Effects of Water-Soluble and Fat-Soluble Antioxidant Combinations in Oil-in-Water Emulsions on the Oxidative Stability of Walnut Kernels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of O/W Antioxidant Emulsion and Coating of Walnut Kernels with Antioxidants
2.3. Accelerated Oxidation Treatment and Extraction of Walnut Oil
2.4. Determination of Peroxide Value
2.5. Determination of Acid Value
2.6. Determination of TBARS
2.7. Determination of DPPH Radical Scavenging Ability
2.8. Fatty Acid Composition
2.9. Thermogravimetric Analysis
2.10. Detection of Intermediary Radicals by ESR Spectroscopy
2.11. Statistical Analysis
3. Results
3.1. Peroxide Value
3.2. Acid Value
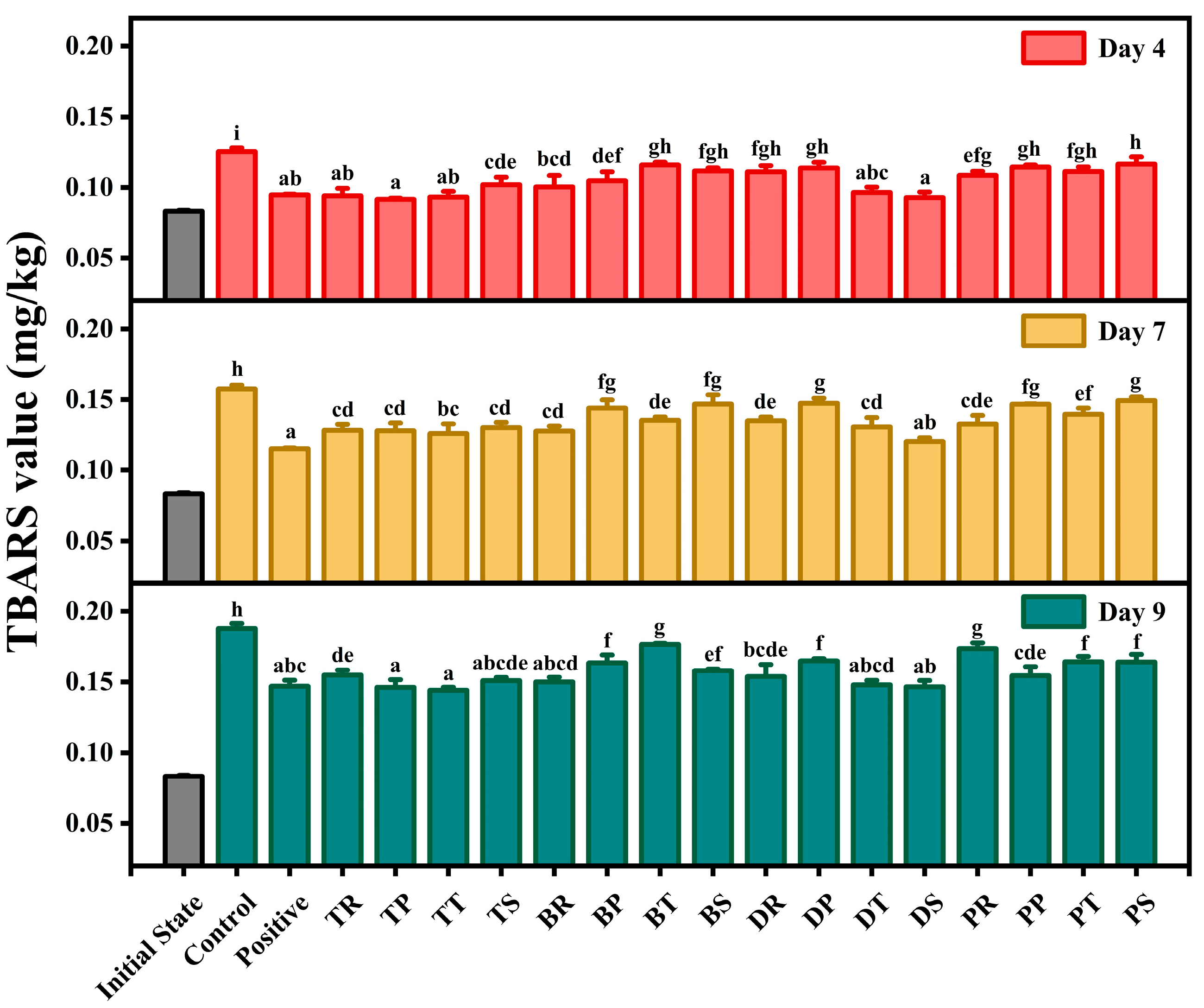
3.3. TBARS
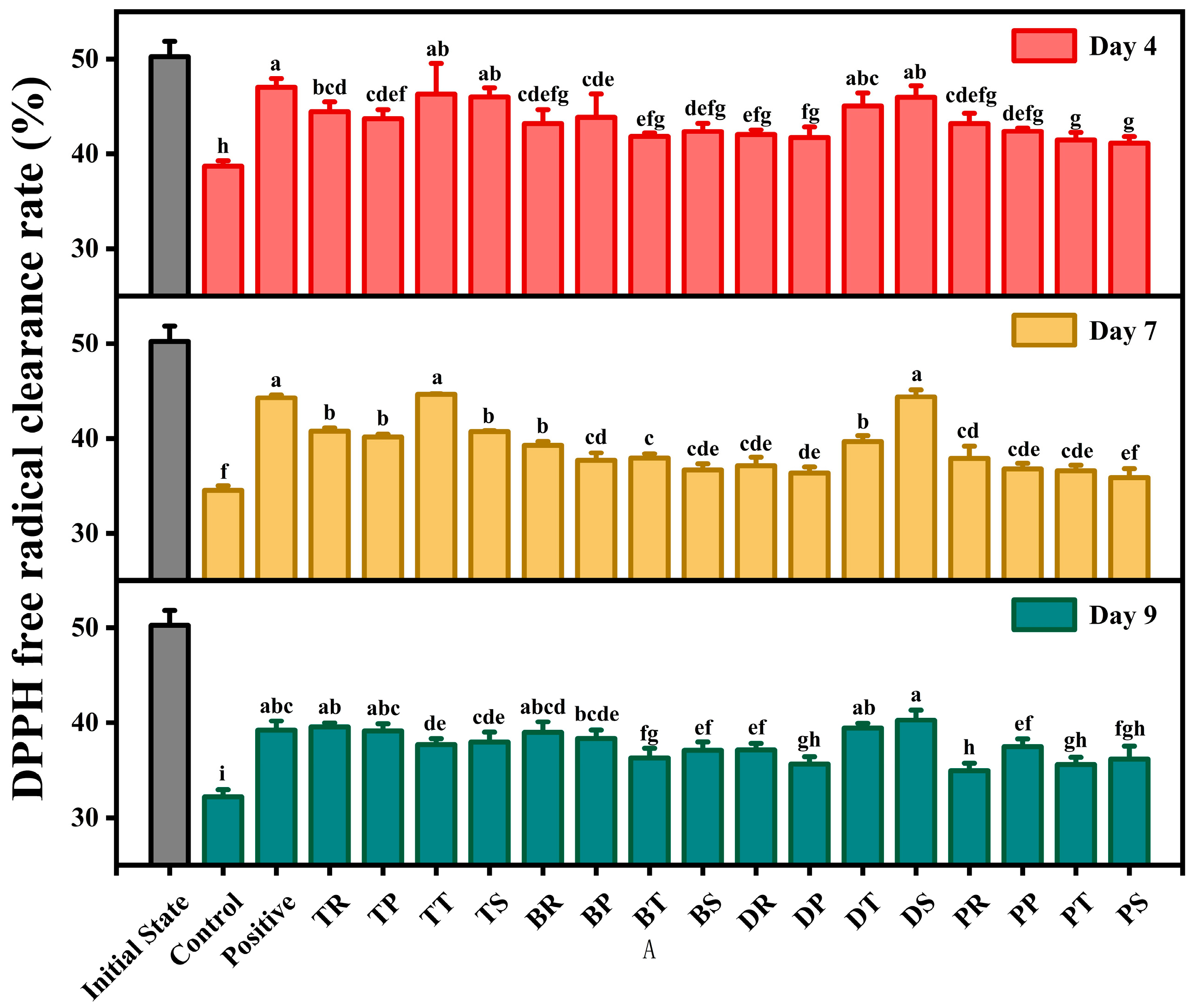
3.4. DPPH Radical Scavenging Ability
3.5. Fatty Acid
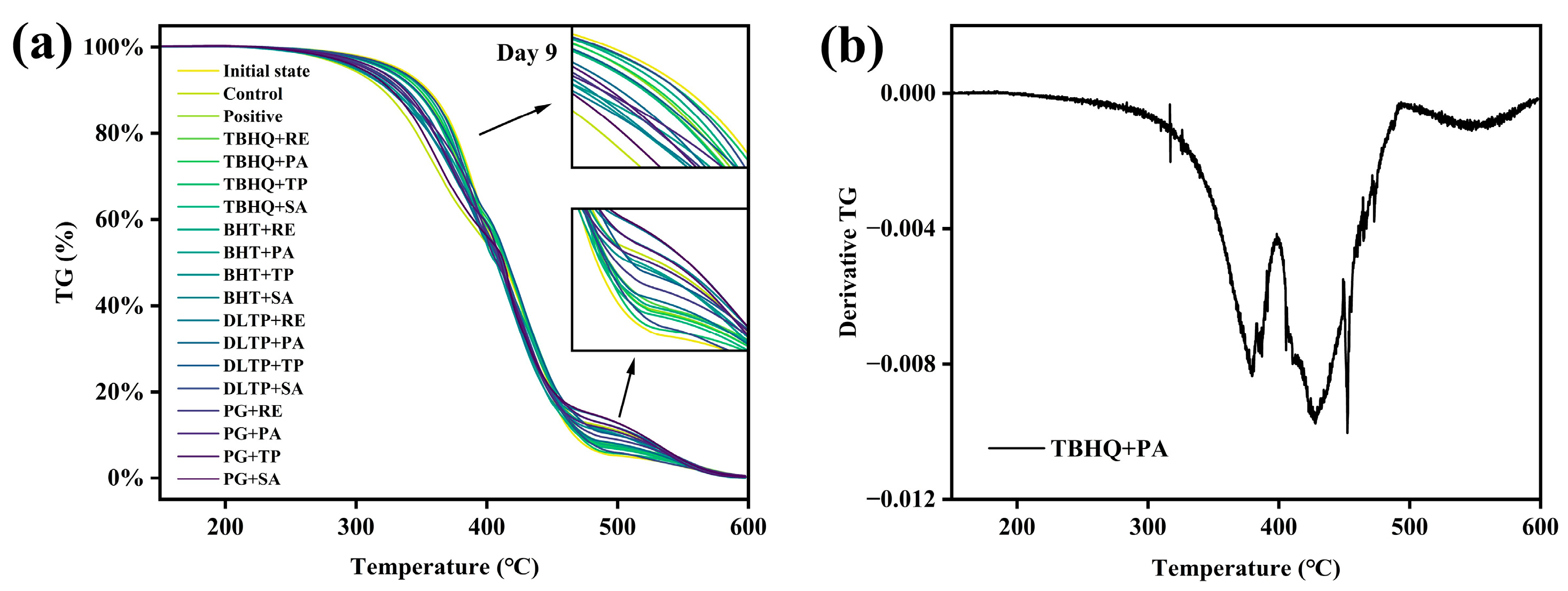
3.6. Thermogravimetric Analysis
3.7. Electron Spin Resonance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UFAs | Unsaturated fatty acids |
| POV | Peroxide value |
| AV | Acid value |
| TBARS | Thiobarbituric acid reactive substances |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl radical |
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| FA | Fatty acid composition |
| TG | Thermogravimetric analysis |
| ESR | Electron spin resonance |
| O/W | Oil in water |
| FAMEs | Fatty acid methyl esters |
References
- Rébufa, C.; Artaud, J.; Le Dréau, Y. Walnut (Juglans regia L.) oil chemical composition depending on variety, locality, extraction process and storage conditions: A comprehensive review. J. Food Compos. Anal. 2022, 110, 104534. [Google Scholar] [CrossRef]
