Effect of Temperature, Surface, and Medium Qualities on the Biofilm Formation of Listeria monocytogenes and Their Influencing Effects on the Antibacterial, Biofilm-Inhibitory, and Biofilm-Degrading Activities of Essential Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Media
2.2. Essential Oils
2.3. Drop-Plate Testing
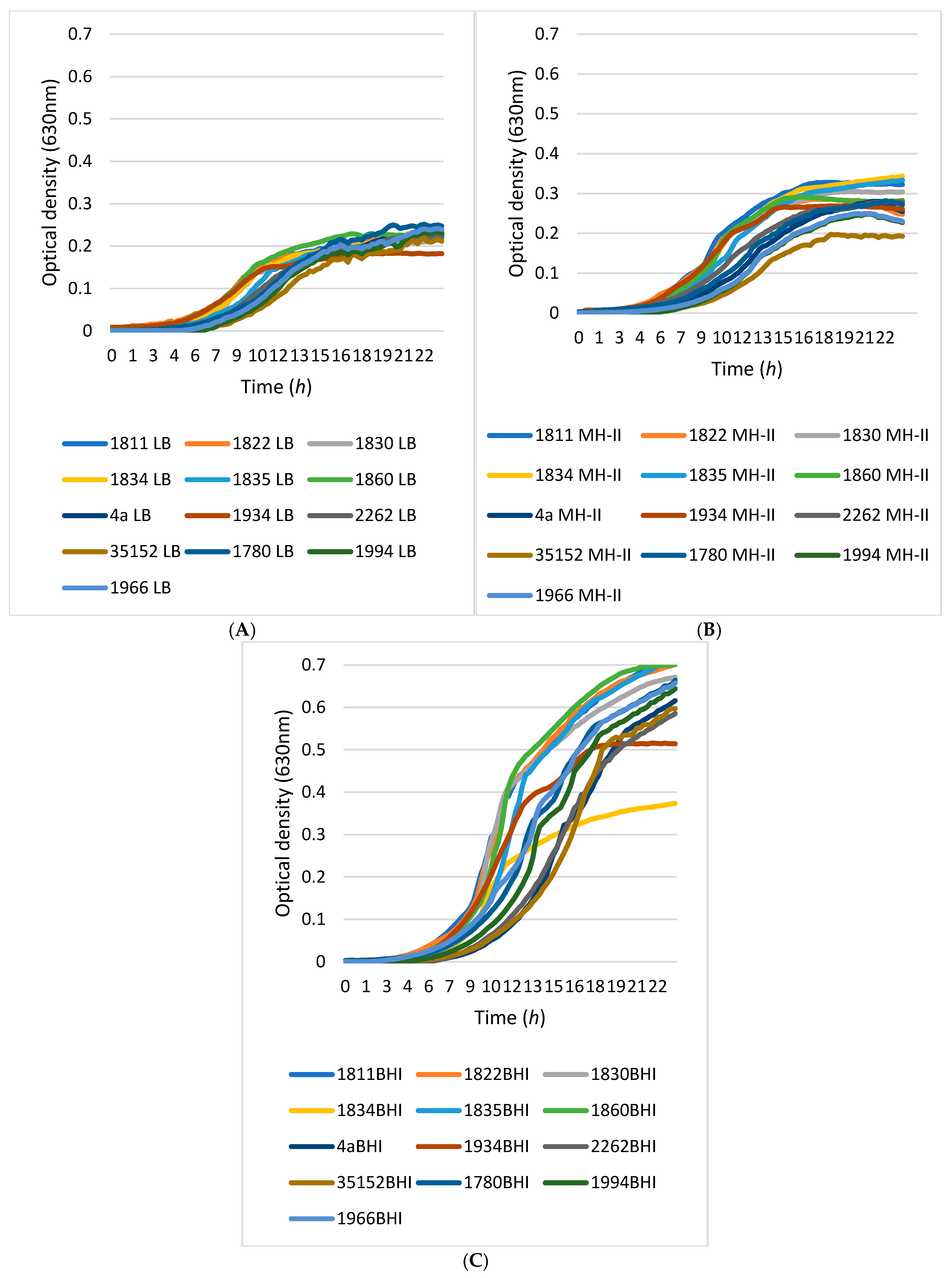
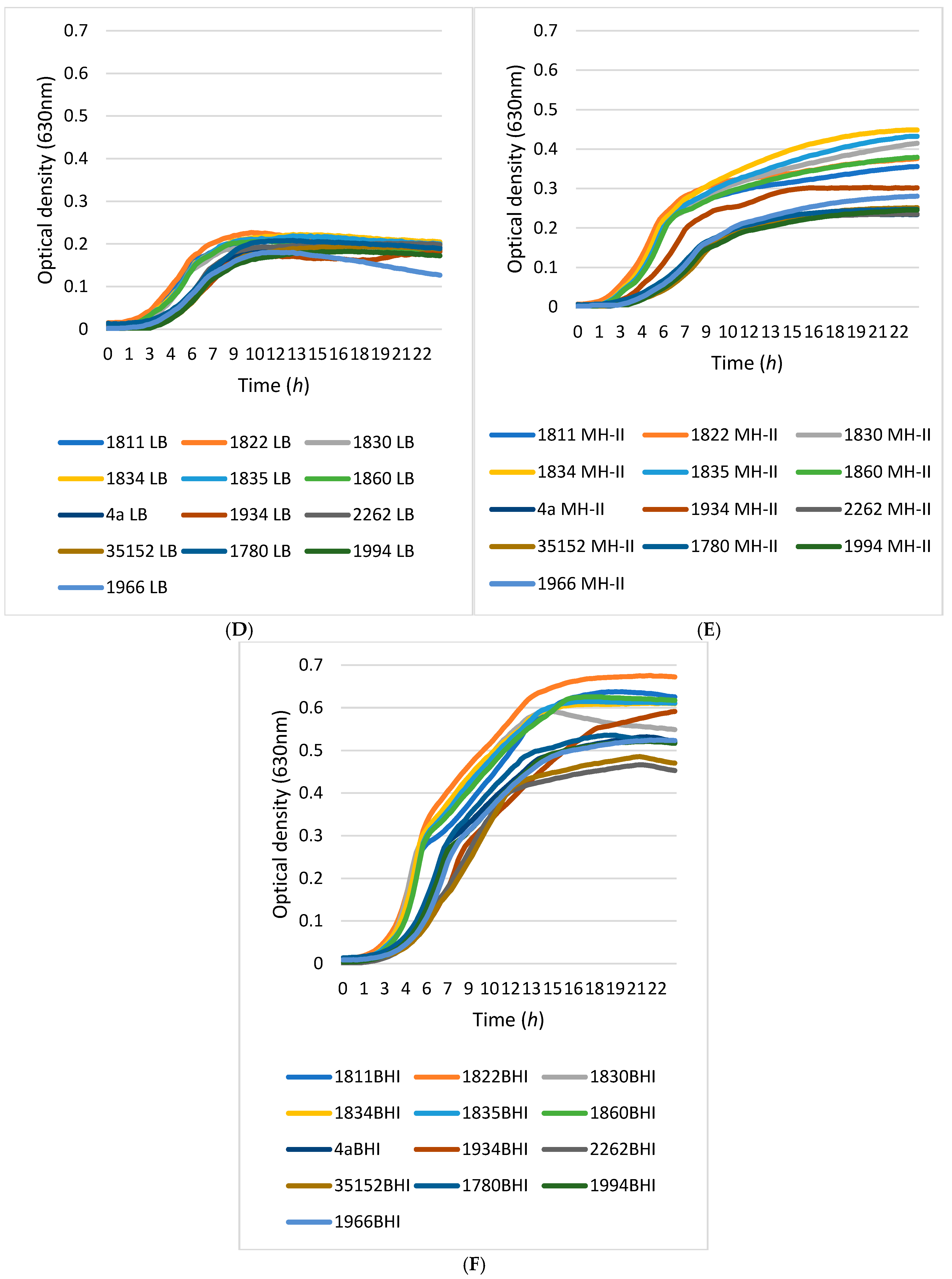
2.4. Comparative Growth Kinetics of L. monocytogenes Isolates
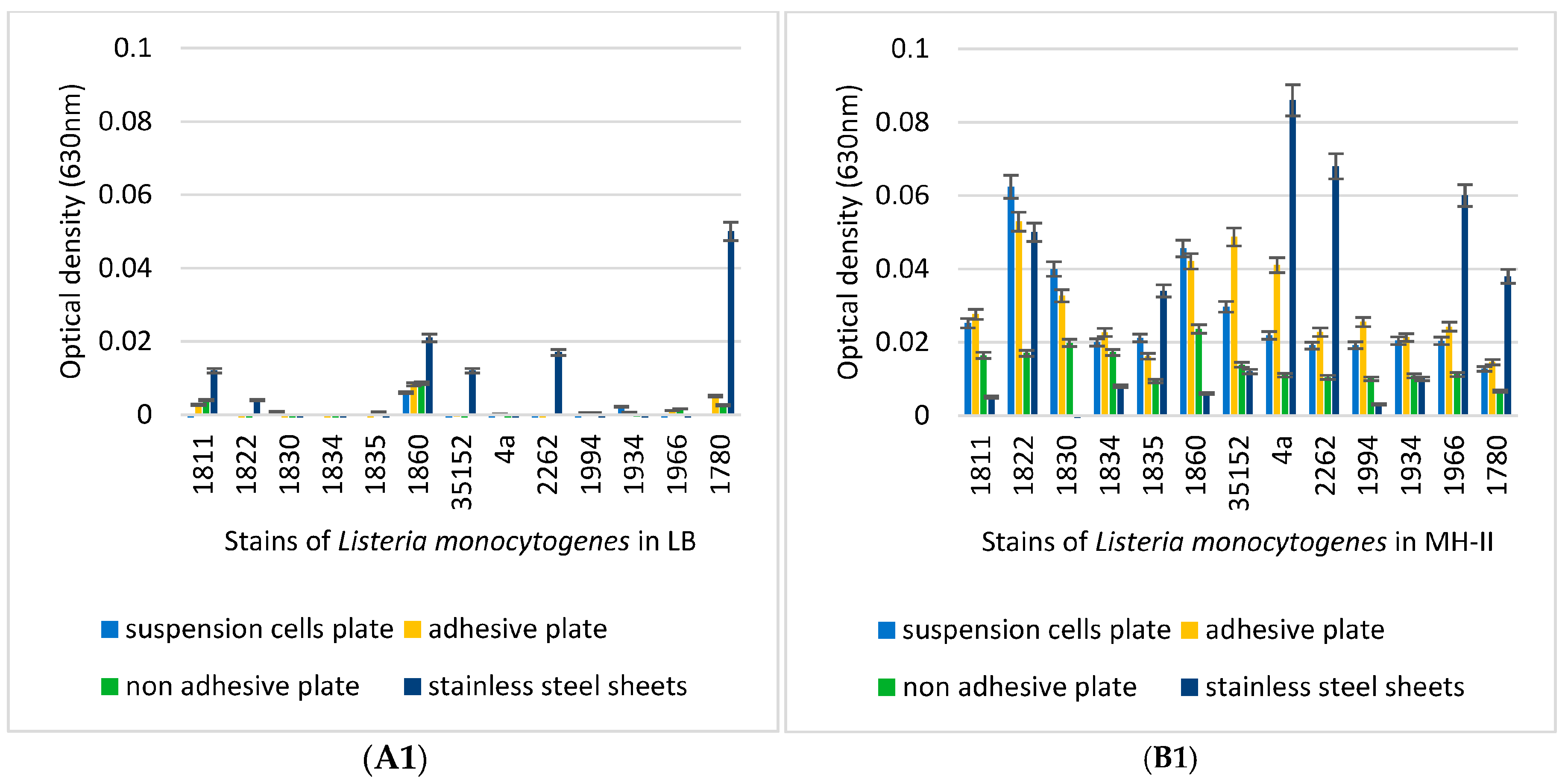
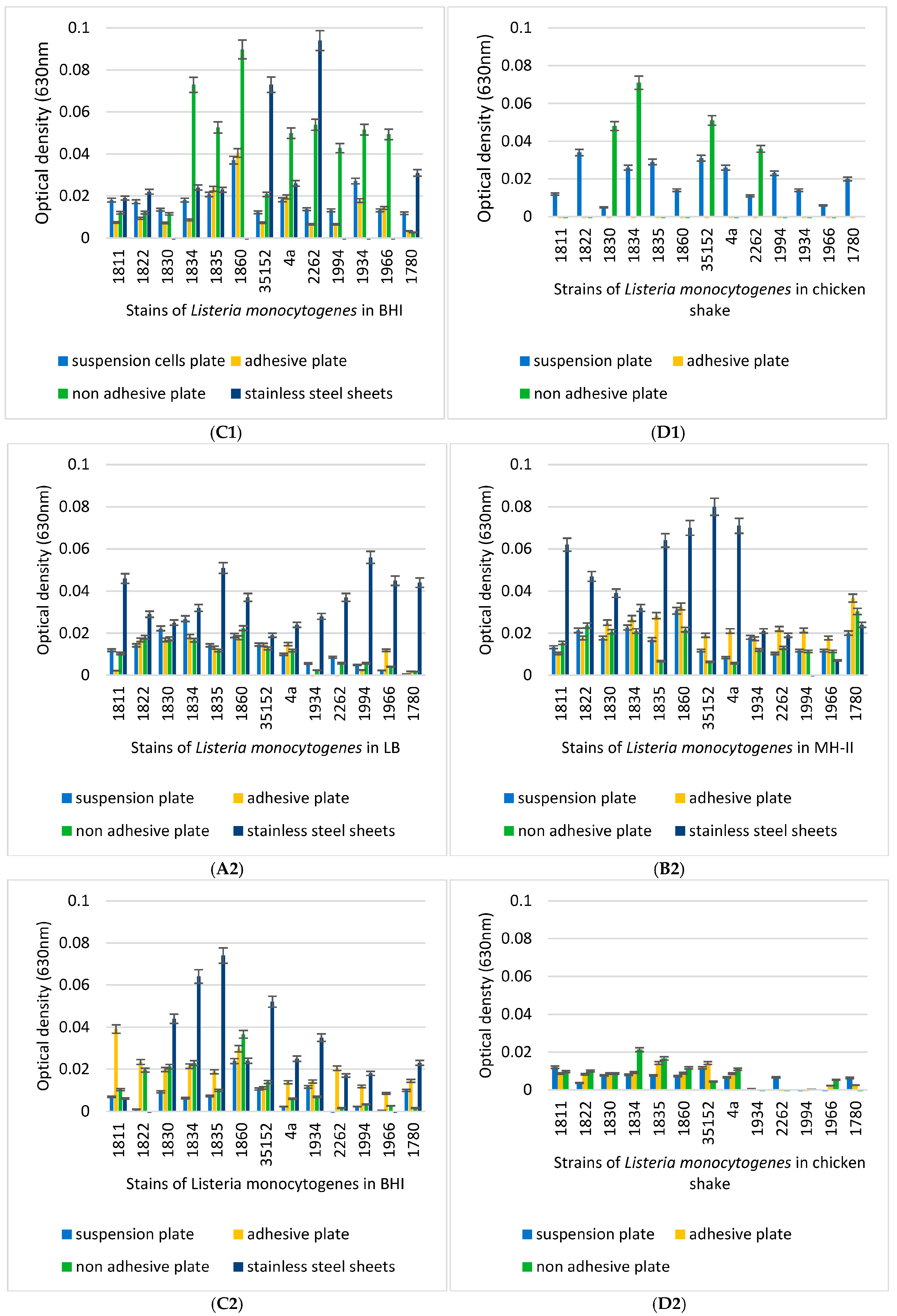
2.5. Biofilm-Formation Tests
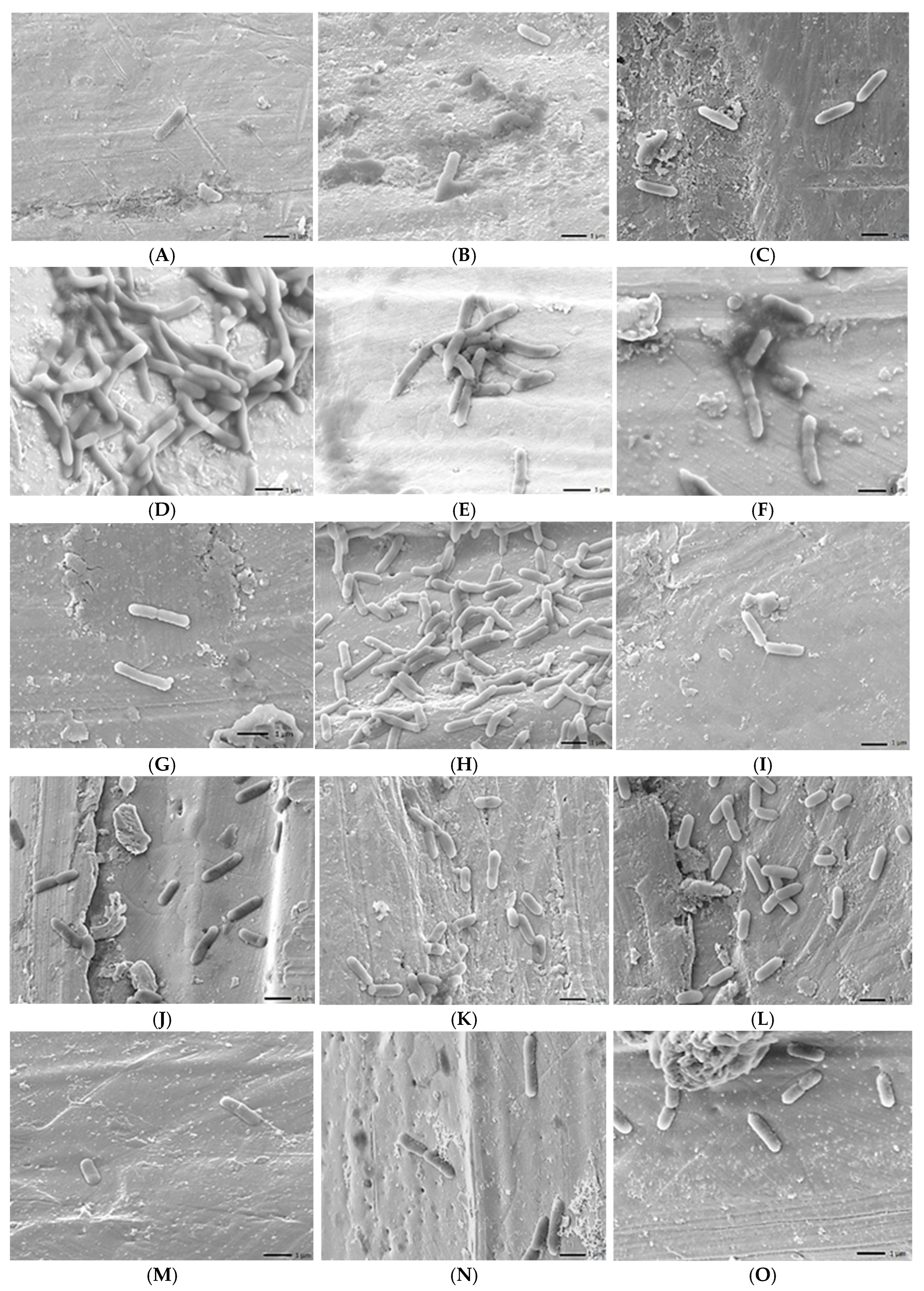
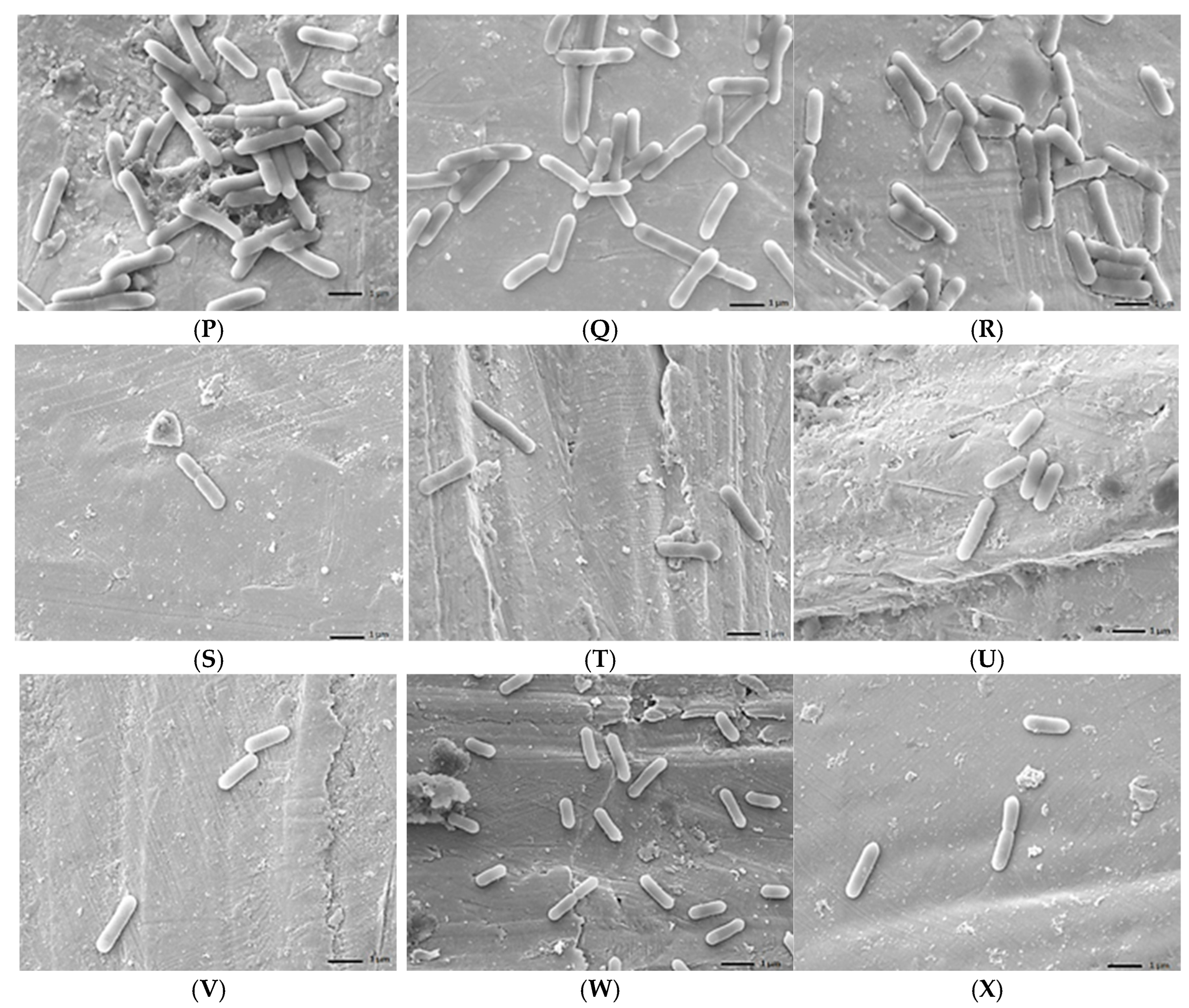
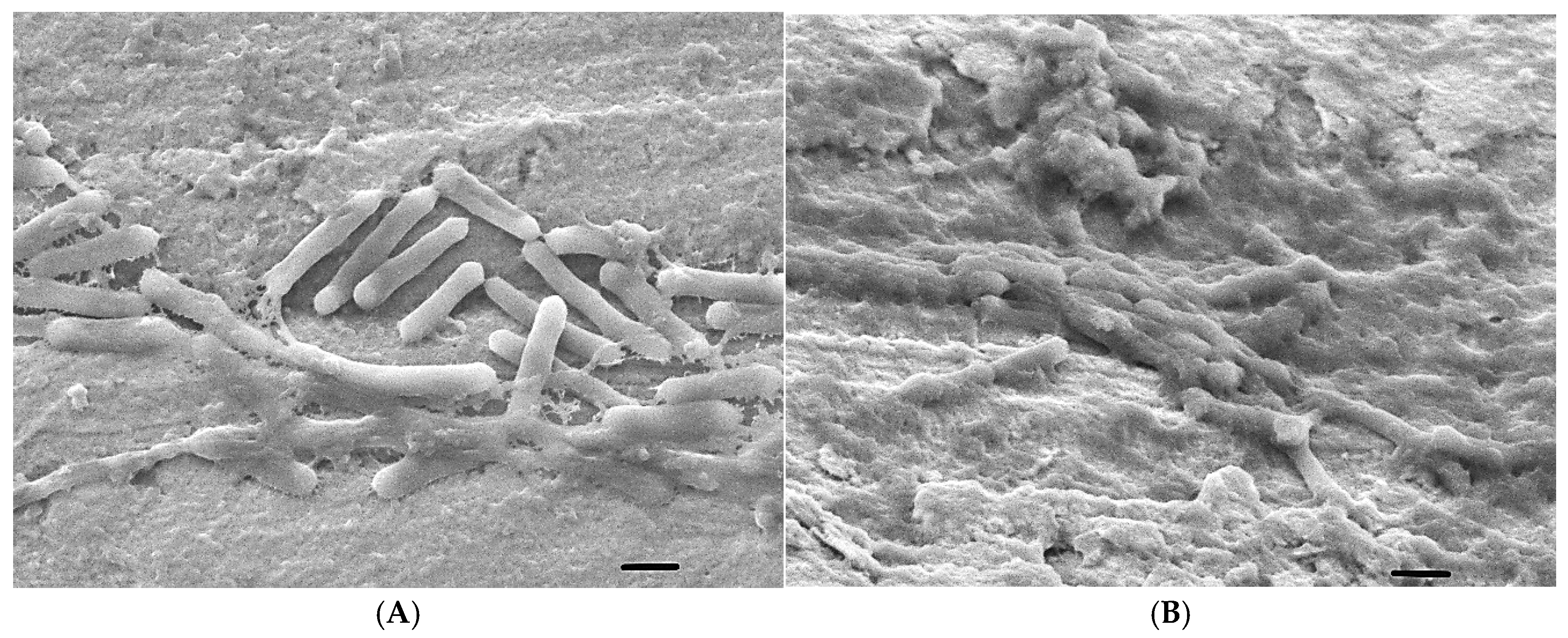
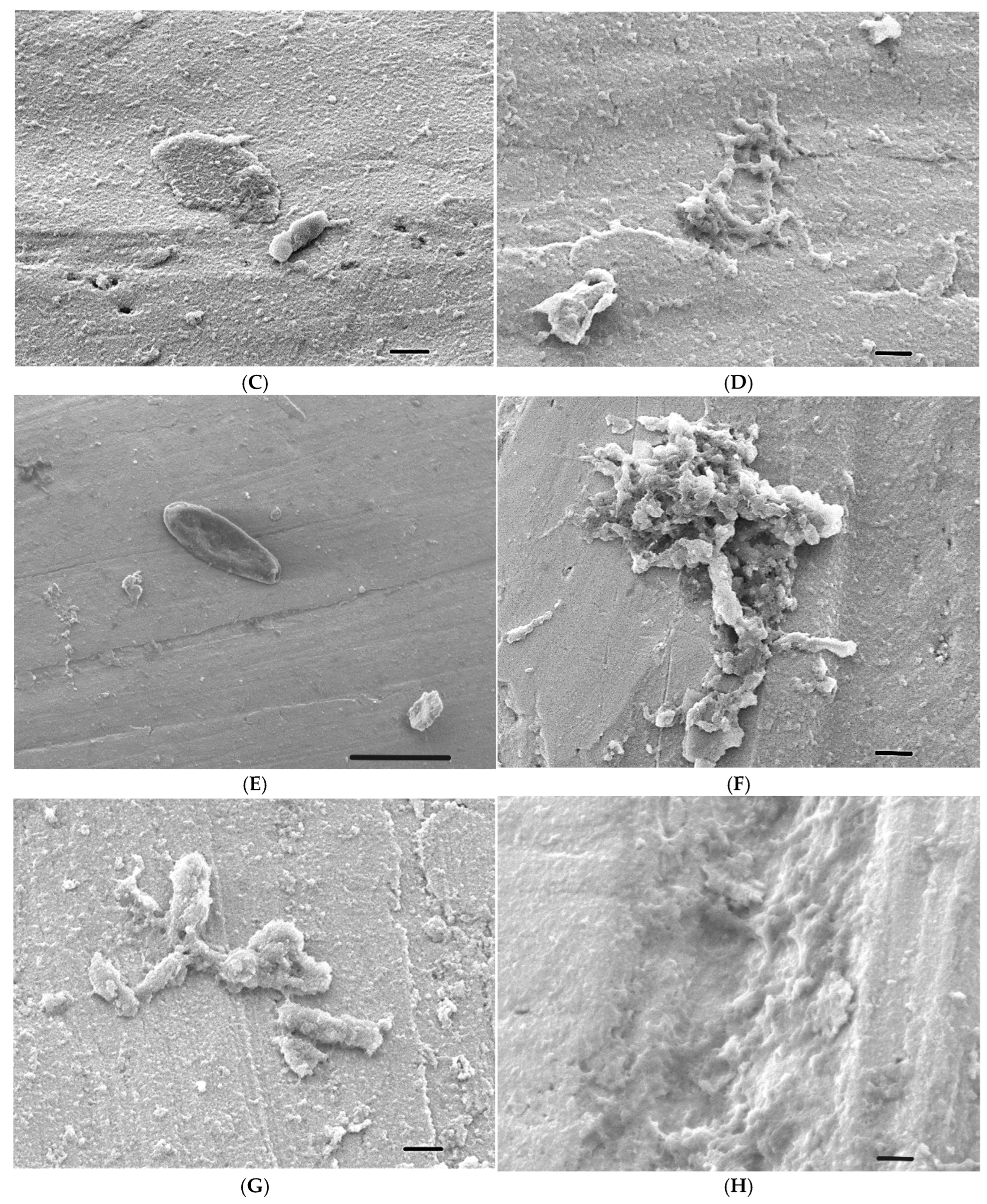
2.6. Scanning Electron Microscope (SEM) Analysis
2.7. Biofilm-Inhibition Capacity of EOs
2.8. Mature Biofilm Eradication Capacity of EOs
2.9. Statistical Analysis
2.10. Flow Chart of the Experimental Design
3. Results
3.1. Drop-Plate Testing
3.2. Growth Kinetics
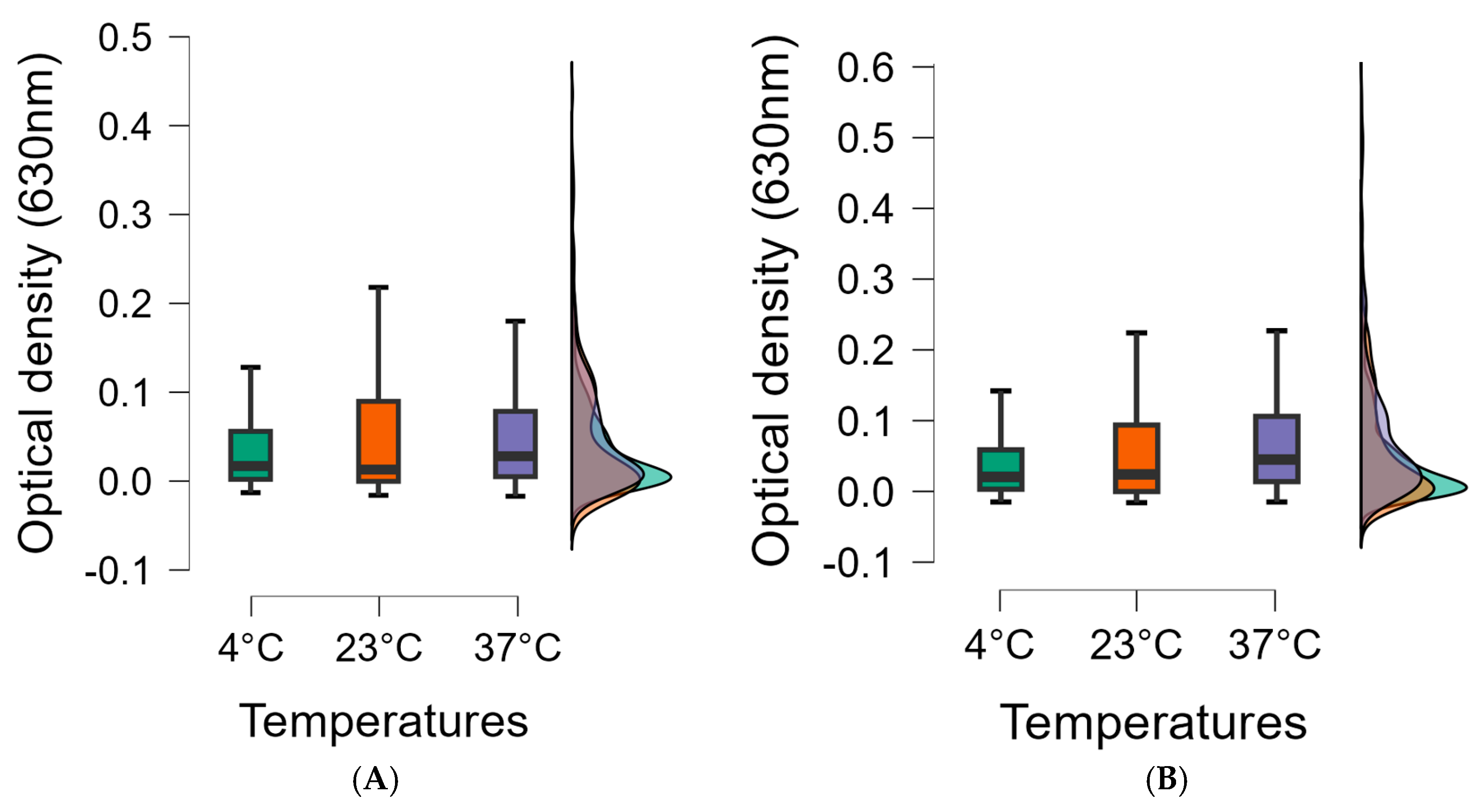
3.3. Biofilm-Forming Capacity of the L. monocytogenes Isolates on Polystyrene Surfaces
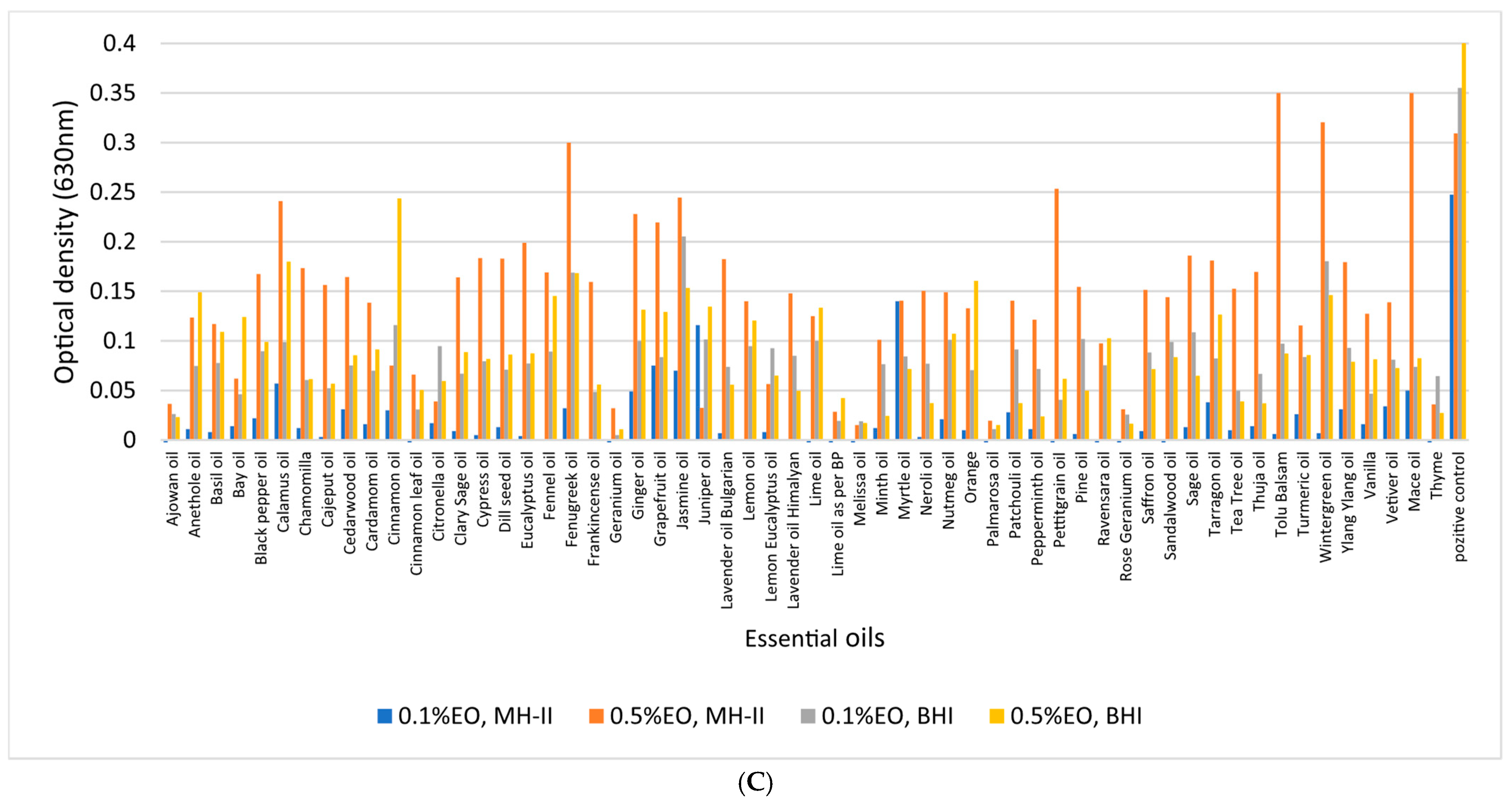
3.4. Biofilm-Inhibition Assay
3.5. Biofilm Eradication from Polystyrene Surfaces
3.6. Biofilm Eradication from SSC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McLaughlin, H.P.; Casey, P.G.; Cotter, J.; Gahan, C.G.; Hill, C. Factors affecting survival of Listeria monocytogenes and Listeria innocua in soil samples. Arch. Microbiol. 2011, 193, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Taherkhani, A.; Attar, H.M.; Moazzam, M.M.A.; Mirzaee, S.A.; Jalali, M. Prevalence of Listeria monocytogenes in the river receiving the effluent of municipal wastewater treatment plant. Int. J. Environ. Health Eng. 2013, 2, 49. [Google Scholar]
- Sleator, R.D.; Watson, D.; Hill, C.; Gahan, C.G. The interaction between Listeria monocytogenes and the host gastrointestinal tract. Microbiology 2009, 155, 2463–2475. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, N.D.; Ayaz, Y.; Kaplan, Y.Z.; Dogru, A.K.; Aksoy, M.H. Rapid detection of Listeria monocytogenes in chicken carcasses by IMS-PCR. Ann. Microbiol. 2009, 59, 741–744. [Google Scholar] [CrossRef]
- Kanuganti, S.R.; Wesley, I.V.; Reddy, P.G.; McKean, J.; Hurd, H.S. Detection of Listeria monocytogenes in pigs and pork. J. Food Prot. 2002, 65, 1470–1474. [Google Scholar] [CrossRef] [PubMed]
- Terentjeva, M.; Šteingolde, Ž.; Meistere, I.; Elferts, D.; Avsejenko, J.; Streikiša, M.; Gradovska, S.; Alksne, L.; Ķibilds, J.; Bērziņš, A. Prevalence, genetic diversity and factors associated with distribution of Listeria monocytogenes and other Listeria spp. in cattle farms in Latvia. Pathogens 2021, 10, 851. [Google Scholar] [CrossRef]
- Weindl, L.; Frank, E.; Ullrich, U.; Heurich, M.; Kleta, S.; Ellerbroek, L.; Gareis, M. Listeria monocytogenes in different specimens from healthy red deer and wild boars. Foodborne Pathog. Dis. 2016, 13, 391–397. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, L.; Lan, R.; Salazar, J.K.; Liu, J.; Xu, J.; Ye, C. Isolation and characterization of Listeria species from rodents in natural environments in China: Isolation and characterization of Listeria species. Emerg. Microbes Infect. 2017, 6, 1–6. [Google Scholar]
- Farber, J. Listeria monocytogenes in fish products. J. Food Prot. 1991, 54, 922–925. [Google Scholar] [CrossRef]
