Study on Nutritional Characteristics, Antioxidant Activity, and Volatile Compounds in Non-Saccharomyces cerevisiae–Lactiplantibacillus plantarum Co-Fermented Prune Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Physicochemical Properties Analysis
2.4. Determination of TPC and TFC Contents
2.5. Determination of Antioxidant Capacity
2.5.1. DPPH Radical Scavenging Assay
2.5.2. ABTS Radical Scavenging Assay
2.6. Determination of Volatile Compounds
2.7. Determination of Organic Acids
2.8. Determination of Amino Acids
2.9. Statistical Analysis
3. Results and Discussion
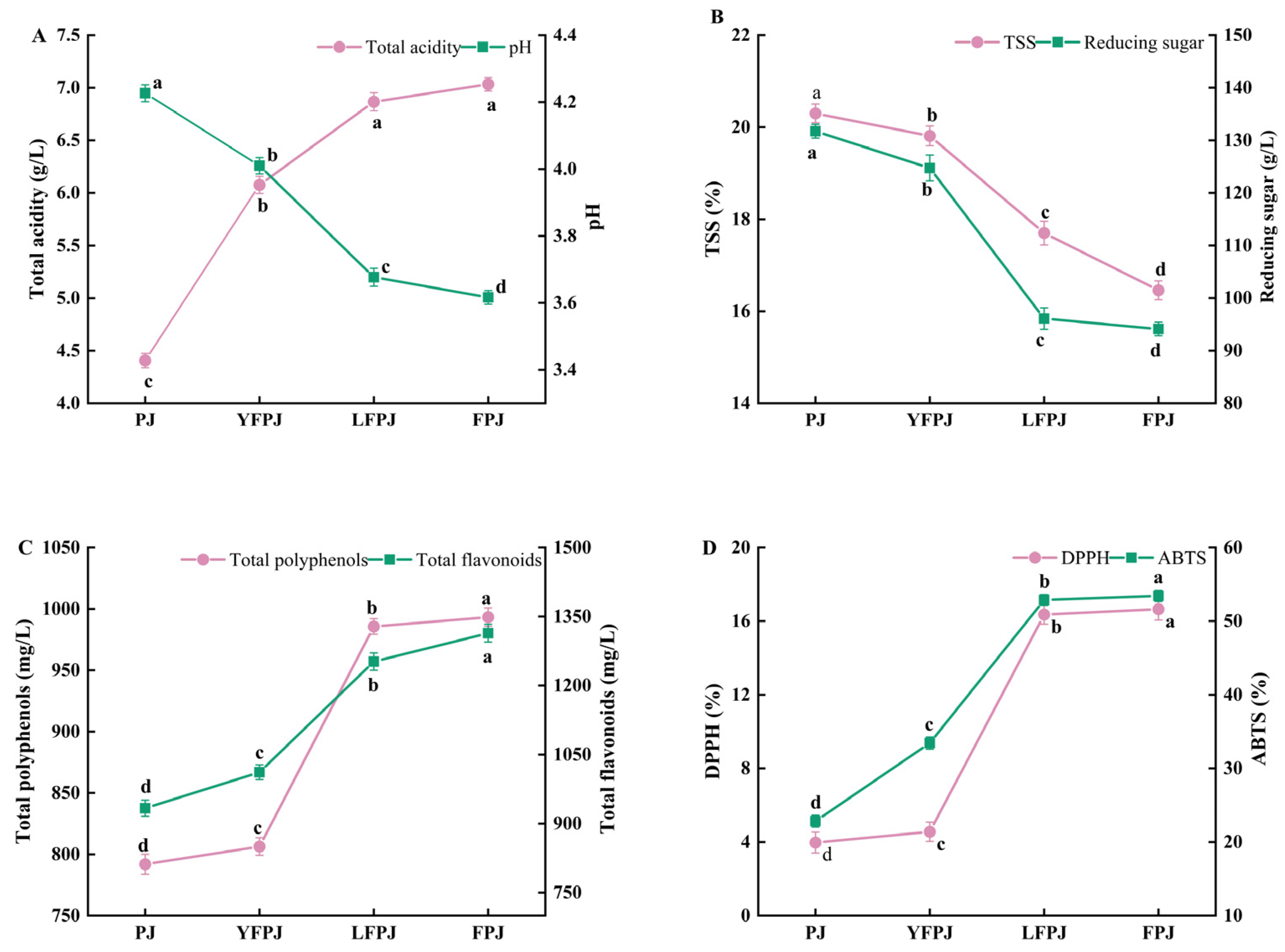
3.1. Changes in TA and pH of FPJ During Different Fermentation Stages
3.2. Changes in TSS and RS of FPJ During Different Fermentation Stages
3.3. Changes in TFC and TPC of FPJ During Different Fermentation Stages
3.4. Changes in DPPH and ABTS of FPJ During Different Fermentation Stages
3.5. Analysis of Volatile Compounds in Different Fermentation Stages of FPJ
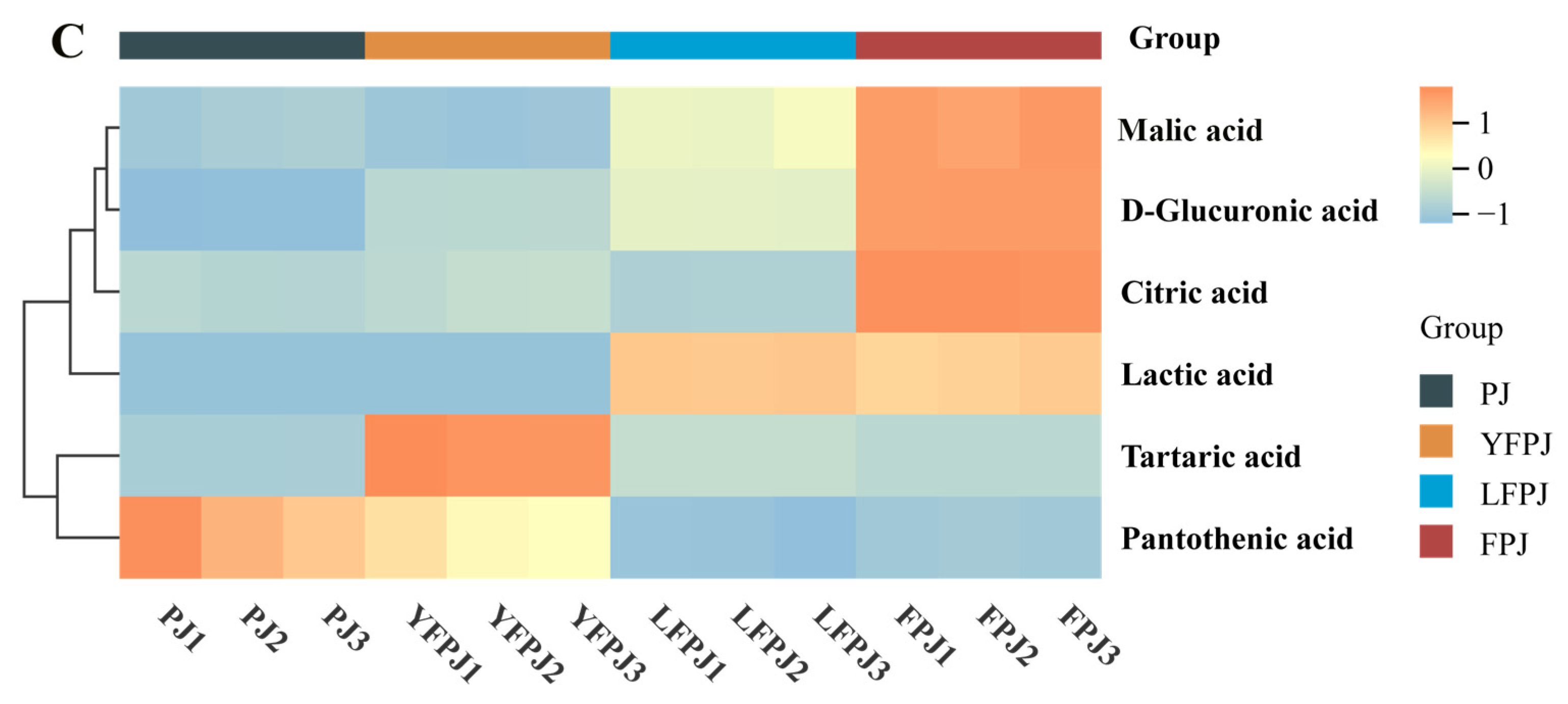
3.6. Analysis of Organic Acids in FPJ During Fermentation
3.7. Analysis of Amino Acids in FPJ During Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PJ | Prune juice |
| YFPJ | Yeast fermentation prune juice |
| LFPJ | Lactic acid fermentation prune juice |
| FPJ | Fermented prune juice |
| ABTS | 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonate acid) |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| GC-IMS | Gas chromatography–ion mobility spectrometry |
| LC-MS | Liquid chromatography–mass spectrometry |
