Unveiling the Impact of Processing Methods on In Vitro Protein Digestibility: A Focus on Highland Barley and Its Application in Wine Production
Abstract
1. Introduction
2. Nutritional and Functional Properties of Highland Barley Protein
2.1. Nutritional Value of Highland Barley Protein
2.1.1. Composition of Highland Barley Protein
2.1.2. Digestibility of Highland Barley Protein and Its Influencing Factors
2.2. Functional Properties of Highland Barley Protein
3. In Vitro Digestion Test of Highland Barley Proteins
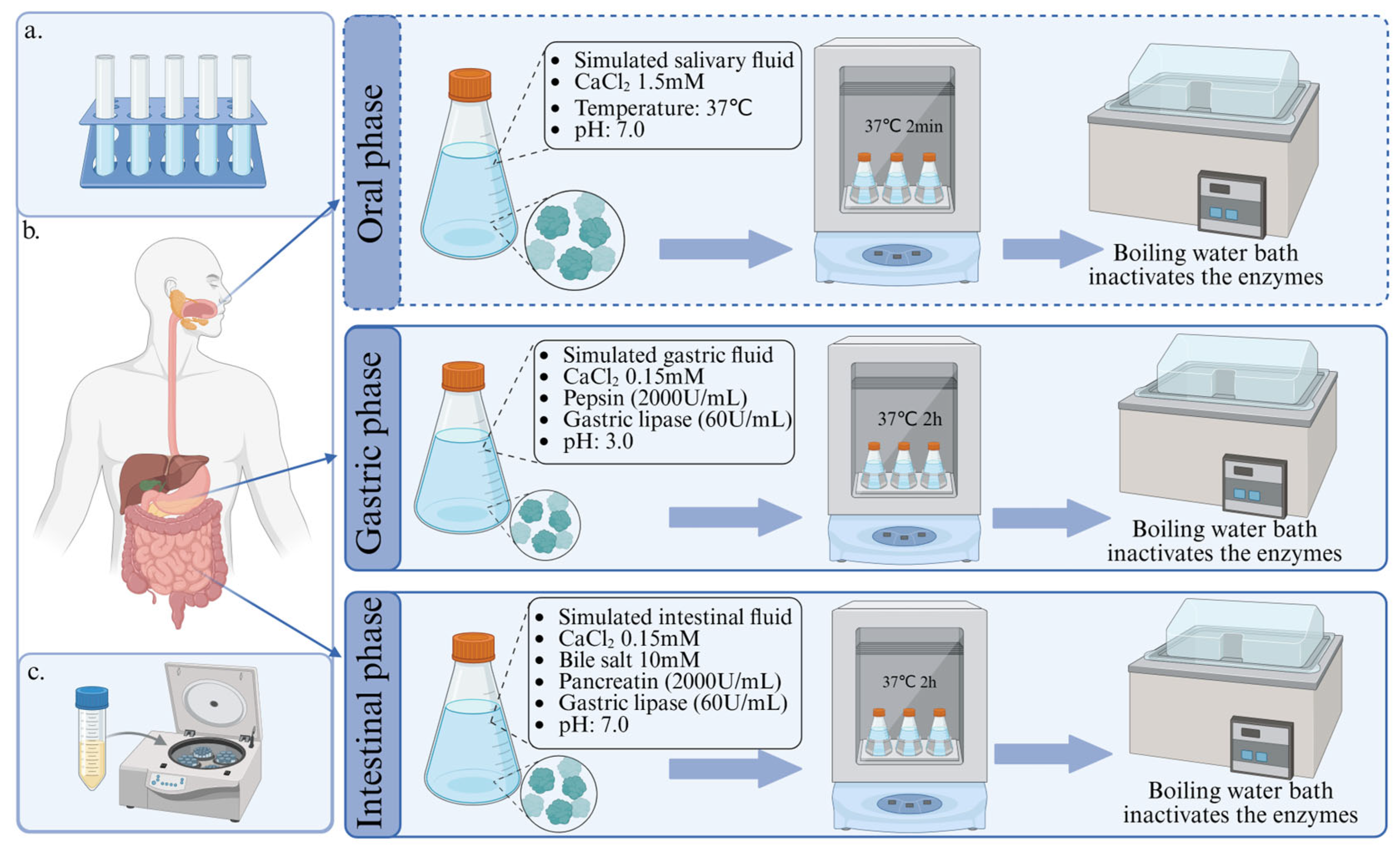
3.1. Commonly Used In Vitro Digestive Models
3.1.1. Static Digestion Model
3.1.2. Dynamic Digestive Modelling
3.2. Key Factors in the Digestive System Affecting In Vitro Digestion of Highland Barley Proteins
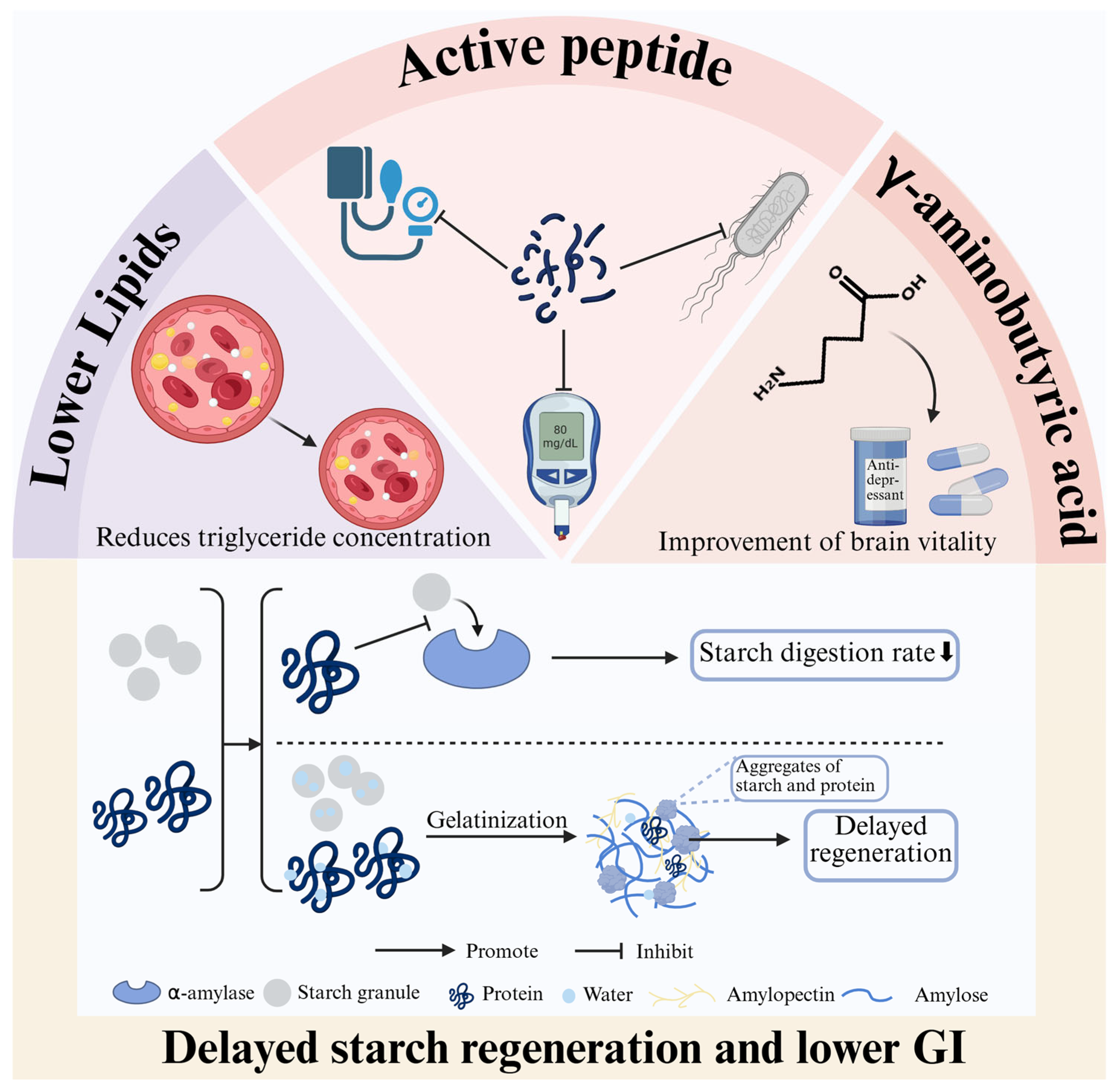
3.3. Digestive Characteristics of Highland Barley Protein
4. Application of Highland Barley Protein in Winemaking
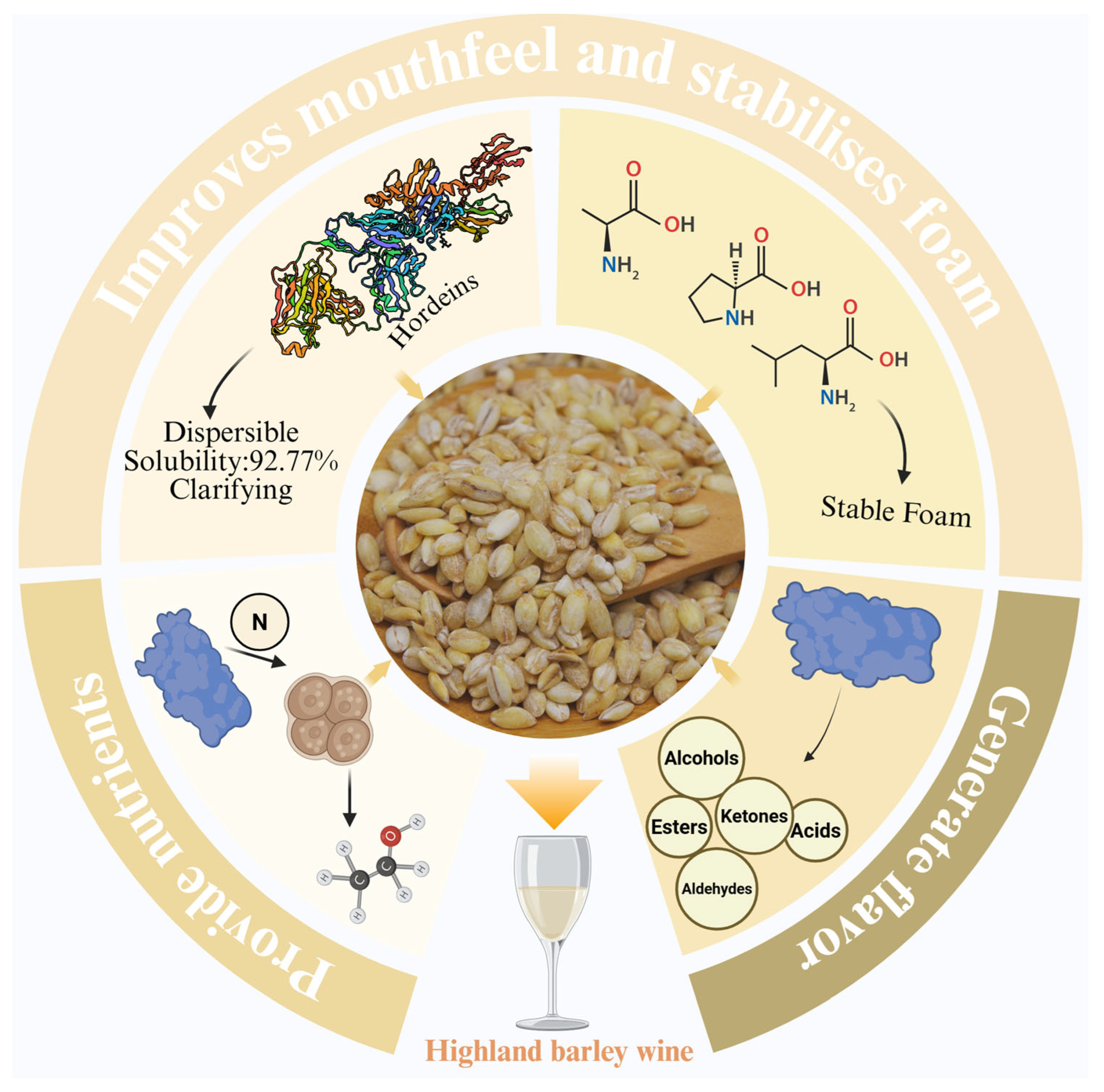
4.1. The Unique Role of Highland Barley Protein in Highland Barley Wine Production
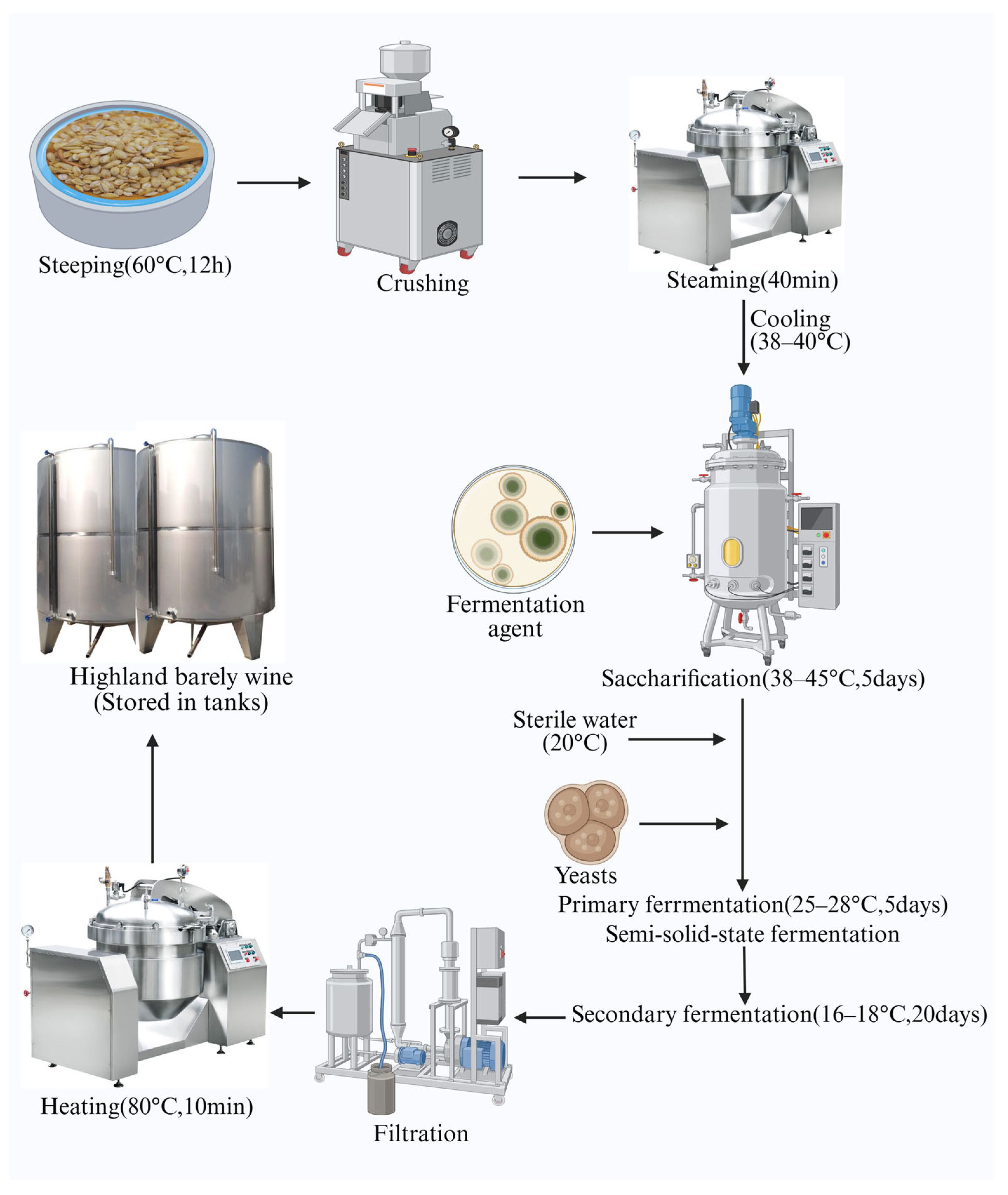
4.2. Traditional and Modern Brewing Processing Techniques
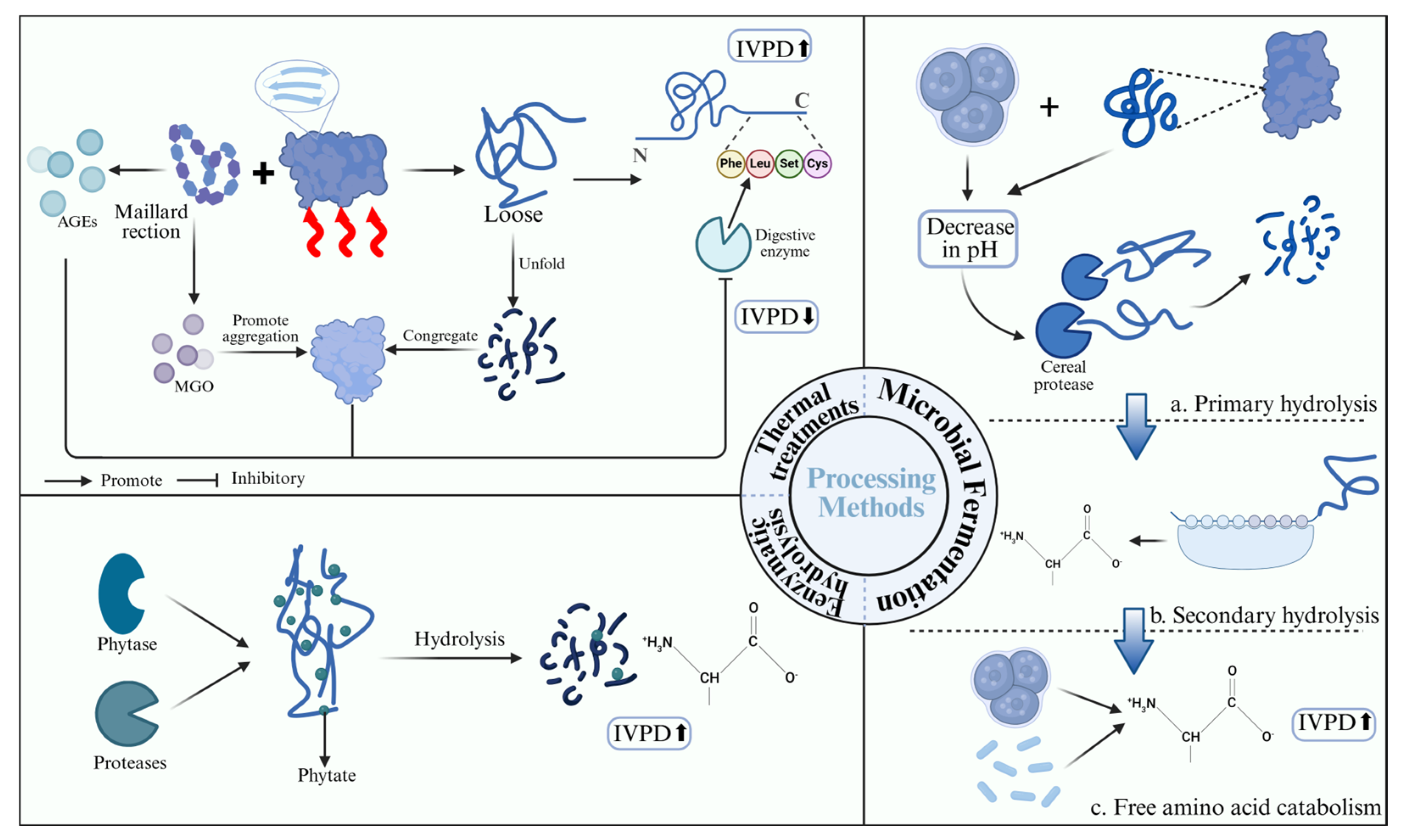
5. Effect of Different Processing Methods on Highland Barley Protein Digestibility in Highland Barley Wine Production
5.1. The Effect of Key Processing Techniques on Highland Barley Protein Digestibility During Winemaking
5.1.1. Thermal Processing
5.1.2. Enzymatic Treatment
5.1.3. Fermentation
5.2. Comparative Analysis of Different Processing Methods
5.3. The Significance of Increased Protein Digestibility for Highland Barley Wine Production
6. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, T.; Cao, J.; Cao, J.; Zhu, F.; Cao, F.; Su, E. Almond (Amygdalus communis L.) kernel protein: A review on the extraction, functional properties and nutritional value. Food Res. Int. 2023, 167, 11. [Google Scholar] [CrossRef] [PubMed]
- Dolganyuk, V.; Sukhikh, S.; Kalashnikova, O.; Ivanova, S.; Kashirskikh, E.; Prosekov, A.; Michaud, P.; Babich, O. Food Proteins: Potential Resources. Sustainability 2023, 15, 5863. [Google Scholar] [CrossRef]
- Rodriguez, N.R. Introduction to Protein Summit 2.0: Continued exploration of the impact of high-quality protein on optimal health. Am. J. Clin. Nutr. 2023, 101, 1317S–1319S. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Horvath, C.; Chen, L.; Chen, J.; Zheng, B. Understanding the nutrient composition and nutritional functions of highland barley (Qingke): A review. Trends Food Sci. Technol. 2020, 103, 109–117. [Google Scholar] [CrossRef]
- Du, Y.; Liang, F.; Chen, Z.; Zhou, W.; Tu, Z.; Li, J. Effects of decolorization on aggregation behavior of highland barley proteins: Comparison with wheat proteins. Food Res. Int. 2022, 160, 10. [Google Scholar] [CrossRef]
- Xie, J.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C.; Ban, X. Highland Barley Starch: Structures, Properties, and Applications. Foods 2023, 12, 387. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, J.; Qiao, Z.; Cao, X.; Luo, Q.; Zhao, J.; Wang, F.; Zhang, W. Assessment of β-glucans, phenols, flavor and volatile profiles of hulless barley wine originating from highland areas of China. Food Chem. 2019, 293, 32–40. [Google Scholar] [CrossRef]
- Mengxiao, Y.; Zhi, Y.; David, W.E.; Elliot Paul, G.; Harjinder, S.; Aiqian, Y. Digestion of food proteins: The role of pepsin. Crit. Rev. Food Sci. Nutr. 2025, 22, 1–22. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Food processing for the improvement of plant proteins digestibility. Crit. Rev. Food Sci. Nutr. 2019, 60, 3367–3386. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; El-Din, A.; Bekhit, A.; Kumar, S.; Bhat, H.F. Emerging processing technologies for improved digestibility of muscle proteins. Trends Food Sci. Technol. 2021, 110, 226–239. [Google Scholar] [CrossRef]