- Hansen, C.H.; Vogel, B.F.; Gram, L. Prevalence and survival of Listeria monocytogenes in Danish aquatic and fish-processing environments. J. Food Prot. 2006, 69, 2113–2122. [Google Scholar] [CrossRef]
- Trias, R.; Badosa, E.; Montesinos, E.; Bañeras, L. Bioprotective Leuconostoc strains against Listeria monocytogenes in fresh fruits and vegetables. Int. J. Food Microbiol. 2008, 127, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Beuchat, L.R. Listeria monocytogenes: Incidence on vegetables. Food Control 1996, 7, 223–228. [Google Scholar] [CrossRef]
- Schneider, G.; Steinbach, A.; Putics, A.; Solti-Hodovan, A.; Palkovics, T. Potential of Essential Oils in the Control of Listeria monocytogenes. Microorganisms 2023, 11, 1364. [Google Scholar] [CrossRef]
- Schoder, D.; Guldimann, C.; Märtlbauer, E. Asymptomatic carriage of Listeria monocytogenes by animals and humans and its impact on the food chain. Foods 2022, 11, 3472. [Google Scholar] [CrossRef]
- Jordan, K.; McAuliffe, O. Listeria monocytogenes in foods. Adv. Food Nutr. Res. 2018, 86, 181–213. [Google Scholar]
- Kljujev, I.; Raicevic, V.; Jovicic-Petrovic, J.; Vujovic, B.; Mirkovic, M.; Rothballer, M. Listeria monocytogenes–Danger for health safety vegetable production. Microb. Pathog. 2018, 120, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, T.; Peter, K.; Chen, Y.; Jin, Q.; Zhang, G.; LaBorde, L.F.; Macarisin, D. Prevalence and distribution of Listeria monocytogenes in three commercial tree fruit packinghouses. Front. Microbiol. 2021, 12, 652708. [Google Scholar] [CrossRef] [PubMed]
- Farber, J.M.; Peterkin, P. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 1991, 55, 476–511. [Google Scholar] [CrossRef]
- Khan, S.A.; Khalid, S.; Siddiqui, R. The effect of pH and temperature on haemolysin production by Listeria species. Lett. Appl. Microbiol. 1993, 17, 14–16. [Google Scholar] [CrossRef]
- Hoffmann, S.; White, A.E.; McQueen, R.B.; Ahn, J.-W.; Gunn-Sandell, L.B.; Scallan Walter, E.J. Economic Burden of Foodborne Illnesses Acquired in the United States. Foodborne Pathog. Dis. 2024; ahead of print. [Google Scholar] [CrossRef]
- Ivanek, R.; Gröhn, Y.T.; Tauer, L.W.; Wiedmann, M. The cost and benefit of Listeria monocytogenes food safety measures. Crit. Rev. Food Sci. Nutr. 2004, 44, 513–523. [Google Scholar] [CrossRef]
- Donnelly, C.W. Listeria monocytogenes: A continuing challenge. Nutr. Rev. 2001, 59, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Archer, D.L. The evolution of FDA’s policy on Listeria monocytogenes in ready-to-eat foods in the United States. Curr. Opin. Food Sci. 2018, 20, 64–68. [Google Scholar] [CrossRef]
- Brandt, A.L.; Castillo, A.; Harris, K.B.; Keeton, J.T.; Hardin, M.D.; Taylor, T.M. Inhibition of Listeria monocytogenes by food antimicrobials applied singly and in combination. J. Food Sci. 2010, 75, M557–M563. [Google Scholar] [CrossRef] [PubMed]
- Ramos, B.; Brandão, T.R.; Teixeira, P.; Silva, C.L. Biopreservation approaches to reduce Listeria monocytogenes in fresh vegetables. Food Microbiol. 2020, 85, 103282. [Google Scholar] [CrossRef]
- Lis-Balchin, M.; Deans, S.G. Bioactivity of selected plant essential oils against Listeria monocytogenes. J. Appl. Microbiol. 1997, 82, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Møretrø, T.; Langsrud, S. Listeria monocytogenes: Biofilm formation and persistence in food-processing environments. Biofilms 2004, 1, 107–121. [Google Scholar] [CrossRef]