| PCA | Principal component analysis |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| TA | Titratable acidity |
| TSS | Total soluble solids |
| RS | Reducing sugars |
| TPC | Total phenolic content |
| TFC | Total flavonoid content |
| VIP | Variable importance in projection |
| M | Monomer |
| D | Dimer |
| P | Polymer |
References
- Sebastià, N.; Montoro, A.; Mañes, J.; Soriano, J.M. A Preliminary Study of Presence of Resveratrol in Skins and Pulps of European and Japanese Plum Cultivars. J. Sci. Food Agric. 2012, 92, 3091–3094. [Google Scholar] [CrossRef] [PubMed]
- Ionica, M.E.; Nour, V.; Trandafir, I.; Cosmulescu, S.; Botu, M. Physical and Chemical Properties of Some European Plum Cultivars (Prunus domestica L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 499–503. [Google Scholar] [CrossRef]
- Zhao, Y. Xinjiang: Rapid Expansion of Production Value in Jiashi’s New Plum Industry. China Fruit Ind. Inf. 2024, 41, 69. [Google Scholar]
- Simsek, S.; El, S.N.; Kancabas Kilinc, A.; Karakaya, S. Vegetable and Fermented Vegetable Juices Containing Germinated Seeds and Sprouts of Lentil and Cowpea. Food Chem. 2014, 156, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qin, Z.; Zhang, R.; Hu, Y.; Xie, J. Preparation and Flavor Characteristics of Plant-Based Meat Flavoring by Mixed Fermentation of Multiple Strains. J. Food Compos. Anal. 2024, 136, 106841. [Google Scholar] [CrossRef]
- Phytochemical and Flavor Characteristics of Mulberry Juice Fermented with Lactiplantibacillus Plantarum BXM2. Available online: https://www.mdpi.com/2304-8158/13/17/2648 (accessed on 19 April 2025).
