Preparation, Characterization, and In Vitro Stability Analysis of Deer Sinew Peptide-Zinc Chelate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Deer Tendon Peptides
2.3. Optimization of Zinc Chelation Conditions for Deer Tendon Peptides
2.4. Analysis of Zinc Content
2.5. Characterization of DSPs Versus DSPs-Zn Chelates
2.5.1. UV-Vis Analysis
2.5.2. FT-IR Analysis
2.5.3. Analysis of Particle Size and Zeta Potential
2.5.4. SEM and EDS Analysis
2.5.5. Molecular Weight Distribution
2.5.6. Amino Acid Composition
2.6. Solubility Analysis of Zinc at Different pH Conditions
2.7. Simulated Gastrointestinal Digestion Experiment
2.8. Statistical Analysis
3. Results and Discussion
3.1. Process Optimization of DSPs-Zn Preparation Conditions
3.1.1. One-Factor Analysis
3.1.2. Response Surface Optimization
3.2. Characterization of DSPs and DSPs-Zn
3.2.1. UV-Vis Analysis
3.2.2. FT-IR Analysis
3.2.3. Analysis of Particle Size and Zeta Potential
3.2.4. Scanning Electron Microscopy and Elemental Composition Analysis
3.2.5. Molecular Weight Distribution
3.2.6. Amino Acid Composition
3.3. Solubility Analysis of Zinc at Different pH Conditions
3.4. Simulated Gastrointestinal Digestion Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DSPs | deer tendon peptide |
| DSPs-Zn | deer tendon peptide-zinc chelate |
| BBD | Box-Behnken Design |
| UV-vis | ultraviolet-visible absorption spectroscopy |
| FT-IR | Fourier transform infrared |
| SEM | scanning electron microscopy |
| EDS | energy dispersive spectrometer |
References
- Huang, C.; Hightower, K.E.; Fierke, C.A. Mechanistic Studies of Rat Protein Farnesyltransferase Indicate an Associative Transition State. Biochemistry 2000, 39, 2593–2602. [Google Scholar] [CrossRef] [PubMed]
- Halsted, C.H. Dietary Supplements and the American Journal of Clinical Nutrition. Am. J. Clin. Nutr. 2023, 71, 399–400. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.I.; Sarmento-Ribeiro, A.B.; Gonçalves, A.C. Zinc: From Biological Functions to Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 4822. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Jiang, W.; Wang, X.; Shahid, S.; Saba, N.; Ahmad, M.; Dar, A.; Masood, S.U.; Imran, M.; Mustafa, A. Mechanistic Impact of Zinc Deficiency in Human Development. Front. Nutr. 2022, 9, 717064. [Google Scholar] [CrossRef]