- Da Silva, E.P.; De Martinis, E.C.P. Current knowledge and perspectives on biofilm formation: The case of Listeria monocytogenes. Appl. Microbiol. Biotechnol. 2013, 97, 957–968. [Google Scholar] [CrossRef]
- Fan, Y.; Qiao, J.; Lu, Z.; Fen, Z.; Tao, Y.; Lv, F.; Zhao, H.; Zhang, C.; Bie, X. Influence of different factors on biofilm formation of Listeria monocytogenes and the regulation of cheY gene. Food Res. Int. 2020, 137, 109405. [Google Scholar] [CrossRef]
- Chae, M.S.; Schraft, H.; Hansen, L.T.; Mackereth, R. Effects of physicochemical surface characteristics of Listeria monocytogenes strains on attachment to glass. Food Microbiol. 2006, 23, 250–259. [Google Scholar] [CrossRef]
- Reis-Teixeira, F.B.d.; Alves, V.F.; Martinis, E.C.P.d. Growth, viability and architecture of biofilms of Listeria monocytogenes formed on abiotic surfaces. Braz. J. Microbiol. 2017, 48, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Skowron, K.; Hulisz, K.; Gryń, G.; Olszewska, H.; Wiktorczyk, N.; Paluszak, Z. Comparison of selected disinfectants efficiency against Listeria monocytogenes biofilm formed on various surfaces. Int. Microbiol. 2018, 21, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Maggio, F.; Chaves-López, C.; Valbonetti, L.; Berrettoni, M.; Paparella, A.; Serio, A. Effectiveness of selected essential oils and one hydrolate to prevent and remove Listeria monocytogenes biofilms on polystyrene and stainless steel food-contact surfaces. J. Appl. Microbiol. 2022, 132, 1866–1876. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.; Cervellieri, S.; Netti, T.; Lippolis, V.; Baruzzi, F. Antibacterial activity of oregano (Origanum vulgare L.) essential oil vapors against microbial contaminants of food-contact surfaces. Antibiotics 2024, 13, 371. [Google Scholar] [CrossRef]
- Baumann, A.R.; Martin, S.E.; Feng, H. Removal of Listeria monocytogenes biofilms from stainless steel by use of ultrasound and ozone. J. Food Prot. 2009, 72, 1306–1309. [Google Scholar] [CrossRef]
- Skowron, K.; Wałecka-Zacharska, E.; Grudlewska, K.; Gajewski, P.; Wiktorczyk, N.; Wietlicka-Piszcz, M.; Dudek, A.; Skowron, K.J.; Gospodarek-Komkowska, E. Disinfectant susceptibility of biofilm formed by Listeria monocytogenes under selected environmental conditions. Microorganisms 2019, 7, 280. [Google Scholar] [CrossRef]
- Soni, K.A.; Nannapaneni, R. Removal of Listeria monocytogenes biofilms with bacteriophage P100. J. Food Prot. 2010, 73, 1519. [Google Scholar] [CrossRef]
- Aureli, P.; Costantini, A.; Zolea, S. Antimicrobial activity of some plant essential oils against Listeria monocytogenes. J. Food Prot. 1992, 55, 344–348. [Google Scholar] [CrossRef]
- Tomičić, R.M.; Čabarkapa, I.S.; Varga, A.O.; Tomičić, Z.M. Antimicrobial activity of essential oils against Listeria monocytogenes. Food Feed Res. 2018, 45, 37–44. [Google Scholar] [CrossRef]
- Leonard, C.; Virijevic, S.; Regnier, T.; Combrinck, S. Bioactivity of selected essential oils and some components on Listeria monocytogenes biofilms. S. Afr. J. Bot. 2010, 76, 676–680. [Google Scholar] [CrossRef]
- Vasilijević, B.; Mitić-Ćulafić, D.; Djekic, I.; Marković, T.; Knežević-Vukčević, J.; Tomasevic, I.; Velebit, B.; Nikolić, B. Antibacterial effect of Juniperus communis and Satureja montana essential oils against Listeria monocytogenes in vitro and in wine marinated beef. Food Control 2019, 100, 247–256. [Google Scholar] [CrossRef]
