A Comparative Analysis on the Biochemical Composition and Nutrition Evaluation of Crayfish (Procambarus clarkii) Cultivated in Saline-Alkali and Fresh Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Sample Collection
2.3. Determination of Meat Yield (MY) of Crayfish
2.4. Determination of Nutritional Components in Crayfish
2.5. Determination of Trace Elements in Crayfish
2.6. Determination of Muscle Dehydration Rate (MDR) and Steaming Loss Rate (SLR) in Crayfish
2.7. Analysis of Amino Acid Composition in Crayfish
2.8. Analysis of Fatty Acid Composition in Crayfish
2.9. Determination of Betaine in Crayfish
2.10. Determination of Nucleotides in Crayfish
2.11. Evaluation of Nutritional Quality of Crayfish
2.12. Geosmin Detection
2.13. Data Analysis
3. Results and Discussion
3.1. Nutritional Profiles of Crayfish
3.2. Meat Yield, MDR, and SLR in Crayfish
3.3. Trace Elements in Crayfish
3.4. Fatty Acids Profiles in Crayfish
3.5. Amino Acid Composition and Content in Crayfish
3.6. Analysis of AAS, CS, and EAAI in Crayfish
3.7. Betaine, Geosmin, and Nucleotide Content in Crayfish
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Longshaw, M. Diseases of crayfish: A review. J. Invertebr. Pathol. 2011, 106, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Xu, X.; Chen, Y.; Xie, H.; Zhang, T.; Mao, X. Red Swamp Crayfish (Procambarus clarkii) as a Growing Food Source: Opportunities and Challenges in Comprehensive Research and Utilization. Foods 2024, 13, 3780. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Peng, G.; Peng, B.; Tan, Y.; Bai, X. Robust strategy for disease resistance and increasing production breeding in red swamp crayfish (Procambarus clarkii). Fish Shellfish Immunol. 2022, 122, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Alvanou, M.V.; Feidantsis, K.; Staikou, A.; Apostolidis, A.P.; Michaelidis, B.; Giantsis, I.A. Probiotics, Prebiotics, and Synbiotics Utilization in Crayfish Aquaculture and Factors Affecting Gut Microbiota. Microorganisms 2023, 11, 1232. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Huang, J.N.; Wen, B.; Gao, J.Z.; Chen, Z.Z. Comprehensive assessment of three crayfish culture modes: From production performance to environmental sustainability. Sci Total Environ. 2024, 954, 176470. [Google Scholar] [CrossRef]
- Liu, L.; Wang, B. Protection of Halophytes and Their Uses for Cultivation of Saline-Alkali Soil in China. Biology 2021, 10, 353. [Google Scholar] [CrossRef]
