Comparative Study of Chemical Compositions and Antioxidant Capacities of Oils Obtained from Sixteen Oat Cultivars in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Proximate Analysis of Oat Seeds
2.3. Oat Oil Extraction Process
2.4. Fatty Acid and Triacylglycerol Compositions Analysis
2.5. Lipid Concomitants Analysis
2.5.1. Tocochromanols Analysis
2.5.2. Phytosterols and Squalene Analysis
2.5.3. The Total Polyphenol Content Analysis
2.6. Determination of Antioxidant Capacity
2.7. Statistical Analysis
3. Results and Discussion
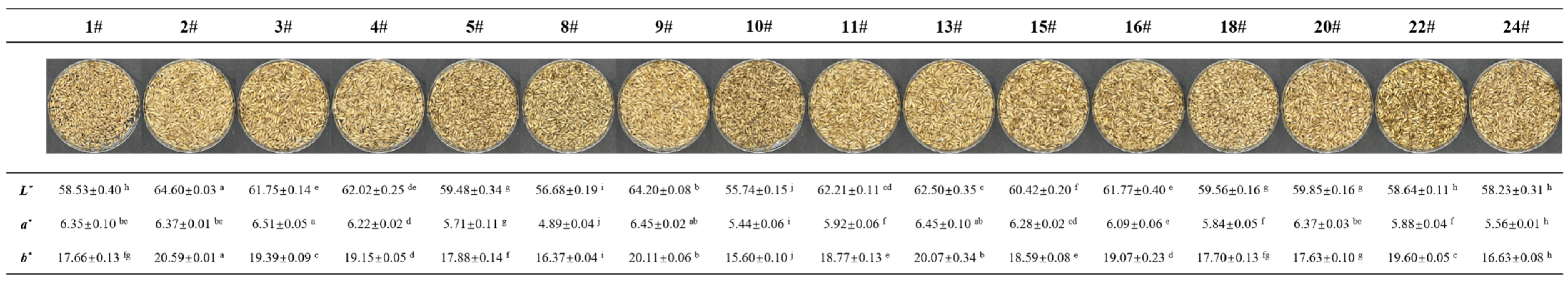
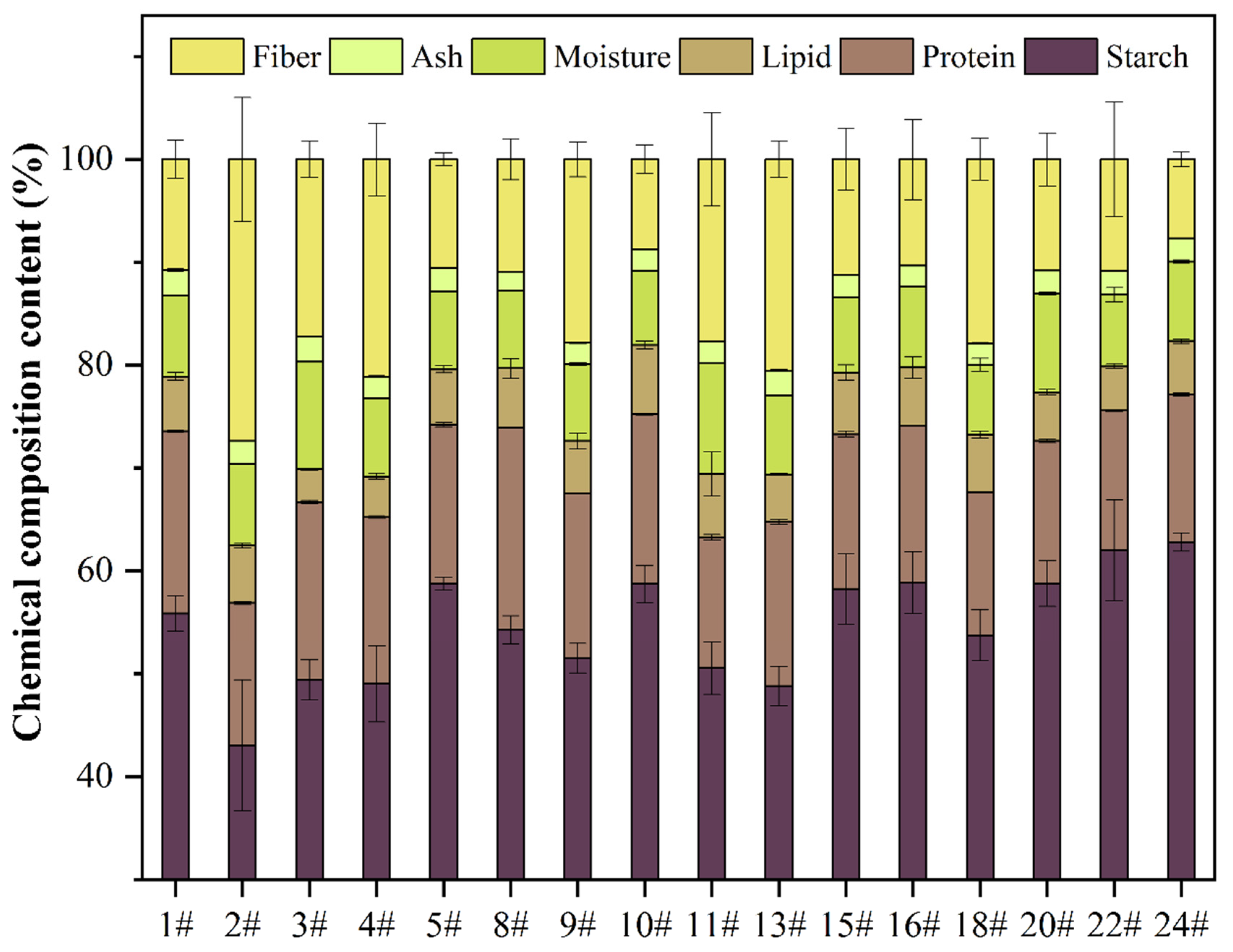
3.1. The Appearance and Proximate Composition Analysis of Oat Seed
3.2. Fatty Acid and Triacylglycerol Compositions
3.2.1. Fatty Acid Compositions
3.2.2. Triacylglycerol Compositions
3.3. Lipid Concomitants Content
3.3.1. Tocochromanols
3.3.2. Phytosterols
3.3.3. Squalenes
3.3.4. Polyphenols
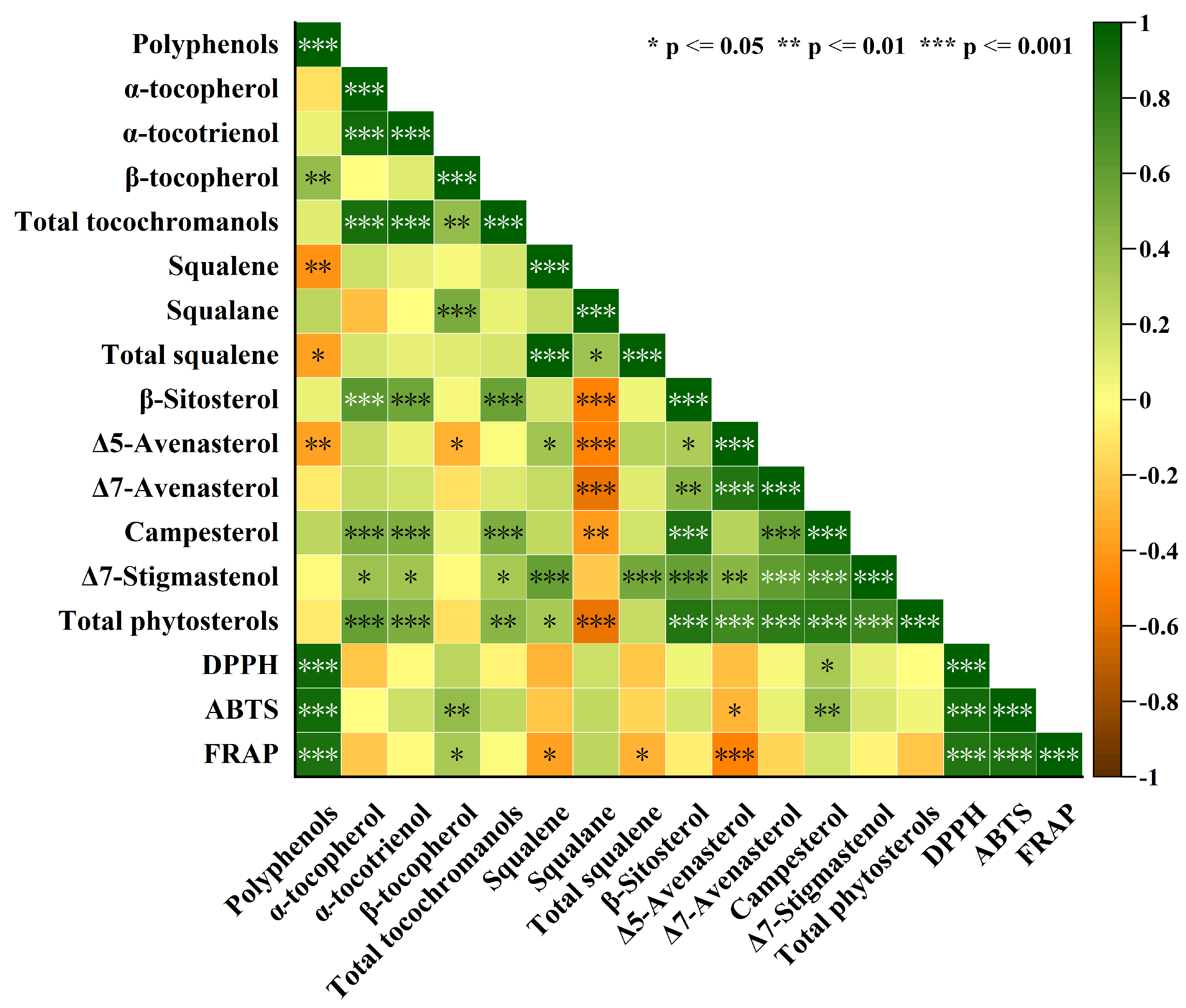
3.4. Antioxidant Capacity and Correlations Between Antioxidant Capacity and Lipid Concomitants
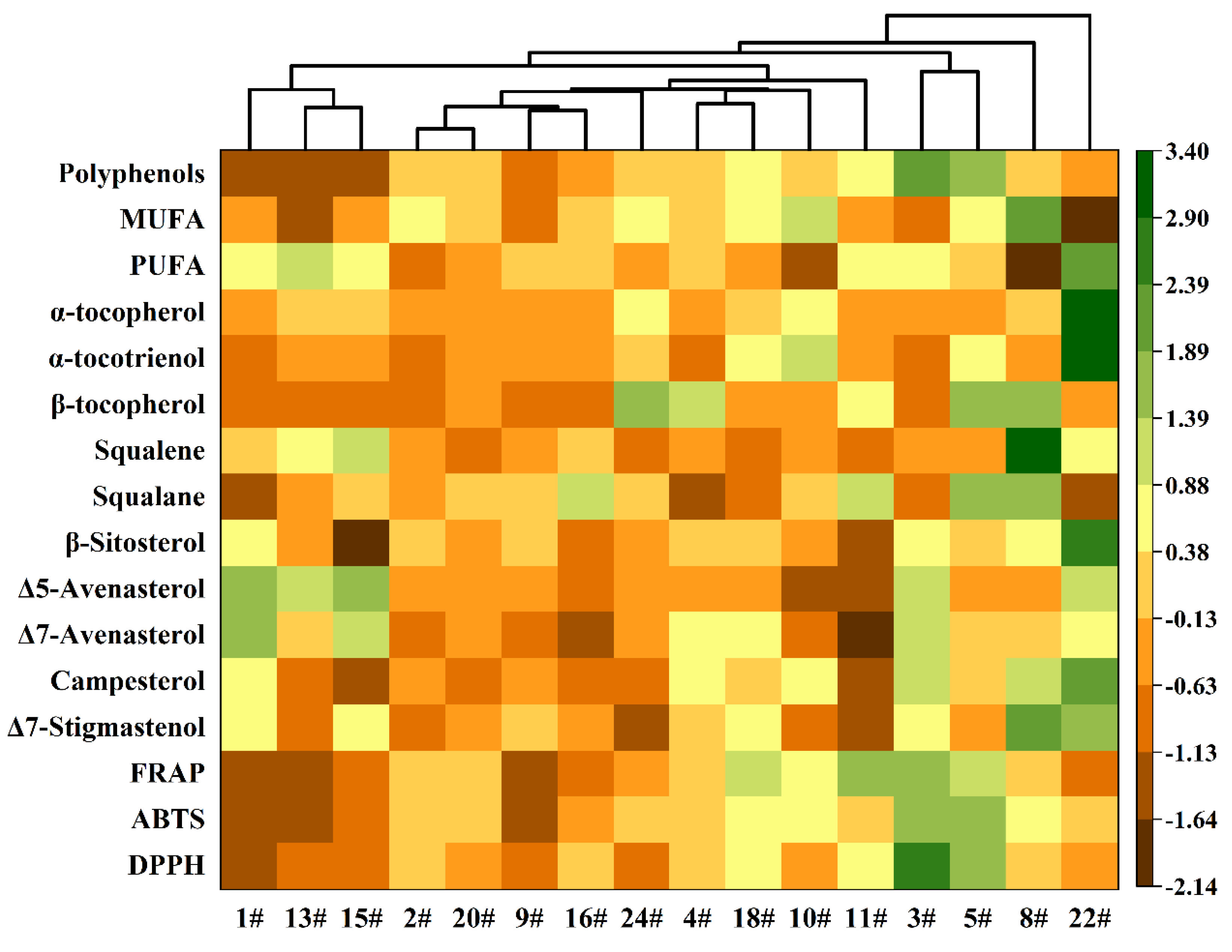
3.5. Hierarchical Cluster Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, Y.; Yan, H.; Guo, L.; Deng, C.; Wang, C.; Wang, Y.; Kang, L.; Zhou, P.; Yu, K.; Dong, X.; et al. Reference genome assemblies reveal the origin and evolution of allohexaploid oat. Nat. Genet. 2022, 54, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Shuai, X.; Dai, T.; Chen, M.; Liu, C.; Ruan, R.; Liu, Y.; Chen, J. Characterization of lipid compositions, minor components and antioxidant capacities in macadamia (Macadamia integrifolia) oil from four major areas in China. Food Biosci. 2022, 50, 102009. [Google Scholar] [CrossRef]
- Zhou, Z.; Kaur, R.; Donoso, T.; Ohm, J.B.; Gupta, R.; Lefsrud, M.; Singh, J. Metabolic engineering-induced transcriptome reprogramming of lipid biosynthesis enhances oil composition in oat. Plant Biotechnol. J. 2024, 22, 3459–3472. [Google Scholar] [CrossRef]
- Sang, S.; Chu, Y. Whole grain oats, more than just a fiber: Role of unique phytochemicals. Mol. Nutr. Food Res. 2017, 61, 1600715. [Google Scholar] [CrossRef] [PubMed]
- Banaś, K.; Harasym, J. Current Knowledge of Content and Composition of Oat Oil—Future Perspectives of Oat as Oil Source. Food Bioprocess Technol. 2020, 14, 232–247. [Google Scholar] [CrossRef]
- Gorash, A.; Armonienė, R.; Mitchell Fetch, J.; Liatukas, Ž.; Danytė, V. Aspects in oat breeding: Nutrition quality, nakedness and disease resistance, challenges and perspectives. Ann. Appl. Biol. 2017, 171, 281–302. [Google Scholar] [CrossRef]
- Halima, N.B.; Saad, R.B.; Khemakhem, B.; Fendri, I.; Abdelkafi, S. Oat (Avena sativa L.): Oil and Nutriment Compounds Valorization for Potential Use in Industrial Applications. J. Oleo Sci. 2015, 64, 915–932. [Google Scholar] [CrossRef]
- Grimberg, Å. Preferred carbon precursors for lipid labelling in the heterotrophic endosperm of developing oat (Avena sativa L.) grains. Plant Physiol. Biochem. 2014, 83, 346–355. [Google Scholar] [CrossRef]
- Li, N.; Huang, Y.; Zhao, Y.; Yang, Z.; Jia, Q.; Feng, B.; Taylor, D.C.; Du, C.; Zhang, M. Lipidomics studies reveal dynamic changes in polar lipids of developing endosperm of oat and wheat varieties with differing oil contents. Food Chem. 2024, 444, 138597. [Google Scholar] [CrossRef]
- Pokhrel, K.; Kouřimská, L.; Rudolf, O.; Tilami, S.K. Oxidative stability of crude oils relative to tocol content from eight oat cultivars: Comparing the Schaal oven and Rancimat tests. J. Food Compos. Anal. 2024, 126, 105918. [Google Scholar] [CrossRef]
