The Impact of Imidacloprid in Dietary Residues on Intestinal Damage and the Increased Risk of Enterotoxigenic Escherichia coli Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Chemicals
2.2. Design of the Experiment
2.3. In Vitro Intestinal Permeability Assay
2.4. Histopathological Examination
2.5. Short-Chain Fatty Acid Analysis
2.6. ETEC Infection
2.7. ETEC Burden in Fecal Samples
2.8. Flow Cytometry Analysis of T Lymphocytes Located in Intestinal Lamina Propria
2.9. Gene Expression Analysis
2.10. Inflammatory Cytokine Measurements
2.11. Statistical Analysis
3. Results
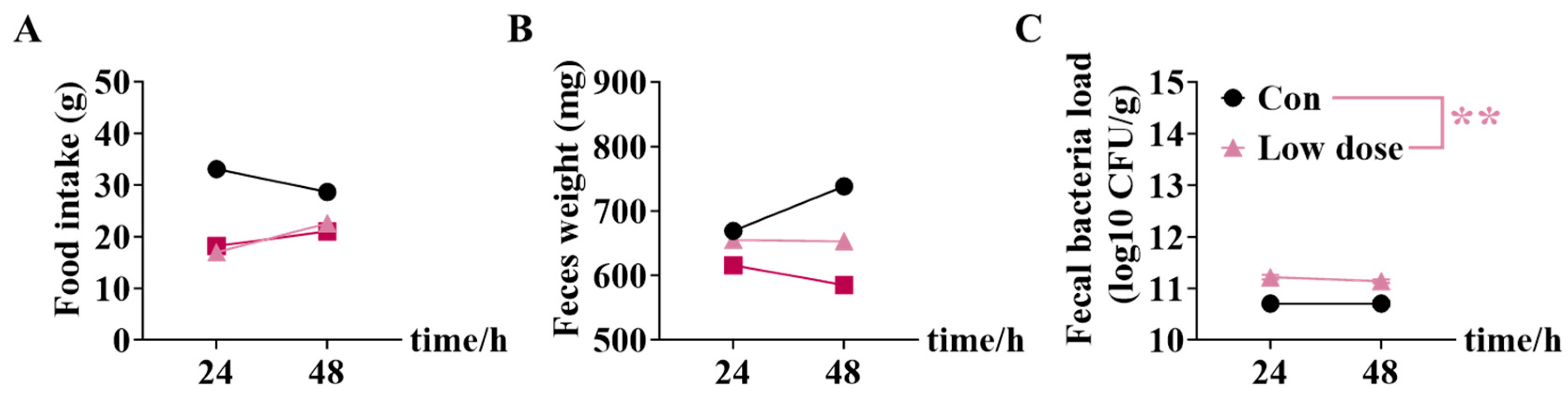
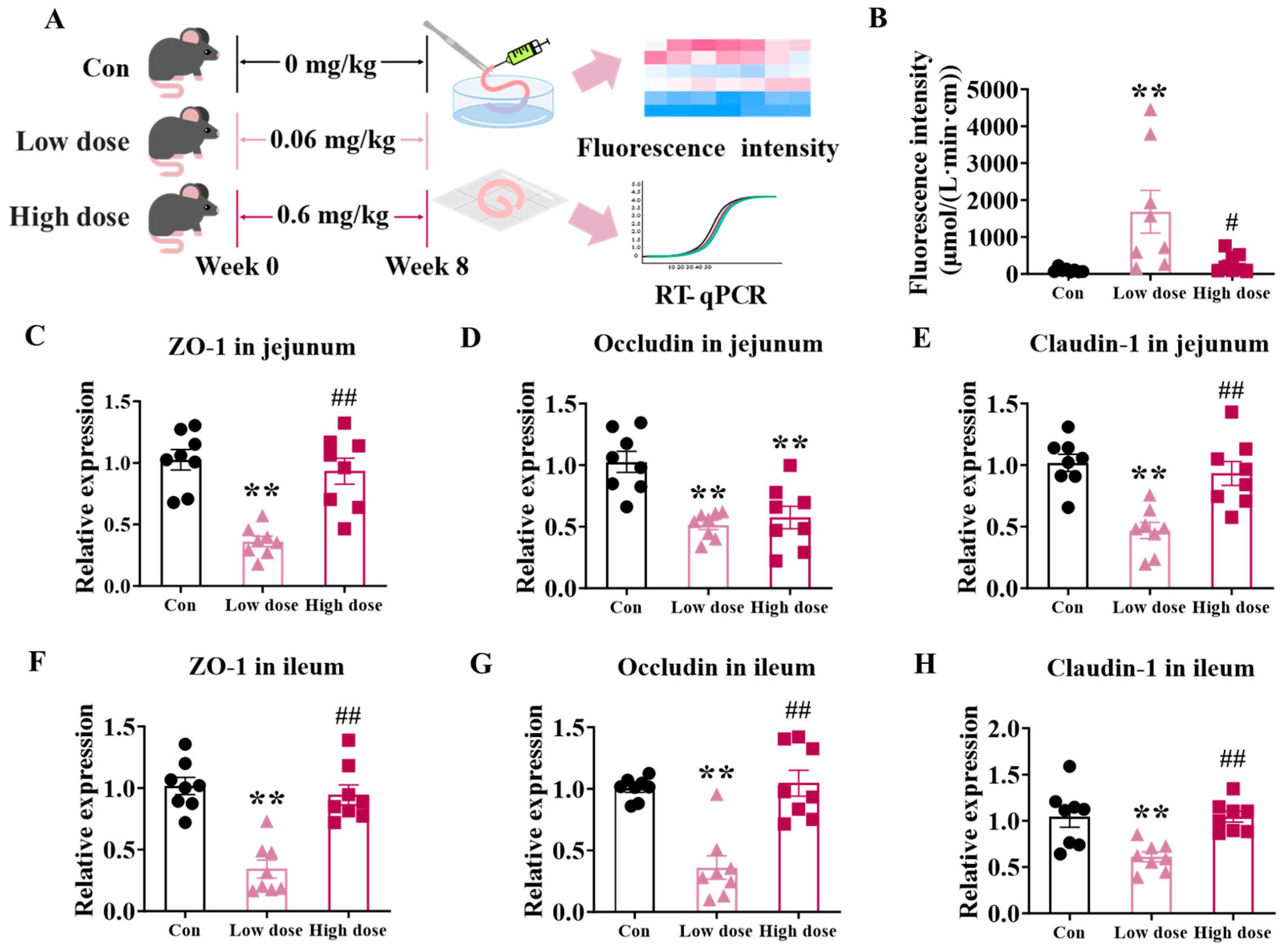
3.1. Exposure to Low Dose of IMI Significantly Damaged Intestinal Integrity Whereas High Dose of IMI Just Induced Inflammation
3.2. IMI Exposure Significantly Damaged the Structure of Villi and Proliferation, but Only the Low Dose Significantly Disturbed Intestinal Stem Cells
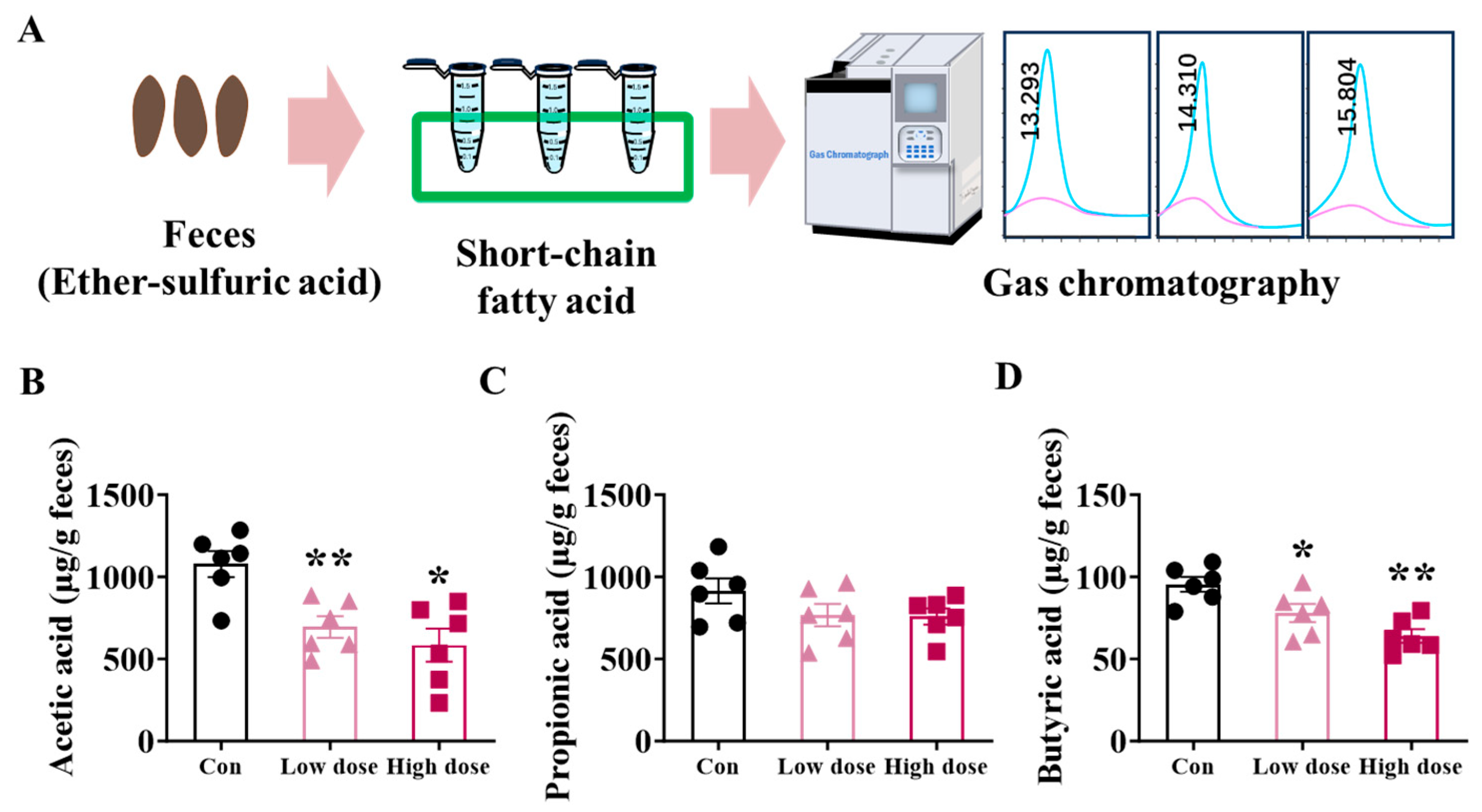
3.3. IMI Exposure Decreased SCFA Production and Mucus Layer in the Colon at Both Doses
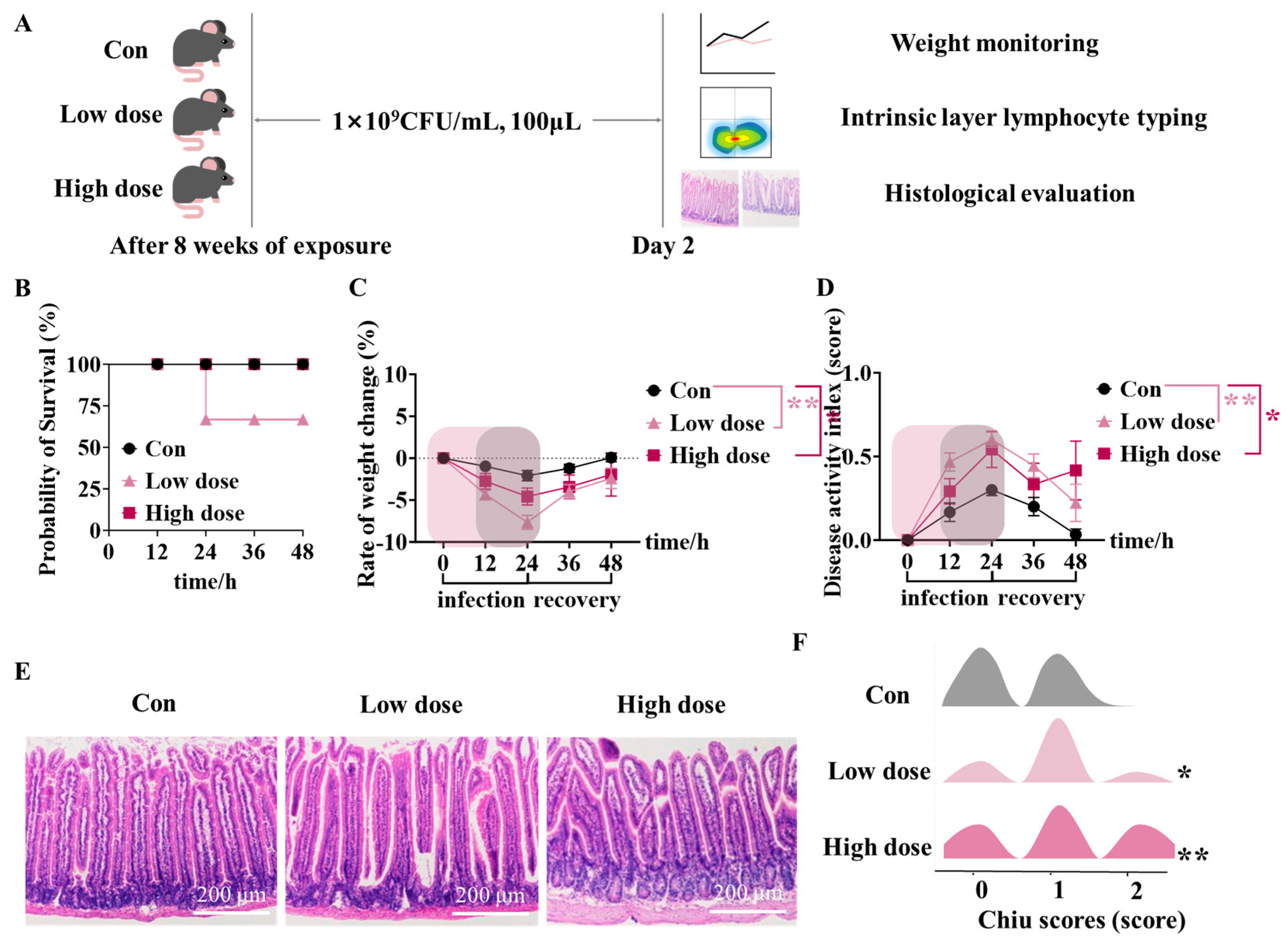
3.4. IMI Exposure Led to Susceptibility to ETEC Infection and Even Death in Mice of the Low Dose Group
3.5. Mice Survived Due to Activation of Intestinal Lamina Propria Immunity at High Dose
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IMI | Imidacloprid |
| ETEC | Escherichia coli |
| ZO-1 | Zonula occludens-1 |
| ISCs | Intestinal stem cells |
| Treg | Regulatory T |
| AB-PAS | Alcian blue-picric acid Schiff |
| H&E | Hematoxylin–eosin |
| SCFAs | Short-chain fatty acids |
| DAI | Disease activity index |
| CFUs | Colony-forming units |
| IL-1β | Interleukin-1 beta |
| TNF-α | Tumor necrosis factor-α |
| ROS | Reactive oxygen species |
| IBD | Inflammatory bowel disease |
Appendix A


References
- Agace, W.W.; McCoy, K.D. Regionalized Development and Maintenance of the Intestinal Adaptive Immune Landscape. Immunity 2017, 46, 532–548. [Google Scholar] [CrossRef] [PubMed]