- Che, C.; Yang, P.; Qin, K.; Li, Y.; Fan, Z.; Li, W.; Gao, S.; Wang, C.; Mu, C.; Wang, H. Based on metabolomics analysis: Metabolic mechanism of intestinal tract of Scylla paramamosain under low-salt saline-alkali water aquaculture environment. BMC Genom. 2024, 25, 1232. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hong, Y.; He, Y.; Liu, Y. Growth and high-valued products accumulation characteristics of microalgae in saline-alkali leachate from Inner Mongolia. Environ. Sci. Pollut. Res. Int. 2019, 26, 36985–36992. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhong, Z.; Hou, J.; You, L.; Zhao, Z.; Kwok, L.Y.; Bilige, M. Metagenomic analysis revealed the potential of lactic acid bacteria in improving natural saline-alkali land. Int. Microbiol. 2024, 27, 311–324. [Google Scholar] [CrossRef]
- Qin, K.; Feng, W.; Ji, Z.; Jiang, X.; Hu, Y.; Li, Y.; Che, C.; Wang, C.; Mu, C.; Wang, H. Shrimp Cultured in Low-Salt Saline-Alkali Water has a Better Amino Acid Nutrition and Umami─Comparison of Flavors between Saline-Alkali Water- and Seawater-Cultured Litopenaeus vannamei. J. Agric. Food Chem. 2024, 72, 6585–6592. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, E.; Xu, C.; Chen, L.; Wang, X. Effects of Diets With Different Carbohydrate to Lipid Ratios on the Growth Performance, Ion Transport, and Carbohydrate, Lipid and Ammonia Metabolism of Nile Tilapia (Oreochromis niloticus) Under Long-Term Saline-Alkali Stress. Aquac. Nutr. 2024, 2024, 9388755. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jin, Y.; Guo, W.; Xue, Y.; Yu, L. Metabolic and Physiological Changes in the Roots of Two Oat Cultivars in Response to Complex Saline-Alkali Stress. Front. Plant Sci. 2022, 13, 835414. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Tang, Z.; Yu, J.; Wang, G.; An, W.; Zhang, Y.; Yang, Q. Effects of exogenous melatonin on the growth and photosynthetic characteristics of tomato seedlings under saline-alkali stress. Sci. Rep. 2025, 15, 5172. [Google Scholar] [CrossRef]
- Jia, X.M.; Zhu, Y.F.; Hu, Y.; Zhang, R.; Cheng, L.; Zhu, Z.L.; Zhao, T.; Zhang, X.; Wang, Y.X. Integrated physiologic, proteomic, and metabolomic analyses of Malus halliana adaptation to saline-alkali stress. Hortic. Res. 2019, 6, 91. [Google Scholar] [CrossRef]
- Jia, Y.; Du, J.; Xi, R.; Zhang, Q.; Li, L.; Li, D.; Takagi, Y.; Zhang, X. Effects of different culture salinities on the growth and muscle quality of grass carp (Ctenopharyngodon idellus). J. Anim. Sci. 2024, 102, skae281. [Google Scholar] [CrossRef]
- Zheng, Z.; Nie, Z.; Zheng, Y.; Tang, X.; Sun, Y.; Zhu, H.; Gao, J.; Xu, P.; Xu, G. Effects of Submerged Macrophytes on the Growth, Morphology, Nutritional Value, and Flavor of Cultured Largemouth Bass (Micropterus salmoides). Molecules 2022, 27, 4927. [Google Scholar] [CrossRef]
