/norm/din-55474/228004598 (accessed on 19 June 2025).
- ASTM D3985–17:2017; Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. ASTM International: West Conshohocken, PA, USA, 2017.
- BS EN 1186-3:2022; Materials and Articles in Contact with Foodstuffs. Plastics Test Methods for Overall Migration in Evaporable Simulants. Available online: https://www.dinmedia.de/de/norm/din-en-1186-3/351605746 (accessed on 19 June 2025).
- Dobrucka, R.; Urbaniak, M.; Kozak, W.; Szymański, M. Innovative method of environmental safety research of starch-based films with silver nanoparticles. Environ. Prog. Sustain. Energy 2024, 43, e14480. [Google Scholar] [CrossRef]
- Rrucaj, E.; Carpentieri, S.; Scognamiglio, M.; Siano, F.; Ferrari, G.; Pataro, G. Sustainable Valorization of Industrial Cherry Pomace: A Novel Cascade Approach Using Pulsed Electric Fields and Ultrasound Assisted-Extraction. Foods 2024, 13, 1043. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, F.M.; Karaaslan, M.; Vardin, H. Optimization of extraction parameters on the isolation of phenolic compounds from sour cherry (Prunus cerasus L.) pomace. J. Food Sci. Technol. 2015, 52, 2851–2859. [Google Scholar] [CrossRef]
- Carpentieri, S.; Ferrari, G.; Pataro, G. Optimization of Pulsed Electric Fields-Assisted Extraction of Phenolic Compounds From White Grape Pomace Using Response Surface Methodology. Front. Sustain. Food Syst. 2022, 6, 854968. [Google Scholar] [CrossRef]
- Carpentieri, S.; Mazza, L.; Nutrizio, M.; Jambrak, A.R.; Ferrari, G.; Pataro, G. Pulsed Electric Fields- and Ultrasound-Assisted Green Extraction of Valuable Compounds from Origanum vulgare L. and Thymus serpyllum L. Int. J. Food Sci. Technol. 2021, 56, 4834–4842. [Google Scholar] [CrossRef]