- Chen, H.; Qiu, S.; Gan, J.; Li, Z.; Nirasawa, S.; Yin, L. New insights into the antioxidant activity and components in crude oat oil and soybean oil. J. Food Sci. Technol. 2015, 53, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Luo, J.; Nawaz, M.A.; Stockmann, R.; Buckow, R.; Barrow, C.; Dunshea, F.; Rasul Suleria, H.A. Phytochemistry, Bioaccessibility, and Bioactivities of Sesame Seeds: An Overview. Food Rev. Int. 2024, 40, 309–335. [Google Scholar] [CrossRef]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Comparative study of chemical compositions and antioxidant capacities of oils obtained from two species of walnut: Juglans regia and Juglans sigillata. Food Chem. 2019, 279, 279–287. [Google Scholar] [CrossRef]
- Lakhlifi El Idrissi, Z.; El Guezzane, C.; Boujemaa, I.; El Bernoussi, S.; Sifou, A.; El Moudden, H.; Ullah, R.; Bari, A.; Goh, K.W.; Goh, B.H.; et al. Blending cold-pressed peanut oil with omega-3 fatty acids from walnut oil: Analytical profiling and prediction of nutritive attributes and oxidative stability. Food Chem. X 2024, 22, 101453. [Google Scholar] [CrossRef]
- Ma, F.Y.; Wei, Z.F.; Zhang, M.; Shuai, X.X.; Du, L.Q. Optimization of Aqueous Enzymatic Microwave Assisted Extraction of Macadamia Oil And Evaluation of Its Chemical Composition, Physicochemical Properties, and Antioxidant Activities. Eur. J. Lipid Sci. Technol. 2021, 124, 2100079. [Google Scholar] [CrossRef]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Comparison of Different Processing Methods of Iron Walnut Oils (Juglans sigillata): Lipid Yield, Lipid Compositions, Minor Components, and Antioxidant Capacity. Eur. J. Lipid Sci. Technol. 2018, 120, 1800151. [Google Scholar] [CrossRef]
- Shuai, X.; Dai, T.; Chen, M.; Liang, R.; Du, L.; Chen, J.; Liu, C. Comparative study on the extraction of macadamia (Macadamia integrifolia) oil using different processing methods. LWT 2022, 154, 112614. [Google Scholar] [CrossRef]
- Shuai, X.; Dai, T.; Chen, M.; Liang, R.; Du, L.; Chen, J.; Liu, C. Comparative Study of Chemical Compositions and Antioxidant Capacities of Oils Obtained from 15 Macadamia (Macadamia integrifolia) Cultivars in China. Foods 2021, 10, 1031. [Google Scholar] [CrossRef]
- Belhoussaine, O.; El Kourchi, C.; Amakhmakh, M.; Ullah, R.; Iqbal, Z.; Goh, K.W.; Gallo, M.; Harhar, H.; Bouyahya, A.; Tabyaoui, M. Oxidative stability and nutritional quality of stored Linum usitatissmium L. and Argania spinosa L., oil blends: Chemical compositions, properties and nutritional value. Food Chem. X 2024, 23, 101680. [Google Scholar] [CrossRef]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Effects of processing methods on the chemical composition and antioxidant capacity of walnut (Juglans regia L.) oil. LWT 2021, 135, 109958. [Google Scholar] [CrossRef]
- Hao, J.W.; Wang, W.T.; Chen, N.D.; Shen, Y. Identification of 13 natural antioxidants in green calyx plum using AAPH, ABTS, and FRAP-coupled HPLC-DAD via QTOF-MS/MS. Food Chem. 2025, 477, 143568. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Fang, H.; Liu, J.; Zhang, B.; Bao, Y.; Hou, W.; Yang, K.Q. Analysis of the nutritional components in the kernels of yellowhorn (Xanthoceras sorbifolium Bunge) accessions. J. Food Compos. Anal. 2021, 100, 103925. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, J.; Su, X.; Li, Q.; Su, C.; Li, Y.; Ma, G.; Zhang, S.; Yu, X. Extraction, chemical components, bioactive functions and adulteration identification of walnut oils: A review. Grain Oil Sci. Technol. 2024, 7, 30–41. [Google Scholar] [CrossRef]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Comparison of solvents for extraction of walnut oils: Lipid yield, lipid compositions, minor-component content, and antioxidant capacity. LWT 2019, 110, 346–352. [Google Scholar] [CrossRef]