- Martens, E.C.; Neumann, M.; Desai, M.S. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat. Rev. Microbiol. 2018, 16, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Wang, G.; Han, R.; Xie, X. Effects of chlorpyrifos on the gut microbiome and urine metabolome in mouse (Mus musculus). Chemosphere 2016, 153, 287–293. [Google Scholar] [CrossRef]
- Fang, B.; Li, J.W.; Zhang, M.; Ren, F.Z.; Pang, G.F. Chronic chlorpyrifos exposure elicits diet-specific effects on metabolism and the gut microbiome in rats. Food Chem. Toxicol. 2018, 111, 144–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Q.; Hu, C.; Han, M.; Guo, Q.; Li, S.; Bo, C.; Zhang, Y.; Qi, X.; Sai, L.; et al. Effects of chlorpyrifos exposure on liver inflammation and intestinal flora structure in mice. Toxicol. Res. 2021, 10, 141–149. [Google Scholar] [CrossRef]
- Sun, Y.; Pei, J.; Chen, X.; Lin, M.; Pan, Y.; Zhang, Y.; Bai, W.; Zhou, X.; Zhang, W. The role of the gut microbiota in depressive-like behavior induced by chlorpyrifos in mice. Ecotoxicol. Environ. Saf. 2023, 250, 114470. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Malagelada, J.R. Gut flora in health and disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- DuPont, A.W.; DuPont, H.L. The intestinal microbiota and chronic disorders of the gut. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 523–531. [Google Scholar] [CrossRef]
- Woodhouse, C.A.; Patel, V.C.; Singanayagam, A.; Shawcross, D.L. Review article: The gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment. Pharmacol. Ther. 2018, 47, 192–202. [Google Scholar] [CrossRef]
- Hou, J.; Hu, M.; Zhang, L.; Gao, Y.; Ma, L.; Xu, Q. Dietary Taxifolin Protects Against Dextran Sulfate Sodium-Induced Colitis via NF-kappaB Signaling, Enhancing Intestinal Barrier and Modulating Gut Microbiota. Front. Immunol. 2020, 11, 631809. [Google Scholar]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y.; Wang, W.; Bu, W.; Song, J.; Tan, X.; Sun, E.; Ning, Q.; et al. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2021, 166, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Huang, J.; Sun, M.; Jiang, Y.; Wang, S.; Wang, L.; Yu, N.; Peng, D.; Wang, Y.; Chen, W.; et al. Poria cocos polysaccharide improves intestinal barrier function and maintains intestinal homeostasis in mice. Int. J. Biol. Macromol. 2023, 249, 125953. [Google Scholar] [CrossRef]