- Martín-García, B.; Tylewicz, U.; Verardo, V.; Pasini, F.; Gómez-Caravaca, A.M.; Caboni, M.F.; Dalla Rosa, M. Pulsed Electric Field (PEF) as Pre-Treatment to Improve the Phenolic Compounds Recovery from Brewers’ Spent Grains. Innov. Food Sci. Emerg. Technol. 2020, 64, 102402. [Google Scholar] [CrossRef]
- Van Der Sluis, A.A.; Dekker, M.; Skrede, G.; Jongen, W.M. Activity and concentration of polyphenolic antioxidants in apple juice. 1. Effect of existing production methods. J. Agric. Food Chem. 2002, 50, 7211–7219. [Google Scholar] [CrossRef]
- Cheng, V.J.; Bekhit, A.A.; McConnell, M.; Mros, S.; Zhao, J. Effect of extraction solvent, waste fraction and grape variety on the antimicrobial and antioxidant activities of extracts from wine residue from cool climate. Food Chem. 2012, 134, 474–482. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, H.; Holland, B.; Barrow, C.J.; Suleria, H.A.R. An Optimization of the Extraction of Phenolic Compounds from Grape Marc: A Comparison between Conventional and Ultrasound-Assisted Methods. Chemosensors 2024, 12, 177. [Google Scholar] [CrossRef]
- Kashyap, P.; Riar, C.S.; Jindal, N. Polyphenol Bio-Accessibility and Antioxidant Activity of in Vitro Digested Ultrasound-Assisted Meghalayan Cherry (Prunus nepalensis) Pomace Extract. Biomass Conv. Bioref. 2023, 13, 14071–14085. [Google Scholar] [CrossRef]
