An Elucidation of the Anti-Photoaging Efficacy and Molecular Mechanisms of Epigallocatechin Gallate Nanoparticles in a Balb/c Murine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Epigallocatechin Gallate Nanoparticles (EGCG-NPs)
2.1.1. Preparation of Blank Nanoparticles
2.1.2. Preparation of EGCG-NPs
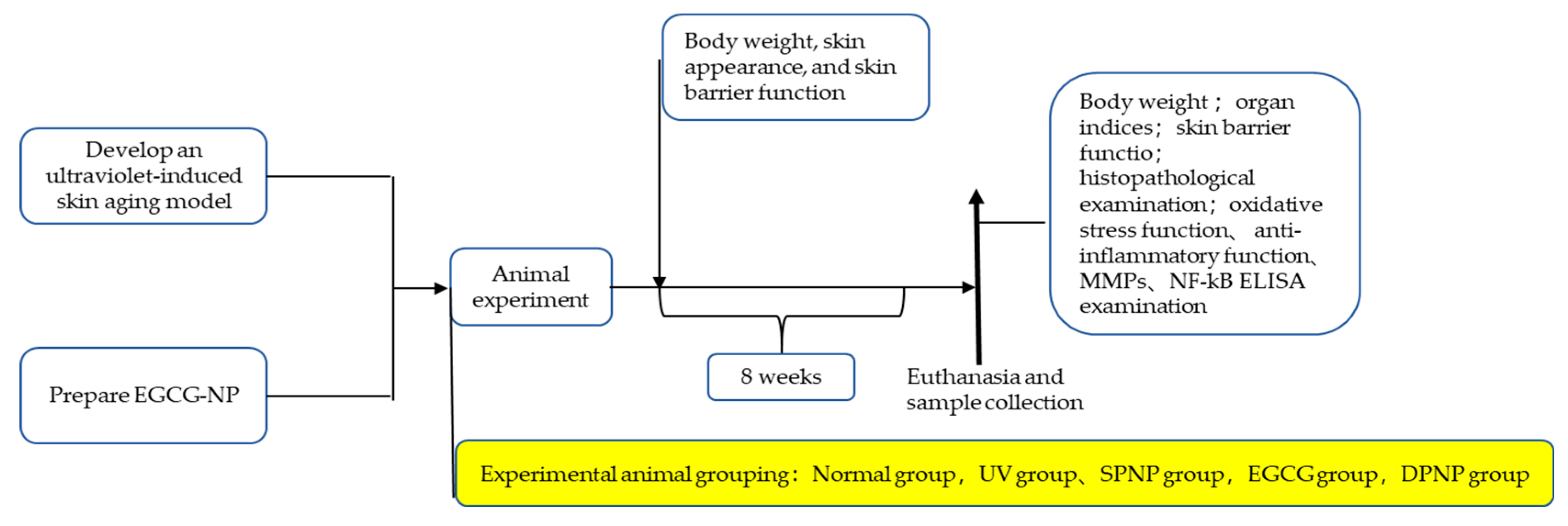
2.2. Animal Care and Skin Aging Model
2.3. Assessment of Skin Barrier Function
2.4. Tissue Harvesting and Organ Index Calculation
2.5. H&E Staining of Liver and Skin Tissues
2.6. Determination of Oxidative Stress Markers in Skin Tissue
2.7. Determination of Inflammatory Markers in Skin Tissue
2.8. Determination of Matrix Metalloproteinase (MMP) Markers in Skin Tissue
2.9. Measurement of NF-κB Signaling Pathway Activity
2.10. Statistics
3. Results
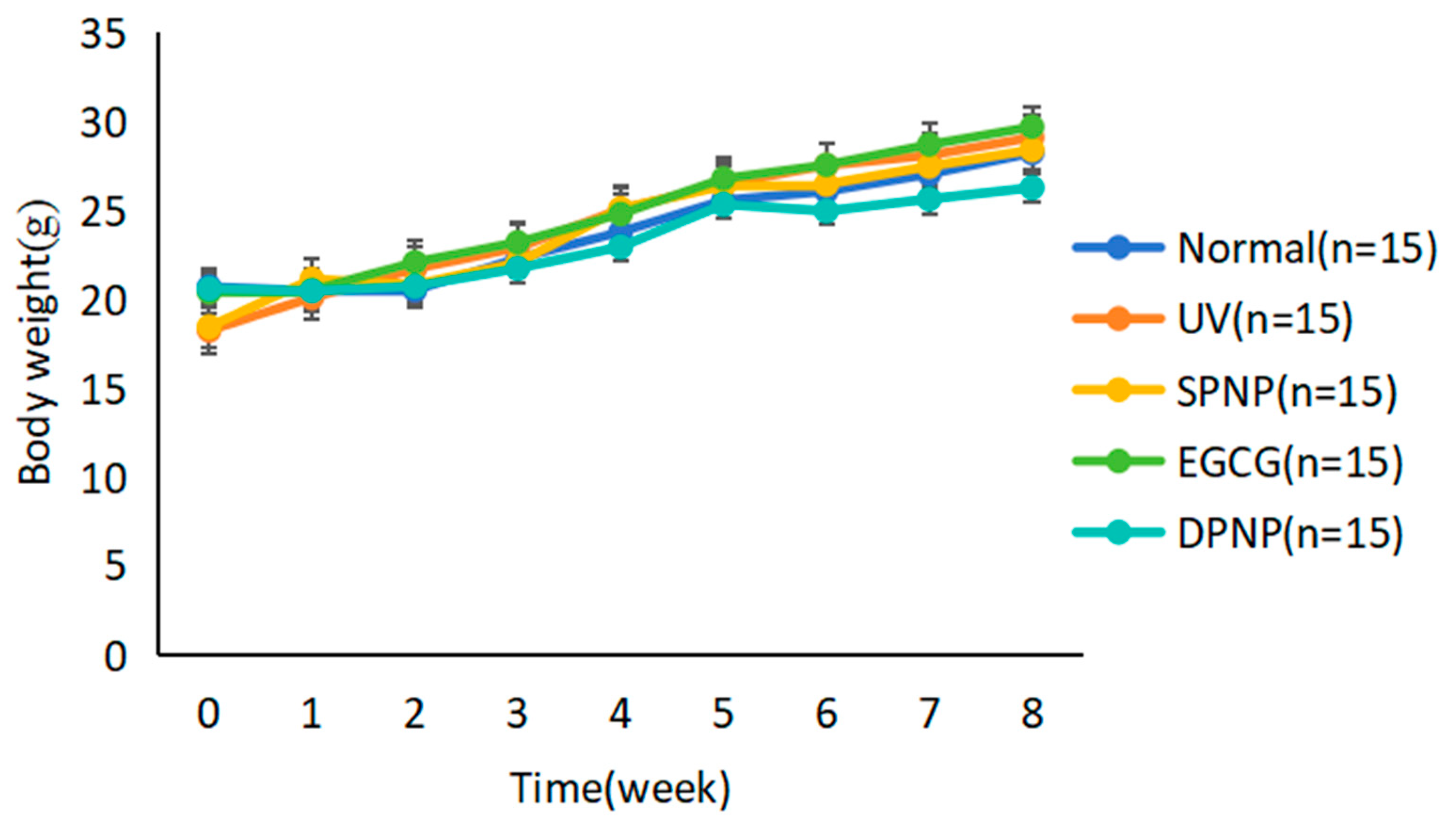
3.1. Effects of EGCG-NPs on General Condition and Body Weight Changes
3.2. Effects of EGCG-NPs on Organ Indices and Hepatic Function
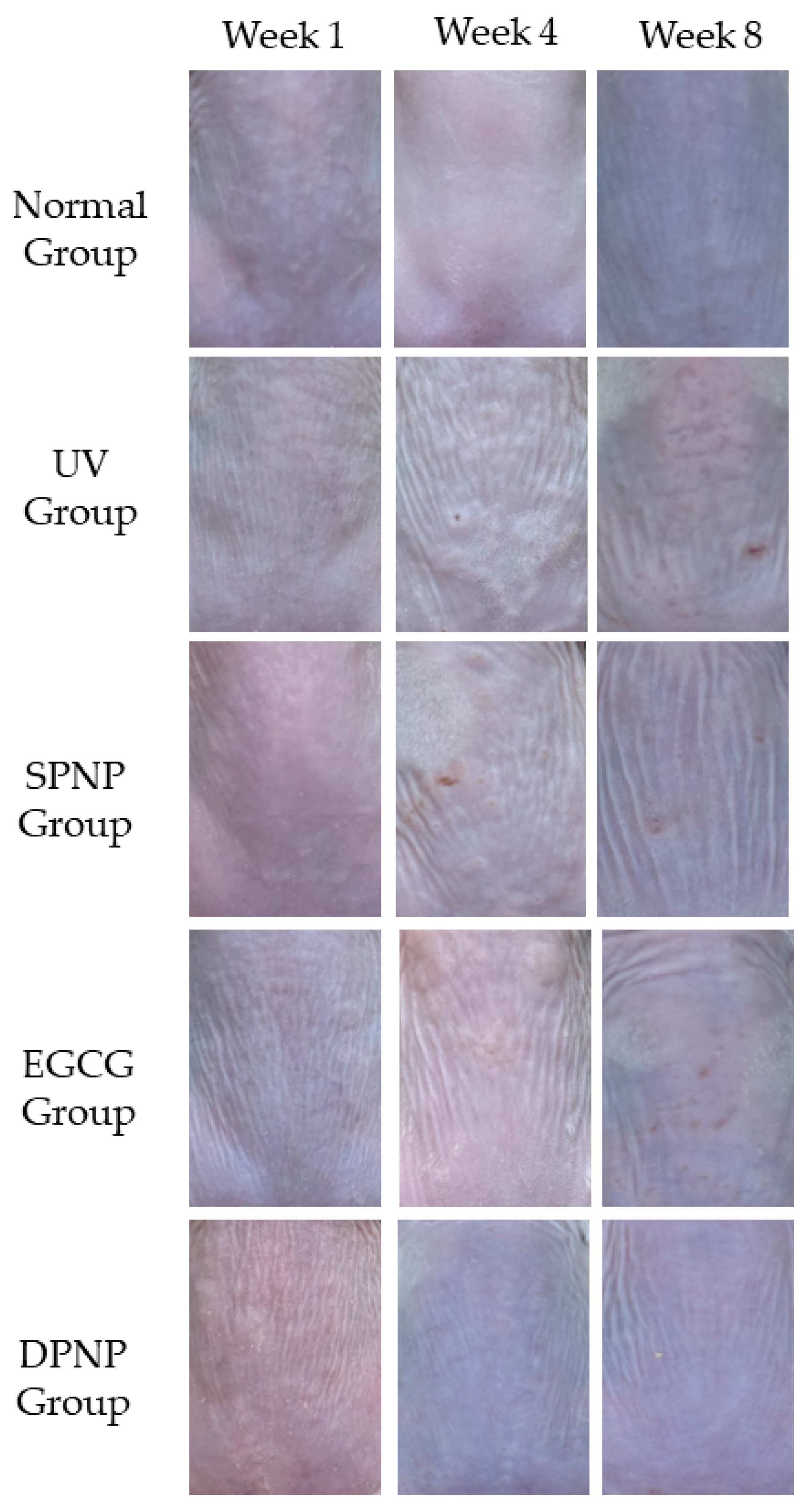
3.3. Effects of EGCG-NPs on Recovery of Dorsal Skin Lesions
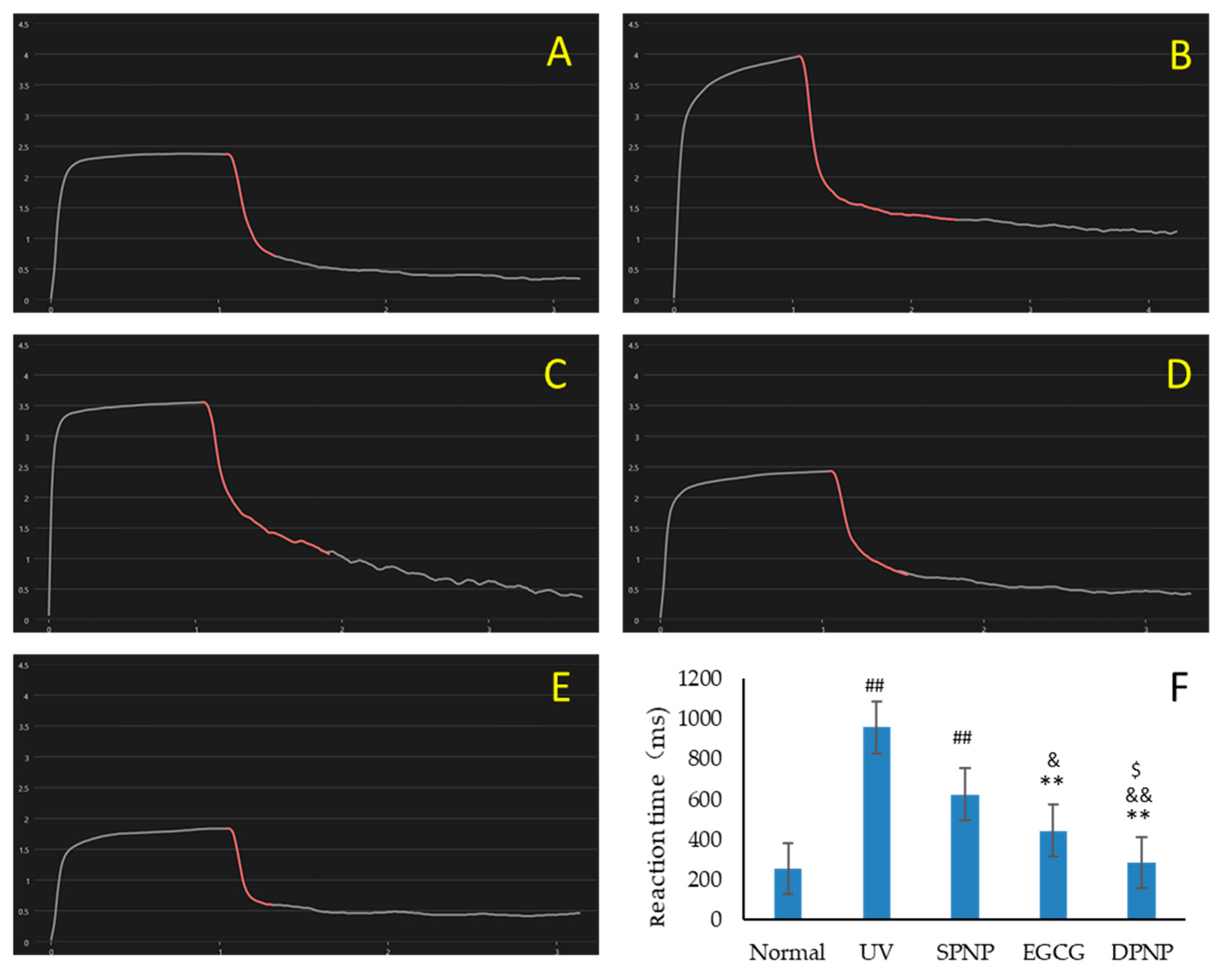
3.4. Effects of EGCG-NPs on Improvement of Skin Barrier Function
3.5. Effects of EGCG-NPs on Pathological Architecture of Skin and Collagen Content
3.6. Effects of EGCG-NPs on Oxidative Stress Function of Skin
3.7. Effects of EGCG-NPs on Anti-Inflammatory Function of Skin
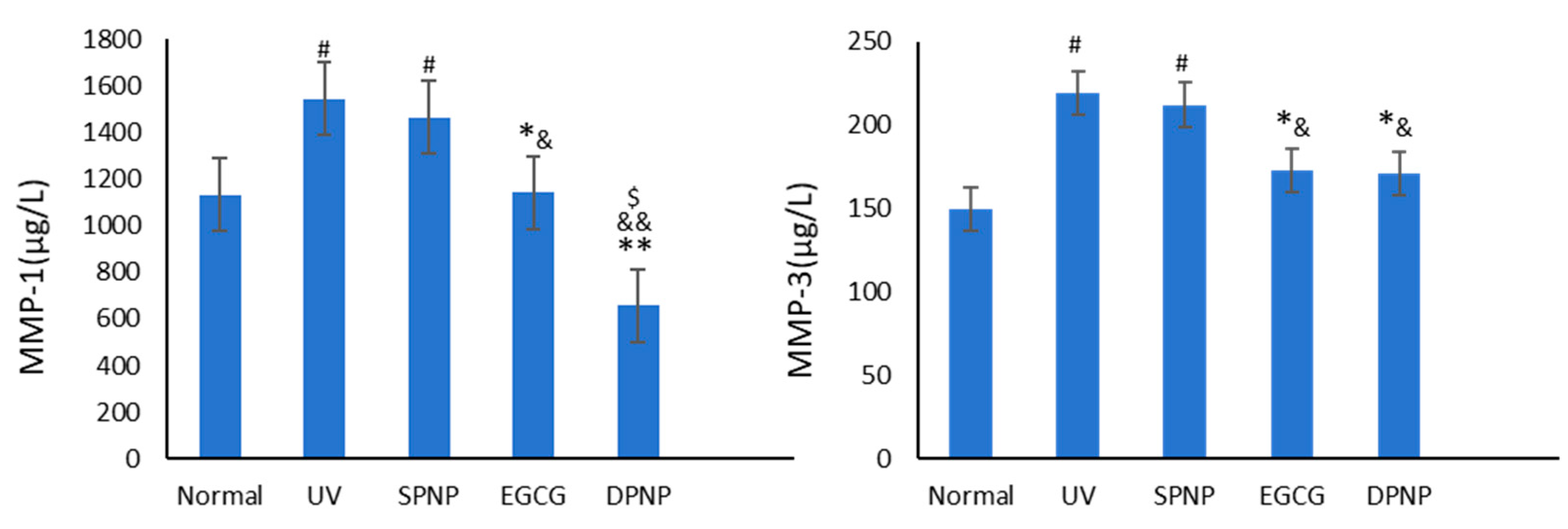
3.8. Effects of EGCG-NPs on Expression Level of MMPs in Skin
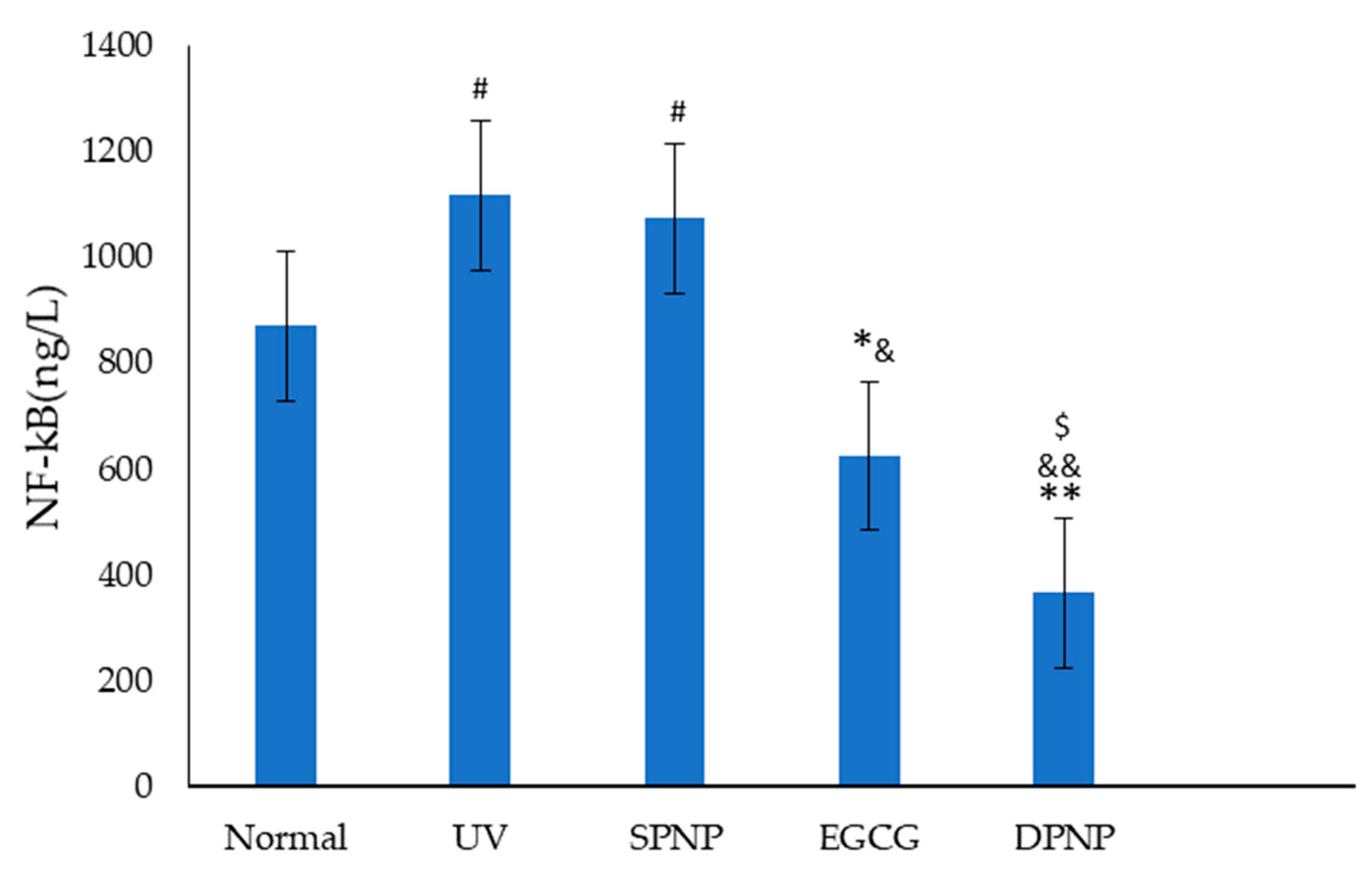
3.9. Effects of EGCG-NPs on NF-κB Signal Transduction Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAT | Catalase |
| ECM | Extracellular matrix |
| EGCG | Epigallocatechin gallate |
| ELISA | Enzyme-linked immunosorbent assay |
| GSH-Px | Glutathione peroxidase |
| H&E | Hematoxylin–eosin |
| IL | Interleukin |
| MDA | Malondialdehyde |
| MED | Minimal erythema dose |
| MMPs | Matrix metalloproteinases |