- Liu, Y.; Wan, Z.; Yohannes, K.W.; Yu, Q.; Yang, Z.; Li, H.; Liu, J.; Wang, J. Functional Characteristics of Lactobacillus and Yeast Single Starter Cultures in the Ripening Process of Dry Fermented Sausage. Front. Microbiol. 2021, 11, 611260. [Google Scholar] [CrossRef]
- Effect of Lactic Acid Fermentation on Volatile Compounds and Sensory Characteristics of Mango (Mangifera indica) Juices. Available online: https://www.mdpi.com/2304-8158/11/3/383 (accessed on 19 April 2025).
- Salas-Millán, J.Á.; Aguayo, E. Bioaccessibility and Unravelling of Polyphenols, Sulforaphane, and Indoles Biotransformation after in Vitro Gastrointestinal Digestion of a Novel Lactofermented Broccoli Beverage. Food Funct. 2024, 15, 11949–11960. [Google Scholar] [CrossRef]
- Bi, J.; Yang, Z.; Li, Y.; Li, B.; Gao, Y.; Ping, C.; Chen, Z.; Li, C. Effects of Different Cooking Methods on Volatile Flavor Compounds in Garlic. Int. J. Gastron. Food Sci. 2023, 31, 100642. [Google Scholar] [CrossRef]
- Minnaar, P.P.; du Plessis, H.W.; Paulsen, V.; Ntushelo, N.; Jolly, N.P.; du Toit, M. Saccharomyces Cerevisiae, Non-Saccharomyces Yeasts and Lactic Acid Bacteria in Sequential Fermentations: Effect on Phenolics and Sensory Attributes of South African Syrah Wines. S. Afr. J. Enol. Vitic. 2017, 38, 237–244. [Google Scholar] [CrossRef]
- Aihaiti, A.; Zhao, L.; Maimaitiyiming, R.; Wang, L.; Liu, R.; Mu, Y.; Chen, K.; Wang, Y. Changes in Volatile Flavors during the Fermentation of Tomato (Solanum lycopersicum L.) Juice and Its Storage Stabilization. Food Chem. 2025, 463, 141077. [Google Scholar] [CrossRef]
- Liu, N.; Shen, S.; Huang, L.; Deng, G.; Wei, Y.; Ning, J.; Wang, Y. Revelation of Volatile Contributions in Green Teas with Different Aroma Types by GC–MS and GC–IMS. Food Res. Int. 2023, 169, 112845. [Google Scholar] [CrossRef]
- Jikang, J.; Wenxiang, L.; Shuping, Y. The Effect of Inoculation Leuconostoc Mesenteroides and Lactiplantibacillus Planetarium on the Quality of Pleurotus Eryngii Jiaosu. LWT 2022, 163, 113445. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Tao, Y.; Li, D.; Han, Y.; Show, P.L.; Wen, G.; Zhou, J. Fermentation of Blueberry and Blackberry Juices Using Lactobacillus Plantarum, Streptococcus Thermophilus and Bifidobacterium Bifidum: Growth of Probiotics, Metabolism of Phenolics, Antioxidant Capacity in vitro and Sensory Evaluation. Food Chem. 2021, 348, 129083. [Google Scholar] [CrossRef]
- Impact of Fermentation Conditions on Physicochemical Properties, Antioxidant Activity, and Sensory Properties of Apple–Tomato Pulp. Available online: https://www.mdpi.com/1420-3049/28/11/4363 (accessed on 19 April 2025).
- Li, H.; Huang, J.; Wang, Y.; Wang, X.; Ren, Y.; Yue, T.; Wang, Z.; Gao, Z. Study on the Nutritional Characteristics and Antioxidant Activity of Dealcoholized Sequentially Fermented Apple Juice with Saccharomyces Cerevisiae and Lactobacillus Plantarum Fermentation. Food Chem. 2021, 363, 130351. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Zeng, M.; Duan, F.; Wang, J. Quantified Low Voltage Electrostatic Field: The Effects of Intensity on Cherry Tomato Preservation and Mechanism. Food Chem. 2025, 463, 141100. [Google Scholar] [CrossRef]
- Fiori, J.; Amadesi, E.; Fanelli, F.; Tropeano, C.V.; Rugolo, M.; Gotti, R. Cellular and Mitochondrial Determination of Low Molecular Mass Organic Acids by LC-MS/MS. J. Pharm. Biomed. Anal. 2018, 150, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Klupczynska, A.; Plewa, S.; Sytek, N.; Sawicki, W.; Dereziński, P.; Matysiak, J.; Kokot, Z.J. A Study of Low-Molecular-Weight Organic Acid Urinary Profiles in Prostate Cancer by a New Liquid Chromatography-Tandem Mass Spectrometry Method. J. Pharm. Biomed. Anal. 2018, 159, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Virág, D.; Király, M.; Drahos, L.; Édes, A.E.; Gecse, K.; Bagdy, G.; Juhász, G.; Antal, I.; Klebovich, I.; Dalmadi Kiss, B.; et al. Development, Validation and Application of LC–MS/MS Method for Quantification of Amino Acids, Kynurenine and Serotonin in Human Plasma. J. Pharm. Biomed. Anal. 2020, 180, 113018. [Google Scholar] [CrossRef]
- Liyanaarachchi, G.V.V.; Mahanama, K.R.R.; Somasiri, H.P.P.S.; Punyasiri, P.A.N. Development and Validation of a Method for Direct, Underivatized Analysis of Free Amino Acids in Rice Using Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 2018, 1568, 131–139. [Google Scholar] [CrossRef]