- Jia, Y.; Yuan, B.; Yang, Y.; Zheng, C.; Zhou, Q. Flavor characteristics of peeled walnut kernels under two-steps roasting processes. Food Chem. 2023, 423, 136290. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Tan, W.; Liu, W.; Wei, C.; Chen, J.; Zhao, Z.; Tian, J. Inhibited the walnut oil oxidation through the microcapsules that consisted of (−)-Epigallocatechin gallate and sodium caseinate. Food Biosci. 2024, 61, 104601. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Xu, X.; Zeng, Q.; Li, C.; Xi, B.-N.; Shu, Y.; Ma, T.; Dong, H.; Shen, Y. Lipidomics and metabolomics reveal the molecular mechanisms underlying the effect of thermal treatment on composition and oxidative stability of walnut oil. Food Res. Int. 2024, 191, 114695. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, J.; Zhao, Z.; Tian, J.; Fu, X.; Zhao, Y.; Wei, C.; Liu, W. Roasting treatments affect oil extraction rate, fatty acids, oxidative stability, antioxidant activity, and flavor of walnut oil. Front. Nutr. 2022, 9, 1077081. [Google Scholar] [CrossRef]
- Neale, E.P.; Guan, V.; Tapsell, L.C.; Probst, Y.C. Effect of walnut consumption on markers of blood glucose control: A systematic review and meta-analysis. Br. J. Nutr. 2020, 124, 641–653. [Google Scholar] [CrossRef]
- Ni, Z.J.; Zhang, Y.G.; Chen, S.X.; Thakur, K.; Wang, S.; Zhang, J.G.; Shang, Y.F.; Wei, Z.J. Exploration of walnut components and their association with health effects. Crit. Rev. Food Sci. Nutr. 2022, 62, 5113–5129. [Google Scholar] [CrossRef]
- Adelakun, S.A.; Ukwenya, V.O.; Ogunlade, B.S.; Aniah, A.J.; Ibiayo, G.A. Nitrite-induced testicular toxicity in rats: Therapeutic potential of walnut oil. JBRA Assist. Reprod. 2019, 23, 15–23. [Google Scholar] [CrossRef]
- Bhat, A.A.; Shakeel, A.; Rafiq, S.; Farooq, I.; Malik, A.Q.; Alghuthami, M.E.; Alharthi, S.; Qanash, H.; Alharthy, S.A. Juglans regia Linn.: A Natural Repository of Vital Phytochemical and Pharmacological Compounds. Life 2023, 13, 380. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, C.; Zhou, Q.; Huang, C.; Wang, W.; Huang, Y.; Liu, C. Comparison evaluation pretreatments on the quality characteristics, oxidative stability, and volatile flavor of walnut oil. Food Chem. 2024, 448, 139124. [Google Scholar] [CrossRef]