- Kasapoğlu, E.D.; Kahraman, S.; Tornuk, F. Optimization of ultrasound assisted antioxidant extraction from apricot pomace using response surface methodology. Food Meas. 2021, 15, 5277–5287. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Ahmad, M.H.; Buntat, Z.; Nawawi, Z.; Pavan Kumara, C.L.G.; Abdulameer, A.Z.; Sidik, M.A.B. Coaxial Treatment Chamber for Liquid Food Treatment through Pulsed Electric Field. Indones. J. Electr. Eng. Comput. Sci. 2020, 19, 1169–1176. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, Y.; Yu, X.; Zhu, J.; Chen, K.; Kuang, Y.; Wu, K.; Jiang, F. Konjac glucomannan films incorporated pectin-stabilized Mandarin oil emulsions: Structure, properties, and application in fruit preservation. Int. J. Biol. Macromol. 2024, 267, 131292. [Google Scholar] [CrossRef]
- Pirsa, S.; Karimi Sani, I.; Pirouzifard, M.K.; Erfani, A. Smart film based on chitosan/Melissa officinalis essences/pomegranate peel extract to detect cream cheeses spoilage. Food Addit. Contam. Part A 2020, 37, 634–648. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef]
- Karim, R.; Nahar, K.; Zohora, F.T.; Islam, M.M.; Bhuiyan, R.H.; Jahan, M.S.; Shaikh, M.A.A. Pectin from lemon and mango peel: Extraction, characterisation and application in biodegradable film. Carbohydr. Polym. Technol. Appl. 2022, 4, 100258. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Y.; Yang, T.X.; Zhao, Q.S.; Zhao, B. Development and characterization of pectin-based composite film incorporated with cannabidiol/2, 6-di-O-methyl-β-cyclodextrin inclusion complex for food packaging. Int. J. Biol. Macromol. 2024, 277, 133525. [Google Scholar] [CrossRef]
- Chaudhary, B.U.; Lingayat, S.; Banerjee, A.N.; Kale, R.D. Development of multifunctional food packaging films based on waste Garlic peel extract and Chitosan. Int. J. Biol. Macromol. 2021, 192, 479–490. [Google Scholar] [CrossRef]
