Isolation and Characterization of Aeromonas salmonicida Phage TSW001 and Its Application on Large Yellow Croaker
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Phage Isolation, Purification, and Enrichment
2.3. Morphological Analysis via Transmission Electron Microscopy (TEM)
2.4. Genomic DNA Isolation and Analysis of the Genome
2.5. Phage Lytic Range
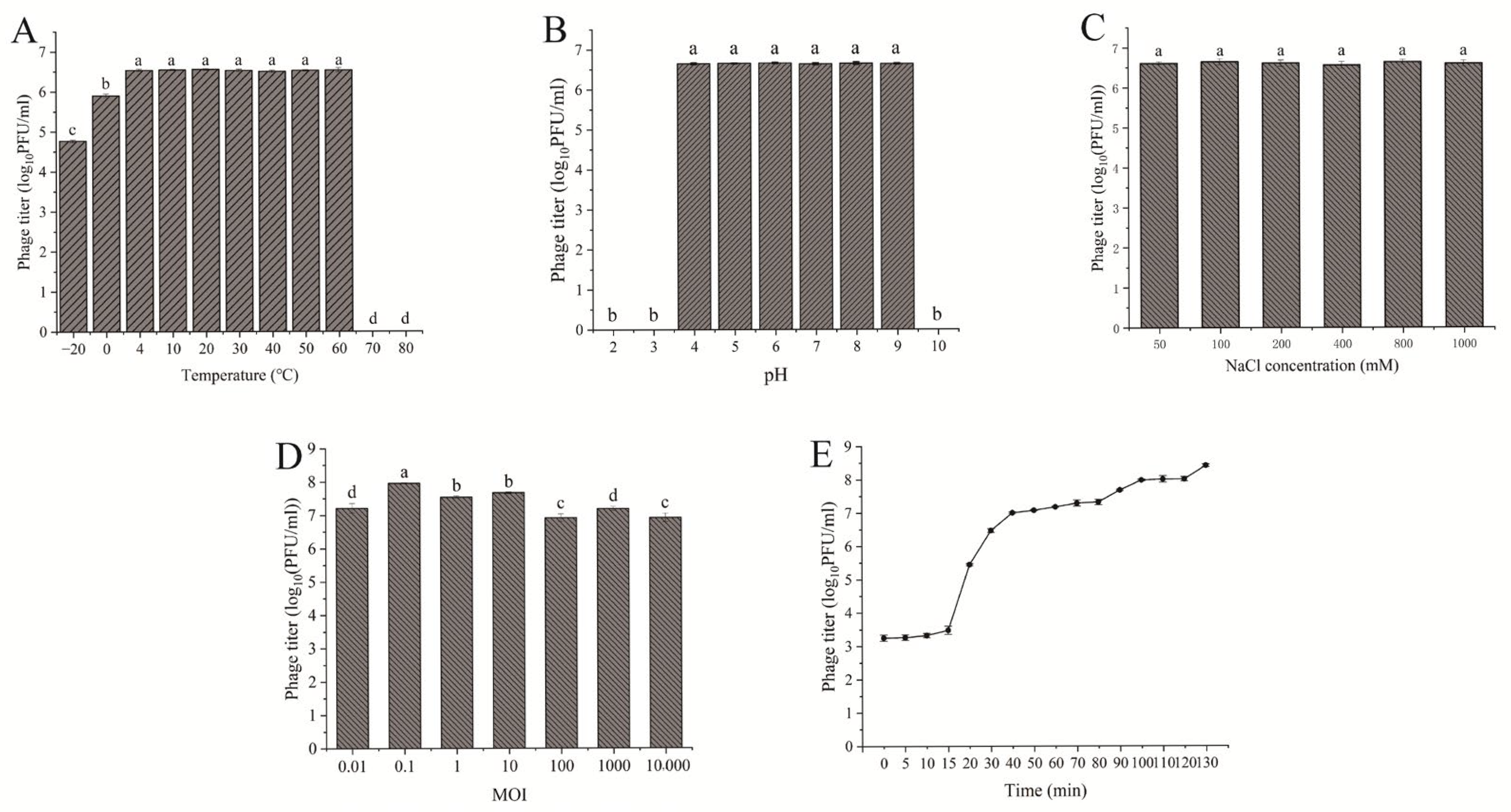
2.6. Stability Tests of Bacteriophage TSW001
2.7. Multiplicity of Infection Evaluation
2.8. One-Step Growth Assay of Phage TSW001
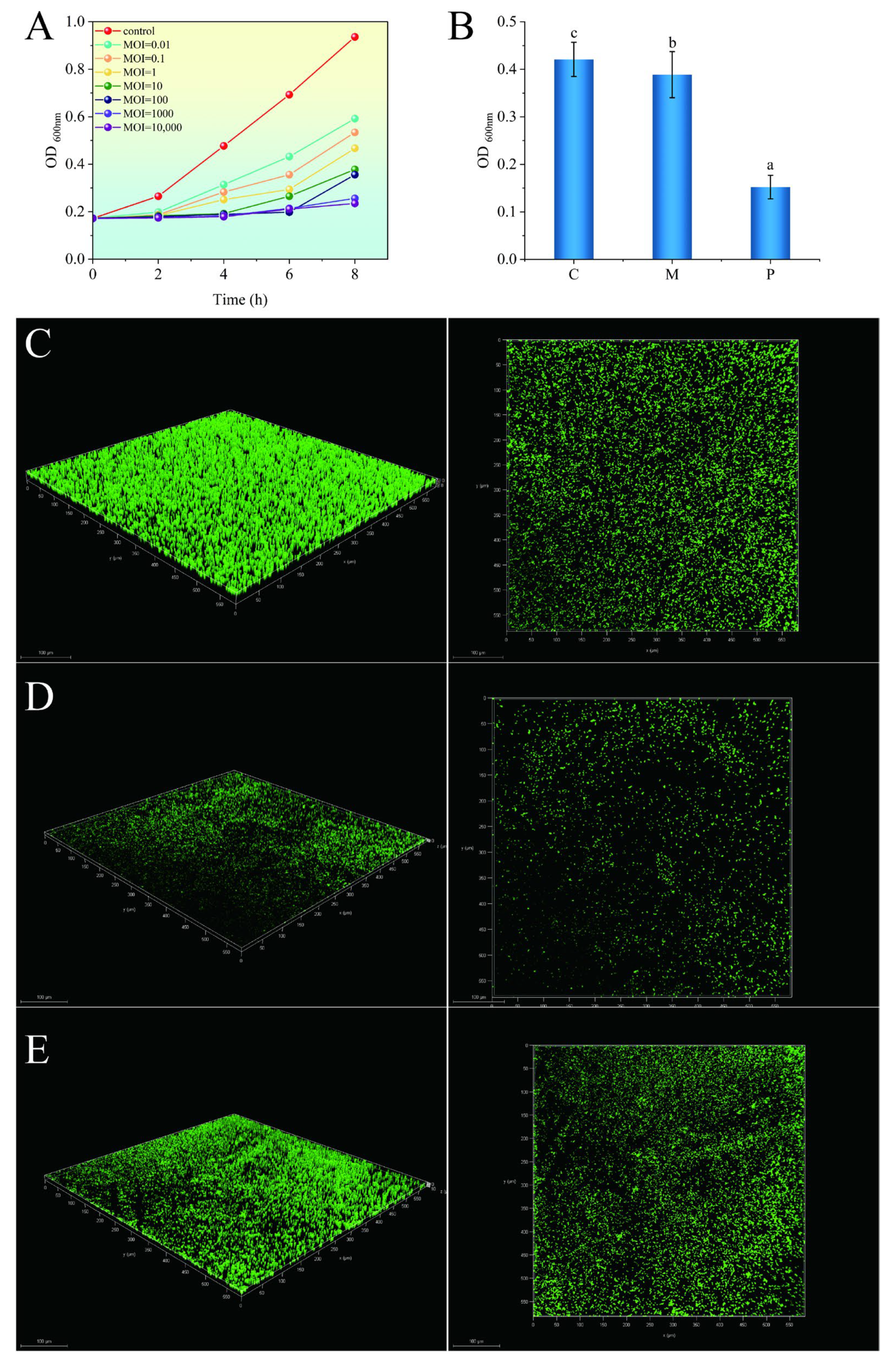
2.9. In Vitro Bactericidal Effects of TSW001
2.10. Effect of the Phage on A. salmonicida Biofilm
2.10.1. Biofilm Formation and Quantification
2.10.2. Visualization by Confocal Laser Scanning Microscopy (CLSM)
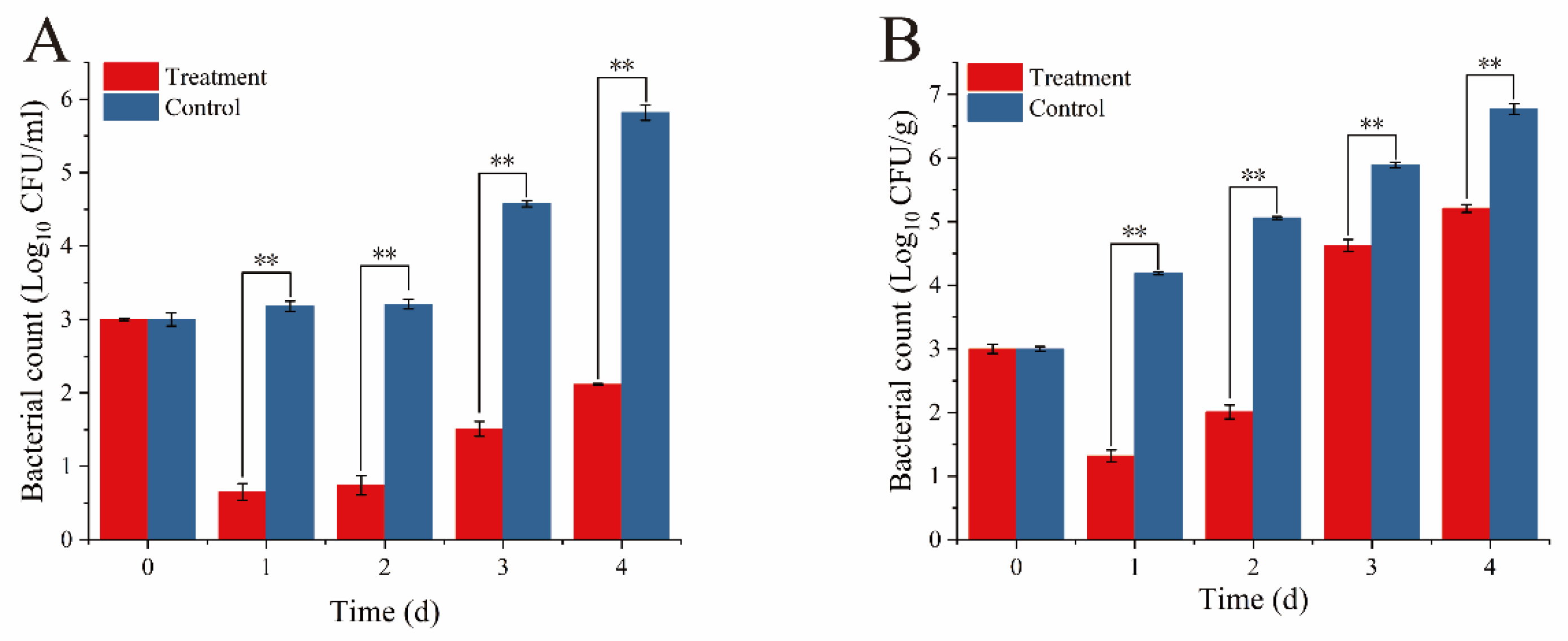
2.11. Effect of Phage TSW001 Against A. salmonicida on Large Yellow Croaker
2.12. Statistical Analysis
3. Results
3.1. Isolation and Morphological Characterization of Phage TSW001
3.2. Host Range of Phage TSW001
3.3. The Stability and Lysis Kinetics of Phage TSW001
3.4. Genetic Characterization of TSW001
3.5. Phage Treatment Inhibits the Growth of Bacteria and Their Biofilms
3.6. Antibacterial Activity of Phage TSW001 Against AS08 in Large Yellow Croaker
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.; Liu, X.; Li, H.; Hu, T.; Jatt, A.-N.; Liu, Y. Antibacterial mechanism of lactobionic acid against Aeromonas salmonicida by transcriptomic analysis and its application in refrigerated grass carp. Food Control 2024, 158, 110255. [Google Scholar] [CrossRef]
- Shao, L.; Dong, Y.; Chen, S.; Sheng, J.; Cai, L.; Xu, X.; Wang, H. Revealing extracellular protein profile and excavating spoilage-related proteases of Aeromonas salmonicida based on multi-omics investigation. Int. J. Biol. Macromol. 2024, 265, 130916. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yan, Y.; Feng, L.; Zhu, J. Quorum sensing asaI mutants affect spoilage phenotypes, motility, and biofilm formation in a marine fish isolate of Aeromonas salmonicida. Food Microbiol. 