- Hur, S.J.; Lim, B.O.; Decker, E.A.; McClements, D.J. In vitro human digestion models for food applications. Food Chem. 2011, 125, 1–12. [Google Scholar] [CrossRef]
- Li, C.; Yu, W.; Wu, P.; Chen, X.D. Current in vitro digestion systems for understanding food digestion in human upper gastrointestinal tract. Trends Food Sci. Technol. 2020, 96, 114–126. [Google Scholar] [CrossRef]
- Aghababaei, F.; McClements, D.J.; Hadidi, M. Ultrasound processing for enhanced digestibility of plant proteins. Food Hydrocoll. 2024, 155, 110188. [Google Scholar] [CrossRef]
- Loveday, S.M. Food Proteins: Technological, Nutritional, and Sustainability Attributes of Traditional and Emerging Proteins. Annu. Rev. Food Sci. Technol. 2019, 10, 311–339. [Google Scholar] [CrossRef]
- Salazar-Villanea, S.; Hendriks, W.H.; Bruininx, E.M.A.M.; Gruppen, H.; van der Poel, A.F.B. Protein structural changes during processing of vegetable feed ingredients used in swine diets: Implications for nutritional value. Nutr. Res. Rev. 2016, 29, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zhang, Q.; Shen, L.; Luo, J.; Zhu, R.; Mao, J.; Zhao, M.; Cai, C. Phenolic profile and antioxidant activity of Chinese rice wine fermented with different rice materials and starters. LWT-Food Sci. Technol. 2019, 111, 226–234. [Google Scholar] [CrossRef]
- Chen, L.; Peng, Q.; Liu, B.; Zhang, Y.; Feng, S. Study on the dynamic changes of nutrient components and flavor compounds during the fermentation process of high-quality highland barley wine. Int. J. Gastron. Food Sci. 2023, 35, 13. [Google Scholar] [CrossRef]
- Wang, C.-P.; Pan, Z.-F.; Nima, Z.-X.; Tang, Y.-W.; Cai, P.; Liang, J.-J.; Deng, G.-B.; Long, H.; Yu, M.-Q. Starch granule-associated proteins of hull-less barley (Hordeum vulgare L.) from the Qinghai-Tibet Plateau in China. J. Sci. Food Agric. 2011, 91, 616–624. [Google Scholar] [CrossRef]
- Hui-sheng, Z.; Qiao, L.; Yu-hong, Z.; Xing-quan, Z.; Ya-wei, T.; Tashi, N.; Guang-bing, D.; Hai, L.; Zhi-fen, P.; Mao-qun, Y. Evaluation of the Amino Acids Composition and Nutrition Value of 72 Hulless Barley. J. Plant Genet. Resour. 2021, 22, 121–129. [Google Scholar] [CrossRef]
- Huang, H.J.; Gao, X.L.; Li, Y.; Tian, P.J.; Nima, Y.Z.; Laba, Z.X.; Ci, Z.; Wei, X.H.; Qu, J.; Guan, W.X.; et al. Content analysis of vitamins, dietary fibers and amino acids in a wide collection of barley (Hordeum vulgare L.) from Tibet, China. Bioinformation 2020, 16, 314–321. [Google Scholar] [CrossRef]
- Tomičić, Z.; Pezo, L.; Spasevski, N.; Lazarević, J.; Cabarkapa, I.; Tomičić, R. Diversity of amino acids composition in cereals. Food Feed Res. 2022, 49, 11–22. [Google Scholar] [CrossRef]
- Huang, K.; Wang, Q.Y.; Song, H.D.; Cao, H.W.; Guan, X. In vitro gastrointestinal digestion of highland barley protein: Identification and characterization of novel bioactive peptides involved in gut cholecystokinin secretion. J. Sci. Food Agric. 2023, 103, 7869–7876. [Google Scholar] [CrossRef]
- Xing, J.; Li, Z.; Zhang, W.; Wang, P. The Composition, Structure, and Functionalities of Prolamins from Highland Barley. Molecules 2023, 28, 5334. [Google Scholar] [CrossRef]
- Hongwei, W.; Jingjing, W.; Jianquan, K. Comparison of structure characteristics of gliadin and glutenin in highland barley and wheat. Food Sci. China 2016, 37, 43–48. [Google Scholar]
- Tavano, O.L.; de Miguel Amista, M.J.; Del Ciello, G.; Martini Rodrigues, M.C.; Bono Nishida, A.M.; Valadares, L.A.; Siqueira, B.M.; da Silva Gomes, R.A.; Parolini, M.T.; da Silva Junior, S.I. Isolation and evaluation of quinoa (Chenopodium quinoa Willd.) protein fractions. A nutritional and bio-functional approach to the globulin fraction. Curr. Res. Food Sci. 2022, 5, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.; Bin, D.; Jiwei, K.; Ping, M.; Jing, Y.; Xijuan, Y. Study on the Structure and Functional Properties of Highland Barley Glutenin. J. Nucl. Agric. Sci. 2024, 38, 1539–1549. [Google Scholar] [CrossRef]