- Demir, M.K.; Ünver, A.; Arslan, D.; Üçok, G.; Terlemez, F.; Türker, S. Characterisation of einkorn (Triticum monococcum L. subsp. monococcum) wheat oil. Qual. Assur. Saf. Crops Foods 2015, 7, 707–712. [Google Scholar] [CrossRef]
- Kostadinović Veličkovska, S.; Naumova Letia, G.; Čočevska, M.; Brühl, L.; Silaghi-Dumitrescu, R.; Mirhosseini, H.; Ilieva, F.; Mihajlov, L.; Dimovska, V.; Kovacevič, B.; et al. Effect of bioactive compounds on antiradical and antimicrobial activity of extracts and cold-pressed edible oils from nutty fruits from Macedonia. J. Food Meas. Charact. 2018, 12, 2545–2552. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, N.; Li, X.; Qu, L.; Li, Y.; Yu, X. Volatile, glycerides, tocopherol, phytosterols, and triterpene alcohol in 31 commercial walnut oils from three countries. J. Food Compos. Anal. 2024, 136, 106831. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Fukui, K. α-Tocotrienol Protects Neurons by Preventing Tau Hyperphosphorylation via Inhibiting Microtubule Affinity-Regulating Kinase Activation. Int. J. Mol. Sci. 2024, 25, 8428. [Google Scholar] [CrossRef]
- Nieto-Salazar, M.A. Neurological Dysfunction Associated with Vitamin Deficiencies: A Narrative Review. J. Cardiol. Cardiovasc. Ther. 2023, 18, 555979. [Google Scholar] [CrossRef]
- Yang, F.; Wang, J.; Han, Y.; Li, Y.; Wang, S. Identification of Adulteration of Flaxseed Oil From QINGHAI Area Using GC–MS Profiling of Phytosterol Composition and Chemometrics. J. Food Prot. 2024, 87, 100221. [Google Scholar] [CrossRef]
- Khan, Z.; Nath, N.; Rauf, A.; Emran, T.B.; Mitra, S.; Islam, F.; Chandran, D.; Barua, J.; Khandaker, M.U.; Idris, A.M.; et al. Multifunctional roles and pharmacological potential of β-sitosterol: Emerging evidence toward clinical applications. Chem. Biol. Interact. 2022, 365, 110117. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, M.; Zhai, L.; Zhang, H.; Shen, L. Qualitative and quantitative analysis of β-sitosterol marker in virgin camellia oil and virgin olive oil. Food Qual. Saf. 2023, 7, fyad034. [Google Scholar] [CrossRef]
- Cheng, L.; Ji, T.; Zhang, M.; Fang, B. Recent advances in squalene: Biological activities, sources, extraction, and delivery systems. Trends Food Sci. Technol. 2024, 146, 104392. [Google Scholar] [CrossRef]
- Maguire, L.S.; O’Sullivan, S.M.; Galvin, K.; O’Connor, T.P.; O’Brien, N.M. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int. J. Food Sci. Nutr. 2009, 55, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Bai, Y.; Tian, H.; Zhao, X. The Chemical Composition and Health-Promoting Benefits of Vegetable Oils—A Review. Molecules 2023, 28, 6393. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, M.; Liu, C.; Liang, R.; Shuai, X.; Chen, J. Characterization of a novel squalene-rich oil: Pachira macrocarpa seed oil. J. Food Sci. 2022, 87, 1696–1707. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, L.; Li, P.; Yu, L.; Mao, J.; Wang, X.; Zhang, Q. A review of chemical composition and nutritional properties of minor vegetable oils in China. Trends Food Sci. Technol. 2018, 74, 26–32. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Q.; Zhang, Q.; Zhang, C.; Liu, D.; Fan, D.; Yang, H. Comparative study on composition, physicochemical and antioxidant characteristics of different varieties of kiwifruit seed oil in China. Food Chem. 2018, 264, 411–418. [Google Scholar] [CrossRef]
- Chang, M.; Wang, Z.; Zhang, T.; Wang, T.; Liu, R.; Wang, Y.; Jin, Q.; Wang, X. Characterization of fatty acids, triacylglycerols, phytosterols and tocopherols in peony seed oil from five different major areas in China. Food Res. Int. 2020, 137, 109416. [Google Scholar] [CrossRef]