- Lv, Y.; Chu, Y.; Zhou, P.; Mei, J.; Xie, J. Effects of Different Freezing Methods on Water Distribution, Microstructure and Protein Properties of Cuttlefish during the Frozen Storage. Appl. Sci. 2021, 11, 6866. [Google Scholar] [CrossRef]
- Khan, I.; Khan, M.I.; Hashim, S.; Fawad, M.; Jamal, A.; Seleiman, M.F.; Khan, H.; Gul, B.; Hussain, Z.; Saeed, M.F.; et al. Managing Weed–Crop Interactions Enhances Chickpea (Cicer arietinum L.) Chemical Components. Plants 2023, 12, 3073. [Google Scholar] [CrossRef]
- Chi, H.; Fu, Z.; Wang, P.; Yu, D.; Zhao, L.; Li, L.; Liu, Y.; Zheng, J. Process Optimization for Antarctic Krill (Euphausia superba) Sauce Based on Back Propagation Neural Network Combined with Genetic Algorithm. Appl. Sci. 2024, 14, 7337. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Y.; Xu, R.; Tian, J.; Li, T.; Chen, S. Comparative Analysis of the Nutrient Composition of Caulerpa lentillifera from Various Cultivation Sites. Foods 2025, 14, 474. [Google Scholar] [CrossRef]
- Ran, Z.; Li, S.; Zhang, R.; Xu, J.; Liao, K.; Yu, X.; Zhong, Y.; Ye, M.; Yu, S.; Ran, Y.; et al. Proximate, amino acid and lipid compositions in Sinonovacula constricta (Lamarck) reared at different salinities. J. Sci. Food Agric. 2017, 97, 4476–4483. [Google Scholar] [CrossRef] [PubMed]
- Servillo, L.; D’Onofrio, N.; Giovane, A.; Casale, R.; Cautela, D.; Ferrari, G.; Castaldo, D.; Balestrieri, M.L. The betaine profile of cereal flours unveils new and uncommon betaines. Food Chem. 2018, 239, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yu, T.; Du, Y.; Sun, X.; Feng, Z.; Ma, X.; Ding, W.; Chen, C. An organic polymer monolith modified with an amino acid ionic liquid and graphene oxide for use in capillary electrochromatography: Application to the separation of amino acids, β-blockers, and nucleotides. Mikrochim Acta. 2019, 186, 636. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.L.; Haworth-Duff, A.; Young, I.S.; Myers, P.; Hampson, M.R.; Williams, J.; Maher, S. An automated micro solid phase extraction gas chromatography-mass spectrometry (μSPE-GC-MS) detection method for geosmin and 2-methylisoborneol in drinking water. Sci Rep. 2023, 13, 1768. [Google Scholar] [CrossRef]
- Hsieh, Y.; Shiao, J.C.; Lin, S.W.; Iizuka, Y. Quantitative reconstruction of salinity history by otolith oxygen stable isotopes: An example of a euryhaline fish Lateolabrax japonicus. Rapid Commun. Mass Spectrom. 2019, 33, 1344–1354. [Google Scholar] [CrossRef]