- Barker, N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Bio. 2014, 15, 19–33. [Google Scholar] [CrossRef]
- Huels, D.J.; Bruens, L.; Hodder, M.C.; Cammareri, P.; Campbell, A.D.; Ridgway, R.A.; Gay, D.M.; Solar-Abboud, M.; Faller, W.J.; Nixon, C.; et al. Wnt ligands influence tumour initiation by controlling the number of intestinal stem cells. Nat. Commun. 2018, 9, 1132. [Google Scholar] [CrossRef] [PubMed]
- Peeters, T.; Vantrappen, G. The Paneth cell: A source of intestinal lysozyme. Gut 1975, 16, 553–558. [Google Scholar] [CrossRef]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.Y.; Ko, H.J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends. Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef]
- Gustafsson, J.K.; Johansson, M.E.V. The role of goblet cells and mucus in intestinal homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 785–803. [Google Scholar] [CrossRef]
- Chen, D.W.; Zhang, Y.P.; Lv, B.; Liu, Z.B.; Han, J.J.; Li, J.G.; Zhao, Y.F.; Wu, Y.N. Dietary exposure to neonicotinoid insecticides and health risks in the Chinese general population through two consecutive total diet studies. Environ. Int. 2020, 135, 105399. [Google Scholar] [CrossRef]
- Lu, C.S.; Chang, C.H.; Palmer, C.; Zhao, M.R.; Zhang, Q. Neonicotinoid Residues in Fruits and Vegetables: An Integrated Dietary Exposure Assessment Approach. Environ. Sci. Technol. 2018, 52, 3175–3184. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Zhang, Y.Y.; Li, J.H.; Su, G.Y.; Chen, Q.; Ding, Z.; Sun, H. Serum concentrations of neonicotinoids, and their associations with lipid molecules of the general residents in Wuxi City, Eastern China. J. Hazard. Mater. 2021, 413, 125235. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Abdourahime, H.; Anastassiadou, M.; Brancato, A.; Brocca, D.; Carrasco Cabrera, L.; De Lentdecker, C.; Ferreira, L.; Greco, L.; Jarrah, S.; et al. Review of the existing maximum residue levels for imidacloprid according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2019, 17, e05570. [Google Scholar]