- Esposito, M.; Di Pierro, P.; Regalado-Gonzales, C.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R. Polyamines as new cationic plasticizers for pectin-based edible films. Carbohydr. Polym. 2016, 153, 222–228. [Google Scholar] [CrossRef]
- Tristanto, N.A.; Cao, W.; Chen, N.; Suryoprabowo, S.; Soetaredjo, F.E.; Ismadji, S.; Hua, X. Pectin extracted from red dragon fruit (Hylocereus polyrhizus) peel and its usage in edible film. Int. J. Biol. Macromol. 2024, 276, 133804. [Google Scholar] [CrossRef] [PubMed]
- Ursachi, V.F.; Oroian, M.; Spinei, M. Development and characterization of biodegradable films based on cellulose derivatives and citrus pectin: A comparative study. Ind. Crops Prod. 2024, 219, 119052. [Google Scholar] [CrossRef]
- Sivarooban, T.; Hettiarachchy, N.S.; Johnson, M.G. Physical and antimicrobial properties of grape seed extract, nisin, and EDTA incorporated soy protein edible films. Food Res. Int. 2008, 41, 781–785. [Google Scholar] [CrossRef]
- Adilah, A.N.; Jamilah, B.; Noranizan, M.A.; Hanani, Z.N. Utilization of mango peel extracts on the biodegradable films for active packaging. Food Packag. Shelf Life 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Sorde, K.L.; Ananthanarayan, L. Effect of transglutaminase treatment on properties of coconut protein-guar gum composite film. LWT 2019, 115, 108422. [Google Scholar] [CrossRef]