- Gregory, P.J.; Wahbi, A.; Adu-Gyamfi, J.; Heiling, M.; Gruber, R.; Joy, E.J.M.; Broadley, M.R. Approaches to Reduce Zinc and Iron Deficits in Food Systems. Glob. Food Secur. 2017, 15, 1–10. [Google Scholar] [CrossRef]
- Maret, W.; Sandstead, H.H. Zinc Requirements and the Risks and Benefits of Zinc Supplementation. J. Trace Elements Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef]
- Sandstead, H.H.; Smith, J.C. Deliberations and Evaluations of Approaches, Endpoints and Paradigms for Determining Zinc Dietary Recommendations. J. Nutr. 2018, 126, 2410S–2418S. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, F.; Liu, X.; Zhao, M. Particulate Nanocomposite from Oyster (Crassostrea rivularis) Hydrolysates Via Zinc Chelation Improves Zinc Solubility and Peptide Activity. Food Chem. 2018, 258, 269–277. [Google Scholar] [CrossRef]
- Tanishige, H.; Yamanaka, H.; Masuyama, R. Early Regulation of Intestinal Calcium Transport in Response to Luminal Calcium. Clin. Nutr. ESPEN 2024, 63, 1164. [Google Scholar] [CrossRef]
- Udechukwu, M.C.; Collins, S.A.; Udenigwe, C.C. Prospects of Enhancing Dietary Zinc Bioavailability with Food-Derived Zinc-Chelating Peptides. Food Funct. 2016, 7, 4137–4144. [Google Scholar] [CrossRef]
- Guo, L.; Harnedy, P.A.; Li, B.; Hou, H.; Zhang, Z.; Zhao, X.; FitzGerald, R.J. Food Protein-Derived Chelating Peptides: Biofunctional Ingredients for Dietary Mineral Bioavailability Enhancement. Trends Food Sci. Technol. 2014, 37, 92–105. [Google Scholar] [CrossRef]
- Miquel, E.; Farré, R. Effects and Future Trends of Casein Phosphopeptides on Zinc Bioavailability. Trends Food Sci. Technol. 2007, 18, 139–143. [Google Scholar] [CrossRef]
- Li, J.; Gong, C.; Wang, Z.; Gao, R.; Ren, J.; Zhou, X.; Wang, H.; Xu, H.; Xiao, F.; Cao, Y.; et al. Oyster-Derived Zinc-Binding Peptide Modified by Plastein Reaction via Zinc Chelation Promotes the Intestinal Absorption of Zinc. Mar. Drugs 2019, 17, 341. [Google Scholar] [CrossRef]