- Bakkalbaşı, E.; Yılmaz, Ö.M.; Javidipour, I.; Artık, N. Effects of packaging materials, storage conditions and variety on oxidative stability of shelled walnuts. LWT—Food Sci. Technol. 2012, 46, 203–209. [Google Scholar] [CrossRef]
- Ma, W.P.; Yin, S.N.; Chen, J.P.; Geng, X.C.; Liu, M.F.; Li, H.H.; Liu, M.; Liu, H.B. Stimulating the Hematopoietic Effect of Simulated Digestive Product of Fucoidan from Sargassum fusiforme on Cyclophosphamide-Induced Hematopoietic Damage in Mice and Its Protective Mechanisms Based on Serum Lipidomics. Mar. Drugs 2022, 20, 201. [Google Scholar] [CrossRef]
- Yuan, B.; Zheng, C.; Yue, Y.; Zhou, Q. Identification of potential aroma indicators in light and strong intensity flaxseed oil by molecular sensory science technology. J. Food Compos. Anal. 2025, 140, 107189. [Google Scholar] [CrossRef]
- Grilo, F.S.; Wang, S.C. Walnut (Juglans regia L.) Volatile Compounds Indicate Kernel and Oil Oxidation. Foods 2021, 10, 329. [Google Scholar] [CrossRef]
- Mexis, S.F.; Badeka, A.V.; Riganakos, K.A.; Karakostas, K.X.; Kontominas, M.G. Effect of packaging and storage conditions on quality of shelled walnuts. Food Control 2009, 20, 743–751. [Google Scholar] [CrossRef]
- Christopoulos, M.V.; Tsantili, E. Effects of temperature and packaging atmosphere on total antioxidants and colour of walnut (Juglans regia L.) kernels during storage. Sci. Hortic. 2011, 131, 49–57. [Google Scholar] [CrossRef]
- Shaukat, M.N.; Palmeri, R.; Restuccia, C.; Parafati, L.; Fallico, B. Glycerol ginger extract addition to edible coating formulation for preventing oxidation and fungal spoilage of stored walnuts. Food Biosci. 2023, 52, 102420. [Google Scholar] [CrossRef]
- Gull, A.; Masoodi, F.A.; Masoodi, L.; Gani, A.; Muzaffar, S. Effect of sodium alginate coatings enriched with α-tocopherol on quality of fresh walnut kernels. Food Chem. Adv. 2023, 2, 100169. [Google Scholar] [CrossRef]