- Crentsil, K.B.; Akwasi, A.A.; Felicia, A.; Samuel, F.; Achibold, B. Dietary exposure to chlorinated pesticide residues in fruits and vegetables from Ghanaian markets. J. Food Compos. Anal. 2016, 46, 103–113. [Google Scholar]
- Arfat, Y.; Mahmood, N.; Tahir, M.U.; Rashid, M.; Anjum, S.; Zhao, F.; Li, D.J.; Sun, Y.L.; Hu, L.; Zhihao, C.; et al. Effect of imidacloprid on hepatotoxicity and nephrotoxicity in male albino mice. Toxicol. Rep. 2014, 1, 554–561. [Google Scholar] [CrossRef]
- Khalil, S.R.; Awad, A.; Mohammed, H.H.; Nassan, M.A. Imidacloprid insecticide exposure induces stress and disrupts glucose homeostasis in male rats. Environ. Toxicol. Pharmacol. 2017, 55, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.P.; Wang, X.Y.; Li, J.W.; Wang, R.; Ren, F.Z.; Pang, G.F.; Li, Y.X. Imidacloprid increases intestinal permeability by disrupting tight junctions. Ecotoxicol. Environ. Saf. 2021, 222, 112476. [Google Scholar] [CrossRef]
- Craddock, H.A.; Huang, D.; Turner, P.C.; Quirós-Alcalá, L.; Payne-Sturges, D.C. Trends in neonicotinoid pesticide residues in food and water in the United States, 1999–2015. Environ. Health. 2019, 18, 1–16. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Z.B.; Chang, C.H.; Yu, C.; Wang, X.M.; Lu, C.S. Dietary risk of neonicotinoid insecticides through fruit and vegetable consumption in school-age children. Environ. Int. 2019, 126, 672–681. [Google Scholar] [CrossRef]
- Klingensmith, N.J.; Fay, K.T.; Swift, D.A.; Bazzano, J.M.; Lyons, J.D.; Chen, C.W.; Meng, M.; Ramonell, K.M.; Liang, Z.; Burd, E.M.; et al. Junctional adhesion molecule-A deletion increases phagocytosis and improves survival in a murine model of sepsis. J. Clin. Insight. 2022, 7, e156255. [Google Scholar] [CrossRef]