- Kwaw, E.; Tchabo, W.; Ma, Y.; Apaliya, M.T.; Sackey, A.S.; Mintah, B.K.; Farooq, M.; Ma, S. Effect of Storage on Quality Attributes of Lactic-Acid-Fermented Mulberry Juice Subjected to Combined Pulsed Light and Ultrasonic Pasteurization Treatment. J. Food Meas. Charact. 2018, 12, 1763–1771. [Google Scholar] [CrossRef]
- Sharma, H.; Ozogul, F.; Bartkiene, E.; Rocha, J.M. Impact of Lactic Acid Bacteria and Their Metabolites on the Techno-Functional Properties and Health Benefits of Fermented Dairy Products. Crit. Rev. Food Sci. Nutr. 2021, 63, 4819–4841. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Sevilla, A.J.; Mena, P.; Martí, N.; Viguera, C.G.; Carbonell-Barrachina, Á.A. Volatile Composition and Descriptive Sensory Analysis of Pomegranate Juice and Wine. Food Res. Int. 2013, 54, 246–254. [Google Scholar] [CrossRef]
- Chen, D.; Liu, S. Chemical and Volatile Composition of Lychee Wines Fermented with Four Commercial S Accharomyces Cerevisiae Yeast Strains. Int. J. Food Sci. Technol. 2014, 49, 521–530. [Google Scholar] [CrossRef]
- Handbook of Scholarly Publications from the Air Force Institute of Technology (AFIT), Volume 1, 2000-2020, 1st ed.; Badiru, A.B., Ciarallo, F.W., Mbonimpa, E., Air Force Institute of Technology, (U.S.), Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2023; ISBN 978-1-003-22097-8. [Google Scholar]
- Chen, C.; Lu, Y.; Yu, H.; Chen, Z.; Tian, H. Influence of 4 Lactic Acid Bacteria on the Flavor Profile of Fermented Apple juiceInfluence of 4 Lactic Acid Bacteria. Food Biosci. 2019, 27, 30–36. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, C.; Pu, X.; Li, T.; Shi, X.; Wang, B.; Cheng, W. Flavor and Functional Analysis of Lactobacillus Plantarum Fermented Apricot Juice. Fermentation 2022, 8, 533. [Google Scholar] [CrossRef]
- Ziarno, M.; Zaręba, D.; Ścibisz, I.; Kozłowska, M. Texture and Water Holding Capacity of Oat Drinks Fermented with Lactic Acid Bacteria, Bifidobacteria and Propionibacterium. Int. J. Food Prop. 2023, 27, 106–122. [Google Scholar] [CrossRef]
- Effect of Substrate Composition and Inoculum on the Fermentation Kinetics and Flavour Compound Profiles of Potentially Non-Dairy Probiotic Formulations, LWT—Food Science and Technology—X-MOL. Available online: https://www.x-mol.com/paper/1235827838049566720?adv (accessed on 19 April 2025).
- Kowalska, E.; Ziarno, M. Characterization of Buckwheat Beverages Fermented with Lactic Acid Bacterial Cultures and Bifidobacteria. Foods 2020, 9, 1771. [Google Scholar] [CrossRef] [PubMed]
- Fermentation of Kiwifruit Juice from Two Cultivars by Probiotic Bacteria: Bioactive Phenolics, Antioxidant Activities and Flavor Volatiles, Food Chemistry—X-MOL. Available online: https://www.x-mol.com/paper/1452483969976008704?adv (accessed on 19 April 2025).
- Drużyńska, B.; Wołosiak, R.; Grzebalska, M.; Majewska, E.; Ciecierska, M.; Worobiej, E. Comparison of the Content of Selected Bioactive Components and Antiradical Properties in Yoghurts Enriched with Chia Seeds (Salvia hispanica L.) and Chia Seeds Soaked in Apple Juice. Antioxidants 2021, 10, 1989. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation Transforms the Phenolic Profiles and Bioactivities of Plant-Based Foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef]
- Mantzourani, I.; Kazakos, S.; Terpou, A.; Mallouchos, A.; Kimbaris, A.; Alexopoulos, A.; Bezirtzoglou, E.; Plessas, S. Assessment of Volatile Compounds Evolution, Antioxidant Activity, and Total Phenolics Content during Cold Storage of Pomegranate Beverage Fermented by Lactobacillus Paracasei K5. Fermentation 2018, 4, 95. [Google Scholar] [CrossRef]
- Li, H.; Jiang, D.; Liu, W.; Yang, Y.; Zhang, Y.; Jin, C.; Sun, S. Comparison of Fermentation Behaviors and Properties of Raspberry Wines by Spontaneous and Controlled Alcoholic Fermentations. Food Res. Int. 2020, 128, 108801. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Wu, J.; Wang, X.; Sun, X.; Hackman, R.M.; Li, Z.; Feng, X. Evaluation of Antioxidant Capacity and Flavor Profile Change of Pomegranate Wine during Fermentation and Aging Process. Food Chem. 2017, 232, 777–787. [Google Scholar] [CrossRef]
- Biotransformation of Phenolic Profiles and Improvement of Antioxidant Capacities in Jujube Juice by Select Lactic Acid Bacteria.,Food Chemistry—X-MOL. Available online: https://www.x-mol.com/paper/1295419587190988800?adv (accessed on 19 April 2025).