- Jia, H.; Ren, F.; Liu, H. Innovative non-thermal processing: Unraveling structural and functional transformations in food macromolecules-Starch, proteins, and lipids. Food Res. Int. 2025, 212, 116500. [Google Scholar] [CrossRef]
- Joye, I. Protein Digestibility of Cereal Products. Foods 2019, 8, 199. [Google Scholar] [CrossRef]
- Becker, P.M.; Yu, P. What makes protein indigestible from tissue-related, cellular, and molecular aspects? Mol. Nutr. Food Res. 2013, 57, 1695–1707. [Google Scholar] [CrossRef]
- Osko, J.; Nasierowska, K.; Grembecka, M. Application of In Vitro Digestion Models in the Evaluation of Dietary Supplements. Foods 2024, 13, 2135. [Google Scholar] [CrossRef]
- Nikmaram, N.; Leong, S.Y.; Koubaa, M.; Zhu, Z.; Barba, F.J.; Greiner, R.; Oey, I.; Roohinejad, S. Effect of extrusion on the anti-nutritional factors of food products: An overview. Food Control 2017, 79, 62–73. [Google Scholar] [CrossRef]
- Gänzle, M.G. Food fermentations for improved digestibility of plant foods–an essential ex situ digestion step in agricultural societies? Curr. Opin. Food Sci. 2020, 32, 124–132. [Google Scholar] [CrossRef]
- Lopez, H.W.; Leenhardt, F.; Coudray, C.; Remesy, C. Minerals and phytic acid interactions: Is it a real problem for human nutrition? Int. J. Food Sci. Technol. 2002, 37, 727–739. [Google Scholar] [CrossRef]
- Laleg, K.; Cassan, D.; Barron, C.; Prabhasankar, P.; Micard, V. Structural, Culinary, Nutritional and Anti-Nutritional Properties of High Protein, Gluten Free, 100% Legume Pasta. PLoS ONE 2016, 11, 19. [Google Scholar] [CrossRef]
- Duodu, K.G.; Taylor, J.R.N.; Belton, P.S.; Hamaker, B.R. Factors affecting sorghum protein digestibility. J. Cereal Sci. 2003, 38, 117–131. [Google Scholar] [CrossRef]
- Marina, M.; Patricia, L.-S.; Diana, O.; Karin, L.; Anna, S. Oat protein in vitro digestion is not influenced by pectin in dispersion or gel systems. Food Hydrocoll. 2025, 163, 10. [Google Scholar] [CrossRef]
- Annor, G.A.; Tyl, C.; Marcone, M.; Ragaee, S.; Marti, A. Why do millets have slower starch and protein digestibility than other cereals? Trends Food Sci. Technol. 2017, 66, 73–83. [Google Scholar] [CrossRef]
- Jinjin, P.; Zhenzhen, F.; Ting, R.; Wengang, J.; Xinsheng, L.; Dejing, C.; Yanduo, T.; Jun, D. Selectively screen the antibacterial peptide from the hydrolysates of highland barley. Eng. Life Sci. 2018, 18, 48–54. [Google Scholar] [CrossRef]
- Chung, H.-J.; Jang, S.-H.; Cho, H.Y.; Lim, S.-T. Effects of steeping and anaerobic treatment on GABA (γ-aminobutyric acid) content in germinated waxy hull-less barley. LWT-Food Sci. Technol. 2009, 42, 1712–1716. [Google Scholar] [CrossRef]
- Jia, H.B.; Ren, F.Y.; Liu, H.Z. Development of low glycemic index food products with wheat resistant starch: A review. Carbohydr. Polym. 2025, 361, 16. [Google Scholar] [CrossRef]
- Jiaxin, L.; Ran, L.; Mengzi, N.; Aixia, W.; Xue, G.; Lili, W.; Liya, L.; Bin, D.; Xijuan, Y.; Fengzhong, W.; et al. Effect of four highland barley proteins on the retrogradation and in vitro digestion properties of highland barley starch. Food Chem. X 2024, 24, 101915. [Google Scholar] [CrossRef]
- Bravo-Núñez, Á.; Garzón, R.; Rosell, C.M.; Gómez, M. Evaluation of Starch–Protein Interactions as A Function of pH. Foods 2019, 8, 155. [Google Scholar] [CrossRef]
- Bohn, T.; Carriere, F.; Day, L.; Deglaire, A.; Egger, L.; Freitas, D.; Golding, M.; Le Feunteun, S.; Macierzanka, A.; Menard, O.; et al. Correlation between in vitro and in vivo data on food digestion. What can we predict with static in vitro digestion models? Crit. Rev. Food Sci. Nutr. 2017, 58, 2239–2261. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, Z.; Shi, A.; Xiang, Z.; Zhang, H. Design and construction of in vitro digestive simulation model. Int. J. Food Sci. Technol. 2024, 59, 6856–6865. [Google Scholar] [CrossRef]