- Udechukwu, M.C.; Downey, B.; Udenigwe, C.C. Influence of Structural and Surface Properties of Whey-Derived Peptides on Zinc-Chelating Capacity, and in Vitro Gastric Stability and Bioaccessibility of the Zinc-Peptide Complexes. Food Chem. 2017, 240, 1227–1232. [Google Scholar] [CrossRef]
- Suo, H.; Lu, L.; Zhang, L.; Zhang, X.; Li, H.; Lu, Y.; Luo, X. Relative Bioavailability of Zinc-Methionine Chelate for Broilers Fed a Conventional Corn–Soybean Meal Diet. Biol. Trace Element Res. 2015, 165, 206–213. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, Y.; Qi, B.; Liu, L.; Zhou, G.; Bai, X.; Yang, C.; Zhao, D.; Zhao, Y. Preventive Effects of Collagen Peptide from Deer Sinew on Bone Loss in Ovariectomized Rats. Evidence-Based Complement. Altern. Med. 2014, 2014, 627285. [Google Scholar] [CrossRef] [PubMed]
- Her, C.; Thompson, A.R.; Karim, C.B.; Thomas, D.D. Probing the Calcium-Dependent Structural States of Calmodulin-RyR using Bifunctional Spin Labels and Deer. Biophys. J. 2018, 114, 159A. [Google Scholar] [CrossRef]
- Wen, C.; Wang, D.; Zhang, Z.; Liu, G.; Liang, L.; Liu, X.; Zhang, J.; Li, Y.; Xu, X. Intervention Effects of Deer-Tendon Collagen Hydrolysates on Osteoporosis In Vitro and In Vivo. Molecules 2023, 28, 6275. [Google Scholar] [CrossRef]
- Cao, S.; Wang, Y.; Xing, L.; Zhang, W.; Zhou, G. Structure and Physical Properties of Gelatin from Bovine Bone Collagen Influenced by Acid Pretreatment and Pepsin. Food Bioprod. Process. 2020, 121, 213–223. [Google Scholar] [CrossRef]
- Xie, N.; Huang, J.; Li, B.; Cheng, J.; Wang, Z.; Yin, J.; Yan, X. Affinity Purification and Characterisation of Zinc Chelating Peptides from Rapeseed Protein Hydrolysates: Possible Contribution of Characteristic Amino Acid Residues. Food Chem. 2014, 173, 210–217. [Google Scholar] [CrossRef]
- Xu, B.; Wang, X.; Zheng, Y.; Shi, P.; Zhang, Y.; Liu, Y.; Long, N. Millet Bran Globulin Hydrolysate Derived Tetrapeptide-Ferrous Chelate: Preparation, Structural Characterization, Security Prediction in Silico, and Stability against Different Food Processing Conditions. LWT—Food Sci. Technol. 2022, 165, 113673. [Google Scholar] [CrossRef]