- Chen, R.Z.; Xu, Y.; Wu, P.; Zhou, H.; Lasanajak, Y.; Fang, Y.Y.; Tang, L.; Ye, L.; Li, X.; Cai, Z.; et al. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol. Res. 2019, 148, 104403. [Google Scholar] [CrossRef]
- Li, M.; Zhao, D.; Meng, J.; Pan, T.; Li, J.; Guo, J.; Huang, H.; Wang, N.; Zhang, D.; Wang, C.; et al. Bacillus halotolerans attenuates inflammation induced by enterotoxigenic Escherichia coli infection in vivo and in vitro based on its metabolite soyasaponin I regulating the p105-Tpl2-ERK pathway. Food. Funct. 2024, 15, 6743–6758. [Google Scholar] [CrossRef]
- Qiu, J.; Heller, J.J.; Guo, X.H.; Chen, Z.M.E.; Fish, K.; Fu, Y.X.; Zhou, L. The Aryl Hydrocarbon Receptor Regulates Gut Immunity through Modulation of Innate Lymphoid Cells. Immunity 2012, 36, 92–104. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Lv, K.; Zhao, W.; Zhang, N.; Yang, F.; Wen, X.; Jiang, X.; Tian, J.; Liu, X.; et al. Pterostilbene Ameliorates DSS-Induced Intestinal Epithelial Barrier Loss in Mice via Suppression of the NF-kappaB-Mediated MLCK-MLC Signaling Pathway. J. Agric. Food Chem. 2021, 69, 3871–3878. [Google Scholar] [CrossRef] [PubMed]
- Roche, K.C.; Gracz, A.D.; Liu, X.F.; Newton, V.; Akiyama, H.; Magness, S.T. SOX9 maintains reserve stem cells and preserves radioresistance in mouse small intestine. Gastroenterology 2015, 149, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Liu, Y.; Zhang, M.; Yuan, X.; Ji, T.; Jin, Y.; Li, Y.; Wang, R.; Hao, Y.; Fang, B. Effects of 1-oleate-2-palmitate-3-linoleate glycerol supplementation on the small intestinal development and gut microbial composition of neonatal mice. Food Res. Int. 2024, 195, 114993. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Leong, R.W.; Wasinger, V.C.; Ip, M.; Yang, M.; Phan, T.G. Impaired Intestinal Permeability Contributes to Ongoing Bowel Symptoms in Patients with Inflammatory Bowel Disease and Mucosal Healing. Gastroenterology 2017, 153, 723–731. [Google Scholar] [CrossRef]