- Du, X.; Zhang, W.; He, J.; Zhao, M.; Wang, J.; Dong, X.; Fu, Y.; Xie, X.; Miao, S. The Impact of Rearing Salinity on Flesh Texture, Taste, and Fatty Acid Composition in Largemouth Bass Micropterus salmoides. Foods 2022, 11, 3261. [Google Scholar] [CrossRef]
- Tocher, D.R.; Castell, J.D.; Dick, J.R.; Sargent, J.R. Effects of salinity on the growth and lipid composition of Atlantic salmon (Salmo salar) and turbot (Scophthalmus maximus) cells in culture. Fish Physiol. Biochem. 1994, 13, 451–461. [Google Scholar] [CrossRef]
- Lazarević, J.; Čabarkapa, I.; Rakita, S.; Banjac, M.; Tomičić, Z.; Škrobot, D.; Radivojević, G.; Kalenjuk Pivarski, B.; Tešanović, D. Invasive Crayfish Faxonius limosus: Meat Safety, Nutritional Quality and Sensory Profile. Int. J. Environ. Res. Public Health 2022, 19, 16819. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, T.; Lu, J.; Zhu, J.; Ren, T.; Cong, R.; Lu, Z.; Zhang, Y.; Li, X. Long-term rice-crayfish farming alters soil dissolved organic carbon quality and biodegradability by regulating microbial metabolism and iron oxidation. J. Environ. Manag. 2024, 370, 122777. [Google Scholar] [CrossRef]
- Sun, Y.; Shan, X.; Li, D.; Liu, X.; Han, Z.; Qin, J.; Guan, B.; Tan, L.; Zheng, J.; Wei, M.; et al. Analysis of the Differences in Muscle Nutrition among Individuals of Different Sexes in Redclaw Crayfish, Cherax quadricarinatus. Metabolites 2023, 13, 190. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Gong, F.; Yang, F.; Jiang, Q.; Xu, Y.; Xia, W. A comparison of eating safety and quality of live and dead freshwater crayfish (Procambarus clarkii) at different stages. Food Res. Int. 2022, 159, 111630. [Google Scholar] [CrossRef] [PubMed]
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—A review. Meat Sci. 2011, 89, 111–124. [Google Scholar] [CrossRef]
- Rao, M.; Li, X.; Xu, X.; Zhang, D.; Ma, J.; Huang, J.; Xu, J.; Zheng, Q.; Ji, J.; Lu, S. Trace elements in aquatic products from Shenzhen, China and their implications for human exposure. Sci. Total. Environ. 2023, 885, 163726. [Google Scholar] [CrossRef]
- Kovacik, A.; Tvrda, E.; Tomka, M.; Revesz, N.; Arvay, J.; Fik, M.; Harangozo, L.; Hleba, L.; Kovacikova, E.; Jambor, T.; et al. Seasonal assessment of selected trace elements in grass carp (Ctenopharyngodon idella) blood and their effects on the biochemistry and oxidative stress markers. Environ. Monit Assess. 2023, 195, 1522. [Google Scholar] [CrossRef]