- Zhou, H.; Tan, Y.; McClements, D.J. Applications of the INFOGEST In Vitro Digestion Model to Foods: A Review. Annu. Rev. Food Sci. Technol. 2022, 14, 135–156. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assuncao, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carriere, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Sanchón, J.; Fernández-Tomé, S.; Miralles, B.; Hernández-Ledesma, B.; Tomé, D.; Gaudichon, C.; Recio, I. Protein degradation and peptide release from milk proteins in human jejunum. Comparison with in vitro gastrointestinal simulation. Food Chem. 2017, 239, 486–494. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Z.; Zhou, G.; Xu, X. Comparative study on the in vitro digestibility of chicken protein after different modifications. Food Chem. 2022, 385, 132652. [Google Scholar] [CrossRef]
- Scemama, A.; Lunetto, S.; Tailor, A.; Di Cio, S.; Dibble, M.; Gautrot, J.; Biddle, A. Development of an in vitro microfluidic model to study the role of microenvironmental cells in oral cancer metastasis. F1000Research 2024, 12, 439. [Google Scholar] [CrossRef]
- Morelli, M.; Kurek, D.; Ng, C.P.; Queiroz, K. Gut-on-a-Chip Models: Current and Future Perspectives for Host–Microbial Interactions Research. Biomedicines 2023, 11, 619. [Google Scholar] [CrossRef]
- Govindaraju, I.; Das, A.R.; Chakraborty, I.; Mal, S.S.; Sarmah, B.; Baruah, V.J.; Mazumder, N. Investigation of the physicochemical factors affecting the in vitro digestion and glycemic indices of indigenous indica rice cultivars. Sci. Rep. 2025, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jo, K.; Jeong, H.G.; Choi, Y.-S.; Yong, H.I.; Jung, S. Understanding protein digestion in infants and the elderly: Current in vitro digestion models. Crit. Rev. Food Sci. Nutr. 2021, 63, 975–992. [Google Scholar] [CrossRef]
- Minekus, M.; Marteau, P.; Havenaar, R.; Veld, J.H.J.H.i.t. A Multicompartmental Dynamic Computer-controlled Model Simulating the Stomach and Small Intestine. Altern. Lab. Anim. 1995, 23, 197–209. [Google Scholar] [CrossRef]
- Kong, F.; Singh, R.P. A Human Gastric Simulator (HGS) to Study Food Digestion in Human Stomach. J. Food Sci. 2010, 75, E627–E635. [Google Scholar] [CrossRef]
- van der Lugt, T.; Venema, K.; van Leeuwen, S.; Vrolijk, M.F.; Opperhuizen, A.; Bast, A. Gastrointestinal digestion of dietary advanced glycation endproducts using an in vitro model of the gastrointestinal tract (TIM-1). Food Funct. 2020, 11, 6297–6307. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, X.; Wang, D.; Yao, Q.; Ma, G.-L.; Fan, X. Simulator of the Human Intestinal Microbial Ecosystem (SHIME®): Current Developments, Applications, and Future Prospects. Pharmaceuticals 2024, 17, 1639. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xing, M.; Wang, K.; Wei, Q.; Zhao, J.; Wang, Y.; Ji, K.; Song, S. Application of Fucoidan in Caco-2 Model Establishment. Pharmaceuticals 2022, 15, 418. [Google Scholar] [CrossRef]
- Funata, M.; Nio, Y.; Erion, D.M.; Thompson, W.L.; Takebe, T. The promise of human organoids in the digestive system. Cell Death Differ. 2021, 28, 84–94. [Google Scholar] [CrossRef]
- Coles, L.T.; Moughan, P.J.; Darragh, A.J. In vitro digestion and fermentation methods, including gas production techniques, as applied to nutritive evaluation of foods in the hindgut of humans and other simple-stomached animals. Anim. Feed Sci. Technol. 2005, 123, 421–444. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M. Effects of baking on protein digestibility of organic spelt products determined by two in vitro digestion methods. Lwt-Food Sci. Technol. 2008, 41, 1282–1288. [Google Scholar] [CrossRef]