2018, 76, 40–51. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm matrixome: Extracellular components in structured microbial communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Sharan, M.; Vijay, D.; Dhaka, P.; Bedi, J.S.; Gill, J.P.S. Biofilms as a microbial hazard in the food industry: A scoping review. J. Appl. Microbiol. 2022, 133, 2210–2234. [Google Scholar] [CrossRef]
- Tian, Y.; Tian, X.; Li, T.; Wang, W. Overview of the effects and mechanisms of NO and its donors on biofilms. Crit. Rev. Food Sci. Nutr. 2023, 65, 647–666. [Google Scholar] [CrossRef]
- Ling, X.-D.; Lv, J.; Chen, F.-J.; Qin, X.-T.; Wu, M.-S.; Bai, F.; Luo, H.-Q. Expression characteristics and in vitro antibacterial properties of C-type lysozyme in crucian carp infected with Aeromonas salmonicida. Heliyon 2024, 10, e24044. [Google Scholar] [CrossRef]
- Yan, J.; Guo, Z.; Zhao, Z.; Yuan, J.; Wang, X.; Xie, J. High-value development and utilization of functional peptides from seafood by-products and discards: A case study of antimicrobial peptides. Food Biosci. 2024, 59, 104246. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Paidari, S.; Baghbaderani, S.A.; Nateghi, L.; Al-Hassan, A.A.; Ariffin, F. Essential oils as natural antimicrobial agents in postharvest treatments of fruits and vegetables: A review. Food Meas. 2022, 16, 507–522. [Google Scholar] [CrossRef]
- Papadochristopoulos, A.; Kerry, J.P.; Fegan, N.; Burgess, C.M.; Duffy, G. Natural Anti-Microbials for Enhanced Microbial Safety and Shelf-Life of Processed Packaged Meat. Foods 2021, 10, 1598. [Google Scholar] [CrossRef]
- Kim, B.H.; Ashrafudoulla, M.; Shaila, S.; Park, H.J.; Sul, J.D.; Park, S.H.; Ha, S.-D. Isolation, characterization, and application of bacteriophage on Vibrio parahaemolyticus biofilm to control seafood contamination. Int. J. Antimicrob. Agents 2024, 64, 107194. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Lee, H.; Park, M.-K. Isolation, characterization, and application of a novel, lytic phage vB_SalA_KFSST3 with depolymerase for the control of Salmonella and its biofilm on cantaloupe under cold temperature. Food Res. Int. 2023, 172, 113062. [Google Scholar] [CrossRef] [PubMed]