- Qi, J.; Huang, H.; Wang, J.; Liu, N.; Chen, X.; Jiang, T.; Xu, H.; Lei, H. Insights into the Improvement of Bioactive Phytochemicals, Antioxidant Activities and Flavor Profiles in Chinese Wolfberry Juice by Select Lactic Acid Bacteria. Food Biosci. 2021, 43, 101264. [Google Scholar] [CrossRef]
- Perjéssy, J.; Hegyi, F.; Nagy-Gasztonyi, M.; Zalán, Z. Effect of the Lactic Acid Fermentation by Probiotic Strains on the Sour Cherry Juice and Its Bioactive Compounds. Food Sci. Technol. Int. 2021, 28, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Baldwin, E.; Plotto, A.; Cameron, R.; Manthey, J.; Dorado, C.; Bai, J. The Effect of Cultivar and Processing Method on the Stability, Flavor, and Nutritional Properties of Winter Melon Juice. LWT—Food Sci. Technol. 2018, 97, 223–230. [Google Scholar] [CrossRef]
- Jia, X.; Katsuno, N.; Nishizu, T. Effects of Temperature Fluctuations on the Development of Surface Sugar Crystals and the Quality Characteristics of Dried Persimmon during Frozen Storage. Food Bioprocess Technol. 2022, 15, 561–570. [Google Scholar] [CrossRef]
- Markakiou, S.; Gaspar, P.; Johansen, E.; Zeidan, A.A.; Neves, A.R. Harnessing the Metabolic Potential of Streptococcus Thermophilus for New Biotechnological Applications. Curr. Opin. Biotechnol. 2020, 61, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Božanić, R.; Lovković, S.; Jeličić, I. Optimising Fermentation of Soymilk with Probiotic Bacteria. Czech J. Food Sci. 2011, 29, 51–56. [Google Scholar] [CrossRef]
- Claus, H.; Mojsov, K. Enzymes for Wine Fermentation: Current and Perspective Applications. Fermentation 2018, 4, 52. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Sada, A.; Orlando, P. Synbiotic Potential of Carrot Juice Supplemented with Lactobacillus Spp. and Inulin or Fructooligosaccharides. J. Sci. Food Agric. 2008, 88, 2271–2276. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Shah, N.P.; Wang, M.-F.; Lui, W.-Y.; Corke, H. Lactobacillus Plantarum WCFS1 Fermentation Differentially Affects Antioxidant Capacity and Polyphenol Content in Mung Bean (Vigna radiata) and Soya Bean (Glycine max) Milks. J. Food Process Preserv. 2016, 41, e12944. [Google Scholar] [CrossRef]
- Li, S.; Ma, C.; Gong, G.; Liu, Z.; Chang, C.; Xu, Z. The Impact of Onion Juice on Milk Fermentation by Lactobacillus Acidophilus. LWT—Food Sci. Technol. 2016, 65, 543–548. [Google Scholar] [CrossRef]
- Solid Phase Microextraction (SPME) of Orange Juice Flavor: Odor Representativeness by Direct Gas Chromatography Olfactometry (D-GC-O). J. Agric. Food Chem. 2003, 51, 7092–7099. [CrossRef] [PubMed]
- Rozada-Sánchez, R.; Sattur, A.P.; Thomas, K.; Pandiella, S.S. Evaluation of Bifidobacterium spp. for the Production of a Potentially Probiotic Malt-Based Beverage. Process Biochem. 2008, 43, 848–854. [Google Scholar] [CrossRef]
- Siebert, T.E.; Barter, S.R.; Lopes, M.A.d.B.; Herderich, M.J.; Francis, I.L. Investigation of ‘Stone Fruit’ Aroma in Chardonnay, Viognier and Botrytis Semillon Wines. Food Chem. 2018, 256, 286–296. [Google Scholar] [CrossRef]