- Turpin, W.; Lee, S.H.; Garay, J.A.R.; Madsen, K.L.; Meddings, J.B.; Bedrani, L.; Power, N.; Espin-Garcia, O.; Xu, W.; Smith, M.I.; et al. Increased Intestinal Permeability Is Associated with Later Development of Crohn’s Disease. Gastroenterology 2020, 159, 2092–2100. [Google Scholar] [CrossRef]
- Yang, F.; Chen, Y.; Xiao, Y.; Jiang, H.; Jiang, Z.; Yang, M.; Li, M.; Su, Y.; Yan, Z.; Lin, Y.; et al. pH-sensitive molybdenum (Mo)-based polyoxometalate nanoclusters have therapeutic efficacy in inflammatory bowel disease by counteracting ferroptosis. Pharmacol. Res. 2023, 188, 106645. [Google Scholar] [CrossRef]
- Xiao, K.; Zhou, M.; Lv, Q.; He, P.; Qin, X.; Wang, D.; Zhao, J.; Liu, Y. Protocatechuic acid and quercetin attenuate ETEC-caused IPEC-1 cell inflammation and injury associated with inhibition of necroptosis and pyroptosis signaling pathways. J. Anim. Sci. Biotechnol. 2023, 14, 5. [Google Scholar] [CrossRef]
- Lee, M.J.; Bing, S.J.; Choi, J.; Jang, M.; Lee, G.; Lee, H.; Chang, B.S.; Jee, Y.; Lee, S.J.; Cho, I.H. IKKβ-mediated inflammatory myeloid cell activation exacerbates experimental autoimmune encephalomyelitis by potentiating Th1/Th17 cell activation and compromising blood brain barrier. Mol. Neurodegener. 2016, 11, 1–21. [Google Scholar] [CrossRef]
- Fagarasan, S.; Kawamoto, S.; Kanagawa, O.; Suzuki, K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu. Rev. Immunol. 2010, 28, 243–273. [Google Scholar] [CrossRef]
- Brown, E.M.; Kenny, D.J.; Xavier, R.J. Gut Microbiota Regulation of T Cells During Inflammation and Autoimmunity. Annu. Rev. Immunol. 2019, 37, 599–624. [Google Scholar] [CrossRef] [PubMed]
- Kosiewicz, M.M.; Nast, C.C.; Krishnan, A.; Rivera-Nieves, J.; Moskaluk, C.A.; Matsumoto, S.; Kozaiwa, K.; Cominelli, F. Th1-type responses mediate spontaneous ileitis in a novel murine model of Crohn’s disease. J. Clin. Invest. 2001, 107, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lopez, A.; Behnsen, J.; Nuccio, S.P.; Raffatellu, M. Mucosal immunity to pathogenic intestinal bacteria. Nat. Rev. Immunol. 2016, 16, 135–148. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, S.; Zhang, W.; Cui, H.; Zhang, J.; Yin, X.; Zheng, X.; Shen, T.; Ying, H.; Chen, L.; et al. Cordycepin mitigates dextran sulfate sodium-induced colitis through improving gut microbiota composition and modulating Th1/Th2 and Th17/Treg balance. Biomed. Pharmacother. 2024, 180, 117394. [Google Scholar] [CrossRef]