- Na, S.; Jin, Z.; Li, D.; Yin, H.; Lin, S. An Exploration of the Calcium-Binding Mode of Egg White Peptide, Asp-His-Thr-Lys-Glu, and In Vitro Calcium Absorption Studies of Peptide–Calcium Complex. J. Agric. Food Chem. 2017, 165, 113673. [Google Scholar]
- Zhao, F.; Hou, W.; Guo, L.; Wang, C.; Liu, Y.; Liu, X.; Min, W. Novel Strategy to the Characterization and Enhance the Glycemic Control Properties of Walnut-Derived Peptides Via Zinc Chelation. Food Chem. 2023, 441, 138288. [Google Scholar] [CrossRef]
- Fang, S.; Ruan, G.; Hao, J.; Regenstein, J.M.; Wang, F. Characterization and Antioxidant Properties of Manchurian Walnut Meal Hydrolysates After Calcium Chelation. LWT 2020, 130, 109632. [Google Scholar] [CrossRef]
- Zhai, W.; Lin, D.; Mo, R.; Zou, X.; Zhang, Y.; Zhang, L.; Ge, Y. Process Optimization, Structural Characterization, and Calcium Release Rate Evaluation of Mung Bean Peptides-Calcium Chelate. Foods 2023, 12, 1058. [Google Scholar] [CrossRef]
- Wu, W.; He, L.; Liang, Y.; Yue, L.; Peng, W.; Jin, G.; Ma, M. Preparation Process Optimization of Pig Bone Collagen Peptide-Calcium Chelate Using Response Surface Methodology and Its Structural Characterization and Stability Analysis. Food Chem. 2019, 284, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Lan, Y.; Liao, W.; Lin, L.; Liu, G.; Xu, H.; Xue, J.; Guo, B.; Cao, Y.; Miao, J. Preparation, Characterization and Biological Activities of Egg White Peptides-Calcium Chelate. LWT 2021, 149, 112035. [Google Scholar] [CrossRef]
- Cui, P.; Sun, N.; Jiang, P.; Wang, D.; Lin, S. Optimised Condition for Preparing Sea Cucumber Ovum Hydrolysate–Calcium Complex and Its Structural Analysis. Int. J. Food Sci. Technol. 2017, 52, 1914–1922. [Google Scholar] [CrossRef]
- Peng, B.; Chen, Z.; Wang, Y. Preparation and Characterization of an Oyster Peptide–Zinc Complex and Its Antiproliferative Activity on Hepg2 Cells. Mar. Drugs 2023, 21, 542. [Google Scholar] [CrossRef]