- Fematt-Flores, G.E.; Aguiló-Aguayo, I.; Marcos, B.; Camargo-Olivas, B.A.; Sánchez-Vega, R.; Soto-Caballero, M.C.; Salas-Salazar, N.A.; Flores-Córdova, M.A.; Rodríguez-Roque, M.J. Milk Protein-Based Edible Films: Influence on Mechanical, Hydrodynamic, Optical and Antioxidant Properties. Coatings 2022, 12, 196. [Google Scholar] [CrossRef]
- Zahran, M. Carbohydrate polymer-supported metal and metal oxide nanoparticles for constructing electrochemical sensors. Mater. Adv. 2024, 5, 68–82. [Google Scholar] [CrossRef]
- Chen, H.; Shang, K.; Bian, X.; Zhao, Z.; Liu, Y.; Lin, X.; Wang, L.; Zhang, W.; Hu, X.; Guo, X. Enhanced functional pectin films incorporated with olive fruit extracts prepared by deep eutectic solvents. Food Packag. Shelf Life 2024, 46, 101361. [Google Scholar] [CrossRef]
- Biratu, G.; Woldemariam, H.W.; Gonfa, G. Development of active edible films from coffee pulp pectin, propolis, and honey with improved mechanical, functional, antioxidant, and antimicrobial properties. Carbohydr. Polym. Technol. Appl. 2024, 8, 100557. [Google Scholar] [CrossRef]
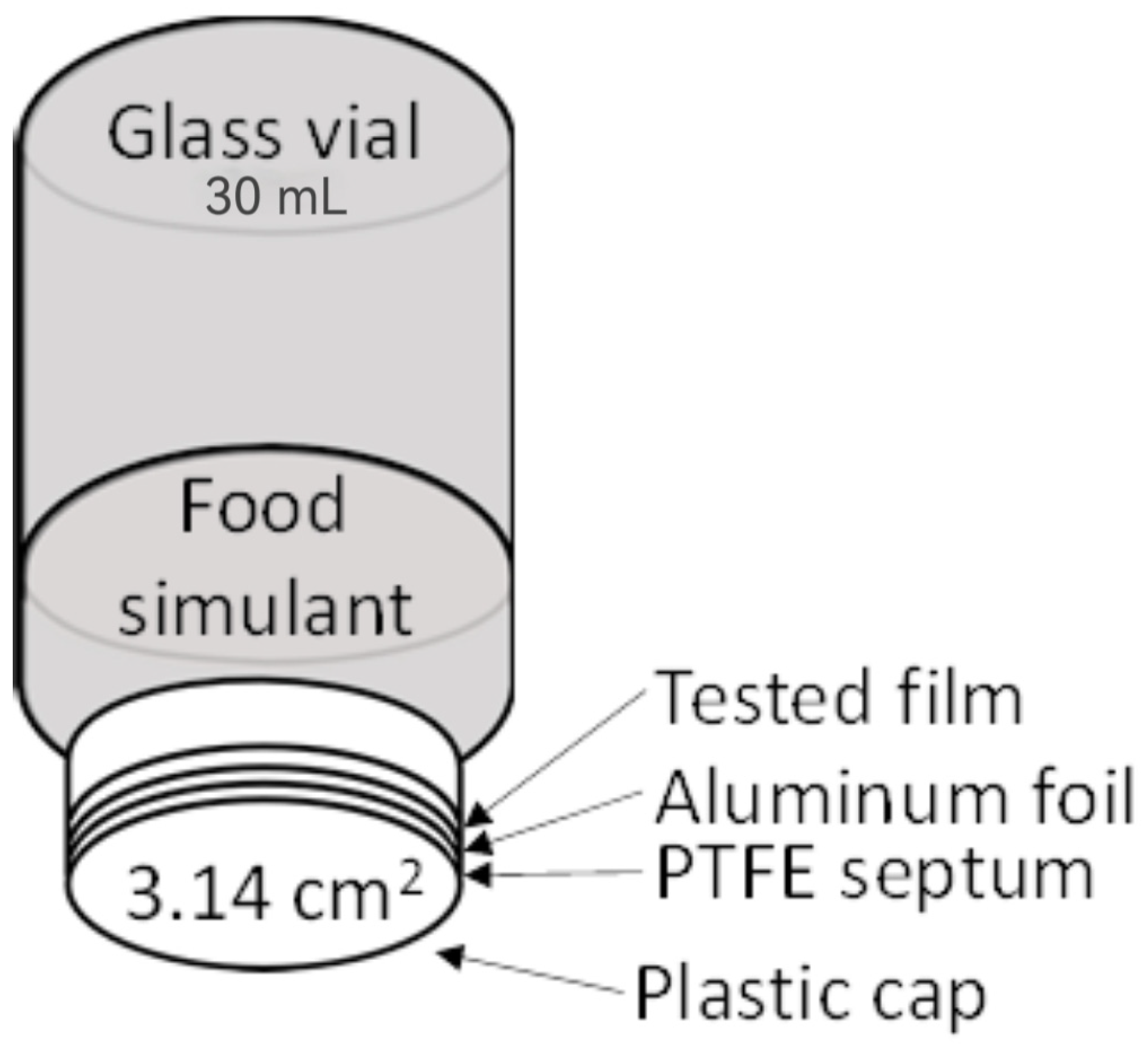
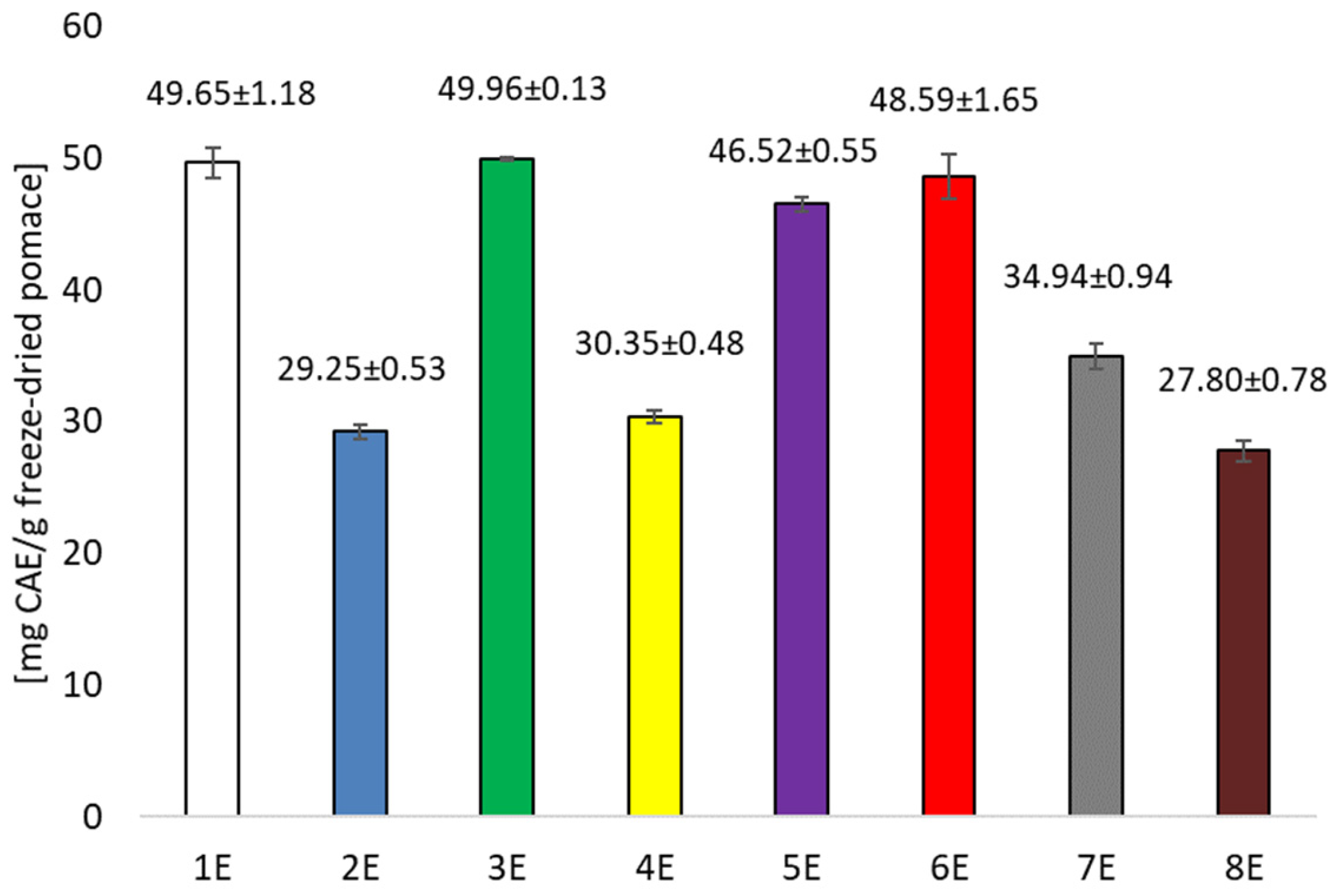
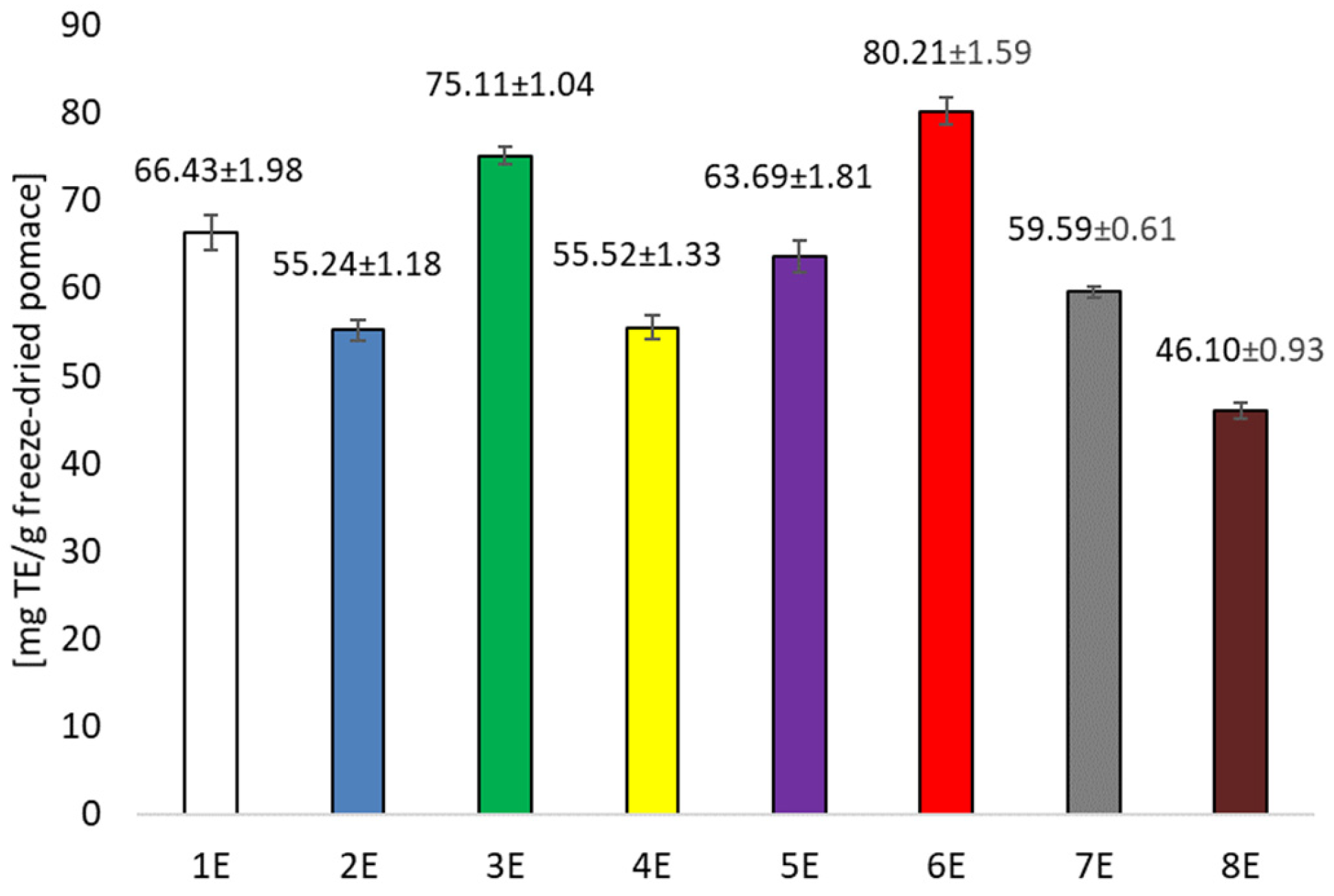
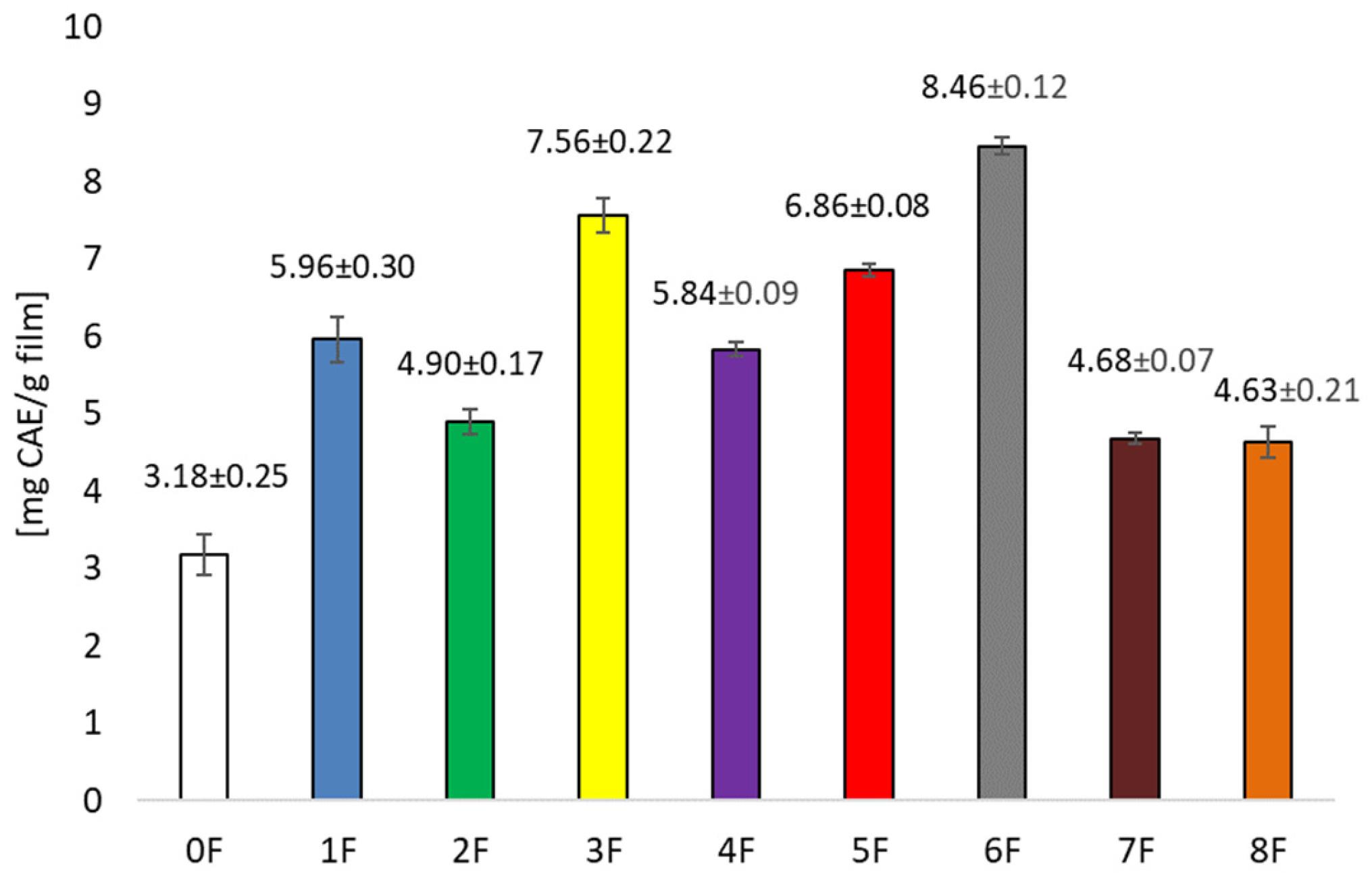
| Sample | Conditions | Extraction Solvent |
|---|---|---|
| 1 (E/F) | Ultrasonic bath (80 W, 0.5 h) | ethanol 50% |
| 2 (E/F) | Ultrasonic bath (80 W, 0.5 h) | water |
| 3 (E/F) | Ultrasonic bath (80 W, 2 h) | ethanol 50% |
| 4 (E/F) | Ultrasonic bath (80 W, 2 h) | water |
| 5 (E/F) | Shaking (130 rpm, 25 °C, 3 h) | ethanol 50% |
| 6 (E/F) | Shaking (130 rpm, 60 °C, 3 h) | ethanol 50% |
| 7 (E/F) | Shaking (130 rpm, 25 °C, 3 h) | water |
| 8 (E/F) | Shaking (130 rpm, 60 °C, 3 h) | water |