- Laserna-Mendieta, E.J.; Clooney, A.G.; Carretero-Gomez, J.F.; Moran, C.; Sheehan, D.; Nolan, J.A.; Hill, C.; Gahan, C.G.M.; Joyce, S.A.; Shanahan, F.; et al. Determinants of Reduced Genetic Capacity for Butyrate Synthesis by the Gut Microbiome in Crohn’s Disease and Ulcerative Colitis. J. Crohns. Colitis. 2018, 12, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Lee, M.; Chang, E.B. The Gut Microbiome and Inflammatory Bowel Diseases. Annu. Rev. Med. 2022, 73, 455–468. [Google Scholar] [CrossRef]
- Sideri, A.; Bakirtzi, K.; Shih, D.Q.; Koon, H.W.; Fleshner, P.; Arsenescu, R.; Arsenescu, V.; Turner, J.R.; Karagiannides, I.; Pothoulakis, C. Substance P mediates pro-inflammatory cytokine release form mesenteric adipocytes in Inflammatory Bowel Disease patients. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 420–432. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, J.E.; Cho, M.L. Immunological pathogenesis of inflammatory bowel disease. Intest. Res. 2018, 16, 26–42. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, D.H.; Park, H.J.; Ma, H.W.; Park, I.S.; Son, M.; Ro, S.Y.; Hong, S.; Han, H.K.; Lim, S.J.; et al. Nanocomposites-based targeted oral drug delivery systems with infliximab in a murine colitis model. J. Nanobiotechnol. 2020, 18, 133. [Google Scholar] [CrossRef]
- Chiu, C.J.; McArdle, A.H.; Brown, R.; Scott, H.J.; Gurd, F.N. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch. Surg. 1970, 101, 478–483. [Google Scholar] [CrossRef]
- Avalos-de León, C.G.; Jiménez-Castro, M.B.; Cornide-Petronio, M.E.; Casillas-Ramírez, A.; Peralta, C. The Role of GLP1 in Rat Steatotic and Non-Steatotic Liver Transplantation from Cardiocirculatory Death Donors. Cells 2019, 8, 1599. [Google Scholar] [CrossRef] [PubMed]