- Fu, T.; Zhang, S.; Sheng, Y.; Feng, Y.; Jiang, Y.; Zhang, Y.; Yu, M.; Wang, C. Isolation and Characterization of Zinc-Binding Peptides from Mung Bean Protein Hydrolysates. Eur. Food Res. Technol. 2019, 246, 113–124. [Google Scholar] [CrossRef]
- Sun, R.; Liu, X.; Yu, Y.; Miao, J.; Leng, K.; Gao, H. Preparation Process Optimization, Structural Characterization and In Vitro Digestion Stability Analysis of Antarctic Krill (Euphausia superba) Peptides-Zinc Chelate. Food Chem. 2020, 340, 128056. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, J.; Pei, H.; Shi, M.; Chen, W.; Zong, Y.; Zhao, Y.; Li, J.; Du, R.; He, Z. Structural Characterisation of Deer Sinew Peptides as Calcium Carriers, Their Promotion of MC3T3-E1 Cell Proliferation and Their Effect on Bone Deposition in Mice. Food Funct. 2024, 15, 2587–2603. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, Q.; Huang, S.; Lin, J.; Wang, S.; Huang, Y.; Hong, J.; Rao, P. Novel Peptide with a Specific Calcium-Binding Capacity from Whey Protein Hydrolysate and the Possible Chelating Mode. J. Agric. Food Chem. 2014, 62, 10274–10282. [Google Scholar] [CrossRef]
- Qu, W.; Feng, Y.; Xiong, T.; Li, Y.; Wahia, H.; Ma, H. Preparation of Corn Ace Inhibitory Peptide-Ferrous Chelate By Dual-Frequency Ultrasound and Its Structure and Stability Analyses. Ultrason. Sonochem. 2022, 83, 105937. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Li, M.; Mao, X.; Zhou, J.; Ren, F. Preparation and Characteristics of Yak Casein Hydrolysate–Iron Complex. Int. J. Food Sci. Technol. 2011, 46, 1705–1710. [Google Scholar] [CrossRef]
- Xiang, H.; Huang, H.; Sun-Waterhouse, D.; Hu, X.; Li, L.; Waterhouse, G.I.N.; Tang, R.; Xiong, J.; Cui, C. Enzymatically Synthesized Γ-[Glu](N≥1)-Gln As Novel Calcium-Binding Peptides to Deliver Calcium with Enhanced Bioavailability. Food Chem. 2022, 387, 132918. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, J.; Tong, P.S.; Mao, X.Y. Zinc-Binding Capacity of Yak Casein Hydrolysate and the Zinc-Releasing Characteristics of Casein Hydrolysate-Zinc Complexes. J. Dairy Sci. 2011, 94, 2731–2740. [Google Scholar] [CrossRef]