| NF-kB | Nuclear factor kappa B |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| UV | Ultraviolet |
References
- Shin, J.W.; Kwon, S.H.; Choi, J.Y. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Min, M.; Egli, C.; Sivamani, R.K. The Gut and Skin Microbiome and Its Association with Aging Clocks. Int. J. Mol. Sci. 2024, 25, 7471. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, S.; Yang, H. Mechanisms and Treatments of Skin Photoaging by Ultraviolet Radiation. Med. Recapitul. 2018, 24, 341–346. Available online: https://link.cnki.net/urlid/11.3553.R.20180117.1846.050 (accessed on 19 January 2018).
- Ma, R.; Lin, Y.; Chen, H. Research Advances on Preventive and Curative Effect of Tea Polyphenols on Skin Photoaging. J. Tea Commun. 2012, 39, 25–28. [Google Scholar]
- Ter Haar, G. HIFU Tissue Ablation: Concept and Devices. Adv. Exp. Med. Biol. 2016, 880, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, P.S. Soft tissue filler and botulinum toxin treatment techniques. G. Ital. Dermatol. Venereol. 2012, 147, 29–44. [Google Scholar] [PubMed]
- He, L.; Lin, X.; Chen, H. Update of the treatments for skin photoaging. China J. Lepr. Ski. Dis. 2020, 36, 687–689+696. [Google Scholar]
- Dréno, B.; Alexis, A.; Chuberre, B.; Marinovich, M. Safety of titanium dioxide nanoparticles in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33 (Suppl. S7), 34–46. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A. Glucocorticoids Inhibit Wound Healing: Novel Mechanism of Action. J. Investig. Dermatol. 2017, 137, 1012–1014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baran, R.; Maibach, H.I. Textbook of Cosmetic Dermatology, 3rd ed.; Informa Healthcare: London, UK, 2013; pp. 342–344. [Google Scholar]