- Worms, I.; Simon, D.F.; Hassler, C.S.; Wilkinson, K.J. Bioavailability of trace metals to aquatic microorganisms: Importance of chemical, biological and physical processes on biouptake. Biochimie 2006, 88, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, W.; Tang, R.; Li, L.; Refaey, M.M.; Li, D. Thermally processed diet greatly affects profiles of amino acids rather than fatty acids in the muscle of carnivorous Silurus meridionalis. Food Chem. 2018, 256, 244–251. [Google Scholar] [CrossRef]
- Putra, C.; Konow, N.; Gage, M.; York, C.G.; Mangano, K.M. Protein Source and Muscle Health in Older Adults: A Literature Review. Nutrients 2021, 13, 743. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.M.; Waagbø, R.; Espe, M. Functional amino acids in fish nutrition, health and welfare. Front. Biosci. 2016, 8, 143–169. [Google Scholar] [CrossRef]
- Rathod, N.B.; Ranveer, R.C.; Bhagwat, P.K.; Ozogul, F.; Benjakul, S.; Pillai, S.; Annapure, U.S. Cold plasma for the preservation of aquatic food products: An overview. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4407–4425. [Google Scholar] [CrossRef]
- Hou, M.; Sun, W.; Ma, Y.; Ye, H.; Zhai, X.; Xue, Y.; Tang, R.; Teng, S.; Wu, R.; Luo, H. Comparative analysis for nutrients, flavor compounds, and lipidome revealed the edible value of pond-cultured male Pelodiscus sinensis with different ages. Food Chem. 2024, 454, 139795. [Google Scholar] [CrossRef]
- Ge, K.Y. Encyclopedia of Nutrition Science; People’s Medical Publishing House: Beijing, China, 2004. [Google Scholar]
- Takeno, S.; Hirata, Y.; Kitamura, K.; Ohtake, T.; Aoki, K.; Murata, N.; Hayashi, M.; Ikeda, M. Metabolic engineering to produce palmitic acid or palmitoleic acid in an oleic acid-producing Corynebacterium glutamicum strain. Metab. Eng. 2023, 78, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Petzinger, C.; Heatley, J.J.; Bailey, C.A.; Bauer, J.E. Lipid metabolic dose response to dietary alpha-linolenic acid in monk parrot (Myiopsitta monachus). Lipids 2014, 49, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Chen, H.; Wu, X.; Liu, L.; Chen, Y.; Wang, Z. Insights into the Seasonal Olfactory Mechanism of Geosmin in Raw Water of Huangpu River. Toxics 2022, 10, 485. [Google Scholar] [CrossRef]
- Hammond, D.; Murri, A.; Mastitsky, S.; Yang, Z.; Foster, R.; Schweitzer, L. Geosmin reduction by algaecide application to drinking water: Field scale efficacy and mechanistic insights. Heliyon 2021, 7, e07706. [Google Scholar] [CrossRef]
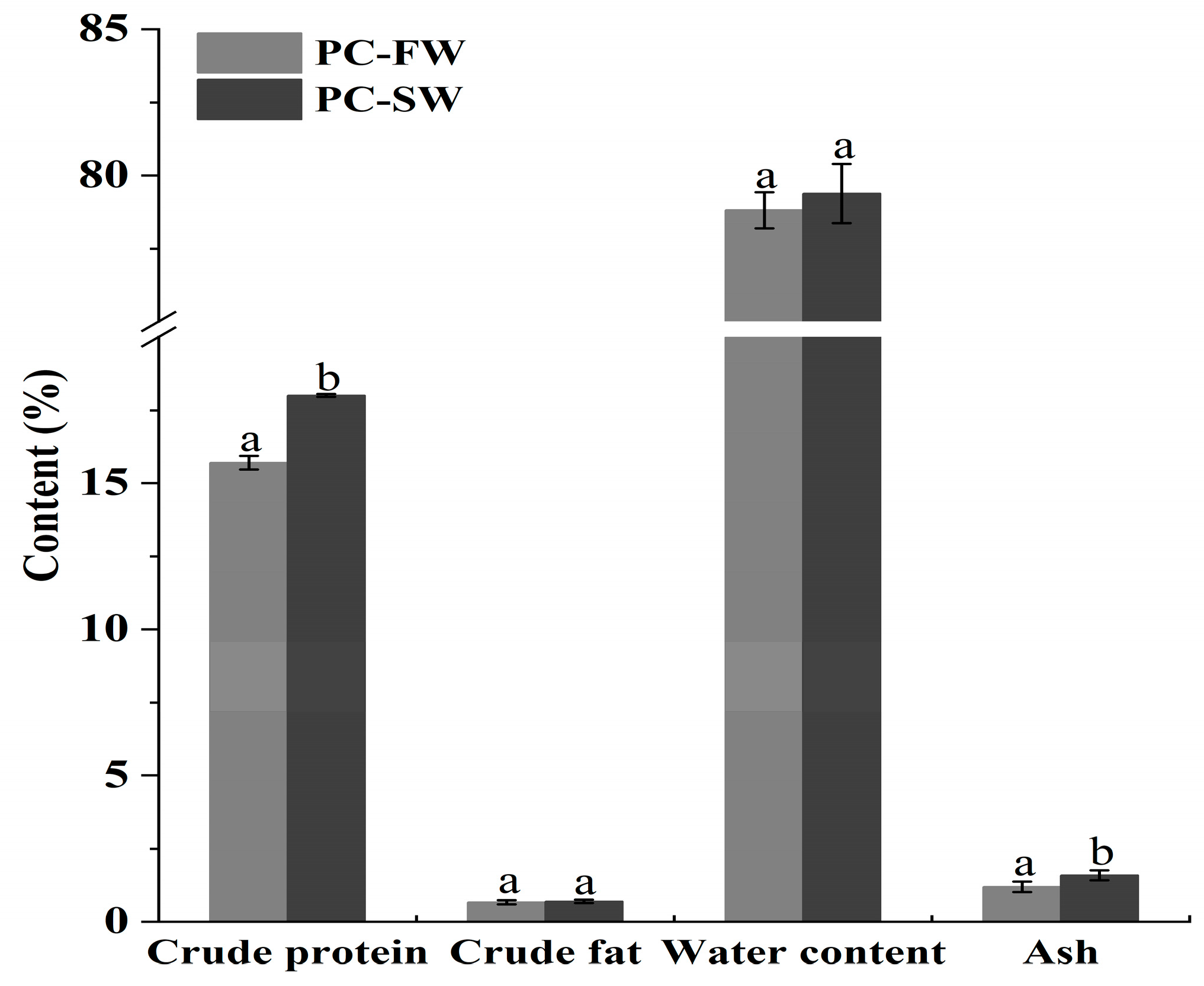

| Meat Yield | Head Weight Ratio | Hepatopancreas Weight Ratio | MDR | SLR | |
|---|---|---|---|---|---|
| PC-FW | 13.41 ± 1.51 a | 74.52 ± 0.10 a | 5.97 ± 0.32 a | 79.93 ± 2.17 a | 23.93 ± 2.00 a |