- Fu, L.; Gao, S.; Li, B. Impact of Processing Methods on the In Vitro Protein Digestibility and DIAAS of Various Foods Produced by Millet, Highland Barley and Buckwheat. Foods 2023, 12, 1714. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, E.G.; Albarracín, G.d.J.; Uñates, M.A.; Piola, H.D.; Camiña, J.M.; Escudero, N.L. Evaluation of the Nutritional Quality of the Grain Protein of New Amaranths Varieties. Plant Foods Hum. Nutr. 2014, 70, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Wei, J.; Sankuan, X.; Jie, X.; Fuchen, J. Influence of highland barley varieties on the nutritional and sensory quality of highland barley wine. Food Sci. China 2023, 44, 38–44. [Google Scholar]
- Obadi, M.; Sun, J.; Xu, B. Highland barley: Chemical composition, bioactive compounds, health effects, and applications. Food Res. Int. 2021, 140, 21. [Google Scholar] [CrossRef]
- Jaeckels, N.; Meier, M.; Dietrich, H.; Will, F.; Decker, H.; Fronk, P. Influence of polysaccharides on wine protein aggregation. Food Chem. 2016, 200, 38–45. [Google Scholar] [CrossRef]
- Di Gaspero, M.; Ruzza, P.; Hussain, R.; Honisch, C.; Biondi, B.; Siligardi, G.; Marangon, M.; Curioni, A.; Vincenzi, S. The Secondary Structure of a Major Wine Protein is Modified upon Interaction with Polyphenols. Molecules 2020, 25, 1646. [Google Scholar] [CrossRef] [PubMed]
- González-Neves, G.; Favre, G.; Gil, G. Effect of fining on the colour and pigment composition of young red wines. Food Chem. 2014, 157, 385–392. [Google Scholar] [CrossRef]
- Marangon, M.; Vincenzi, S.; Curioni, A. Wine Fining with Plant Proteins. Molecules 2019, 24, 2186. [Google Scholar] [CrossRef]
- Malalgoda, M.; Simsek, S. Celiac disease and cereal proteins. Food Hydrocoll. 2017, 68, 108–113. [Google Scholar] [CrossRef]
- Chen, L.H.; Wang, S.X.; Li, D.N.; Feng, S.B. Correlations between microbes and metabolites of hulless barley wines fermented with varieties of hulless barley and different starters. LWT-Food Sci. Technol. 2021, 152, 10. [Google Scholar] [CrossRef]
- Lingxi, G.; Yeming, L.; Yuan, Z.; Ciren, B.; Hui, G.; Yemeng, Z.; Hongfei, F. Exploring microbial dynamics associated with flavours production during highland barley wine fermentation. Food Res. Int. 2020, 130, 108971. [Google Scholar] [CrossRef]
- Chen, X.D.; Song, C.; Zhao, J.; Xiong, Z.; Peng, L.X.; Zou, L.; Liu, B.L.; Li, Q. Effect of a New Fermentation Strain Combination on the Fermentation Process and Quality of Highland Barley Yellow Wine. Foods 2024, 13, 2193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.-Z.; Deng, K.; Luo, H.-B.; Zhou, J.; Wu, Z.-Y.; Zhang, W.-X. Antioxidant properties and phenolic profiles of four Chinese Za wines produced from hull-less barley or maize. J. Inst. Brew. 2013, 119, 182–190. [Google Scholar] [CrossRef]
- Gong, Y.; Feng, M.; Sun, J. Effect of different thermal processing methods and thermal core temperatures on the protein structure and in vitro digestive characteristics of beef. Food Chem. 2025, 464, 141751. [Google Scholar] [CrossRef] [PubMed]
- Orlien, V.; Aalaei, K.; Poojary, M.M.; Nielsen, D.S.; Ahrné, L.; Carrascal, J.R. Effect of processing on in vitro digestibility (IVPD) of food proteins. Crit. Rev. Food Sci. Nutr. 2021, 63, 2790–2839. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, N.; Liu, Y. Effect of Different Heat Treatments on In Vitro Digestion of Egg White Proteins and Identification of Bioactive Peptides in Digested Products. J. Food Sci. 2018, 83, 1140–1148. [Google Scholar] [CrossRef]
- Nørgaard, J.V.; Malla, N.; Dionisio, G.; Madsen, C.K.; Pettersson, D.; Lærke, H.N.; Hjortshøj, R.L.; Brinch-Pedersen, H. Exogenous xylanase or protease for pigs fed barley cultivars with high or low enzyme inhibitors. Anim. Feed Sci. Technol. 2019, 248, 59–66. [Google Scholar] [CrossRef]