- Quan, T. Human Skin Aging and the Anti-Aging Properties of Retinol. Biomolecules 2023, 13, 1614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiao, L.; Tsutsui, T. The lipophilic vitamin C derivative, 6-o-palmitoylascorbate, protects human lymphocytes, preferentially over ascorbate, against X-ray-induced DNA damage, lipid peroxidation, and protein carbonylation. Mol. Cell. Biochem. 2014, 394, 247–259. [Google Scholar] [CrossRef]
- Zhang, Y.; Rao, T.; Liu, H. Research Progress on the Anticancer Mechanism of Tea Polyphenol and EGCG Nanocarrier Technology. Sci. Technol. Food Ind. 2019, 40, 343–348. [Google Scholar] [CrossRef]
- Lai, W.; Baig, M.M.F.A.; Wong, W.-T.; Zhu, B.T. Epigallocatechin-3-gallate in functional food development: From concept to reality. Trends Food Sci. Technol. 2020, 102, 271–279. [Google Scholar] [CrossRef]
- Kim, E.; Hwang, K.; Lee, J. Skin Protective Effect of Epigallocatechin Gallate. Int. J. Mol. Sci. 2018, 19, 173. [Google Scholar] [CrossRef]
- Pires, F.; Geraldo, V.P.N.; Antunes, A. On the role of epigallocatechin-3-gallate in protecting phospholipid molecules against UV irradiation. Colloids Surf. B Biointerfaces 2019, 173, 312–319. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Jing, H.; Ma, C.; Wang, H. Bilosomes as effective delivery systems to improve the gastrointestinal stability and bioavailability of epigallocatechin gallate (EGCG). Food Res. Int. 2021, 149, 110631. [Google Scholar] [CrossRef]
- Bianchi, A.; Marchetti, N.; Scalia, S. Photodegradation of (−)-epigallocatechin-3-gallate in topical cream formulations and its photostabilization. J. Pharm. Biomed. Anal. 2011, 56, 692–697. [Google Scholar] [CrossRef]
- Hong, Z.; Xu, Y.; Yin, J.F.; Jin, J.; Jiang, Y.; Du, Q. Improving the Effectiveness of (−)-Epigallocatechin Gallate (EGCG) against Rabbit Atherosclerosis by EGCG-Loaded Nanoparticles Prepared from Chitosan and Polyaspartic Acid. J. Agric. Food Chem. 2014, 62, 12603–12609. [Google Scholar] [CrossRef]
- Panday, R.; Poudel, A.J.; Li, X.; Adhikari, M.; Ullah, M.W.; Yang, G. Amphiphilic core-shell nanoparticles: Synthesis, biophysical properties, and applications. Colloids Surf. B Biointerfaces 2018, 172, 68–81. [Google Scholar] [CrossRef]