| PC-SW | 16.18 ± 0.74 b | 75.21 ± 2.19 a | 3.69 ± 0.37 b | 81.89 ± 0.60 a | 12.10 ± 1.50 b |
| PC-FW | PC-SW | |
|---|---|---|
| C14:0 | 0.38 ± 0.06 a | 0.41 ± 0.06 a |
| C15:0 | 1.47 ± 0.06 a | 1.73 ± 0.05 b |
| C16:0 | 14.45 ± 0.42 a | 16.69 ± 0.63 b |
| C17:0 | 0.40 ± 0.04 a | 0.46 ± 0.05 a |
| C18:0 | 8.32 ± 0.15 a | 8.74 ± 0.08 a |
| C20:0 | 0.67 ± 0.05 a | 0.70 ± 0.06 a |
| C22:0 | 0.67 ± 0.04 a | 0.67 ± 0.10 a |
| C23:0 | 3.31 ± 0.17 a | 3.32 ± 0.30 a |
| ΣSFA | 29.68 ± 0.34 a | 32.72 ± 0.35 b |
| C14:1n5 | 0.03 ± 0.01 a | 0.03 ± 0.03 a |
| C16:1n7 | 4.95 ± 0.19 b | 3.87 ± 0.45 a |
| C17:1n7 | 0.02 ± 0.01 a | 0.02 ± 0.01 a |
| C18:1n9 | 25.42 ± 0.23 a | 26.18 ± 1.02 b |
| C20:1 | 0.55 ± 0.10 a | 0.56 ± 0.05 a |
| C22:1 | 0.23 ± 0.09 b | 0.13 ± 0.02 a |
| ΣMUFA | 31.20 ± 0.25 a | 30.79 ± 1.43 a |
| C18:2n6 (LA) | 14.32 ± 0.49 b | 13.42 ± 1.29 a |
| C18:3n3 (LNA) | 4.65 ± 0.19 b | 2.46 ± 0.34 a |
| C18:3n6 | 0.18 ± 0.01 a | 0.19 ± 0.04 a |
| C20:2n9 | 0.95 ± 0.09 b | 0.73 ± 0.20 a |
| C20:4n6 (ARA) | 0.92 ± 0.04 b | 0.65 ± 0.14 a |
| C20:5n3 (EPA) | 12.16 ± 0.09 a | 14.06 ± 1.10 a |
| C22:5n3 (DPA) | 1.14 ± 0.32 a | 1.35 ± 0.18 b |
| C22:6n3 (DHA) | 3.78 ± 0.17 a | 3.63 ± 0.39 a |
| ΣPUFA | 39.12 ± 0.22 a | 36.49 ± 1.71 a |
| Σn-3PUFA | 22.74 ± 0.28 a | 21.50 ± 0.93 a |
| Σn-6PUFA | 15.24 ± 0.45 a | 14.06 ± 1.18 a |
| n-3/n-6PUFA | 1.49 ± 0.06 a | 1.53 ± 0.11 a |
| ΣHUFA | 70.32 ± 0.34 a | 67.28 ± 0.35 a |
| DHA/EPA | 0.29 ± 0.01 a | 0.26 ± 0.05 a |
| Amino Acids | PC-FW | PC-SW |
|---|---|---|
| Tau | 0.17 ± 0.00 a | 0.14 ± 0.00 a |
| Asp ¥ | 8.80 ± 0.06 a | 8.51 ± 0.05 a |
| Glu ¥ | 13.46 ± 0.08 a | 13.21 ± 0.08 a |
| Gly ¥ | 3.70 ± 0.02 a | 3.98 ± 0.02 a |
| Ala ¥ | 4.89 ± 0.04 a | 4.84 ± 0.03 a |
| Thr * | 3.38 ± 0.03 a | 3.26 ± 0.01 a |
| Val * | 3.91 ± 0.02 a | 3.77 ± 0.01 a |
| Met * | 1.69 ± 0.01 a | 1.43 ± 0.00 a |
| Ile * | 3.81 ± 0.04 a | 3.69 ± 0.01 a |
| Leu * | 6.70 ± 0.03 a | 6.44 ± 0.04 a |
| Phe * | 3.59 ± 0.02 a | 3.48 ± 0.03 a |
| Lys * | 5.88 ± 0.02 a | 6.23 ± 0.07 b |
| Trp * | 1.44 ± 0.05 a | 1.94 ± 0.07 b |
| His | 1.94 ± 0.01 a | 1.78 ± 0.01 a |
| Arg | 7.99 ± 0.05 a | 7.48 ± 0.04 a |
| Ser | 3.42 ± 0.01 a | 3.18 ± 0.03 a |
| Tyr | 3.14 ± 0.03 a | 3.01 ± 0.02 a |
| Pro | 2.58 ± 0.01 a | 2.42 ± 0.01 a |
| Cys | 0.53 ± 0.09 a | 0.66 ± 0.05 a |
| ∑TAA | 80.85 ± 0.33 a | 79.29 ± 0.48 a |
| ∑TDAA | 30.85 ± 0.21 a | 30.54 ± 0.19 a |
| ∑TDAA/∑TAA (%) | 38.16 ± 0.10 a | 38.52 ± 0.00 a |
| ∑EAA | 30.41 ± 0.13 a | 30.23 ± 0.25 a |
| ∑NEAA | 40.51 ± 0.14 a | 39.81 ± 0.18 a |