| Water Vapor Transmission Rate | Oxygen Permeability | Overall Migration 10% Ethanol | Overall Migration 95% Ethanol | Overall Migration Tenax | Total Polyphenols | Antioxidants Teac | Elongation at Break | Tensile Strength | Haze | Transmittance | Transparency | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water vapor transmission rate | 1.0000 | 0.7167 | −0.3334 | −0.0504 | 0.5301 | −0.4000 | −0.4667 | −0.2167 | −0.1333 | 0.3833 | 0.3500 | −0.1000 |
| Oxygen permeability | 0.7167 | 1.0000 | −0.5814 | −0.1429 | 0.2650 | −0.2000 | −0.2833 | 0.0833 | −0.1500 | 0.5000 | 0.3000 | −0.6667 |
| Overall migration 10% Ethanol | −0.3334 | −0.5814 | 1.0000 | −0.2543 | −0.3421 | 0.1881 | 0.3163 | −0.3163 | 0.3591 | −0.4275 | −0.4189 | 0.7780 |
| Overall migration 95% Ethanol | −0.0504 | −0.1429 | −0.2543 | 1.0000 | 0.1380 | 0.2605 | 0.3698 | 0.0504 | −0.3361 | 0.2185 | −0.3109 | −0.0756 |
| Overall migration Tenax | 0.5301 | 0.2650 | −0.3421 | 0.1380 | 1.0000 | −0.6327 | −0.7096 | 0.5130 | −0.2565 | 0.4446 | 0.3676 | 0.0085 |
| Total polyphenols | −0.4000 | −0.2000 | 0.1881 | 0.2605 | −0.6327 | 1.0000 | 0.9333 | −0.1333 | −0.3167 | 0.1000 | −0.8333 | −0.1500 |
| Antioxidants TEAC | −0.4667 | −0.2833 | 0.3163 | 0.3698 | −0.7096 | 0.9333 | 1.0000 | −0.2833 | −0.0500 | −0.0167 | −0.8333 | −0.0500 |
| Elongation at break | −0.2167 | 0.0833 | −0.3163 | 0.0504 | 0.5130 | −0.1333 | −0.2833 | 1.0000 | −0.4667 | 0.2333 | 0.1167 | −0.2833 |
| Tensile strength | −0.1333 | −0.1500 | 0.3591 | −0.3361 | −0.2565 | −0.3167 | −0.0500 | −0.4667 | 1.0000 | −0.3667 | 0.2000 | 0.2167 |
| Haze | 0.3833 | 0.5000 | −0.4275 | 0.2185 | 0.4446 | 0.1000 | −0.0167 | 0.2333 | −0.3667 | 1.0000 | −0.3500 | −0.6000 |