- Girotti, A.W. Photosensitized oxidation of membrane lipids: Reaction pathways, cytotoxic effects, and cytoprotective mechanisms. J. Photochem. Photobiol. B 2001, 63, 103–113. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Lo, H.W.; Korivi, M. Dermato-protective properties of ergothioneine through induction of Nrf2/ARE-mediated antioxidant genes in UVA-irradiated Human keratinocytes. Free. Radic. Biol. Med. 2015, 86, 102–117. [Google Scholar] [CrossRef]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (-)-epigallocatechin gallate. Eur. J. Pharm. Sci. 2010, 41, 219–225. [Google Scholar] [CrossRef]
- Mehmood, S.; Maqsood, M.; Mahtab, N.; Khan, M.I.; Sahar, A.; Zaib, S.; Gul, S. Epigallocatechin gallate: Phytochemistry, bioavailability, utilization challenges, and strategies. J. Food Biochem. 2022, 46, e14189. [Google Scholar] [CrossRef]
- Weihao, W.; Hao, H. Research Progress on the Mechanisms of Skin Aging and the Treatment Strategies of Skin Rejuvenation. Chin. J. Aesthetic Med. 2020, 29, 178–182. [Google Scholar] [CrossRef]
- El-Domyati, M.; Attia, S.; Saleh, F. Intrinsic aging vs. photoaging: A comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 2002, 11, 398–405. [Google Scholar] [CrossRef]
- Moloney, S.J.; Edmonds, S.H.; Giddens, L.D. The hairless mouse model of photoaging: Evaluation of the relationship between dermal elastin, collagen, skin thickness and wrinkles. Photochem. Photobiol. 1992, 56, 505–511. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Matsui, M.S.; Mukhtar, H. Kinetics of UV light-induced cyclobutane pyrimidine dimers in human skin in vivo: An immunohistochemical analysis of both epidermis and dermis. Photochem. Photobiol. 2000, 72, 788–793. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Li, L. Comparison of Different Skin Aging Mice Models. Chin. J. Dermatovenereology 2019, 33, 651–656. [Google Scholar] [CrossRef]