| ∑EAA/∑TAA (%) | 37.61 ± 0.01 a | 38.12 ± 0.08 a |
| ∑EAA/∑NEAA (%) | 75.07 ± 0.07 a | 79.93 ± 0.28 a |
| ∑BCAA | 14.42 ± 0.09 a | 13.90 ± 0.06 a |
| ∑AAC | 6.73 ± 0.05 a | 6.49 ± 0.05 a |
| ∑BCAA/∑AAC | 2.14 ± 0.00 a | 2.14 ± 0.01 a |
| Amino Acids | FAO/WHO Standard Mode | Whole Egg Mode | PC-FW | PC-SW | ||||
|---|---|---|---|---|---|---|---|---|
| EEA | Content (mg/g) | Content (mg/g) | Content (mg/g) | AAS | CS | Content (mg/g) | AAS | CS |
| Thr | 250 | 292 | 211 | 0.85 | 0.72 | 204 | 0.82 | 0.70 |
| Val | 310 | 411 | 244 | 0.79 | 0.55 | 236 | 0.76 | 0.53 |
| Met+Cys | 220 | 386 | 139 | 0.63 | 0.36 | 136 | 0.59 | 0.34 |
| Ile | 250 | 331 | 238 | 0.95 | 0.72 | 231 | 0.92 | 0.70 |
| Leu | 440 | 534 | 419 | 0.95 | 0.78 | 403 | 0.91 | 0.75 |
| Phe+Tyr | 380 | 565 | 421 | 1.11 | 0.74 | 406 | 1.07 | 0.72 |
| Lys | 340 | 441 | 368 | 1.08 | 0.83 | 389 | 1.15 | 0.88 |
| Trp | 60 | 99 | 90 | 1.50 | 0.91 | 121 | 2.02 | 1.22 |
| Total | 2250 | 3059 | 2130 | 7.86 | 5.61 | 2126 | 8.24 | 5.84 |
| EAAI | 68.02 | 69.01 | ||||||
| Indexes | PC-FW | PC-SW |
|---|---|---|
| Betaine | 7691.30 ± 51.60 a | 8161.60 ± 138.20 b |
| Geosmin | (4.32 ± 0.09) × 10−3 a | (3.13 ± 0.09) × 10−3 b |
| CMP | 71.70 ± 0.41 a | 172.20 ± 2.87 b |
| UMP | 14.28 ± 0.13 a | 19.30 ± 0.42 b |
| IMP | 1283.69 ± 43.79 a | 2835.06 ± 63.27 b |
| GMP | ND | 20.52 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Gao, P.; Yu, D.; Sun, Z.; Yang, X.; Lai, Q.; Chi, H. A Comparative Analysis on the Biochemical Composition and Nutrition Evaluation of Crayfish (Procambarus clarkii) Cultivated in Saline-Alkali and Fresh Water. Foods 2025, 14, 1997. https://doi.org/10.3390/foods14111997
Huang Y, Gao P, Yu D, Sun Z, Yang X, Lai Q, Chi H. A Comparative Analysis on the Biochemical Composition and Nutrition Evaluation of Crayfish (Procambarus clarkii) Cultivated in Saline-Alkali and Fresh Water. Foods. 2025; 14(11):1997. https://doi.org/10.3390/foods14111997
Chicago/Turabian StyleHuang, Yanqing, Pengcheng Gao, Duanduan Yu, Zhen Sun, Xu Yang, Qifang Lai, and Hai Chi. 2025. "A Comparative Analysis on the Biochemical Composition and Nutrition Evaluation of Crayfish (Procambarus clarkii) Cultivated in Saline-Alkali and Fresh Water" Foods 14, no. 11: 1997. https://doi.org/10.3390/foods14111997
APA StyleHuang, Y., Gao, P., Yu, D., Sun, Z., Yang, X., Lai, Q., & Chi, H. (2025). A Comparative Analysis on the Biochemical Composition and Nutrition Evaluation of Crayfish (Procambarus clarkii) Cultivated in Saline-Alkali and Fresh Water. Foods, 14(11), 1997. https://doi.org/10.3390/foods14111997