- Namonyo, S.; Weynberg, K.D.; Guo, J.; Carvalho, G. The effectiveness and role of phages in the disruption and inactivation of clinical P. aeruginosa biofilms. Environ. Res. 2023, 234, 116586. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, J.; Islam, M.S.; Yan, T.; Zhou, Y.; Liang, L.; Connerton, I.F.; Deng, K.; Li, J. Application of a novel phage vB_SalS-LPSTLL for the biological control of Salmonella in foods. Food Res. Int. 2021, 147, 110492. [Google Scholar] [CrossRef]
- Ning, Z.; Zhang, L.; Cai, L.; Xu, X.; Chen, Y.; Wang, H. Biofilm removal mediated by Salmonella phages from chicken-related sources. Food Sci. Hum. Wellness 2023, 12, 1799–1808. [Google Scholar] [CrossRef]
- Azeredo, J.; Garcia, P.; Drulis-Kawa, Z. Targeting biofilms using phages and their enzymes. Curr. Opin. Biotechnol. 2021, 68, 251–261. [Google Scholar] [CrossRef]
- Górski, A.; Międzybrodzki, R.; Węgrzyn, G.; Jończyk-Matysiak, E.; Borysowski, J.; Weber-Dąbrowska, B. Phage therapy: Current status and perspectives. Med. Res. Rev. 2020, 40, 459–463. [Google Scholar] [CrossRef]
- Xiao, K.; Pan, Q.; Wu, Y.; Ding, Y.; Wu, Q.; Zhang, J.; Wang, Z.; Liu, Z.; Wang, W.; Wang, J. Application of a novel phage vB_CjeM_WX1 to control Campylobacter jejuni in foods. Int. J. Food Microbiol. 2025, 427, 110975. [Google Scholar] [CrossRef]
- Yan, J.; Xie, J. Removal of Shewanella putrefaciens Biofilm by acidic electrolyzed water on food contact surfaces. LWT 2021, 151, 112044. [Google Scholar] [CrossRef]