- Wang, P.; Xue, H.; Wang, L. The Role of n-3 Polyunsaturated Fatty Acids in Skin Photoaging. Chin. J. Dermatovenereology 2021, 35, 721–726. [Google Scholar] [CrossRef]
- Meeran, S.M.; Akhtar, S.; Katiyar, S.K. Inhibition of UVB-induced skin tumor development by drinking green tea polyphenols is mediated through DNA repair and subsequent inhibition of inflammation. J. Investig. Dermatol. 2009, 129, 1258–1270. [Google Scholar] [CrossRef]
- Katiyar Santosh, K. Oxidative Stress and Photocarcinogenesis: Strategies for Prevention. In Oxidative Stress, Disease and Cancer; World Scientific: Singapore, 2006; pp. 933–964. [Google Scholar] [CrossRef]
- Wu, S.; Yuan, M.; Gao, Q. Development of epigallocatechin gallate in the inflammatory response ofskin photoaging. J. Food Saf. Qual. 2021, 12, 2513–2519. [Google Scholar] [CrossRef]
- Birkedal-Hansen, H. Catabolism and turnover of collagens: Collagenases. Methods Enzymol. 1987, 144, 140–171. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, B.; Cui, Y.; Shi, M.; Zhao, Y.; Quan, T.; Voorhees, J.J. Skin aging from the perspective of dermal fibroblasts: The interplay between the adaptation to the extracellular matrix microenvironment and cell autonomous processes. J. Cell Commun. Signal. 2023, 17, 523–529. [Google Scholar] [CrossRef]
- Yao, W. Study on the Anti-Photoaging Effects and Mechanisms of UV/H2O2-Degraded Polysaccharides from Sargassum Fusiforme. Master’s Thesis, South China University of Technology, Guangzhou, China, 2022. Available online: https://link.cnki.net/doi/10.27151/d.cnki.ghnlu.2022.002426 (accessed on 16 October 2024).
- Jin, Y. Study on the Repairing Effect and Mechanism of Acanthopanax Senticosus Glycoprotein on Skin Photoaging Induced by Ultraviolet Rays; Changchun University of Chinese Medicine: Changchun, China, 2022; Available online: https://link.cnki.net/doi/10.26980/d.cnki.gcczc.2022.000005 (accessed on 16 January 2023).