- Singh, A.; Singh, R.; Bhunia, A.; Singh, N. Efficacy of plant essential oils as antimicrobial agents against Listeria monocytogenes in hotdogs. LWT-Food Sci. Technol. 2003, 36, 787–794. [Google Scholar] [CrossRef]
- Oliveira, M.M.M.d.; Brugnera, D.F.; Piccoli, R.H. Essential oils of thyme and Rosemary in the control of Listeria monocytogenes in raw beef. Braz. J. Microbiol. 2013, 44, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Khorshidian, N.; Hosseini, H. Potential application of essential oils for mitigation of Listeria monocytogenes in meat and poultry products. Front. Nutr. 2020, 7, 577287. [Google Scholar] [CrossRef] [PubMed]
- Tomičić, R.M.; Čabarkapa, I.S.; Vukmirović, Đ.M.; Lević, J.D.; Tomičić, Z.M. Influence of growth conditions on biofilm formation of Listeria monocytogenes. Food Feed Res. 2016, 43, 19–24. [Google Scholar] [CrossRef]
- Kadam, S.R.; den Besten, H.M.; van der Veen, S.; Zwietering, M.H.; Moezelaar, R.; Abee, T. Diversity assessment of Listeria monocytogenes biofilm formation: Impact of growth condition, serotype and strain origin. Int. J. Food Microbiol. 2013, 165, 259–264. [Google Scholar] [CrossRef]
- Kowalska, J.; Maćkiw, E.; Stasiak, M.; Kucharek, K.; Postupolski, J. Biofilm-forming ability of pathogenic bacteria isolated from retail food in Poland. J. Food Prot. 2020, 83, 2032–2040. [Google Scholar] [CrossRef]
- Schweitzer, B.; Balázs, V.L.; Molnár, S.; Szögi-Tatár, B.; Böszörményi, A.; Palkovics, T.; Horváth, G.; Schneider, G. Antibacterial effect of lemongrass (Cymbopogon citratus) against the aetiological agents of pitted keratolyis. Molecules 2022, 27, 1423. [Google Scholar] [CrossRef]
- Seres-Steinbach, A.; Schneider-Patkó, B.; Jerzsele, Á.; Veres, A.M.; Sonnevend, Á.; Bányai, K.; Schneider, G. Characterization of Canine Otitis Externa Pseudomonas aeruginosa Isolates and Their Sensitivities to Different Essential Oils. Animals 2025, 15, 826. [Google Scholar] [CrossRef]
- Dang, N.P.; Petrich, C.; Pasztor, D.; Schneider, G.; Calay, R.K. Shewanella baltica in a Microbial Fuel Cell for Sensing of Biological Oxygen Demand (BOD) of Wastewater. Anal. Lett. 2025, 58, 755–767. [Google Scholar] [CrossRef]
- Kovács, J.K.; Felső, P.; Makszin, L.; Pápai, Z.; Horváth, G.; Ábrahám, H.; Palkovics, T.; Böszörményi, A.; Emődy, L.; Schneider, G. Antimicrobial and virulence-modulating effects of clove essential oil on the foodborne pathogen Campylobacter jejuni. Appl. Environ. Microbiol. 2016, 82, 6158–6166. [Google Scholar] [CrossRef] [PubMed]
- Oloketuyi, S.F.; Khan, F. Inhibition strategies of Listeria monocytogenes biofilms—Current knowledge and future outlooks. J. Basic Microbiol. 2017, 57, 728–743. [Google Scholar] [CrossRef]
- Aala, J.; Ahmadi, M.; Golestan, L.; Shahidi, S.-A.; Shariatifar, N. Effect of multifactorial free and liposome-coated of bay laurel (Laurus nobilis) and rosemary (Salvia rosmarinus) extracts on the behavior of Listeria monocytogenes and Vibrio parahaemolyticus in silver carp (Hypophthalmichthys molitrix) stored at 4 C. Environ. Res. 2023, 216, 114478. [Google Scholar] [CrossRef]
- da Silva, B.D.; Bernardes, P.C.; Pinheiro, P.F.; Giannotti, J.D.G.; Roberto, C.D. Plectranthus amboinicus (Lour.) Spreng. essential oil as a natural alternative for the conservation of beef patties stored under refrigeration. Food Biosci. 2022, 49, 101896. [Google Scholar] [CrossRef]