- Alexandra, E.H.; Carmen, I.M. Effect of High Pressure Processing and heat treatment on in vitro digestibility and trypsin inhibitor activity in lentil and faba bean protein concentrates. LWT-Food Sci. Technol. 2021, 152, 9. [Google Scholar] [CrossRef]
- Bailey, H.M.; Fanelli, N.S.; Stein, H.H. Effect of heat treatment on protein quality of rapeseed protein isolate compared with non-heated rapeseed isolate, soy and whey protein isolates, and rice and pea protein concentrates. J. Sci. Food Agric. 2023, 103, 7251–7259. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Wang, S.; Guo, J.; Wang, S. Modification of Glutenin and Associated Changes in Digestibility Due to Methylglyoxal during Heat Processing. J. Agric. Food Chem. 2019, 67, 10734–10743. [Google Scholar] [CrossRef]
- Zhao, D.; Li, L.; Le, T.T.; Larsen, L.B.; Su, G.; Liang, Y.; Li, B. Digestibility of Glyoxal-Glycated β-Casein and β-Lactoglobulin and Distribution of Peptide-Bound Advanced Glycation End Products in Gastrointestinal Digests. J. Agric. Food Chem. 2017, 65, 5778–5788. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.B.; Jia, Y.Q.; Ren, F.Y.; Liu, H.Z. Enhancing bioactive compounds in plant-based foods: Influencing factors and technological advances. Food Chem. 2024, 460, 12. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Sibakov, N.; Re, M.; Karsma, A.; Laitila, A.; Nordlund, E. Phytic Acid Reduction by Bioprocessing as a Tool to Improve the In Vitro Digestibility of Faba Bean Protein. J. Agric. Food Chem. 2018, 66, 10394–10399. [Google Scholar] [CrossRef] [PubMed]
- Gaenzle, M.G.; Loponen, J.; Gobbetti, M. Proteolysis in sourdough fermentations: Mechanisms and potential for improved bread quality. Trends Food Sci. Technol. 2008, 19, 513–521. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Shao, G.; Chang, X.; Liu, Y.; Xiang, T.; Zhu, Q.; Ren, A.; Jiang, A.; He, Q. Bidirectional Solid-State Fermentation of Highland Barley by Edible Fungi to Improve Its Functional Components, Antioxidant Activity and Texture Characteristics. Plant Foods Hum. Nutr. 2024, 79, 308–315. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, S.; Li, Y.; Sun, F.; Huang, D.; Chen, X. Alternations in the multilevel structures of chickpea protein during fermentation and their relationship with digestibility. Food Res. Int. 2023, 165, 112453. [Google Scholar] [CrossRef]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef]
- Kopf-Bolanz, K.A.; Schwander, F.; Gijs, M.; Vergères, G.; Portmann, R.; Egger, L. Impact of milk processing on the generation of peptides during digestion. Int. Dairy J. 2014, 35, 130–138. [Google Scholar] [CrossRef]
- Xiong, K.; Li, M.-m.; Chen, Y.-q.; Hu, Y.-m.; Jin, W. Formation and Reduction of Toxic Compounds Derived from the Maillard Reaction During the Thermal Processing of Different Food Matrices. J. Food Prot. 2024, 87, 19. [Google Scholar] [CrossRef]
- Beaubier, S.; Pineda-Vadillo, C.; Mesieres, O.; Framboisier, X.; Galet, O.; Kapel, R. Improving the in vitro digestibility of rapeseed albumins resistant to gastrointestinal proteolysis while preserving the functional properties using enzymatic hydrolysis. Food Chem. 2023, 407, 10. [Google Scholar] [CrossRef]
- Cho, M.J.; Unklesbay, N.; Hsieh, F.H.; Clarke, A.D. Hydrophobicity of bitter peptides from soy protein hydrolysates. J. Agric. Food Chem. 2004, 52, 5895–5901. [Google Scholar] [CrossRef] [PubMed]
- Yanyan, L.; Min, Z.; Zhongqin, L.; Bhesh, B. A novel combination of enzymatic hydrolysis and fermentation: Effects on the flavor and nutritional quality of fermented Cordyceps militaris beverage. LWT–Food Sci. Technol. 2020, 120, 108934. [Google Scholar] [CrossRef]
- Tao, W.; Fuli, H.; Guibing, C. Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: A concise review. J. Funct. Foods 2014, 7, 101–111. [Google Scholar] [CrossRef]