- Yu, J.; Wang, J.; Su, W. Preventive Role of Extracts from Herba Siegesbeckiae Against Acute Hepatic and Renal Injury Induced by Doxorubicin in Rats. Her. Med. 2014, 33, 422–426. [Google Scholar]
- Castaño, O.; Pérez-Amodio, S.; Navarro-Requena, C.; Mateos-Timoneda, M.Á.; Engel, E. Instructive microenvironments in skin wound healing: Biomaterials as signal releasing platforms. Adv. Drug Deliv. Rev. 2018, 129, 95–117. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A.; Padwad, Y.; Sharma, R. Preadipocyte secretory factors differentially modulate murine macrophage functions during aging which are reversed by the application of phytochemical EGCG. Biogerontology 2020, 21, 325–343. [Google Scholar] [CrossRef]
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The impact of ultraviolet radiation on skin photoaging—Review of in vitro studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.; Shin, D.W. The Molecular Mechanism of Polyphenols with Anti-Aging Activity in Aged Human Dermal Fibroblasts. Molecules 2022, 27, 4351. [Google Scholar] [CrossRef]
- Bang, E.; Kim, D.H.; Chung, H.Y. Protease-activated receptor 2 induces ROS-mediated inflammation through Akt-mediated NF-κB and FoxO6 modulation during skin photoaging. Redox Biol. 2021, 44, 102022. [Google Scholar] [CrossRef]
- Yuan, H.; Li, Y.; Ling, F.; Guan, Y.; Zhang, D.; Zhu, Q.; Liu, J.; Wu, Y.; Niu, Y. The phytochemical epigallocatechin gallate prolongs the lifespan by improving lipid metabolism, reducing inflammation and oxidative stress in high-fat diet-fed obese rats. Aging Cell 2020, 19, e13199. [Google Scholar] [CrossRef]
- Wang, L.; Lee, W.; Cui, Y.R.; Ahn, G.; Jeon, Y.J. Protective effect of green tea catechin against urban fine dust particle-induced skin aging by regulation of NF-κB, AP-1, and MAPKs signaling pathways. Environ. Pollut. 2019, 252 Pt B, 1318–1324. [Google Scholar] [CrossRef]