- Zu, G.; Guo, J.; Zhou, T.; Che, N.; Liu, B.; Wang, D.; Zhang, X. The transcription factor FoxM1 activates Nurr1 to promote intestinal regeneration after ischemia/reperfusion injury. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]



| Gene Name | Forward (5′−>3′) | Reverse (5′−>3′) |
|---|---|---|
| GAPDH | CATCACTGCCACCCAGAAGACTG | ATGCCAGTGAGCTTCCCGTTCAG |
| ZO-1 | GTTGGTACGGTGCCCTGAAAGA | GCTGACAGGTAGGACAGACGAT |
| Occludin | TGGCAAGCGATCATACCCAGAG | CTGCCTGAAGTCATCCACACTC |
| Claudin-1 | AGATACAGTGCAAAGTCTTCGA | CAGGATGCCAATTACCATCAAG |
| IFN-γ | CAGCAACAGCAAGGCGAAAAAGG | TTTCCGCTTCCTGAGGCTGGAT |
| IL-10 | CGGGAAGACAATAACTGCACCC | CGGTTAGCAGTATGTTGTCCAGC |
| IL-4tt | ATCATCGGCATTTTGAACGAGGTC | ACCTTGGAAGCCCTACAGACGA |
| IL-17A | CAGACTACCTCAACCGTTCCAC | TCCAGCTTTCCCTCCGCATTGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Wang, Z.; Wu, F.; Cheng, L.; Jin, Y.; Dong, J.; Zheng, C.; Ma, Y.; Jin, Y.; Fang, B. The Impact of Imidacloprid in Dietary Residues on Intestinal Damage and the Increased Risk of Enterotoxigenic Escherichia coli Infection. Foods 2025, 14, 2119. https://doi.org/10.3390/foods14122119
Yuan X, Wang Z, Wu F, Cheng L, Jin Y, Dong J, Zheng C, Ma Y, Jin Y, Fang B. The Impact of Imidacloprid in Dietary Residues on Intestinal Damage and the Increased Risk of Enterotoxigenic Escherichia coli Infection. Foods. 2025; 14(12):2119. https://doi.org/10.3390/foods14122119
Chicago/Turabian StyleYuan, Xinlei, Zihan Wang, Fang Wu, Le Cheng, Yutong Jin, Jianguo Dong, Chenyan Zheng, Yumeng Ma, Yan Jin, and Bing Fang. 2025. "The Impact of Imidacloprid in Dietary Residues on Intestinal Damage and the Increased Risk of Enterotoxigenic Escherichia coli Infection" Foods 14, no. 12: 2119. https://doi.org/10.3390/foods14122119
APA StyleYuan, X., Wang, Z., Wu, F., Cheng, L., Jin, Y., Dong, J., Zheng, C., Ma, Y., Jin, Y., & Fang, B. (2025). The Impact of Imidacloprid in Dietary Residues on Intestinal Damage and the Increased Risk of Enterotoxigenic Escherichia coli Infection. Foods, 14(12), 2119. https://doi.org/10.3390/foods14122119