| Transmittance | 0.3500 | 0.3000 | −0.4189 | −0.3109 | 0.3676 | −0.8333 | −0.8333 | 0.1167 | 0.2000 | −0.3500 | 1.0000 | −0.0167 |
| Transparency | −0.1000 | −0.6667 | 0.7780 | −0.0756 | 0.0085 | −0.1500 | −0.0500 | −0.2833 | 0.2167 | −0.6000 | −0.0167 | 1.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrucka, R.; Vapenka, L.; Szymański, M.; Pawlik, M.; Lasik-Kurdyś, M.; Gumienna, M. Bio-Packaging Based on Pectin/Tragacanth Gum with Added Extracts of Cherry Waste from the Wine Industry as a New Generation of Active Films for the Food Industry. Foods 2025, 14, 2203. https://doi.org/10.3390/foods14132203
Dobrucka R, Vapenka L, Szymański M, Pawlik M, Lasik-Kurdyś M, Gumienna M. Bio-Packaging Based on Pectin/Tragacanth Gum with Added Extracts of Cherry Waste from the Wine Industry as a New Generation of Active Films for the Food Industry. Foods. 2025; 14(13):2203. https://doi.org/10.3390/foods14132203
Chicago/Turabian StyleDobrucka, Renata, Lukas Vapenka, Marcin Szymański, Mikołaj Pawlik, Małgorzata Lasik-Kurdyś, and Małgorzata Gumienna. 2025. "Bio-Packaging Based on Pectin/Tragacanth Gum with Added Extracts of Cherry Waste from the Wine Industry as a New Generation of Active Films for the Food Industry" Foods 14, no. 13: 2203. https://doi.org/10.3390/foods14132203
APA StyleDobrucka, R., Vapenka, L., Szymański, M., Pawlik, M., Lasik-Kurdyś, M., & Gumienna, M. (2025). Bio-Packaging Based on Pectin/Tragacanth Gum with Added Extracts of Cherry Waste from the Wine Industry as a New Generation of Active Films for the Food Industry. Foods, 14(13), 2203. https://doi.org/10.3390/foods14132203