- Ikehata, H.; Yamamoto, M. Roles of the KEAP1-NRF2 System in Mammalian Skin Exposed to UV Radiation. Toxicol. Appl. Pharmacol. 2018, 360, 69–77. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. The Role of Transcription Factor Nrf2 in Skin Cells Metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef]
- Xian, D.; Xiong, X.; Xu, J.; Xian, L.; Lei, Q.; Song, J. Nrf2 Overexpression for the Protective Effect of Skin-Derived Precursors against UV-Induced Damage: Evidence from a Three-Dimensional Skin Model. Oxidative Med. Cell. Longev. 2019, 2019, 7021428. [Google Scholar] [CrossRef]
- Chen, S.J.; Hseu, Y.C.; Gowrisankar, Y.V.; Chung, Y.T.; Zhang, Y.Z.; Way, T.D. The Anti-melanogenic Effects of 3-O-Ethyl Ascorbic Acid via Nrf2-Mediated α-MSH Inhibition in UVA-Irradiated Keratinocytes and Autophagy Induction in Melanocytes. Free. Radic. Biol. Med. 2021, 173, 151–169. [Google Scholar] [CrossRef]
- Park, C.; Park, J.; Kim, W.-J.; Kim, W.; Cheong, H.; Kim, S.-J. Malonic Acid Isolated from Pinus Densiflora Inhibits UVB-Induced Oxidative Stress and Inflammation in HaCaT Keratinocytes. Polymers 2021, 13, 816. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]

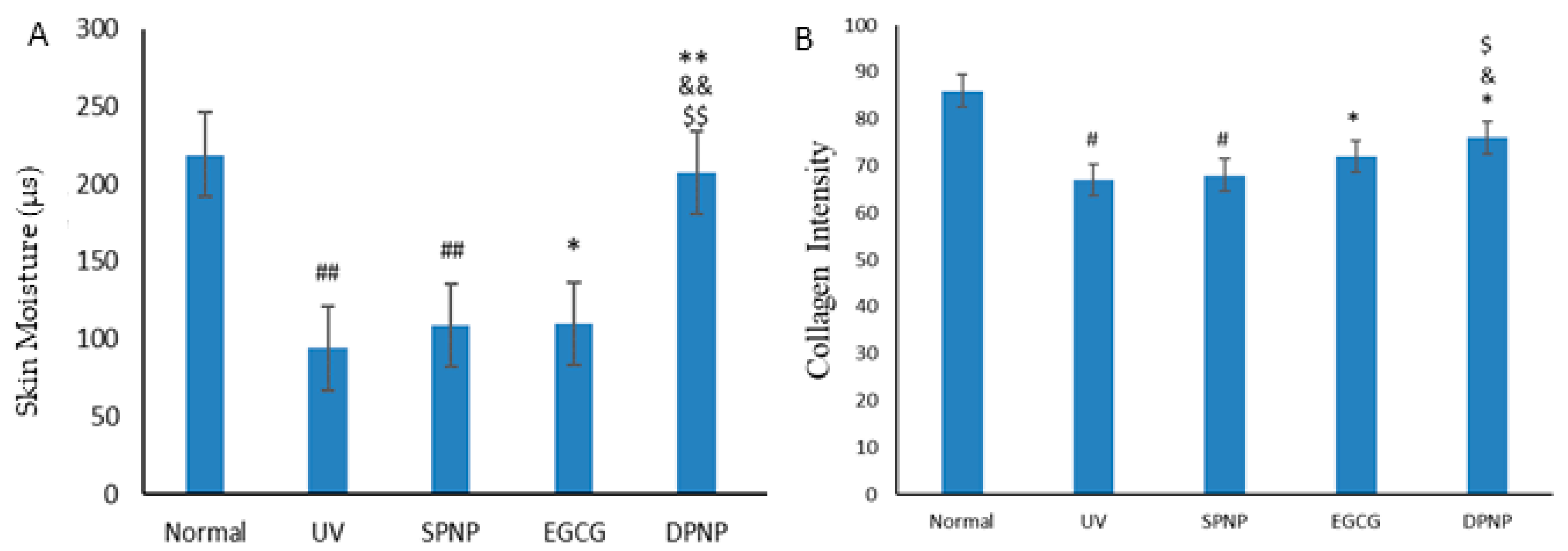


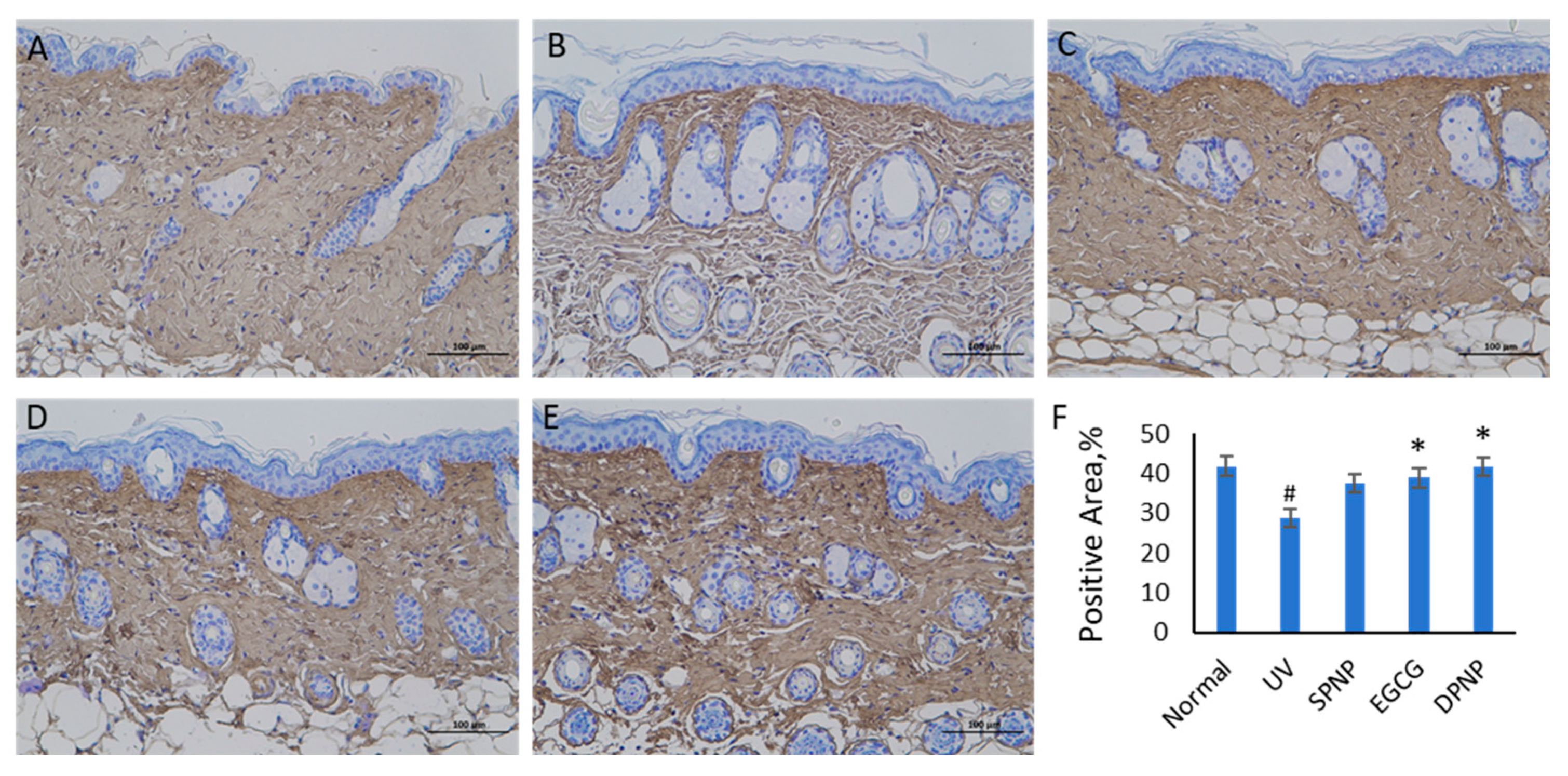
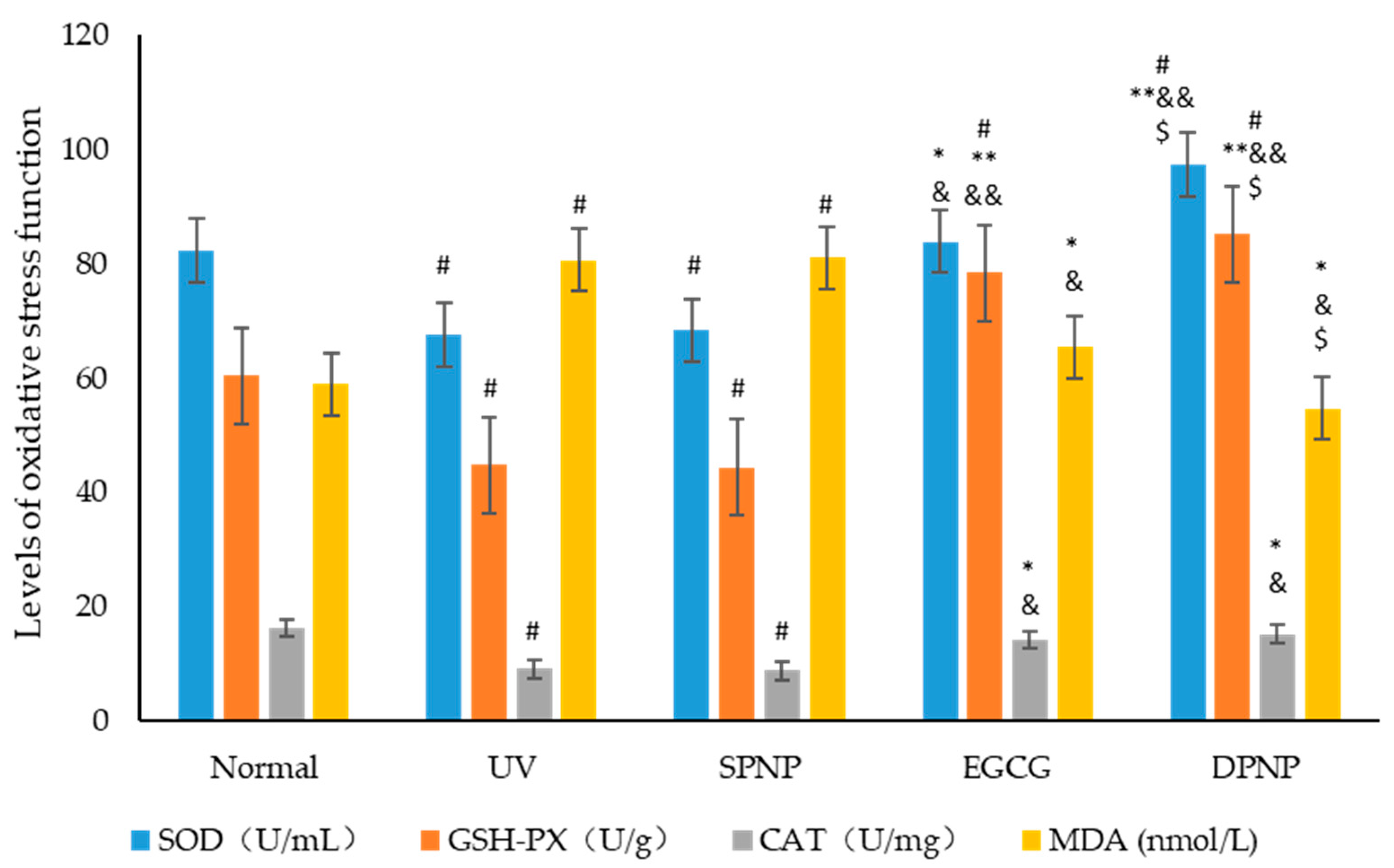
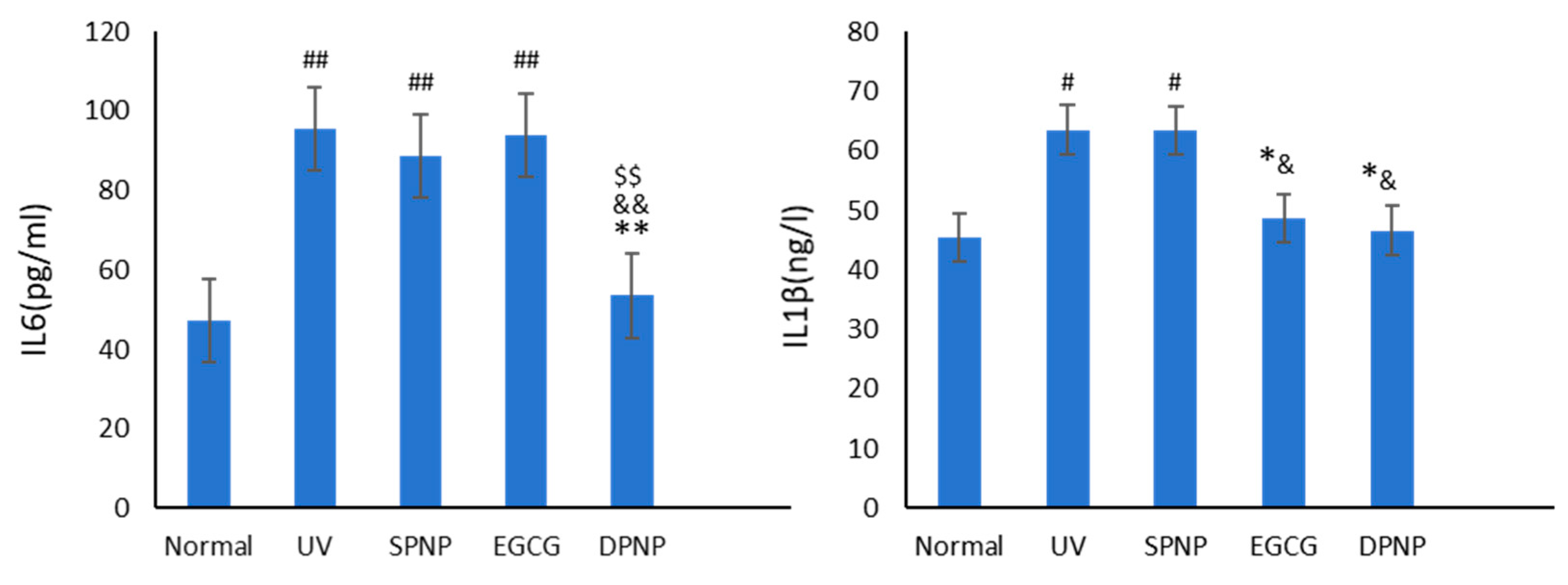
| Group | Number | Intervention Protocol |
|---|---|---|
| Normal | 15 | received no treatment for 8 weeks |
| UV | 15 | UV irradiation for 5 min once daily for 8 weeks |
| SPNP | 15 | UV irradiation for 5 min + oral gavage with 0.1 mL/10 g blank nanoparticle solution once daily for 8 weeks |
| EGCG | 15 | UV irradiation for 5 min + oral gavage with 0.1 mL/10 g EGCG solution (1.1 mg/g.bw) once daily for 8 weeks |