- Xu, D.; Hao, J.; Wang, Z.; Liang, D.; Wang, J.; Ma, Y.; Zhang, M. Physicochemical properties, fatty acid compositions, bioactive compounds, antioxidant activity and thermal behavior of rice bran oil obtained with aqueous enzymatic extraction. LWT 2021, 149, 111817. [Google Scholar] [CrossRef]
- Ge, X.; Jing, L.; Zhao, K.; Su, C.; Zhang, B.; Zhang, Q.; Han, L.; Yu, X.; Li, W. The phenolic compounds profile, quantitative analysis and antioxidant activity of four naked barley grains with different color. Food Chem. 2021, 335, 127655. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhu, X.; Liu, W.; Huang, J.; Xie, Z.; Yang, F.; Shang, Q.; Yang, F.; Wei, Y. Quantitative analysis of the phenolic compounds and antioxidant activities of six quinoa seed grains with different colors. LWT 2024, 203, 116384. [Google Scholar] [CrossRef]
| Name | C14:0 * | C16:0 | C16:1 | C17:0 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | C20:1 | C22:0 | C23:0 | C24:0 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1# | 0.16 ± 0.00 i | 13.38 ± 0.06 j | 0.17 ± 0.00 cd | 0.05 ± 0.00 cd | 2.51 ± 0.01 e | 40.47 ± 0.05 j | 39.87 ± 0.02 c | 1.00 ± 0.00 h | 0.35 ± 0.00 d | 1.24 ± 0.00 cde | 0.20 ± 0.00 b | 0.18 ± 0.01 b | 0.42 ± 0.01 h |
| 2# | 0.20 ± 0.00 f | 14.98 ± 0.14 cd | 0.14 ± 0.00 ef | 0.05 ± 0.00 cd | 2.13 ± 0.01 h | 41.63 ± 0.07 f | 37.49 ± 0.05 l | 0.99 ± 0.00 h | 0.33 ± 0.00 e | 1.22 ± 0.02 def | 0.18 ± 0.01 c | 0.29 ± 0.01 ab | 0.45 ± 0.01 gh |
| 3# | 0.22 ± 0.00 b | 14.18 ± 0.04 h | 0.17 ± 0.00 c | 0.05 ± 0.00 fg | 2.59 ± 0.00 d | 39.48 ± 0.04 m | 39.48 ± 0.05 e | 1.19 ± 0.02 d | 0.39 ± 0.00 b | 1.29 ± 0.00 b | 0.22 ± 0.00 a | 0.20 ± 0.02 a | 0.55 ± 0.04 cd |
| 4# | 0.21 ± 0.00 d | 13.99 ± 0.02 i | 0.14 ± 0.00 ef | 0.05 ± 0.00 ef | 2.68 ± 0.00 c | 40.65 ± 0.04 i | 38.62 ± 0.02 h | 1.10 ± 0.01 ef | 0.40 ± 0.00 ab | 1.20 ± 0.00 fgh | 0.22 ± 0.00 a | 0.15 ± 0.00 cd | 0.60 ± 0.02 ab |
| 5# | 0.15 ± 0.00 j | 13.16 ± 0.01 k | 0.15 ± 0.00 de | 0.05 ± 0.00 de | 2.48 ± 0.00 f | 42.00 ± 0.03 e | 38.91 ± 0.01 f | 1.05 ± 0.00 fg | 0.32 ± 0.00 ef | 1.19 ± 0.00 gh | 0.18 ± 0.01 cd | —— | 0.38 ± 0.00 i |
| 8# | 0.14 ± 0.00 jk | 13.24 ± 0.00 k | 0.09 ± 0.00 k | 0.06 ± 0.00 ab | 3.41 ± 0.00 b | 45.22 ± 0.04 a | 34.13 ± 0.02 n | 1.40 ± 0.01 b | 0.40 ± 0.00 ab | 1.19 ± 0.00 h | 0.16 ± 0.00 e | 0.08 ± 0.01 g | 0.47 ± 0.04 fg |
| 9# | 0.25 ± 0.00 a | 15.05 ± 0.08 bc | 0.16 ± 0.00 d | 0.06 ± 0.00 a | 2.50 ± 0.00 e | 39.53 ± 0.03 m | 38.70 ± 0.02 g | 1.04 ± 0.05 g | 0.41 ± 0.00 a | 1.25 ± 0.00 c | 0.22 ± 0.00 a | 0.16 ± 0.01 c | 0.64 ± 0.00 a |
| 10# | 0.14 ± 0.00 k | 13.97 ± 0.04 i | 0.13 ± 0.00 f | 0.04 ± 0.00 g | 3.57 ± 0.00 a | 43.44 ± 0.02 b | 35.34 ± 0.02 m | 1.17 ± 0.01 d | 0.38 ± 0.00 c | 1.08 ± 0.00 j | 0.14 ± 0.00 gh | 0.06 ± 0.00 hi | 0.58 ± 0.03 bc |
| 11# | 0.18 ± 0.00 g | 14.44 ± 0.03 g | 0.14 ± 0.00 e | 0.05 ± 0.00 fg | 1.82 ± 0.00 m | 40.30 ± 0.04 k | 39.71 ± 0.05 d | 1.16 ± 0.00 d | 0.26 ± 0.00 i | 1.16 ± 0.01 i | 0.14 ± 0.01 fg | 0.11 ± 0.00 f | 0.58 ± 0.02 bc |
| 13# | 0.22 ± 0.00 b | 14.91 ± 0.00 de | 0.20 ± 0.00 b | 0.05 ± 0.00 cd | 2.15 ± 0.00 g | 38.34 ± 0.03 n | 40.68 ± 0.05 b | 1.06 ± 0.01 fg | 0.31 ± 0.00 fg | 1.21 ± 0.00 fgh | 0.18 ± 0.01 c | 0.13 ± 0.00 e | 0.61 ± 0.01 ab |
| 15# | 0.18 ± 0.00 g | 14.14 ± 0.06 h | 0.15 ± 0.02 e | 0.06 ± 0.00 ab | 2.48 ± 0.04 f | 40.02 ± 0.01 l | 39.91 ± 0.13 c | 1.18 ± 0.02 d | 0.28 ± 0.02 h | 1.00 ± 0.03 k | 0.13 ± 0.00 h | 0.09 ± 0.00 fg | 0.47 ± 0.01 fg |