- Liao, W.; Lai, T.; Chen, L.; Fu, J.; Sreenivasan, S.T.; Yu, Z.; Ren, J. Synthesis and Characterization of a Walnut Peptides–Zinc Complex and Its Antiproliferative Activity against Human Breast Carcinoma Cells through the Induction of Apoptosis. J. Agric. Food Chem. 2016, 64, 1509–1519. [Google Scholar] [CrossRef]
- Wu, J.; Cai, X.; Tang, M.; Wang, S. Novel Calcium—Chelating Peptides from Octopus Scraps and Their Corresponding Calcium Bioavailability. J. Sci. Food Agric. 2018, 99, 536–545. [Google Scholar] [CrossRef]
- Li, B.; He, H.; Shi, W.; Hou, T. Effect of Duck Egg White Peptide-Ferrous Chelate on Iron Bioavailability In Vivo and Structure Characterization. J. Sci. Food Agric. 2018, 99, 1834–1841. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Lu, W.; Liu, J.; Zhang, W.; Wang, Y.; Ma, M.; Cao, X.; Guo, Y. Cell Proliferation Stimulation Ability and Osteogenic Activity of Low Molecular Weight Peptides Derived from Bovine Gelatin Hydrolysates. J. Agric. Food Chem. 2020, 68, 7630–7640. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Ren, L.; Jiang, J. Purification of a Histidine-Containing Peptide with Calcium Binding Activity from Shrimp Processing Byproducts Hydrolysate. Eur. Food Res. Technol. 2010, 232, 281–287. [Google Scholar] [CrossRef]
- Chaud, M.V.; Izumi, C.; Nahaal, Z.; Shuhama, T.; Bianchi, M.d.L.P.; de Freitas, O. Iron Derivatives from Casein Hydrolysates as a Potential Source in the Treatment of Iron Deficiency. J. Agric. Food Chem. 2002, 50, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Lao, L.; Jian, H.; Liao, W.; Zeng, C.; Liu, G.; Cao, Y.; Miao, J. Casein Calcium-Binding Peptides: Preparation, Characterization, and Promotion of Calcium Uptake in Caco-2 Cell Monolayers. Process. Biochem. 2023, 130, 78–86. [Google Scholar] [CrossRef]
- Kállay, C.; Ősz, K.; Dávid, A.; Valastyán, Z.; Malandrinos, G.; Hadjiliadis, N.; Sóvágó, I. Zinc(ii) Binding Ability of Tri-, Tetra- and Penta-Peptides Containing Two or Three Histidyl Residues. Dalton Trans. 2007, 36, 4040–4047. [Google Scholar] [CrossRef]
- Suyin, Z.; Zheng, Y.; He, S.; Su, D.; Nag, A.; Zeng, Q.; Yuan, Y. Novel Zn-Binding Peptide Isolated from Soy Protein Hydrolysates: Purification, Structure, and Digestion. J. Agric. Food Chem. 2020, 69, 483–490. [Google Scholar]