| DPNP | 15 | UV irradiation for 5 min + oral gavage with 0.11 mL/10 g EGCG nanoparticle solution (1 mg/mL) once daily for 8 weeks |
| Group | Liver Index | Spleen Index | Kidney Index | Heart Index |
|---|---|---|---|---|
| Normal | 5.4 ± 0.24 | 0.4 ± 0.06 | 1.8 ± 0.13 | 0.7 ± 0.13 |
| UV | 5.4 ± 0.23 | 0.4 ± 0.05 | 1.9 ± 0.12 | 0.6 ± 0.08 |
| SPNP | 5.4 ± 0.23 | 0.4 ± 0.08 | 1.8 ± 0.10 | 0.6 ± 0.15 |
| EGCG | 5.5 ± 0.41 | 0.4 ± 0.06 | 1.9 ± 0.09 | 0.7 ± 0.11 |
| DPNP | 5.6 ± 0.41 | 0.6 ± 0.10 | 1.9 ± 0.12 | 0.8 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, F.; Wang, F.; Kuo, L.; Huang, P.; Liu, A.; Wang, G.; Tang, X.; Guan, K.; Xie, Y.; Wang, J. An Elucidation of the Anti-Photoaging Efficacy and Molecular Mechanisms of Epigallocatechin Gallate Nanoparticles in a Balb/c Murine Model. Foods 2025, 14, 2150. https://doi.org/10.3390/foods14132150
Xia F, Wang F, Kuo L, Huang P, Liu A, Wang G, Tang X, Guan K, Xie Y, Wang J. An Elucidation of the Anti-Photoaging Efficacy and Molecular Mechanisms of Epigallocatechin Gallate Nanoparticles in a Balb/c Murine Model. Foods. 2025; 14(13):2150. https://doi.org/10.3390/foods14132150
Chicago/Turabian StyleXia, Fangzhi, Fei Wang, Liangchun Kuo, Pengyu Huang, Anyi Liu, Gao Wang, Xiaomin Tang, Kun Guan, Ying Xie, and Junbo Wang. 2025. "An Elucidation of the Anti-Photoaging Efficacy and Molecular Mechanisms of Epigallocatechin Gallate Nanoparticles in a Balb/c Murine Model" Foods 14, no. 13: 2150. https://doi.org/10.3390/foods14132150
APA StyleXia, F., Wang, F., Kuo, L., Huang, P., Liu, A., Wang, G., Tang, X., Guan, K., Xie, Y., & Wang, J. (2025). An Elucidation of the Anti-Photoaging Efficacy and Molecular Mechanisms of Epigallocatechin Gallate Nanoparticles in a Balb/c Murine Model. Foods, 14(13), 2150. https://doi.org/10.3390/foods14132150