- Young, P.; Luch, A.; Laux, P. Impact of phosphine and of sulfuryl fluoride fumigation on walnut quality. J. Stored Prod. Res. 2023, 100, 102059. [Google Scholar] [CrossRef]
- Hu, H.; Jing, N.; Peng, Y.; Liu, C.; Ma, H.; Ma, Y. 60Coγ-ray irradiation inhibits germination of fresh walnuts by modulating respiratory metabolism and reducing energy status during storage. Postharvest Biol. Technol. 2021, 182, 111694. [Google Scholar] [CrossRef]
- Mohanan, A.; Nickerson, M.T.; Ghosh, S. Oxidative stability of flaxseed oil: Effect of hydrophilic, hydrophobic and intermediate polarity antioxidants. Food Chem. 2018, 266, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals23. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef]
- Tang, L.; Cao, M.; Liao, C.; Xu, Y.; Karrar, E.; Liu, R.; Chang, M. Prolonging oxidation stability of peony (Paeonia suffruticosa Andr.) seed oil by endogenous lipid concomitants: Phospholipids enhance antioxidant capacity by improving the function of tocopherol. Ind. Crops Prod. 2023, 197, 116552. [Google Scholar] [CrossRef]
- Lu, T.; Shen, Y.; Wang, J.-H.; Xie, H.-K.; Wang, Y.-F.; Zhao, Q.; Zhou, D.-Y.; Shahidi, F. Improving oxidative stability of flaxseed oil with a mixture of antioxidants. J. Food Process. Preserv. 2020, 44, e14355. [Google Scholar] [CrossRef]
- Prodromidis, P.; Katsanidis, E.; Biliaderis, C.G.; Moschakis, T. Effect of Tween 20, emulsification temperature and ultrasonication intensity on structured emulsions with monoglycerides. Food Hydrocoll. 2024, 151, 109772. [Google Scholar] [CrossRef]
- Yin, J.; Becker, E.M.; Andersen, M.L.; Skibsted, L.H. Green tea extract as food antioxidant. Synergism and antagonism with α-tocopherol in vegetable oils and their colloidal systems. Food Chem. 2012, 135, 2195–2202. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xu, Y.; Chang, M.; Tang, L.; Lu, M.; Liu, R.; Jin, Q.; Wang, X. Antioxidant interaction of α-tocopherol, γ-oryzanol and phytosterol in rice bran oil. Food Chem. 2021, 343, 128431. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, M.-J.; Yi, B.; Oh, S.; Lee, J. Effects of relative humidity on the antioxidant properties of α-tocopherol in stripped corn oil. Food Chem. 2015, 167, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.R.; Śārada, R.; Ravishankar, G.A. Stabilization of astaxanthin in edible oils and its use as an antioxidant. J. Sci. Food Agric. 2007, 87, 957–965. [Google Scholar] [CrossRef]
- Ayu, D.F.; Andarwulan, N.; Hariyadi, P.; Purnomo, E.H. Effect of tocopherols, tocotrienols, β-carotene, and chlorophyll on the photo-oxidative stability of red palm oil. Food Sci. Biotechnol. 2016, 25, 401–407. [Google Scholar] [CrossRef]
- Becker, E.M.; Ntouma, G.; Skibsted, L.H. Synergism and antagonism between quercetin and other chain-breaking antioxidants in lipid systems of increasing structural organisation. Food Chem. 2007, 103, 1288–1296. [Google Scholar] [CrossRef]
- Nogala-Kałucka, M.; Dwiecki, K.; Siger, A.; Górnaś, P.; Polewski, K.; Ciosek, S. Antioxidant synergism and antagonism between tocotrienols, quercetin and rutin in model system. Acta Aliment. 2013, 42, 360–370. [Google Scholar] [CrossRef]
- Ma, P.; Wen, H.; Chen, X.; Zhang, W.; Rong, L.; Luo, Y.; Xie, J. Synergistic rosemary extract with TBHQ and citric acid improves oxidative stability and shelf life of peanut. J. Food Sci. 2024, 89, 3591–3602. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhang, Z.; Chen, J.; Niu, J.; Shi, Y.; Wang, Y.; Chen, T.; Sun, Z.; Chen, J.; Luan, M. Physicochemical properties, content, composition and partial least squares models of A. trifoliata seeds oil. Food Chem. X 2021, 12, 100131. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y.; Wen, S.; Sun, Y.; Chen, J.; Gao, Y.; Sagymbek, A.; Yu, X. Analytical methods for determining the peroxide value of edible oils: A mini-review. Food Chem. 2021, 358, 129834. [Google Scholar] [CrossRef]