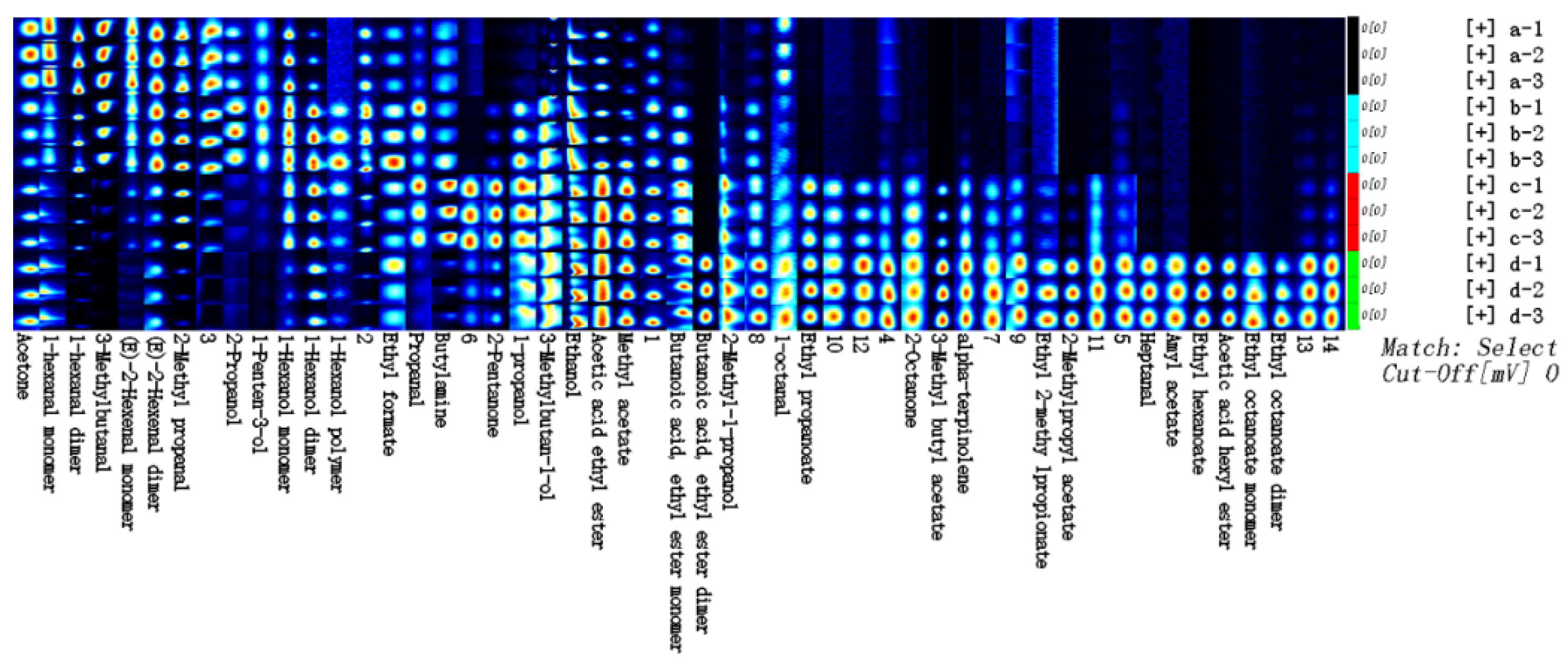





| Volatile Compound | Ri | Rt | Dt | Odor Characteristics | Relative Contents (%) | |||
|---|---|---|---|---|---|---|---|---|
| PJ | YFPJ | LFPJ | FPJ | |||||
| Terpenoids | ||||||||
| (E)-2-Hexenal (D) | 1228.8 | 504.467 | 1.52307 | Banana, fruit fragrance, and cheese | 14.87 ± 0.06 a | 12.91 ± 0.01 b | 5.10 ± 0.01 c | 4.38 ± 0.00 c |
| (E)-2-Hexenal (M) | 1229.6 | 505.801 | 1.18269 | Green leaves, botany aroma, and fat | 8.19 ± 0.04 a | 5.00 ± 0.01 b | 1.26 ± 0.00 c | 0.57 ± 0.00 d |
| alpha-terpinolene | 1294.7 | 627.58 | 1.21139 | Lemon and lime flavor | 0.15 ± 0.16 c | 0.22 ± 0.00 c | 1.04 ± 0.00 b | 1.31 ± 0.00 a |
| 1-Penten-3-ol | 1169.1 | 412.584 | 0.94462 | Green radish, resin, and fruit | 0.29 ± 0.00 b | 0.40 ± 0.00 a | 0.10 ± 0.00 c | 0.04 ± 0.00 d |
| Aldehydes | ||||||||
| 1-hexanal (D) | 1098.3 | 323.086 | 1.56687 | Grassy and fruity | 21.83 ± 0.95 a | 10.46 ± 0.02 b | 3.89 ± 0.00 c | 1.52 ± 0.00 d |
| 1-hexanal (M) | 1098.3 | 323.086 | 1.25783 | Grassy and fruity | 6.89 ± 0.03 a | 3.38 ± 0.00 b | 1.95 ± 0.00 d | 2.24 ± 0.00 c |
| 1-octanal | 1294.5 | 627.164 | 1.40345 | Orange and tangerine | 0.29 ± 0.01 a | 0.21 ± 0.00 b | 0.23 ± 0.00 b | 0.25 ± 0.00 b |
| 2-Methyl propanal | 834.6 | 158.601 | 1.28919 | Green malt | 4.57 ± 0.20 a | 3.21 ± 0.01 b | 1.71 ± 0.00 c | 0.35 ± 0.00 d |
| 3-Methylbutanal | 928.2 | 200.358 | 1.41049 | Cocoa aroma | 5.59 ± 0.24 a | 3.20 ± 0.01 b | 0.59 ± 0.00 c | 0.10 ± 0.00 c |
| Heptanal | 1195 | 451.023 | 1.68912 | Citrusy | 0.12 ± 0.01 b | 0.11 ± 0.00 b | 0.09 ± 0.00 b | 0.71 ± 0.00 a |
| Propanal | 793.3 | 143.092 | 1.04329 | Wine and whiskey | 0.34 ± 0.02 b | 0.56 ± 0.00 a | 0.60 ± 0.00 a | 0.14 ± 0.00 b |
| Alcohols | ||||||||
| 1-Hexanol (D) | 1372.4 | 737.624 | 1.64338 | Fruity and alcoholic | 1.56 ± 0.07 c | 5.42 ± 0.01 a | 3.72 ± 0.01 b | 2.09 ± 0.00 c |
| 1-Hexanol (M) | 1371.8 | 736.612 | 1.32696 | Fruity and alcoholic | 4.16 ± 0.42 b | 6.10 ± 0.00 a | 3.95 ± 0.00 b | 1.18 ± 0.00 c |
| 1-Hexanol (P) | 1370.4 | 734.587 | 2.00646 | Fruity and alcoholic | 0.17 ± 0.02 b | 0.36 ± 0.00 a | 0.22 ± 0.00 b | 0.13 ± 0.00 c |
| 1-propanol | 1051.4 | 280.434 | 1.25231 | Fruity aroma of bubble gum | 0.22 ± 0.02 c | 0.77 ± 0.00 b | 1.28 ± 0.00 a | 0.68 ± 0.00 b |
| 2-Methyl-1-propanol | 1102 | 327.211 | 1.37593 | Whisky | 0.51 ± 0.05 d | 1.50 ± 0.00 c | 3.55 ± 0.00 b | 3.81 ± 0.00 a |
| 2-Propanol | 919.5 | 196.093 | 1.21602 | Alcohol and wood | 0.45 ± 0.04 b | 0.69 ± 0.00 a | 0.12 ± 0.00 c | 0.05 ± 0.00 c |
| 3-Methylbutan-1-ol | 1216.2 | 483.758 | 1.48789 | Potpourri, malt, and empyreumatic | 2.14 ± 0.02 d | 8.27 ± 0.00 b | 9.33 ± 0.00 a | 7.72 ± 0.00 c |
| Ethanol | 951.3 | 212.289 | 1.13151 | Alcoholic | 10.11 ± 0.01 d | 17.93 ± 0.01 a | 13.84 ± 0.00 b | 11.79 ± 0.00 c |
| Amine | ||||||||