- Hu, H.; Cheung, I.W.Y.; Pan, S.Y.; Li-Chan, E.C.Y. Effect of high intensity ultrasound on physicochemical and functional properties of aggregated soybean β-conglycinin and glycinin. Food Hydrocoll. 2015, 45, 102–110. [Google Scholar] [CrossRef]
| Amino Acid Type | Highland Barley [19] | Barley [20] | Wheat [21] | Oat [21] | |
|---|---|---|---|---|---|
| Essential amino acid | Methionine (Met) | 1.52 | 0.5 | 1.9 | 2.8 |
| Lysine (Lys) | 3.64 | 3.9 | 3.6 | 4.4 | |
| Valine (Val) | 4.66 | 0.9 | 5.7 | 6.1 | |
| Isoleucine (Ile) | 3.05 | 3.8 | 5.1 | 4.5 | |
| Phenylalanine (Phe) | 4.66 | 5.5 | 6.6 | 6.5 | |
| Leucine (Leu) | 6.32 | 7.4 | 8.2 | 9.4 | |
| Threonine (Thr) | 3.26 | 4.0 | 3.7 | 4.2 | |
| Overall amount | 27.11 | 26.0 | 28.8 | 37.9 | |
| Non-essential amino acid | Asparaginic acid (Asp) | 5.87 | 5.9 | 7.0 | 8.7 |
| Cystine (Cys) | 1.80 | 5.5 | 2.2 | 4.4 | |
| Proline (Pro) | 9.73 | 9.8 | 12.8 | 7.7 | |
| Arginine (Arg) | 5.26 | 5.6 | 6.5 | 9.7 | |
| Glutamic acid (Glu) | 20.71 | 26.5 | 29.4 | 25.7 | |
| Tyrosine (Tyr) | 2.77 | 3.3 | 2.7 | 3.6 | |
| Serine (Ser) | 3.95 | 4.6 | 6.3 | 5.3 | |
| Glycine (Gly) | 3.95 | 4.6 | 5.2 | 5.8 | |
| Alanine (Ala) | 4.23 | 4.6 | 4.7 | 5.2 | |
| Histidine (His) | 2.08 | 1.2 | 2.5 | 3.3 | |
| Protein Type | Percentage | Molecular Weight (SDS-PAGE Analysis) | Structural Characteristic | Key Amino Acids and Compositional Characteristics | Functional Characteristics |
|---|---|---|---|---|---|
| albumins | 12.95% | 12–60 kDa | Ductile, rough, porous surface, loose texture | high contents of Lys (the main limiting amino acid in the body), Trp, and Met | Its water solubility allows it to compete with starch for more water molecules. |
| globulins | 12.73% | 12–60 kDa | Mainly comprises 7S- and 11S-globulins | high contents of Asp, Glu, Arg, Leu, and Lys | Total water uptake by starch granules can be reduced during the highland barley starch pasting process. |
| hordeins | 16.96% | 30–70 kDa | The main secondary structure is β-folding and β-turning, which is conducive to the formation of a compact globular protein structure and more stable structure. | high contents of Glu, Pro, Trp, and Leu; high contents of hydrophobic amino acids; low contents of essential amino acids | It supplies carbon and hydrogen to highland barley seeds and has a higher emulsion stability index; better stabilisation of oil droplets; higher thermal stability than maize hordeins; and lower surface hydrophobicity. |
| glutelins | 47.83% | 40–300 kDa | The secondary structure was mainly dominated by β-folding; the contents of disulfide bonds and total sulfhydryl groups were 10.3779 μmol/g and 88.2799 μmol/g, respectively, much lower than wheat glutelins. | rich in Glu and Pro, with 34.35% essential/total amino acid content | It has high thermal stability, which is unfavourable for moisture absorption and partial spreading; its hydrate is adhesive and elastic, which provides strength and elasticity to the dough. |
| Model Type | Representative Model | Simulation of Digestive Phase | Main Features | Scope of Application | References |
|---|---|---|---|---|---|
| Static model | INFOGEST | Mouth, stomach, small intestine | Temperature, incubation time, forces, pH, mineral activity, enzyme activity, mucin levels, and bile salt levels were specified for each digestive area; saliva, gastric, and small intestinal fluids were standardised. | Used to measure the final concentration of commonly digested products (e.g., peptides, amino acids, fatty acids, and sugars) after a product has passed through the GI tract, as well as the rate and extent of digestion of a large number of nutrients over time. | [45] |
| Microfluidic chip model | Stomach, intestines | It features a network of transparent polymer microchannels that mimic gastrointestinal tract compartmentalisation, each lined with cells such as intestinal epithelial cells and vascular endothelial cells. A sophisticated pump-valve configuration adjusts hydrodynamic parameters to simulate peristaltic and digestive secretion patterns. Small size. | Real-time monitoring and online analysis of food data in the GI tract. | [44] | |