- Li, C.; Zhou, Y.; Cao, L.; Liu, Y.; Huang, Z.; Li, B.; Xu, L. Characterization and Stability of Soybean Meal Peptide-Zinc Chelate Prepared by Solid State Fermentation with Aspergillus oryzae. Food Biosci. 2023, 56, 103380. [Google Scholar] [CrossRef]
- Ke, X.; Hu, X.; Li, L.; Yang, X.; Chen, S.; Wu, Y.; Xue, C. A Novel Zinc-Binding Peptide Identified from Tilapia (Oreochromis Niloticus) Skin Collagen and Transport Pathway Across Caco-2 Monolayers. Food Biosci. 2021, 42, 101127. [Google Scholar] [CrossRef]
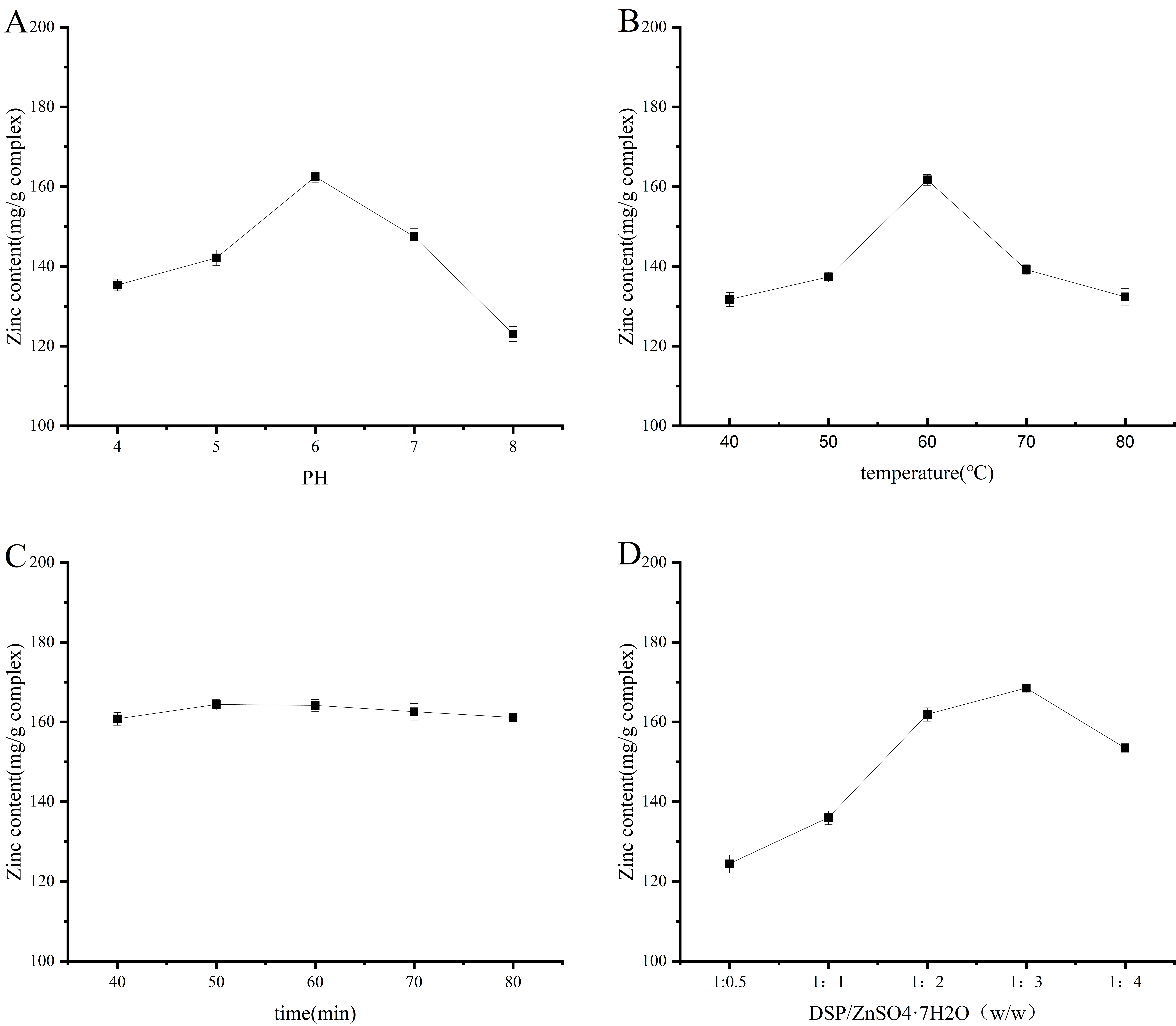
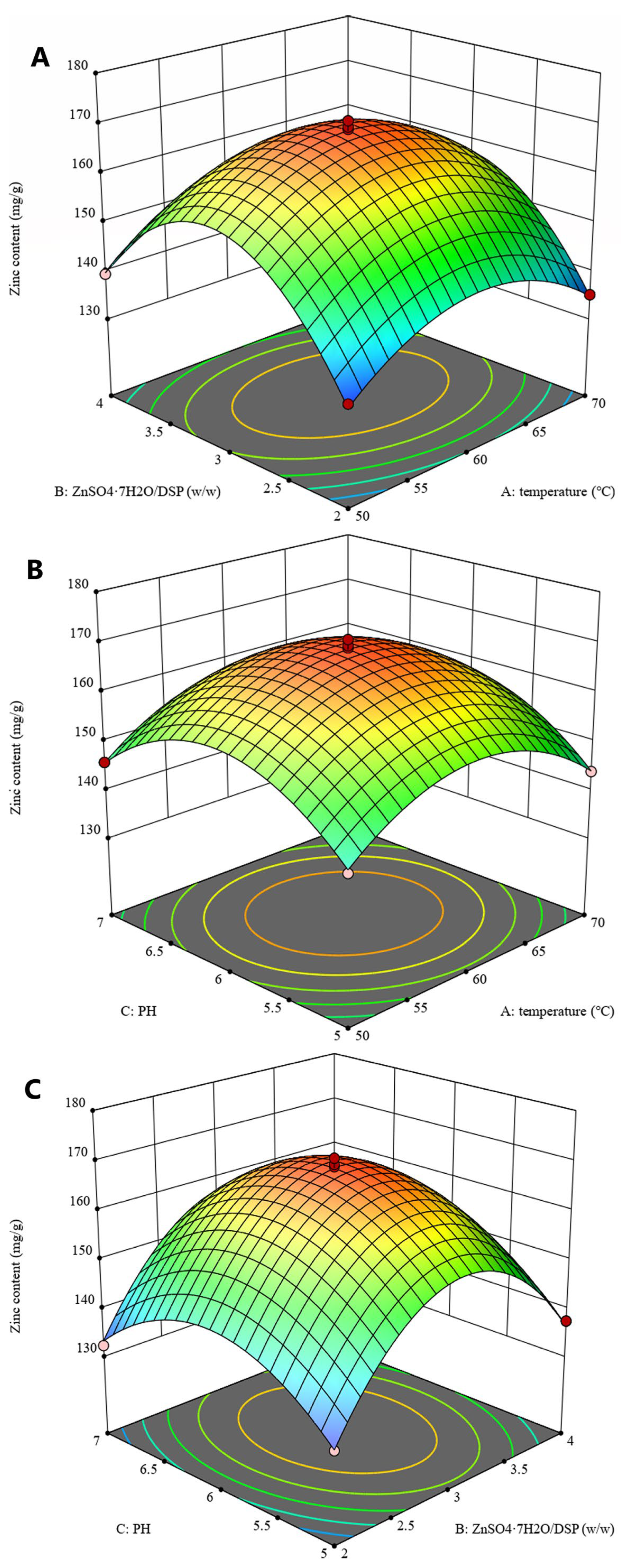
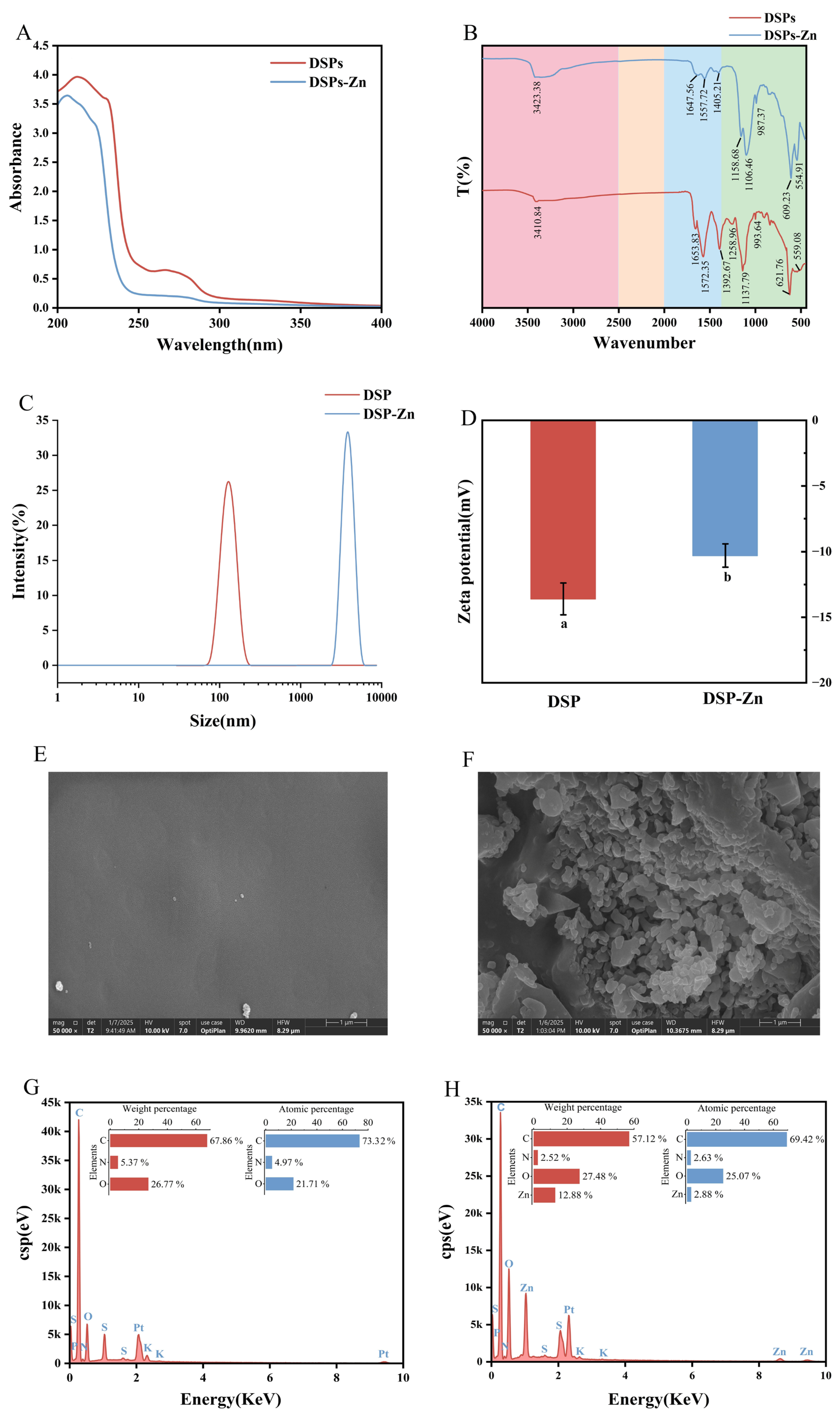

| Coded Levels of Variables | ||||
|---|---|---|---|---|
| No. | A: Temperature/°C | B: Mass Ratio of Zinc: Peptide | C: PH | Zinc Content mg/g |
| 1 | 50 | 2 | 6 | 133.67 |
| 2 | 60 | 3 | 6 | 166.12 |
| 3 | 60 | 2 | 5 | 131.83 |
| 4 | 60 | 3 | 6 | 169.43 |
| 5 | 70 | 3 | 7 | 147.76 |
| 6 | 50 | 4 | 6 | 139.45 |
| 7 | 60 | 4 | 7 | 140.52 |
| 8 | 50 | 3 | 7 | 145.85 |
| 9 | 50 | 3 | 5 | 142.94 |
| 10 | 70 | 3 | 5 | 143.95 |
| 11 | 60 | 3 | 6 | 168.21 |
| 12 | 70 | 4 | 6 | 138.76 |
| 13 | 60 | 4 | 5 | 137.46 |
| 14 | 60 | 3 | 6 | 168.95 |
| 15 | 60 | 2 | 7 | 132.28 |
| 16 | 70 | 2 | 6 | 135.12 |
| 17 | 60 | 3 | 6 | 170.65 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Modle | 3385.60 | 9 | 376.18 | 169.46 | <0.0001 | ** |
| A | 1.75 | 1 | 1.75 | 0.79 | 0.4043 | |
| B | 68.15 | 1 | 68.15 | 30.70 | 0.0009 | ** |
| C | 13.08 | 1 | 13.08 | 5.89 | 0.0456 | * |
| AB | 1.21 | 1 | 1.21 | 0.50 | 0.4845 | |
| AC | 0.20 | 1 | 0.20 | 0.09 | 0.7714 | |
| BC | 1.70 | 1 | 1.70 | 0.78 | 0.4101 | |
| A2 | 525.08 | 1 | 525.08 | 236.54 | <0.0001 | ** |
| B2 | 1816.35 | 1 | 1816.35 | 818.24 | <0.0001 | ** |
| C2 | 645.30 | 1 | 645.30 | 290.70 | <0.0001 | ** |
| Residual | 15.54 | 7 | 2.22 | |||
| Lack of Fit | 4.25 | 3 | 1.42 | 0.50 | 0.7012 | not significant |
| Pure Error | 11.29 | 4 | 2.82 | |||
| Cor Total | 3401.14 | 16 | R2 | 0.9954 |
| Molecular Weight (Da) | DSPs (%) | DSPs-Zn (%) |
|---|---|---|