| 16# | 0.20 ± 0.00 e | 14.82 ± 0.01 e | 0.14 ± 0.00 e | 0.05 ± 0.00 ef | 1.99 ± 0.00 k | 40.83 ± 0.05 h | 38.48 ± 0.01 i | 1.11 ± 0.01 e | 0.31 ± 0.00 g | 1.24 ± 0.01 cde | 0.17 ± 0.01 d | 0.14 ± 0.03 de | 0.45 ± 0.02 gh |
| 18# | 0.16 ± 0.00 i | 14.37 ± 0.04 g | 0.15 ± 0.00 e | 0.04 ± 0.00 h | 1.76 ± 0.00 n | 42.11 ± 0.00 d | 37.95 ± 0.00 j | 1.07 ± 0.01 fg | 0.29 ± 0.00 h | 1.37 ± 0.01 a | 0.18 ± 0.00 cd | 0.13 ± 0.01 e | 0.42 ± 0.00 h |
| 20# | 0.20 ± 0.00 de | 15.13 ± 0.00 b | 0.13 ± 0.00 f | 0.06 ± 0.00 a | 2.04 ± 0.00 j | 40.96 ± 0.02 g | 37.95 ± 0.03 j | 1.24 ± 0.01 cd | 0.32 ± 0.00 ef | 1.21 ± 0.01 efg | 0.17 ± 0.01 cd | 0.09 ± 0.00 g | 0.49 ± 0.04 ef |
| 22# | 0.21 ± 0.00 c | 15.55 ± 0.00 a | 0.37 ± 0.00 a | 0.04 ± 0.00 h | 1.46 ± 0.00 o | 37.03 ± 0.02 o | 41.60 ± 0.01 a | 1.69 ± 0.07 a | 0.69 ± 0.07 j | 1.23 ± 0.01 cde | 0.14 ± 0.00 fg | 0.06 ± 0.00 i | 0.53 ± 0.04 de |
| 24# | 0.17 ± 0.00 h | 14.55 ± 0.15 f | 0.11 ± 0.00 g | 0.05 ± 0.00 bc | 1.97 ± 0.00 l | 42.32 ± 0.00 c | 37.68 ± 0.01 k | 0.93 ± 0.00 i | 0.29 ± 0.01 h | 1.24 ± 0.03 cd | 0.15 ± 0.00 ef | 0.08 ± 0.00 gh | 0.55 ± 0.00 cd |
| Name | 1# | 2# | 3# | 4# | 5# | 8# | 9# | 10# | 11# | 13# | 15# | 16# | 18# | 20# | 22# | 24# |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPP * | 0.44 ± 0.01 f | 0.50 ± 0.01 e | 0.75 ± 0.00 a | 0.51 ± 0.00 d | 0.22 ± 0.00 l | 0.33 ± 0.00 i | 0.58 ± 0.01 c | 0.31 ± 0.00 j | 0.38 ± 0.00 g | 0.60 ± 0.01 b | 0.31 ± 0.00 j | 0.36 ± 0.00 h | 0.36 ± 0.00 h | 0.34 ± 0.01 i | 0.27 ± 0.00 k | 0.38 ± 0.00 g |
| MOP | 0.18 ± 0.00 gh | 0.21 ± 0.01 e | 0.24 ± 0.00 b | 0.23 ± 0.00 c | 0.16 ± 0.00 k | 0.17 ± 0.00 i | 0.27 ± 0.00 a | 0.16 ± 0.00 j | 0.20 ± 0.00 f | 0.23 ± 0.00 c | 0.18 ± 0.01 hi | 0.21 ± 0.00 e | 0.17 ± 0.00 j | 0.21 ± 0.00 e | 0.17 ± 0.05 d | 0.18 ± 0.00 g |
| MLP | 0.19 ± 0.02 c | 0.22 ± 0.01 b | 0.23 ± 0.00 b | 0.22 ± 0.00 b | 0.14 ± 0.00 e | 0.13 ± 0.00 f | 0.26 ± 0.00 a | 0.14 ± 0.00 ef | 0.19 ± 0.02 c | 0.23 ± 0.00 b | 0.17 ± 0.00 d | 0.19 ± 0.00 c | 0.14 ± 0.00 e | 0.19 ± 0.00 c | 0.22 ± 0.00 b | 0.15 ± 0.00 e |
| PPS | 0.22 ± 0.00 e | 0.18 ± 0.00 f | 0.34 ± 0.00 a | 0.24 ± 0.00 d | 0.13 ± 0.00 jk | 0.29 ± 0.00 b | 0.24 ± 0.00 d | 0.27 ± 0.01 c | 0.12 ± 0.01 k | 0.24 ± 0.00 d | 0.16 ± 0.00 g | 0.14 ± 0.00 hi | 0.13 ± 0.01 ij | 0.14 ± 0.01 hi | 0.08 ± 0.00 l | 0.14 ± 0.00 h |
| POP | 4.60 ± 0.03 g | 5.49 ± 0.05 a | 5.10 ± 0.00 d | 5.04 ± 0.11 de | 4.28 ± 0.04 j | 5.01 ± 0.00 e | 5.40 ± 0.01 b | 5.20 ± 0.03 c | 4.98 ± 0.00 e | 4.79 ± 0.02 f | 4.52 ± 0.00 h | 5.03 ± 0.02 de | 4.77 ± 0.05 f | 5.21 ± 0.02 c | 4.45 ± 0.07 i | 5.02 ± 0.00 e |
| MOO | 0.18 ± 0.00 de | 0.22 ± 0.00 b | 0.23 ± 0.00 ab | 0.23 ± 0.01 a | 0.17 ± 0.01 e | 0.19 ± 0.00 c | 0.22 ± 0.00 ab | 0.12 ± 0.05 e | 0.20 ± 0.01 c | 0.14 ± 0.06 c | 0.17 ± 0.01 e | 0.14 ± 0.06 cd | 0.17 ± 0.01 e | 0.21 ± 0.00 b | 0.17 ± 0.00 e | 0.19 ± 0.00 cd |
| PLP | 4.97 ± 0.00 f | 5.53 ± 0.05 c | 5.60 ± 0.01 c | 5.20 ± 0.04 e | 4.34 ± 0.02 i | 3.75 ± 0.02 j | 5.95 ± 0.00 b | 4.42 ± 0.07 i | 5.30 ± 0.00 d | 5.91 ± 0.12 b | 5.29 ± 0.01 d | 5.21 ± 0.11 e | 4.78 ± 0.02 g | 5.24 ± 0.01 de | 6.47 ± 0.02 a | 4.62 ± 0.00 h |
| MLO | 0.39 ± 0.03 f | 0.39 ± 0.01 f | 0.50 ± 0.00 c | 0.45 ± 0.00 d | 0.37 ± 0.01 g | 0.30 ± 0.00 j | 0.52 ± 0.00 b | 0.32 ± 0.01 i | 0.40 ± 0.01 f | 0.52 ± 0.01 b | 0.43 ± 0.01 e | 0.42 ± 0.01 e | 0.35 ± 0.01 h | 0.42 ± 0.00 e | 0.69 ± 0.05 a | 0.36 ± 0.00 h |
| POS | 1.24 ± 0.00 g | 1.18 ± 0.01 h | 1.32 ± 0.01 e | 1.47 ± 0.01 c | 1.28 ± 0.01 f | 2.08 ± 0.01 b | 1.35 ± 0.01 d | 2.18 ± 0.01 a | 0.97 ± 0.00 l | 1.08 ± 0.01 k | 1.10 ± 0.00 j | 1.08 ± 0.00 k | 0.98 ± 0.00 l | 1.16 ± 0.01 i | 0.68 ± 0.01 m | 1.09 ± 0.01 k |