- Yan, J.; Xie, J. Comparative proteome analysis of Shewanella putrefaciens WS13 mature biofilm under cold stress. Front. Microbiol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- López-Cuevas, O.; Medrano-Félix, J.A.; Campo, N.C.-D.; Chaidez, C. Bacteriophage applications for fresh produce food safety. Int. J. Environ. Health Res. 2021, 31, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Callahan, M.T.L.; Woolston, J.W.; Sharma, M.; Sulakvelidze, A. Phage biocontrol for reducing bacterial foodborne pathogens in produce and other foods. Curr. Opin. Biotechnol. 2022, 78, 102805. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Guo, M.; Chen, B.; Zhang, C.; Li, D.; Xie, J. Molecular characterization of spoilage microbiota in high CO2 refrigerated large yellow croaker (Larimichthys crocea) fillets using metagenomic and metabolomic approaches. Food Biosci. 2023, 56, 103227. [Google Scholar] [CrossRef]
- Hosseini, N.; Paquet, V.E.; Marcoux, P.-É.; Alain, C.-A.; Paquet, M.F.; Moineau, S.; Charette, S.J. MQM1, a bacteriophage infecting strains of Aeromonas salmonicida subspecies salmonicida carrying Prophage 3. Virus Res. 2023, 334, 199165. [Google Scholar] [CrossRef]
- Xu, Z.; Jin, P.; Zhou, X.; Zhang, Y.; Wang, Q.; Liu, X.; Shao, S.; Liu, Q. Isolation of a virulent Aeromonas salmonicida subsp. masoucida bacteriophage and its application in phage therapy in turbot (Scophthalmus maximus). Appl. Environ. Microbiol. 2021, 87, e01468-21. [Google Scholar] [CrossRef]
- Mevo, S.I.U.; Ashrafudoulla, M.; Mizan, M.F.R.; Park, S.H.; Ha, S.-D. Promising strategies to control persistent enemies: Some new technologies to combat biofilm in the food industry—A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5938–5964. [Google Scholar] [CrossRef]
- Pires, D.P.; Melo, L.D.R.; Boas, D.V.; Sillankorva, S.; Azeredo, J. Phage therapy as an alternative or complementary strategy to prevent and control biofilm-related infections. Curr. Opin. Microbiol. 2017, 39, 48–56. [Google Scholar] [CrossRef]
- Hu, J.; Miyanaga, K.; Tanji, Y. Diffusion properties of bacteriophages through agarose gel membrane. Biotechnol. Prog. 2010, 26, 1213–1221. [Google Scholar] [CrossRef]
- Flemming, H.-C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The biofilm matrix: Multitasking in a shared space. Nat. Rev. Microbiol. 2023, 21, 70–86. [Google Scholar] [CrossRef]