- Fancello, F.; Petretto, G.L.; Marceddu, S.; Venditti, T.; Pintore, G.; Zara, G.; Mannazzu, I.; Budroni, M.; Zara, S. Antimicrobial activity of gaseous Citrus limon var pompia leaf essential oil against Listeria monocytogenes on ricotta salata cheese. Food Microbiol. 2020, 87, 103386. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Feng, K.; Xiu, Z.; Jiang, A.; Lao, Y. Tandem mass tag-based quantitative proteomic analysis reveal the inhibition mechanism of thyme essential oil against flagellum of Listeria monocytogenes. Food Res. Int. 2019, 125, 108508. [Google Scholar]
- Zakrzewski, A.; Purkiewicz, A.; Jakuć, P.; Wiśniewski, P.; Sawicki, T.; Chajęcka-Wierzchowska, W.; Tańska, M. Effectiveness of various solvent-produced thyme (Thymus vulgaris) extracts in inhibiting the growth of Listeria monocytogenes in frozen vegetables. NFS J. 2022, 29, 26–34. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Tajik, H.; Langroodi, A.M.; Molaei, R.; Mahmoudian, A. Chitosan-starch film containing pomegranate peel extract and Thymus kotschyanus essential oil can prolong the shelf life of beef. Meat Sci. 2020, 163, 108073. [Google Scholar] [CrossRef]
- Jadhav, S.; Shah, R.; Bhave, M.; Palombo, E.A. Inhibitory activity of yarrow essential oil on Listeria planktonic cells and biofilms. Food Control 2013, 29, 125–130. [Google Scholar] [CrossRef]
- Banerji, R.; Mahamune, A.; Saroj, S.D. Aqueous extracts of spices inhibit biofilm in Listeria monocytogenes by downregulating release of eDNA. LWT 2022, 154, 112566. [Google Scholar] [CrossRef]
- Elsherif, W.M.; Shrief, L.M.T.A. Effects of three essential oils and their nano-emulsions on Listeria monocytogenes and Shigella flexneri in Egyptian Talaga cheese. Int. J. Food Microbiol. 2021, 355, 109334. [Google Scholar] [CrossRef] [PubMed]
- Maggio, F.; Rossi, C.; Chaves-López, C.; Valbonetti, L.; Desideri, G.; Paparella, A.; Serio, A. A single exposure to a sublethal concentration of Origanum vulgare essential oil initiates response against food stressors and restoration of antibiotic susceptibility in Listeria monocytogenes. Food Control 2022, 132, 108562. [Google Scholar] [CrossRef]
- Al-Zoreky, N.S.; Al-Taher, A.Y. In vitro and in situ inhibition of some food-borne pathogens by essential oils from date palm (Phoenix dactylifera L.) spathe. Int. J. Food Microbiol. 2019, 299, 64–70. [Google Scholar] [CrossRef]
- Govaris, A.; Botsoglou, E.; Sergelidis, D.; Chatzopoulou, P.S. Antibacterial activity of oregano and thyme essential oils against Listeria monocytogenes and Escherichia coli O157: H7 in feta cheese packaged under modified atmosphere. LWT-Food Sci. Technol. 2011, 44, 1240–1244. [Google Scholar] [CrossRef]
- Radünz, M.; dos Santos Hackbart, H.C.; Camargo, T.M.; Nunes, C.F.P.; de Barros, F.A.P.; Dal Magro, J.; Sanches Filho, P.J.; Gandra, E.A.; Radünz, A.L.; da Rosa Zavareze, E. Antimicrobial potential of spray drying encapsulated thyme (Thymus vulgaris) essential oil on the conservation of hamburger-like meat products. Int. J. Food Microbiol. 2020, 330, 108696. [Google Scholar] [CrossRef]
- Scollard, J.; McManamon, O.; Schmalenberger, A. Inhibition of Listeria monocytogenes growth on fresh-cut produce with thyme essential oil and essential oil compound verbenone. Postharvest Biol. Technol. 2016, 120, 61–68. [Google Scholar] [CrossRef]
- Gil, K.A.; Jerković, I.; Marijanović, Z.; Manca, M.L.; Caddeo, C.; Tuberoso, C.I.G. Evaluation of an innovative sheep cheese with antioxidant activity enriched with different thyme essential oil lecithin liposomes. LWT 2022, 154, 112808. [Google Scholar] [CrossRef]