| POO | 11.55 ± 0.01 k | 13.07 ± 0.09 b | 11.53 ± 0.02 k | 11.91 ± 0.10 i | 11.99 ± 0.02 h | 13.45 ± 0.01 a | 12.05 ± 0.01 h | 12.95 ± 0.01 c | 12.25 ± 0.01 g | 11.21 ± 0.04 l | 11.67 ± 0.01 j | 12.48 ± 0.03 f | 12.71 ± 0.05 de | 12.74 ± 0.02 d | 11.00 ± 0.07 m | 12.66 ± 0.00 e |
| PLS | 1.30 ± 0.01 e | 1.13 ± 0.01 gh | 1.36 ± 0.01 c | 1.34 ± 0.01 d | 1.16 ± 0.00 g | 1.44 ± 0.01 b | 1.29 ± 0.01 e | 1.57 ± 0.00 a | 0.99 ± 0.01 kl | 1.26 ± 0.00 f | 1.12 ± 0.00 h | 1.07 ± 0.01 i | 0.98 ± 0.00 l | 1.02 ± 0.01 j | 1.01 ± 0.05 jk | 0.92 ± 0.01 m |
| PLO | 18.17 ± 0.14 de | 18.31 ± 0.12 c | 18.09 ± 0.03 e | 17.95 ± 0.00 f | 17.51 ± 0.08 g | 16.50 ± 0.01 i | 18.31 ± 0.03 c | 17.30 ± 0.09 h | 18.43 ± 0.01 b | 18.69 ± 0.07 a | 18.14 ± 0.02 de | 18.06 ± 0.01 e | 18.16 ± 0.08 de | 18.23 ± 0.03 cd | 18.59 ± 0.00 a | 18.44 ± 0.01 b |
| PLL | 10.36 ± 0.01 j | 10.53 ± 0.06 i | 10.62 ± 0.03 h | 10.58 ± 0.09 hi | 10.72 ± 0.02 g | 7.87 ± 0.08 m | 11.21 ± 0.01 d | 9.17 ± 0.02 l | 11.20 ± 0.01 d | 11.65 ± 0.03 b | 11.46 ± 0.03 c | 11.01 ± 0.02 e | 10.30 ± 0.06 j | 10.86 ± 0.03 f | 13.73 ± 0.03 a | 9.73 ± 0.03 k |
| SOO | 1.46 ± 0.00 g | 1.49 ± 0.01 f | 1.50 ± 0.00 ef | 1.69 ± 0.00 c | 1.56 ± 0.01 d | 2.25 ± 0.00 a | 1.51 ± 0.01 e | 2.14 ± 0.01 b | 1.24 ± 0.00 l | 1.25 ± 0.00 l | 1.29 ± 0.02 k | 1.37 ± 0.01 j | 1.27 ± 0.01 k | 1.44 ± 0.00 h | 0.93 ± 0.00 m | 1.41 ± 0.01 i |
| OOO | 8.17 ± 0.02 h | 8.49 ± 0.34 fg | 7.70 ± 0.02 i | 7.57 ± 0.36 i | 9.17 ± 0.00 bc | 10.82 ± 0.06 a | 7.71 ± 0.01 i | 9.38 ± 0.06 b | 8.28 ± 0.02 gh | 7.14 ± 0.03 j | 8.20 ± 0.01 h | 8.68 ± 0.01 ef | 9.12 ± 0.03 c | 8.87 ± 0.03 de | 7.20 ± 0.01 j | 8.98 ± 0.06 cd |
| SOL | 2.16 ± 0.01 c | 1.46 ± 0.35 f | 2.18 ± 0.00 c | 1.83 ± 0.37 c | 1.93 ± 0.01 e | 2.53 ± 0.02 a | 2.05 ± 0.10 d | 2.49 ± 0.01 b | 1.64 ± 0.02 j | 2.01 ± 0.02 d | 1.74 ± 0.00 g | 1.76 ± 0.02 g | 1.66 ± 0.02 ij | 1.70 ± 0.02 h | 1.50 ± 0.01 k | 1.68 ± 0.02 hi |
| OLO | 16.87 ± 0.01 e | 15.76 ± 0.18 h | 15.90 ± 0.04 h | 16.94 ± 0.28 e | 17.97 ± 0.09 b | 18.44 ± 0.20 a | 15.14 ± 0.04 j | 17.27 ± 0.08 d | 16.25 ± 0.07 g | 15.50 ± 0.03 i | 16.67 ± 0.04 f | 16.22 ± 0.03 g | 17.16 ± 0.07 d | 16.13 ± 0.01 g | 14.97 ± 0.03 j | 17.49 ± 0.01 c |
| OLL | 12.80 ± 0.06 a | 11.57 ± 0.07 f | 12.32 ± 0.03 b | 12.03 ± 0.06 d | 11.95 ± 0.10 de | 11.00 ± 0.14 i | 11.42 ± 0.02 g | 10.89 ± 0.05 j | 12.15 ± 0.05 c | 12.72 ± 0.08 a | 12.31 ± 0.07 b | 11.85 ± 0.03 e | 12.26 ± 0.04 b | 11.29 ± 0.03 h | 11.95 ± 0.03 de | 12.32 ± 0.03 b |
| LLL | 4.75 ± 0.15 e | 4.28 ± 0.03 g | 4.50 ± 0.02 f | 4.58 ± 0.01 f | 4.95 ± 0.00 c | 3.46 ± 0.00 i | 4.51 ± 0.01 f | 3.73 ± 0.04 h | 4.84 ± 0.01 d | 4.85 ± 0.03 d | 5.08 ± 0.02 b | 4.73 ± 0.02 e | 4.52 ± 0.01 f | 4.57 ± 0.01 f | 5.92 ± 0.13 a | 4.25 ± 0.05 g |
| Name | Antioxidant Capacity (μmol/kg) | Polyphenols (mg/kg) | Total Squalanes (mg/kg) | Total Tocochromanols (mg/kg) | |||||
|---|---|---|---|---|---|---|---|---|---|
| DPPH * | ABTS | FRAP | Squalane | Squalene | α-Tocopherol | α-Tocotrienol | β-Tocopherol | ||
| 1# | 154.34 ± 0.90 m | 124.40 ± 0.95 n | 834.32 ± 3.12 n | 255.47 ± 15.56 k | 28.45 ± 0.14 m | 29.33 ± 0.24 f | —— | —— | —— |
| 2# | 165.22 ± 0.00 g | 232.06 ± 1.25 f | 1908.89 ± 1.18 f | 351.84 ± 4.14 fg | 30.33 ± 0.27 h | 26.60 ± 0.26 g | —— | —— | —— |
| 3# | 189.80 ± 0.20 a | 343.97 ± 0.95 a | 2746.09 ± 3.11 a | 513.99 ± 10.96 a | 29.57 ± 0.04 j | 21.55 ± 0.01 k | —— | —— | —— |
| 4# | 168.90 ± 0.00 d | 236.94 ± 0.62 e | 1852.99 ± 11.23 g | 363.35 ± 5.78 ef | 28.89 ± 0.04 l | 23.45 ± 0.27 j | —— | —— | 20.73 ± 0.18 d |
| 5# | 181.06 ± 0.00 b | 336.38 ± 0.36 b | 2538.68 ± 7.34 c | 492.44 ± 0.73 b | 34.38 ± 0.22 a | 21.83 ± 0.45 k | 2.52 ± 0.01 e | 22.31 ± 1.13 c | 30.50 ± 0.30 b |