| Butylamine | 924.2 | 198.372 | 1.37778 | Ammoniacal fishy smell | 0.92 ± 0.09 a | 0.77 ± 0.00 b | 1.02 ± 0.00 a | 0.18 ± 0.00 c |
| Esters | ||||||||
| 2-Methylpropyl acetate | 1026.6 | 260.21 | 1.62496 | Sweet fruit and banana | 0.08 ± 0.01 c | 0.06 ± 0.00 c | 0.17 ± 0.00 b | 0.82 ± 0.00 a |
| 3-Methyl butyl acetate | 1134.5 | 366.059 | 1.75684 | Sweet fruit and banana | 0.41 ± 0.04 c | 0.33 ± 0.00 c | 4.70 ± 0.00 b | 9.48 ± 0.00 a |
| Acetic acid ethyl ester | 900.2 | 186.837 | 1.34433 | Grape flavor and sweet | 4.74 ± 0.00 c | 4.56 ± 0.01 c | 15.90 ± 0.01 a | 11.90 ± 0.00 b |
| Acetic acid hexyl ester | 1283.6 | 605.021 | 1.9045 | Apple and pear | 0.25 ± 0.00 b | 0.23 ± 0.00 b | 0.27 ± 0.00 b | 1.95 ± 0.00 a |
| Amyl acetate | 1195 | 451.023 | 1.76143 | Banana and sweet | 0.05 ± 0.00 b | 0.05 ± 0.00 b | 0.05 ± 0.00 b | 0.21 ± 0.00 a |
| Butanoic acid, ethyl ester (D) | 1047 | 276.714 | 1.57369 | Apple smell and sweet | 0.20 ± 0.00 b | 0.21 ± 0.00 b | 0.18 ± 0.00 b | 2.09 ± 0.00 a |
| Butanoic acid, ethyl ester (M) | 1049.4 | 278.702 | 1.19216 | Apple smell and sweet | 0.39 ± 0.00 d | 1.23 ± 0.00 b | 1.83 ± 0.00 a | 1.09 ± 0.00 c |
| Ethyl 2-methy lpropionate | 976.6 | 226.106 | 1.57167 | Rum | 0.04 ± 0.00 b | 0.04 ± 0.00 b | 0.04 ± 0.00 b | 0.08 ± 0.00 a |
| Ethyl formate | 814 | 150.648 | 1.07417 | Fruity and winy | 0.47 ± 0.00 b | 0.77 ± 0.00 a | 0.49 ± 0.00 b | 0.54 ± 0.00 b |
| Ethyl hexanoate | 1242.6 | 528.039 | 1.80647 | Banana and pineapple | 0.74 ± 0.00 b | 0.54 ± 0.00 c | 0.41 ± 0.00 c | 10.54 ± 0.00 a |
| Ethyl octanoate (D) | 1443.7 | 855.07 | 2.03485 | Waxy taste and fruity | 0.40 ± 0.00 b | 0.33 ± 0.00 b | 0.32 ± 0.00 b | 2.46 ± 0.01 a |
| Ethyl octanoate (M) | 1441.4 | 851.02 | 1.473 | Waxy taste and fruity | 0.48 ± 0.00 b | 0.37 ± 0.00 c | 0.33 ± 0.00 c | 1.86 ± 0.00 a |
| Ethyl propanoate | 968.4 | 221.547 | 1.46176 | Sweet and fruity | 0.07 ± 0.00 c | 0.07 ± 0.00 c | 0.99 ± 0.00 a | 0.77 ± 0.00 b |
| Methyl acetate | 850.3 | 164.964 | 1.20208 | Fruity and winy | 2.25 ± 0.00 d | 3.69 ± 0.01 c | 9.79 ± 0.00 a | 6.20 ± 0.00 b |
| Ketone | ||||||||
| 2-Octanone | 1294.4 | 626.936 | 1.34411 | Woody fragrance, cheese, and yeast | 0.06 ± 0.00 b | 0.07 ± 0.00 b | 0.30 ± 0.00 a | 0.33 ± 0.00 a |
| 2-Pentanone | 996 | 237.343 | 1.37741 | Fruit and pungent | 0.19 ± 0.00 c | 0.71 ± 0.00 b | 3.06 ± 0.00 a | 0.83 ± 0.00 b |
| Acetone | 831.5 | 157.408 | 1.12158 | Apple and pear | 0.79 ± 0.00 a | 0.36 ± 0.00 b | 0.28 ± 0.00 b | 0.29 ± 0.00 b |
| Organic Acids | Contents (μg/mL) | |||
|---|---|---|---|---|
| PJ | YFPJ | LFPJ | FPJ | |
| Lactic acid | 1.96 ± 0.20 c | 1.59 ± 0.19 c | 2415.17 ± 21.85 a | 2281.41 ± 87.50 b |
| Malic acid | 1411.98 ± 3.58 b | 2571.48 ± 13.39 a | 4.04 ± 0.04 c | 2.59 ± 0.01 d |
| Tartaric acid | 0.33 ± 0.02 d | 4.71 ± 0.10 a | 0.97 ± 0.01 b | 0.72 ± 0.00 c |
| Citric acid | 12.31 ± 0.81 c | 14.94 ± 1.15 b | 10.78 ± 0.29 d | 51.49 ± 0.33 a |