- Rasooli, I.; Rezaei, M.B.; Allameh, A. Ultrastructural studies on antimicrobial efficacy of thyme essential oils on Listeria monocytogenes. Int. J. Infect. Dis. 2006, 10, 236–241. [Google Scholar] [CrossRef]
- Gouveia, A.R.; Alves, M.; Silva, J.A.; Saraiva, C. The antimicrobial effect of rosemary and thyme essential oils against Listeria monocytogenes in sous vide cook-chill beef during storage. Procedia Food Sci. 2016, 7, 173–176. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Gottardo, F.M.; Biduski, B.; dos Santos, L.F.; dos Santos, J.S.; Rodrigues, L.B.; dos Santos, L.R. Microencapsulated oregano and cinnamon essential oils as a natural alternative to reduce Listeria monocytogenes in Italian salami. Food Biosci. 2022, 50, 102146. [Google Scholar] [CrossRef]
- Somrani, M.; Inglés, M.-C.; Debbabi, H.; Abidi, F.; Palop, A. Garlic, onion, and cinnamon essential oil anti-biofilms’ effect against Listeria monocytogenes. Foods 2020, 9, 567. [Google Scholar] [CrossRef]
- Ahmed, J.; Hiremath, N.; Jacob, H. Efficacy of antimicrobial properties of polylactide/cinnamon oil film with and without high-pressure treatment against Listeria monocytogenes and Salmonella typhimurium inoculated in chicken sample. Food Packag. Shelf Life 2016, 10, 72–78. [Google Scholar] [CrossRef]
- Lianou, A.; Moschonas, G.; Nychas, G.-J.E.; Panagou, E.Z. Growth of Listeria monocytogenes in pasteurized vanilla cream pudding as affected by storage temperature and the presence of cinnamon extract. Food Res. Int. 2018, 106, 1114–1122. [Google Scholar] [CrossRef]
- Cava-Roda, R.M.; Taboada-Rodríguez, A.; Valverde-Franco, M.T.; Marín-Iniesta, F. Antimicrobial activity of vanillin and mixtures with cinnamon and clove essential oils in controlling Listeria monocytogenes and Escherichia coli O157: H7 in milk. Food Bioprocess Technol. 2012, 5, 2120–2131. [Google Scholar] [CrossRef]
- Moon, K.-D.; Delaquis, P.; Toivonen, P.; Stanich, K. Effect of vanillin on the fate of Listeria monocytogenes and Escherichia coli O157: H7 in a model apple juice medium and in apple juice. Food Microbiol. 2006, 23, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Tassou, C.; Drosinos, E.; Nychas, G. Effects of essential oil from mint (Mentha piperita) on Salmonella enteritidis and Listeria monocytogenes in model food systems at 4 and 10 C. J. Appl. Bacteriol. 1995, 78, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Farber, J.; Daley, E.; Coates, F.; Emmons, D.; McKellar, R. Factors influencing survival of Listeria monocytogenes in milk in a high-temperature short-time pasteurizer. J. Food Prot. 1992, 55, 946–951. [Google Scholar] [CrossRef]
- Moura-Alves, M.; Gouveia, A.R.; de Almeida, J.M.; Monteiro-Silva, F.; Silva, J.A.; Saraiva, C. Behavior of Listeria monocytogenes in beef Sous vide cooking with Salvia officinalis L. essential oil, during storage at different temperatures. LWT 2020, 132, 109896. [Google Scholar] [CrossRef]
- Dogruyol, H.; Mol, S.; Cosansu, S. Increased thermal sensitivity of Listeria monocytogenes in sous-vide salmon by oregano essential oil and citric acid. Food Microbiol. 2020, 90, 103496. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Hąc-Wydro, K.; Flasiński, M.; Romańczuk, K. Essential oils as food eco-preservatives: Model system studies on the effect of temperature on limonene antibacterial activity. Food Chem. 2017, 235, 127–135. [Google Scholar] [CrossRef]