| 8# | 168.15 ± 0.20 e | 259.90 ± 1.57 d | 1936.68 ± 13.37 e | 358.06 ± 7.95 ef | 33.95 ± 0.25 b | 59.33 ± 0.15 a | 7.85 ± 0.23 d | 8.92 ± 1.07 f | 28.92 ± 0.82 c |
| 9# | 158.87 ± 0.20 k | 134.07 ± 0.95 l | 914.55 ± 9.34 m | 291.34 ± 12.68 i | 31.45 ± 0.25 f | 25.43 ± 0.17 h | —— | 4.28 ± 0.18 h | —— |
| 10# | 164.38 ± 0.20 de | 269.24 ± 0.63 c | 2202.62 ± 2.36 d | 364.63 ± 6.60 ef | 31.74 ± 0.13 e | 24.51 ± 0.12 i | 20.82 ± 0.68 b | 24.09 ± 0.02 b | 5.10 ± 0.03 g |
| 11# | 170.29 ± 0.00 c | 218.20 ± 1.29 h | 2745.37 ± 2.03 a | 418.39 ± 6.42 c | 33.49 ± 0.08 c | 20.87 ± 0.12 l | —— | 2.52 ± 0.36 i | 17.57 ± 0.03 e |
| 13# | 157.96 ± 0.20 l | 131.03 ± 0.95 m | 816.14 ± 12.46 o | 265.69 ± 14.07 jk | 30.63 ± 0.11 g | 36.25 ± 0.46 c | 15.34 ± 0.62 c | 8.98 ± 0.45 f | —— |
| 15# | 158.42 ± 0.34 kl | 172.16 ± 0.72 k | 994.98 ± 14.34 l | 271.62 ± 12.07 j | 31.77 ± 0.09 e | 39.58 ± 0.08 b | 7.79 ± 0.09 d | 7.82 ± 0.18 f | —— |
| 16# | 167.72 ± 0.00 h | 183.91 ± 0.95 j | 1094.51 ± 7.15 k | 327.30 ± 8.76 h | 32.91 ± 0.07 d | 31.07 ± 0.46 e | 2.30 ± 0.01 e | 4.97 ± 0.50 gh | —— |
| 18# | 170.25 ± 0.34 c | 268.61 ± 0.36 c | 2570.06 ± 13.25 b | 407.32 ± 2.55 c | 29.93 ± 0.10 i | 17.39 ± 0.35 n | 15.53 ± 0.84 c | 18.08 ± 0.96 d | 4.28 ± 0.83 h |
| 20# | 163.60 ± 0.52 f | 220.75 ± 0.95 g | 1820.92 ± 5.12 h | 386.96 ± 3.83 d | 31.25 ± 0.06 f | 20.05 ± 0.63 m | —— | 6.12 ± 0.34 g | 4.43 ± 0.17 h |
| 22# | 161.23 ± 0.00 i | 215.98 ± 0.36 i | 1137.01 ± 8.23 j | 337.44 ± 0.36 gh | 29.14 ± 0.17 k | 35.49 ± 0.05 d | 66.43 ± 2.43 a | 52.17 ± 2.97 a | 6.23 ± 0.38 f |
| 24# | 159.97 ± 0.39 j | 215.06 ± 0.62 j | 1337.91 ± 12.48 i | 371.77 ± 11.04 e | 31.86 ± 0.09 e | 16.38 ± 0.11 o | 16.12 ± 0.28 c | 11.21 ± 0.07 e | 31.71 ± 0.11 a |
| Name | 1# | 2# | 3# | 4# | 5# | 8# | 9# | 10# | 11# | 13# | 15# | 16# | 18# | 20# | 22# | 24# |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| brassicasterol | 50.06 ± 0.41 gh | 53.18 ± 2.73 g | 56.51 ± 1.89 f | 51.01 ± 0.13 gh | 63.93 ± 3.10 e | 73.94 ± 0.55 d | 45.72 ± 0.99 i | 60.88 ± 0.74 e | 46.62 ± 0.96 i | 48.66 ± 0.90 hi | 84.76 ± 0.10 b | 51.92 ± 1.74 gh | 79.73 ± 4.38 c | 36.44 ± 1.15 k | 97.27 ± 2.58 a | 40.95 ± 1.34 j |
| 2,4-methylene cholestadienol | 67.57 ± 2.41 e | 50.41 ± 0.36 h | 90.80 ± 2.99 c | 61.89 ± 0.15 f | 61.45 ± 1.91 f | 103.15 ± 1.63 a | 55.67 ± 1.23 g | 85.10 ± 0.19 d | 50.08 ± 0.18 h | 68.51 ± 1.17 e | 96.18 ± 0.63 b | 46.72 ± 0.01 i | 69.69 ± 4.22 e | 43.62 ± 0.62 j | 104.01 ± 1.95 a | 42.86 ± 2.36 j |
| Campesterol | 310.32 ± 0.11 d | 249.17 ± 0.27 j | 355.27 ± 2.20 b | 304.02 ± 0.75 e | 289.67 ± 0.36 g | 339.91 ± 0.53 c | 262.22 ± 0.28 i | 300.40 ± 0.65 f | 201.54 ± 0.41 n | 231.81 ± 1.15 k | 202.035 ± 1.76 n | 215.07 ± 1.10 m | 283.99 ± 3.02 h | 233.42 ± 0.09 k | 399.11 ± 1.31 a | 221.22 ± 2.86 l |
| Campesteranol | 24.77 ± 0.25 b | 14.93 ± 0.08 j | 23.33 ± 0.47 c | 18.78 ± 0.05 gh | 21.52 ± 0.00 e | 19.03 ± 0.55 g | 18.37 ± 0.01 h | 18.71 ± 0.12 gh | 18.34 ± 0.41 h | 20.30 ± 0.09 f | 22.78 ± 0.16 d | 16.64 ± 0.33 i | 20.18 ± 0.13 f | 18.75 ± 0.24 gh | 32.59 ± 0.51 a | 18.51 ± 0.14 gh |
| Stigmasterol | 211.07 ± 9.49 b | 130.32 ± 0.22 h | 174.86 ± 3.88 cd | 137.05 ± 0.34 gh | 167.38 ± 3.91 d | 176.58 ± 1.73 c | 137.91 ± 5.82 g | 156.11 ± 0.64 e | 137.84 ± 1.71 g | 174.44 ± 10.10 cd | 181.90 ± 0.13 c | 140.99 ± 1.76 g | 174.79 ± 0.35 cd | 148.26 ± 2.99 f | 281.66 ± 0.16 a | 117.80 ± 1.66 i |
| Δ7-campesterol | 17.31 ± 0.00 d | 9.33 ± 0.43 g | 15.30 ± 0.49 e | 6.86 ± 0.02 h | 16.18 ± 2.17 de | 26.42 ± 1.89 a | 16.12 ± 1.18 de | 16.15 ± 0.03 de | 10.30 ± 0.70 fg | 8.95 ± 0.73 g | 18.98 ± 0.46 c | 9.68 ± 1.12 fg | 16.23 ± 0.17 de | 11.20 ± 0.00 f | 21.33 ± 1.01 b | 14.68 ± 1.30 e |