| >10,000 | 0.05 ± 0.01 | 0.07 ± 0.01 |
| 10,000–5000 | 7.69 ± 0.06 | 11.29 ± 0.56 ** |
| 5000–3000 | 8.95 ± 0.07 | 17.48 ± 0.75 ** |
| 3000–1000 | 22.84 ± 0.16 | 31.69 ± 0.48 ** |
| 1000–500 | 35.91 ± 0.23 | 24.45 ± 0.14 ** |
| <500 | 24.56 ± 0.18 | 15.02 ± 0.26 ** |
| Amino Acid | Composition (%) | |
|---|---|---|
| DSPs | DSPs-Zn | |
| Asp | 6.46 ± 0.06 | 8.59 ± 0.06 ** |
| Thr | 2.14 ± 0.15 | 1.46 ± 0.14 * |
| Ser | 3.74 ± 0.11 | 3.58 ± 0.19 |
| Glu | 10.71 ± 0.18 | 11.46 ± 0.13 ** |
| Gly | 22.31 ± 0.17 | 22.28 ± 0.21 |
| Ala | 10.34 ± 0.10 | 10.56 ± 0.12 |
| Val | 2.43 ± 0.11 | 2.08 ± 0.12 * |
| Met | 3.29 ± 0.08 | 3.52 ± 0.10 |
| Ile | 1.27 ± 0.13 | 0.95 ± 0.15 |
| Leu | 2.98 ± 0.10 | 2.04 ± 0.11 ** |
| Tyr | 4.54 ± 0.16 | 4.65 ± 0.19 |
| Phe | 1.84 ± 0.11 | 1.59 ± 0.10 |
| Lys | 3.11 ± 0.12 | 3.74 ± 0.16 ** |
| His | 0.69 ± 0.15 | 0.47 ± 0.14 ** |
| Arg | 8.12 ± 0.14 | 9.18 ± 0.11 ** |
| Pro | 15.28 ± 0.15 | 14.31 ± 0.22 ** |
| acidic amino acids (Glu and Asp) | 17.27 ± 0.24 | 20.14 ± 0.26 ** |
| alkaline amino acids (Lys, Arg and His) | 11.98 ± 0.21 | 13.39 ± 0.14 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Liu, T.; Chen, W.; Zong, Y.; Geng, J.; Zhao, Y.; He, Z.; Du, R. Preparation, Characterization, and In Vitro Stability Analysis of Deer Sinew Peptide-Zinc Chelate. Foods 2025, 14, 2131. https://doi.org/10.3390/foods14122131
Yang S, Liu T, Chen W, Zong Y, Geng J, Zhao Y, He Z, Du R. Preparation, Characterization, and In Vitro Stability Analysis of Deer Sinew Peptide-Zinc Chelate. Foods. 2025; 14(12):2131. https://doi.org/10.3390/foods14122131
Chicago/Turabian StyleYang, Shan, Tianyuan Liu, Weijia Chen, Ying Zong, Jianan Geng, Yan Zhao, Zhongmei He, and Rui Du. 2025. "Preparation, Characterization, and In Vitro Stability Analysis of Deer Sinew Peptide-Zinc Chelate" Foods 14, no. 12: 2131. https://doi.org/10.3390/foods14122131
APA StyleYang, S., Liu, T., Chen, W., Zong, Y., Geng, J., Zhao, Y., He, Z., & Du, R. (2025). Preparation, Characterization, and In Vitro Stability Analysis of Deer Sinew Peptide-Zinc Chelate. Foods, 14(12), 2131. https://doi.org/10.3390/foods14122131