- Wiktorczyk-Kapischke, N.; Skowron, K.; Grudlewska-Buda, K.; Wałecka-Zacharska, E.; Korkus, J.; Gospodarek-Komkowska, E. Adaptive response of Listeria monocytogenes to the stress factors in the food processing environment. Front. Microbiol. 2021, 12, 710085. [Google Scholar] [CrossRef] [PubMed]
- Garmyn, D.; Augagneur, Y.; Gal, L.; Vivant, A.-L.; Piveteau, P. Listeria monocytogenes differential transcriptome analysis reveals temperature-dependent Agr regulation and suggests overlaps with other regulons. PLoS ONE 2012, 7, e43154. [Google Scholar] [CrossRef]
- Neunlist, M.R.; Federighi, M.; Laroche, M.; Sohier, D.; Delattre, G.; Jacquet, C.; Chihib, N.-E. Cellular lipid fatty acid pattern heterogeneity between reference and recent food isolates of Listeria monocytogenes as a response to cold stress. Antonie Van Leeuwenhoek 2005, 88, 199–206. [Google Scholar] [CrossRef]
- Russell, N.; Evans, R.; Ter Steeg, P.; Hellemons, J.; Verheul, A.; Abee, T. Membranes as a target for stress adaptation. Int. J. Food Microbiol. 1995, 28, 255–261. [Google Scholar] [CrossRef]
- Püttmann, M.; Ade, N.; Hof, H. Dependence of fatty acid composition of Listeria spp. on growth temperature. Res. Microbiol. 1993, 144, 279–283. [Google Scholar] [CrossRef]
- Beales, N. Adaptation of microorganisms to cold temperatures, weak acid preservatives, low pH, and osmotic stress: A review. Compr. Rev. Food Sci. Food Saf. 2004, 3, 1–20. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential oils and their major components: An updated review on antimicrobial activities, mechanism of action and their potential application in the food industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid.-Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.; Robb, D. Cold chain optimisation models: A systematic literature review. Comput. Ind. Eng. 2025, 204, 110972. [Google Scholar] [CrossRef]
- Pedro, A.-L.; Rodolfo, R.-V.; Arturo, M.-H.P.; Nazmín, T.-G.D.; Antonio, S.-J.L. Cold chain relevance in the food safety of perishable products. Foods Raw Mater. 2023, 11, 116–128. [Google Scholar]
- Khwaza, V.; Aderibigbe, B.A. Antibacterial Activity of Selected Essential Oil Components and Their Derivatives: A Review. Antibiotics 2025, 14, 68. [Google Scholar] [CrossRef]
- Wang, L.-H.; Zhang, Z.-H.; Zeng, X.-A.; Gong, D.-M.; Wang, M.-S. Combination of microbiological, spectroscopic and molecular docking techniques to study the antibacterial mechanism of thymol against Staphylococcus aureus: Membrane damage and genomic DNA binding. Anal. Bioanal. Chem. 2017, 409, 1615–1625. [Google Scholar] [CrossRef]
- Yin, L.; Chen, J.; Wang, K.; Geng, Y.; Lai, W.; Huang, X.; Chen, D.; Guo, H.; Fang, J.; Chen, Z. Study the antibacterial mechanism of cinnamaldehyde against drug-resistant Aeromonas hydrophila in vitro. Microb. Pathog. 2020, 145, 104208. [Google Scholar] [CrossRef]
- Yang, S.-K.; Tan, N.-P.; Chong, C.-W.; Abushelaibi, A.; Lim, S.-H.-E.; Lai, K.-S. The missing piece: Recent approaches investigating the antimicrobial mode of action of essential oils. Evol. Bioinform. 2021, 17, 1176934320938391. [Google Scholar] [CrossRef]
- Rao, A.; Zhang, Y.; Muend, S.; Rao, R. Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrob. Agents Chemother. 2010, 54, 5062–5069. [Google Scholar] [CrossRef]
- Bridier, A.; Briandet, R.; Thomas, V.; Dubois-Brissonnet, F. Resistance of bacterial biofilms to disinfectants: A review. Biofouling 2011, 27, 1017–1032. [Google Scholar] [CrossRef]