| Δ5,23-Stigmastadienol | 87.38 ± 1.44 bc | 78.16 ± 1.05 e | 66.35 ± 0.44 ij | 71.35 ± 0.17 g | 86.08 ± 3.12 c | 69.67 ± 0.46 gh | 66.17 ± 0.53 ij | 69.82 ± 1.17 gh | 83.67 ± 1.23 d | 65.29 ± 1.02 j | 68.91 ± 0.95 h | 74.03 ± 1.36 f | 76.98 ± 1.42 e | 106.53 ± 1.10 a | 89.31 ± 2.04 b | 68.12 ± 1.62 hi |
| Clerosterol | 105.83 ± 0.39 b | 67.70 ± 1.51 jk | 88.67 ± 2.33 d | 75.14 ± 0.18 g | 79.98 ± 1.06 f | 69.53 ± 0.41 ij | 91.55 ± 2.19 c | 66.76 ± 3.25 k | 61.87 ± 0.57 l | 82.78 ± 0.15 e | 104.72 ± 0.94 b | 72.24 ± 0.08 h | 82.99 ± 2.63 e | 67.43 ± 1.00 jk | 117.44 ± 1.13 a | 70.37 ± 1.24 hi |
| β-Sitosterol | 1868.90 ± 0.65 b | 1670.81 ± 6.38 f | 1846.43 ± 17.29 c | 1697.75 ± 4.22 e | 1649.92 ± 2.52 g | 1812.32 ± 12.66 d | 1668.64 ± 0.23 f | 1566.64 ± 5.29 i | 1359.11 ± 3.66 k | 1561.59 ± 3.22 i | 1253.79 ± 0.98 l | 1426.99 ± 4.65 j | 1666.63 ± 1.49 f | 1624.31 ± 6.40 h | 2368.93 ± 1.06 a | 1632.73 ± 6.50 h |
| β-Stiostanol | 71.59 ± 1.28 a | 34.82 ± 0.93 g | 43.23 ± 0.98 e | 42.67 ± 0.11 e | 45.88 ± 0.53 d | 61.34 ± 1.95 b | 38.26 ± 1.15 f | 49.44 ± 0.63 c | 28.31 ± 0.99 h | 46.32 ± 2.18 d | 42.66 ± 1.11 e | 30.40 ± 1.14 h | 40.46 ± 0.00 f | 37.82 ± 0.94 f | 72.64 ± 0.40 a | 49.45 ± 0.92 c |
| Δ5-Avenasterol | 1379.29 ± 5.12 a | 1019.58 ± 10.41 g | 1233.18 ± 15.60 c | 1058.86 ± 2.63 de | 1050.45 ± 4.78 ef | 1063.54 ± 2.57 d | 998.10 ± 8.96 h | 891.35 ± 4.30 j | 852.39 ± 1.38 k | 1256.77 ± 3.12 b | 1382.55 ± 0.25 a | 953.95 ± 1.10 i | 1017.18 ± 2.63 g | 995.43 ± 6.13 h | 1247.56 ± 11.06 b | 1040.89 ± 9.31 f |
| Δ5,24-Stigmastadienol | 150.56 ± 3.20 c | 118.11 ± 0.09 f | 171.35 ± 1.62 b | 126.21 ± 0.31 e | 154.28 ± 4.30 c | 88.09 ± 3.17 h | 126.41 ± 1.33 e | 125.60 ± 2.22 e | 90.12 ± 2.20 h | 140.74 ± 2.45 d | 140.51 ± 3.38 d | 107.90 ± 1.84 g | 139.96 ± 9.39 d | 101.16 ± 9.15 g | 180.09 ± 1.01 a | 102.72 ± 0.14 g |
| Δ7-Stigmastenol | 190.86 ± 1.18 de | 155.95 ± 1.48 h | 198.83 ± 0.17 c | 184.90 ± 0.46 e | 176.26 ± 4.83 g | 234.41 ± 1.60 a | 186.65 ± 3.22 e | 157.69 ± 0.15 gh | 146.45 ± 1.47 i | 155.65 ± 1.73 h | 196.92 ± 9.60 cd | 163.58 ± 0.39 g | 200.91 ± 8.34 c | 164.03 ± 3.90 g | 227.91 ± 0.89 b | 139.80 ± 0.09 j |
| Δ7-Avenasterol | 558.19 ± 3.12 a | 384.74 ± 0.23 k | 519.78 ± 0.75 b | 481.04 ± 1.20 f | 461.71 ± 0.84 g | 447.92 ± 1.35 h | 380.04 ± 3.90 l | 393.38 ± 0.72 j | 294.34 ± 4.68 n | 461.92 ± 4.04 g | 514.39 ± 0.68 c | 342.20 ± 0.13 m | 485.94 ± 1.85 e | 398.99 ± 0.84 i | 496.13 ± 2.83 d | 398.58 ± 1.18 i |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, F.; Dai, T.; Guo, L.; Wang, C.; Li, C.; Li, C.; Chen, J.; Ren, C. Comparative Study of Chemical Compositions and Antioxidant Capacities of Oils Obtained from Sixteen Oat Cultivars in China. Foods 2025, 14, 2007. https://doi.org/10.3390/foods14122007
Ma F, Dai T, Guo L, Wang C, Li C, Li C, Chen J, Ren C. Comparative Study of Chemical Compositions and Antioxidant Capacities of Oils Obtained from Sixteen Oat Cultivars in China. Foods. 2025; 14(12):2007. https://doi.org/10.3390/foods14122007
Chicago/Turabian StyleMa, Feiyue, Taotao Dai, Laichun Guo, Chunlong Wang, Changhong Li, Chunhua Li, Jun Chen, and Changzhong Ren. 2025. "Comparative Study of Chemical Compositions and Antioxidant Capacities of Oils Obtained from Sixteen Oat Cultivars in China" Foods 14, no. 12: 2007. https://doi.org/10.3390/foods14122007
APA StyleMa, F., Dai, T., Guo, L., Wang, C., Li, C., Li, C., Chen, J., & Ren, C. (2025). Comparative Study of Chemical Compositions and Antioxidant Capacities of Oils Obtained from Sixteen Oat Cultivars in China. Foods, 14(12), 2007. https://doi.org/10.3390/foods14122007