| Dynamic modelling | DGM (Dynamic Gastric Modelling) | Stomach | It is a very close reproduction of dynamic conditions based on monogastric animals and the human interior, accurate for gastrointestinal transit, pH, bile salt concentration, and glucose absorption data. | Study of the fate of ingested components (e.g., food, microorganisms, and drugs) during gastrointestinal transport. | [53] |
| HGS (Human Gastric stomach) | Stomach | It simulates the continuous peristalsis of the gastric wall with contractile forces similar in amplitude and frequency to those reported in vivo, and incorporates gastric secretion, emptying systems, and temperature control. | This approach allows for a comprehensive assessment of changes in GI content characteristics through the evaluation of physicochemical parameters, as well as the study of the modulation of digestive matrix breakdown and release of bioactive compounds by biological factors, including changes in pH, patterns of enzyme activity, and muscular motility mechanisms. | [54] | |
| TNO Gastrointestinal tract model (TIM-1) | Stomach, small intestine | Dynamic pH curve fitting, peristaltic mixing, addition of bile and acetyl digestive enzymes, and passive absorption. | For modelling the digestive behaviour of food and drugs in the GI tract with good predictive power. | [55] | |
| SHIME (Simulator of the Human Intestinal Microbial Ecosystem) | Stomach, small intestine | More realistic simulation of microorganisms in the GI tract; multi-compartment design allows simulation and integration of the entire GI tract; real-time monitoring device allows continuous measurement of parameters such as pH, temperature, and so on. | Modelling food digestion and absorption processes, studying nutrient metabolic pathways, and assessing their impact on the gut microbiota. | [56] | |
| New model | Caco-2 cell model | Intestinal tract | Simulation of transmembrane transport and bioavailability of digestive products by intestinal epithelial cells; direct reflection of intestinal absorption potential. | Transport, absorption, and permeability of substances in the gut and the effect of drug dosage forms, precursors, carriers, and structures on absorption. | [57] |
| Organoid models | Intestinal tract | Organoid derived from adult stem cells mimics the human digestive system. | Complex food digestion and absorption, a study on the interaction with human immunity. | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Zhou, X.; Koris, A.; Chen, B.; Zhu, X.; Ren, F.; Liu, H. Unveiling the Impact of Processing Methods on In Vitro Protein Digestibility: A Focus on Highland Barley and Its Application in Wine Production. Foods 2025, 14, 2020. https://doi.org/10.3390/foods14122020
He M, Zhou X, Koris A, Chen B, Zhu X, Ren F, Liu H. Unveiling the Impact of Processing Methods on In Vitro Protein Digestibility: A Focus on Highland Barley and Its Application in Wine Production. Foods. 2025; 14(12):2020. https://doi.org/10.3390/foods14122020
Chicago/Turabian StyleHe, Mengchuan, Xinjing Zhou, András Koris, Bingyu Chen, Xuchun Zhu, Feiyue Ren, and Hongzhi Liu. 2025. "Unveiling the Impact of Processing Methods on In Vitro Protein Digestibility: A Focus on Highland Barley and Its Application in Wine Production" Foods 14, no. 12: 2020. https://doi.org/10.3390/foods14122020
APA StyleHe, M., Zhou, X., Koris, A., Chen, B., Zhu, X., Ren, F., & Liu, H. (2025). Unveiling the Impact of Processing Methods on In Vitro Protein Digestibility: A Focus on Highland Barley and Its Application in Wine Production. Foods, 14(12), 2020. https://doi.org/10.3390/foods14122020