- Drulis-Kawa, Z.; Majkowska-Skrobek, G.; Maciejewska, B. Bacteriophages and phage-derived proteins—Application approaches. Curr. Med. Chem. 2015, 22, 1757–1773. [Google Scholar] [CrossRef]
- Bryan, D.; El-Shibiny, A.; Hobbs, Z.; Porter, J.; Kutter, E.M. Bacteriophage T4 infection of stationary phase E. coli: Life after log from a phage perspective. Front. Microbiol. 2016, 7, 1391. [Google Scholar] [CrossRef]


| Strain | Source | Lytic |
|---|---|---|
| A. salmonicida AS03 | Oreochromis niloticus | + |
| A. salmonicida AS08 | Larimichthys crocea | + |
| Aeromonas hydrophila | Oreochromis niloticus | + |
| Aeromonas sobria | Oreochromis niloticus | − |
| Aeromonas piscicola | Oreochromis niloticus | − |
| Pseudomonas mevalonii | Grouper | − |
| Acinetobacter johnsonii | Thunnus obesus | − |
| Shewanella putrefaciens | Thunnus obesus | − |
| Hafnia paralvei | Grouper | − |
| A. allosaccharophila | Grouper | − |
| Pseudomonas fluorescens | Larimichthys crocea | − |
| Pseudomonas fluorescens | Milk | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Guo, Z.; Xie, J. Isolation and Characterization of Aeromonas salmonicida Phage TSW001 and Its Application on Large Yellow Croaker. Foods 2025, 14, 2082. https://doi.org/10.3390/foods14122082
Yan J, Guo Z, Xie J. Isolation and Characterization of Aeromonas salmonicida Phage TSW001 and Its Application on Large Yellow Croaker. Foods. 2025; 14(12):2082. https://doi.org/10.3390/foods14122082
Chicago/Turabian StyleYan, Jun, Zhenghao Guo, and Jing Xie. 2025. "Isolation and Characterization of Aeromonas salmonicida Phage TSW001 and Its Application on Large Yellow Croaker" Foods 14, no. 12: 2082. https://doi.org/10.3390/foods14122082
APA StyleYan, J., Guo, Z., & Xie, J. (2025). Isolation and Characterization of Aeromonas salmonicida Phage TSW001 and Its Application on Large Yellow Croaker. Foods, 14(12), 2082. https://doi.org/10.3390/foods14122082