| D-Glucuronic acid | 125.86 ± 8.05 d | 693.94 ± 10.48 c | 1272.03 ± 18.57 b | 2965.31 ± 13.92 a |
| Pantothenic acid | 56.38 ± 4.32 a | 45.77 ± 2.52 b | 27.02 ± 0.58 d | 28.88 ± 0.38 c |
| Free Amino Acids | Contents (μg/mL) | |||
|---|---|---|---|---|
| PJ | YFPJ | LFPJ | FPJ | |
| Gly | 0.46 ± 0.03 d | 1.24 ± 0.10 a | 0.51 ± 0.05 c | 0.72 ± 0.06 b |
| Ala | 28.50 ± 0.20 b | 31.55 ± 0.41 a | 22.09 ± 0.18 d | 26.92 ± 0.78 c |
| GABA | 49.71 ± 0.41 c | 63.14 ± 0.20 a | 43.72 ± 0.39 d | 57.01 ± 0.95 b |
| Ser | 24.16 ± 0.49 ab | 26.43 ± 0.22 a | 17.33 ± 0.64 c | 20.65 ± 0.42 b |
| Pro | 179.23 ± 1.50 b | 195.97 ± 0.53 a | 140.98 ± 1.34 c | 177.38 ± 2.64 b |
| Val | 21.83 ± 0.36 b | 31.89 ± 0.23 a | 16.39 ± 0.17 c | 21.29 ± 0.44 b |
| Thr | 26.80 ± 0.20 b | 31.16 ± 1.08 a | 20.22 ± 0.32 c | 22.66 ± 0.49 c |
| Ile | 8.34 ± 0.09 b | 13.89 ± 0.27 a | 6.81 ± 0.07 c | 8.78 ± 0.18 b |
| Leu | 5.04 ± 0.02 c | 9.71 ± 0.23 a | 4.91 ± 0.11 c | 6.91 ± 0.15 b |
| Asn | 1982.76 ± 41.47 c | 3113.73 ± 34.75 a | 1973.04 ± 12.49 c | 2305.85 ± 65.01 b |
| Asp | 152.23 ± 1.88 b | 206.68 ± 0.95 a | 133.36 ± 2.01 c | 158.20 ± 3.33 b |
| Gln | 395.81 ± 3.27 a | 393.55 ± 4.30 a | 236.73 ± 2.89 c | 265.05 ± 7.43 b |
| Lys | 0.80 ± 0.02 d | 2.03 ± 0.04 b | 1.36 ± 0.01 c | 2.50 ± 0.09 a |
| Glu | 110.50 ± 1.43 a | 95.02 ± 1.50 b | 60.40 ± 1.16 d | 68.34 ± 1.14 c |
| His | 3.48 ± 0.15 c | 11.22 ± 0.25 a | 6.85 ± 0.09 b | 6.82 ± 0.20 b |
| Phe | 77.25 ± 0.76 a | 78.92 ± 1.28 a | 40.82 ± 0.46 c | 53.20 ± 1.08 b |
| Tyr | 36.80 ± 0.86 a | 38.93 ± 0.54 a | 21.16 ± 0.11 c | 28.36 ± 0.51 b |
| Trp | 2.30 ± 0.03 d | 6.31 ± 0.08 a | 3.24 ± 0.07 c | 4.35 ± 0.01 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Yang, R.; Wang, W.; Sun, T.; Han, X.; Ai, M.; Huang, S. Study on Nutritional Characteristics, Antioxidant Activity, and Volatile Compounds in Non-Saccharomyces cerevisiae–Lactiplantibacillus plantarum Co-Fermented Prune Juice. Foods 2025, 14, 1966. https://doi.org/10.3390/foods14111966
Zhao Y, Yang R, Wang W, Sun T, Han X, Ai M, Huang S. Study on Nutritional Characteristics, Antioxidant Activity, and Volatile Compounds in Non-Saccharomyces cerevisiae–Lactiplantibacillus plantarum Co-Fermented Prune Juice. Foods. 2025; 14(11):1966. https://doi.org/10.3390/foods14111966
Chicago/Turabian StyleZhao, Yu, Rui Yang, Wei Wang, Tongle Sun, Xinyao Han, Mingxun Ai, and Shihao Huang. 2025. "Study on Nutritional Characteristics, Antioxidant Activity, and Volatile Compounds in Non-Saccharomyces cerevisiae–Lactiplantibacillus plantarum Co-Fermented Prune Juice" Foods 14, no. 11: 1966. https://doi.org/10.3390/foods14111966
APA StyleZhao, Y., Yang, R., Wang, W., Sun, T., Han, X., Ai, M., & Huang, S. (2025). Study on Nutritional Characteristics, Antioxidant Activity, and Volatile Compounds in Non-Saccharomyces cerevisiae–Lactiplantibacillus plantarum Co-Fermented Prune Juice. Foods, 14(11), 1966. https://doi.org/10.3390/foods14111966