- Esbelin, J.; Santos, T.; Hébraud, M. Desiccation: An environmental and food industry stress that bacteria commonly face. Food Microbiol. 2018, 69, 82–88. [Google Scholar] [CrossRef]
- Lee, B.-H.; Cole, S.; Badel-Berchoux, S.; Guillier, L.; Felix, B.; Krezdorn, N.; Hébraud, M.; Bernardi, T.; Sultan, I.; Piveteau, P. Biofilm formation of Listeria monocytogenes strains under food processing environments and pan-genome-wide association study. Front. Microbiol. 2019, 10, 2698. [Google Scholar] [CrossRef] [PubMed]
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Maktabi, S.; Rashnavadi, R.; Tabandeh, M.R.; Sourestani, M.M. Effective Inhibition of Listeria monocytogenes Biofilm Formation by Satureja rechingeri Essential Oil: Mechanisms and Implications. Curr. Microbiol. 2024, 81, 77. [Google Scholar] [CrossRef] [PubMed]


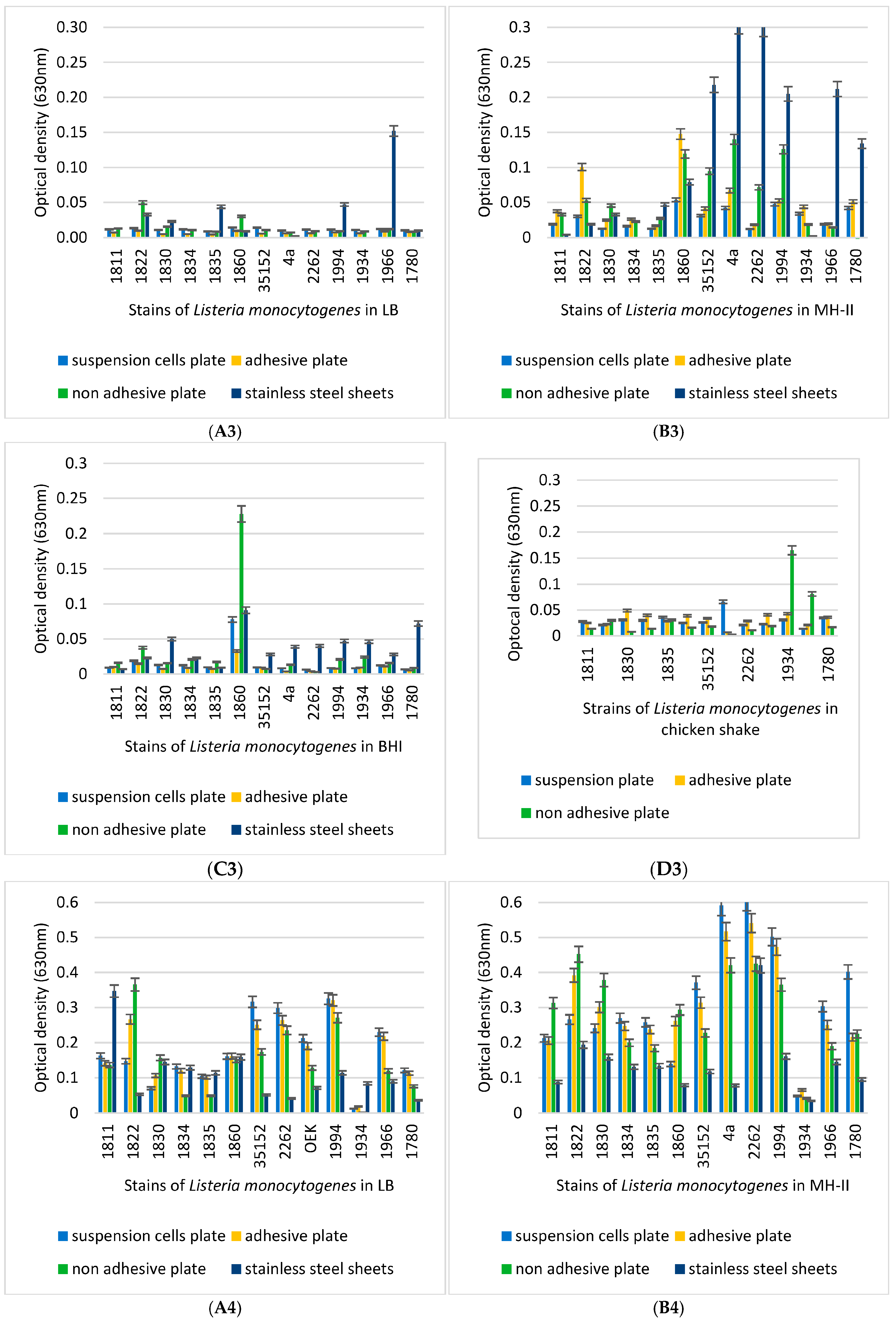
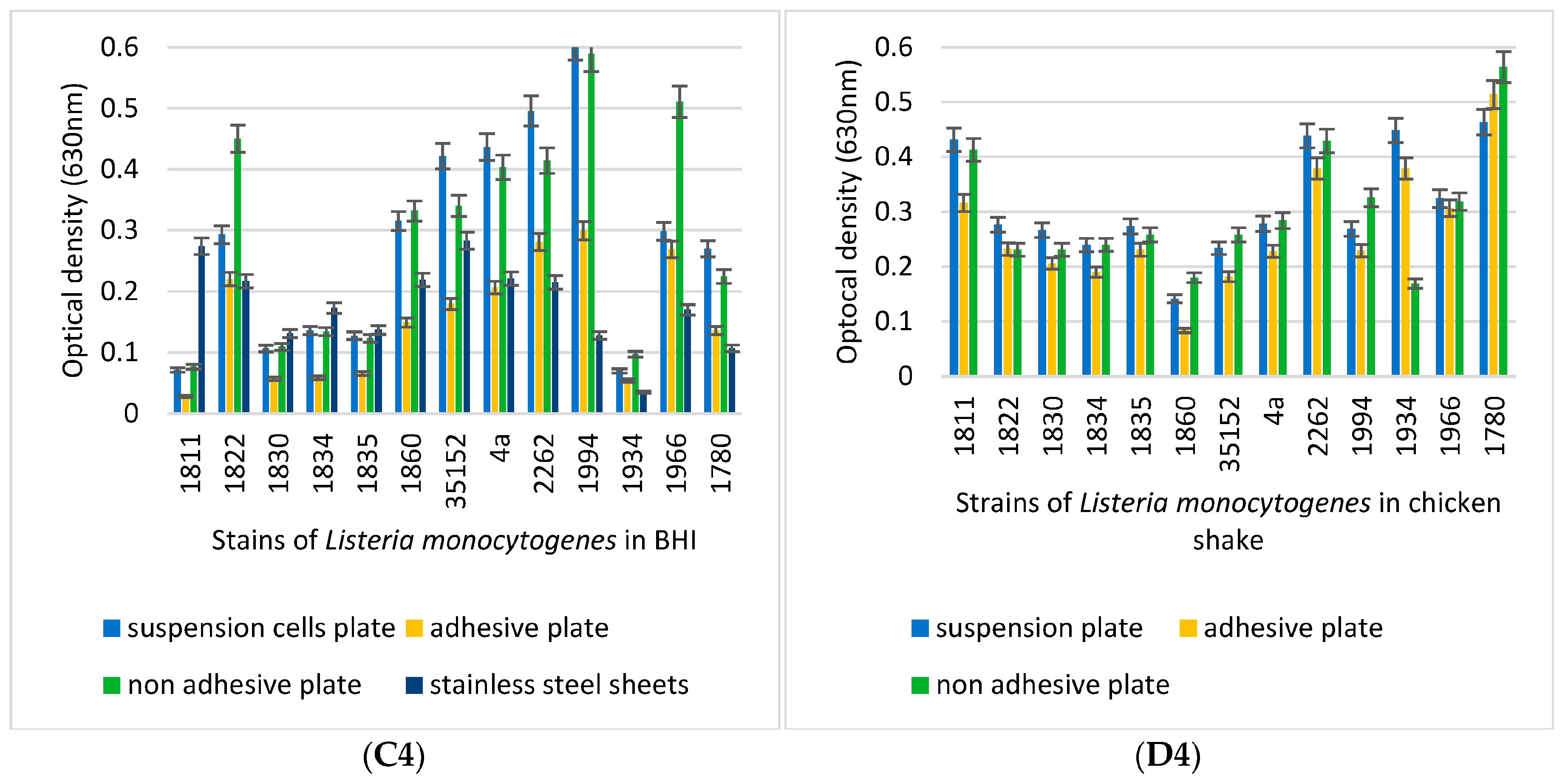

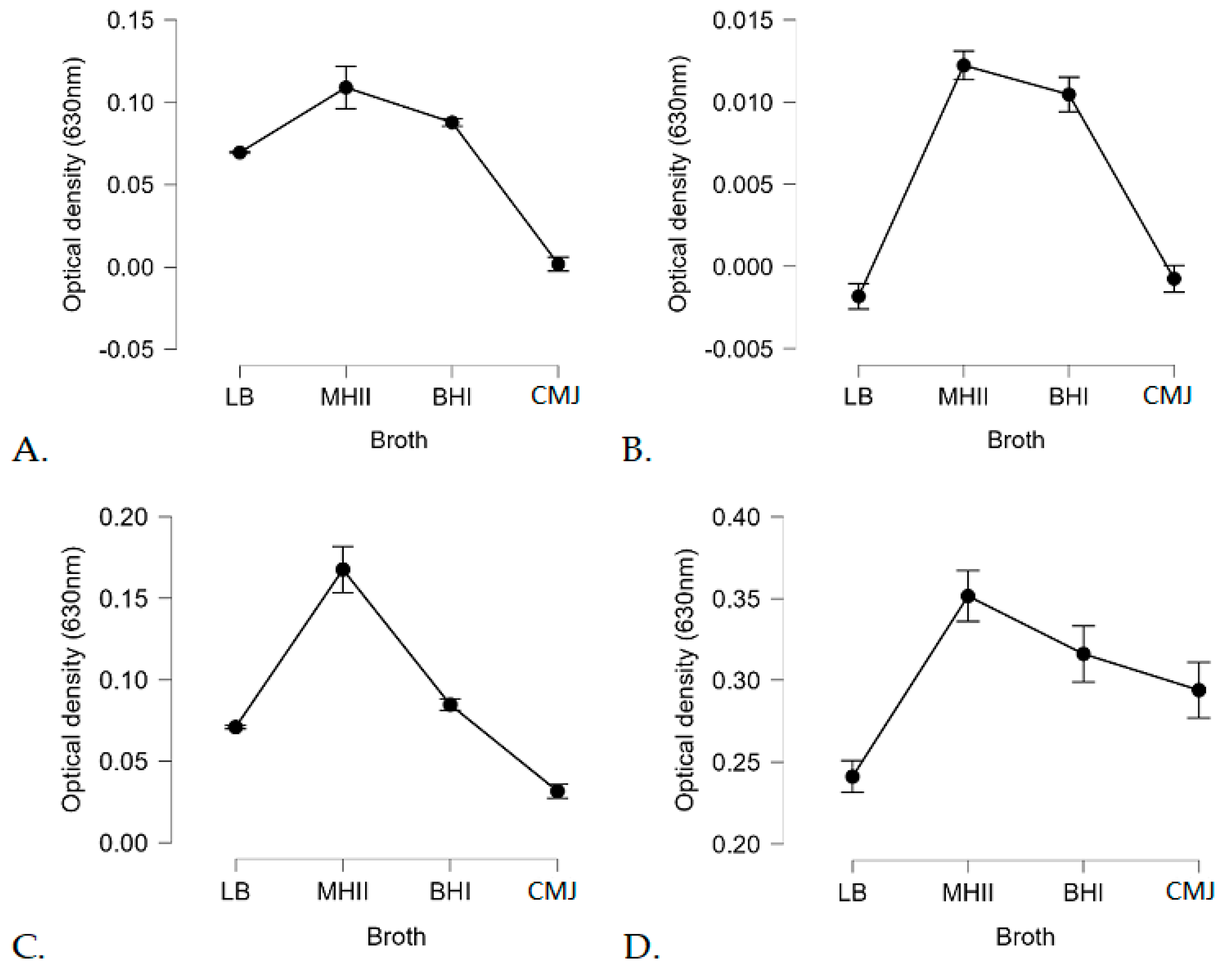
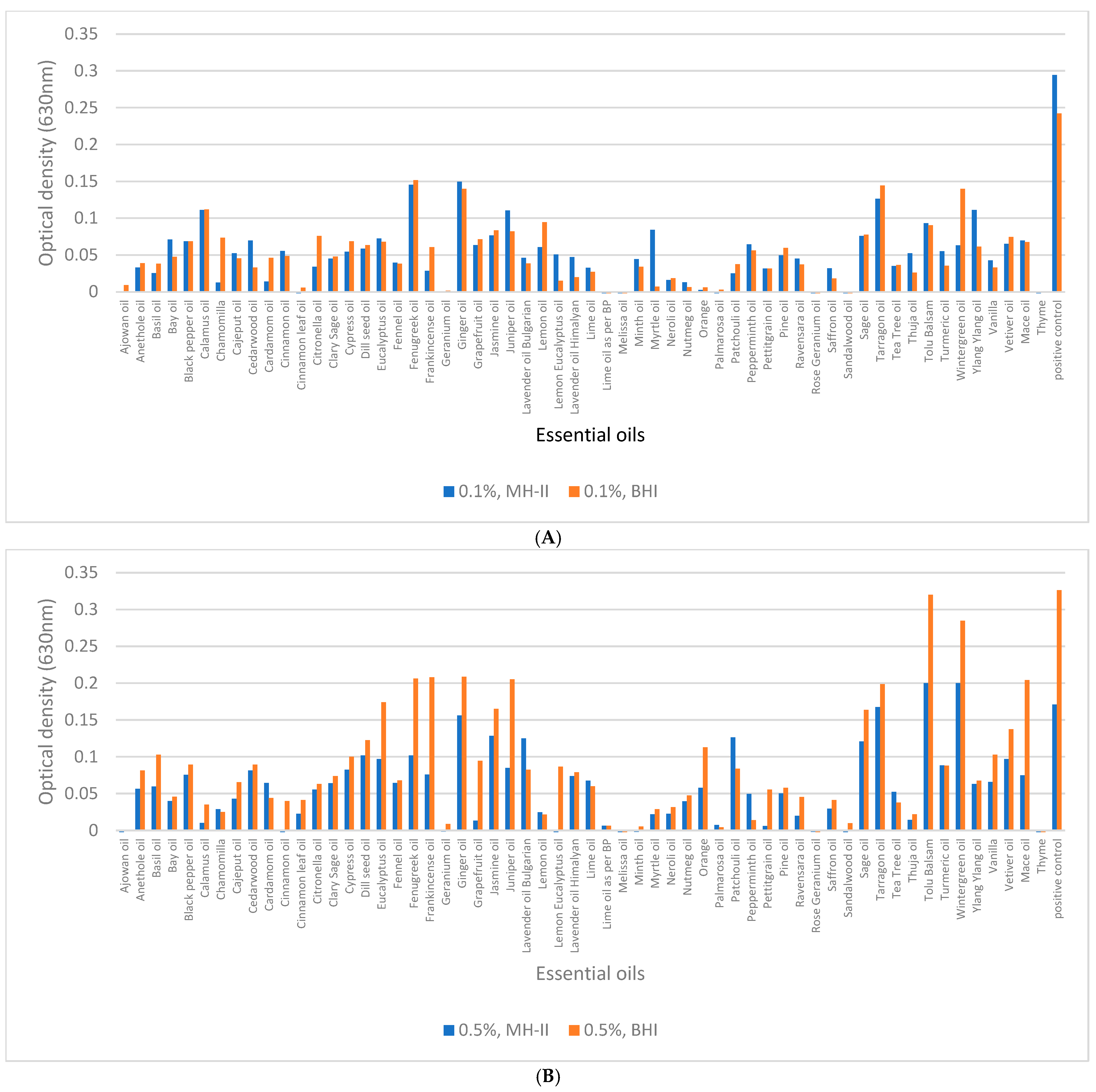
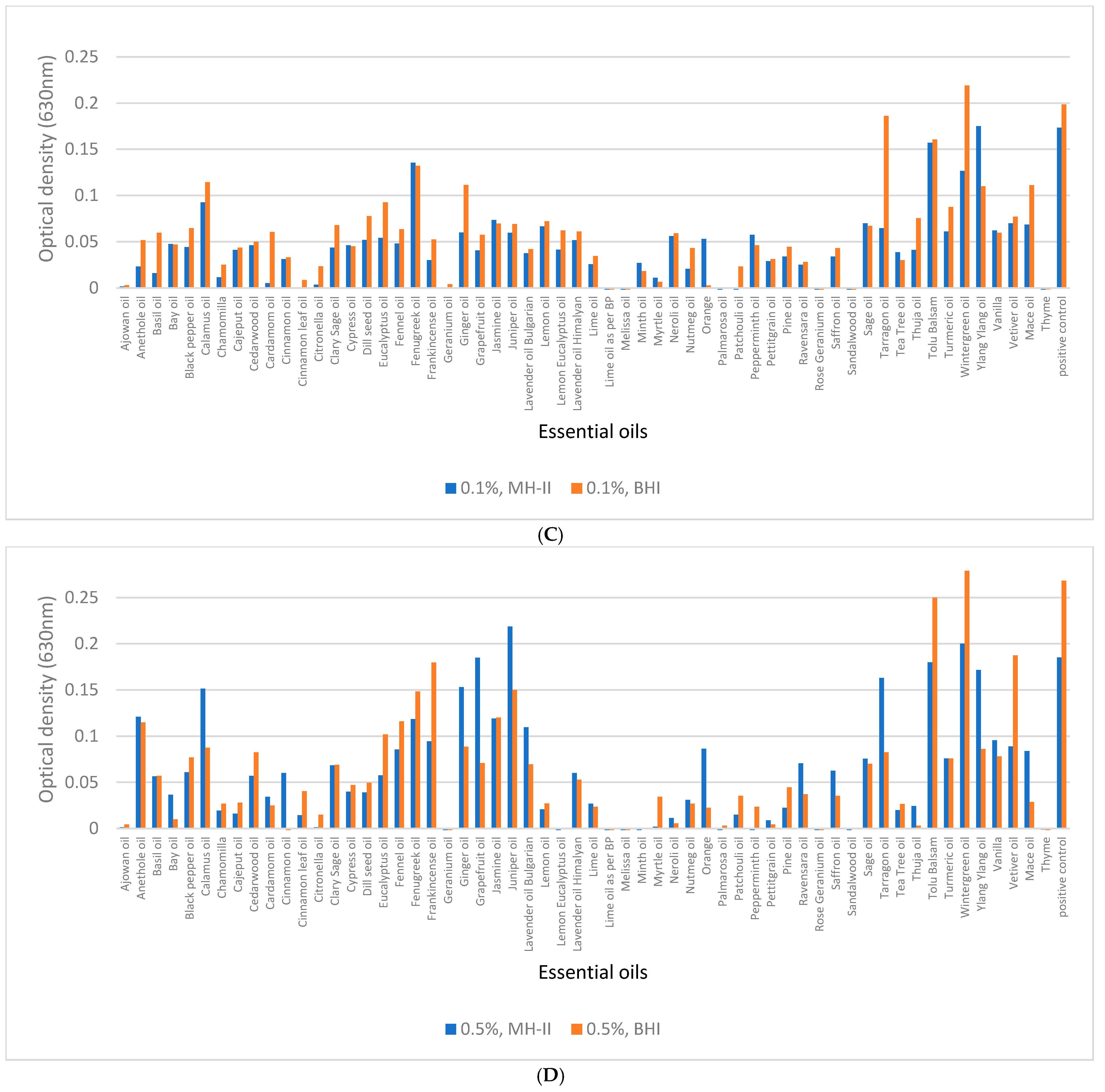
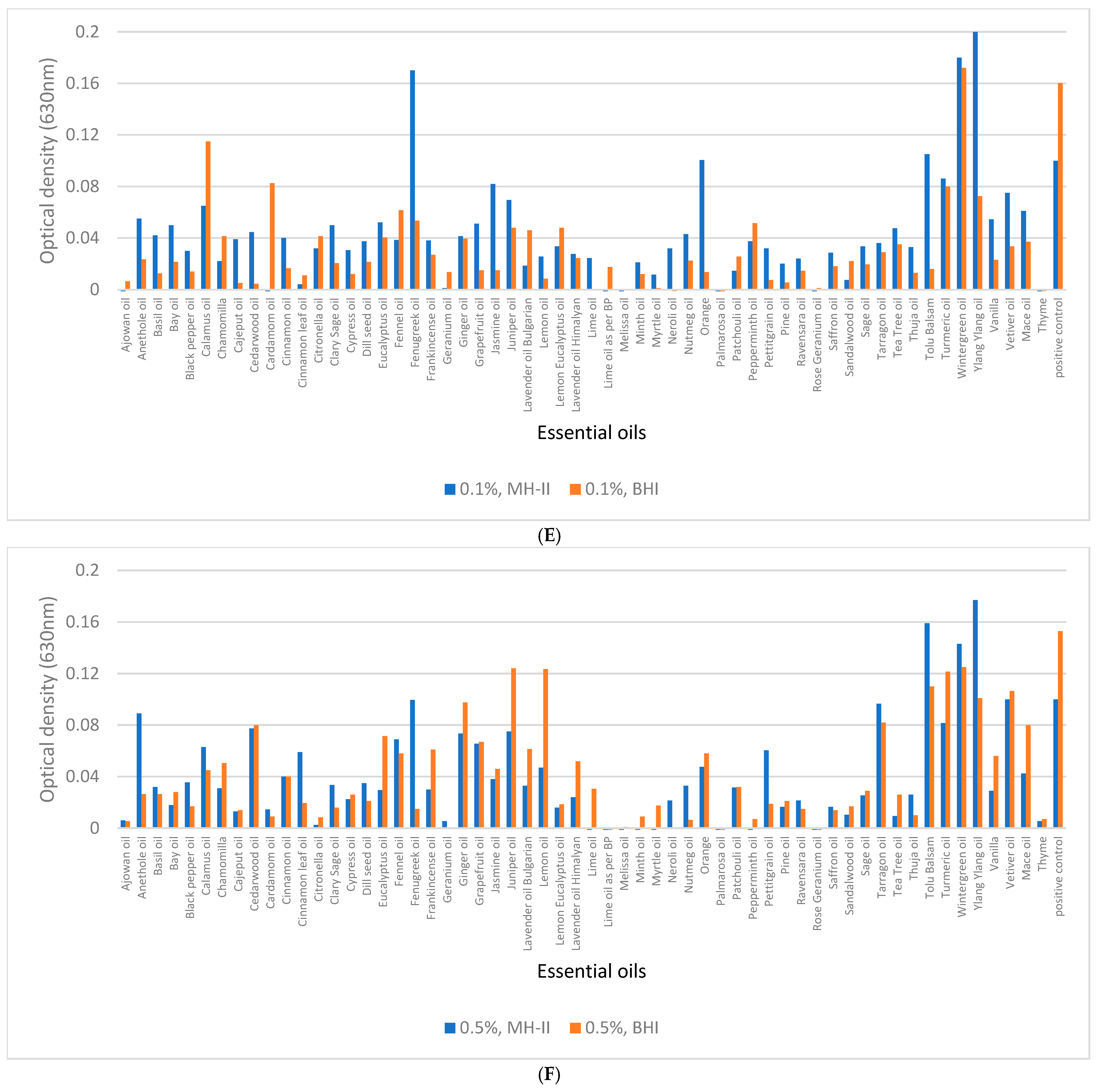




| Isolate Abbreviation | Strains of Listeria monocytogenes | Origin |
|---|---|---|
| 1966 | NCAIM B01966 | NCAIM B01966 |
| 1780 | NCAIM B01780 | NCAIM, LUX |
| 2262 | NCAIM B02262 | NCAIM, ATCC 19111, OKI133001 |
| 1994 | NCAIM B01994 | NCAIM (Murray, 1926) |
| 1934 | NCAIM B 01934 | NCAIM, CIP82.110, ATCC 15313, NCTC10357 |
| 35152 | ATCC 35152 | ATCC type strain (Murray 1926) |
| 4a | LmUP_4a | own isolate_2006, raw meat, chicken |
| 1811 | LmUP_1811 | own isolate_2006, raw meat pork |
| 1822 | LmUP_1822 | own isolate_2006, raw meat, pork |
| 1830 | LmUP_1830 | own isolate_2006, goat cheese |
| 1834 | LmUP_1834 | own isolate_2006, goat cheese |
| 1835 | LmUP_1835 | own isolate_2006, processed meat |
| 1860 | LmUP_1860 | own isolate_2006, processed meat |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seres-Steinbach, A.; Szabó, P.; Bányai, K.; Schneider, G. Effect of Temperature, Surface, and Medium Qualities on the Biofilm Formation of Listeria monocytogenes and Their Influencing Effects on the Antibacterial, Biofilm-Inhibitory, and Biofilm-Degrading Activities of Essential Oils. Foods 2025, 14, 2097. https://doi.org/10.3390/foods14122097
Seres-Steinbach A, Szabó P, Bányai K, Schneider G. Effect of Temperature, Surface, and Medium Qualities on the Biofilm Formation of Listeria monocytogenes and Their Influencing Effects on the Antibacterial, Biofilm-Inhibitory, and Biofilm-Degrading Activities of Essential Oils. Foods. 2025; 14(12):2097. https://doi.org/10.3390/foods14122097
Chicago/Turabian StyleSeres-Steinbach, Anita, Péter Szabó, Krisztián Bányai, and György Schneider. 2025. "Effect of Temperature, Surface, and Medium Qualities on the Biofilm Formation of Listeria monocytogenes and Their Influencing Effects on the Antibacterial, Biofilm-Inhibitory, and Biofilm-Degrading Activities of Essential Oils" Foods 14, no. 12: 2097. https://doi.org/10.3390/foods14122097
APA StyleSeres-Steinbach, A., Szabó, P., Bányai, K., & Schneider, G. (2025). Effect of Temperature, Surface, and Medium Qualities on the Biofilm Formation of Listeria monocytogenes and Their Influencing Effects on the Antibacterial, Biofilm-Inhibitory, and Biofilm-Degrading Activities of Essential Oils. Foods, 14(12), 2097. https://doi.org/10.3390/foods14122097