- Cho, Y.-J.; Kim, T.-E.; Gil, B. Correlation between refractive index of vegetable oils measured with surface plasmon resonance and acid values determined with the AOCS official method. LWT—Food Sci. Technol. 2013, 53, 517–521. [Google Scholar] [CrossRef]
- Li, Y.; Liu, G. Encapsulation of Antarctic krill oil and kaempferol co-loaded nano-liposomes in alginate-chitosan hydrogel beads: Improved stability and modified digestive behaviour. Int. J. Food Sci. Technol. 2024, 59, 3198–3211. [Google Scholar] [CrossRef]
- Xu, M.; Shen, C.; Zheng, H.; Xu, Y.; Xue, C.; Zhu, B.; Hu, J. Metabolomic analysis of acerola cherry (Malpighia emarginata) fruit during ripening development via UPLC-Q-TOF and contribution to the antioxidant activity. Food Res. Int. 2020, 130, 108915. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, S.; Shen, M.; Xie, J.; Yang, J. Evaluation of trans fatty acids, carbonyl compounds and bioactive minor components in commercial linseed oils. Food Chem. 2022, 369, 130930. [Google Scholar] [CrossRef]
- Coni, E.; Podestà, E.; Catone, T. Oxidizability of different vegetables oils evaluated by thermogravimetric analysis. Thermochim. Acta 2004, 418, 11–15. [Google Scholar] [CrossRef]
- Chen, Q.; Xie, Y.; Xi, J.; Guo, Y.; Qian, H.; Cheng, Y.; Chen, Y.; Yao, W. Characterization of lipid oxidation process of beef during repeated freeze-thaw by electron spin resonance technology and Raman spectroscopy. Food Chem. 2018, 243, 58–64. [Google Scholar] [CrossRef]
- Shen, Y.; Lu, T.; Liu, X.-Y.; Zhao, M.-T.; Yin, F.-W.; Rakariyatham, K.; Zhou, D.-Y. Improving the oxidative stability and lengthening the shelf life of DHA algae oil with composite antioxidants. Food Chem. 2020, 313, 126139. [Google Scholar] [CrossRef] [PubMed]
- Szterk, A.; Stefaniuk, I.; Waszkiewicz-Robak, B.; Roszko, M. Oxidative Stability of Lipids by Means of EPR Spectroscopy and Chemiluminescence. J. Am. Oil Chem. Soc. 2011, 88, 611–618. [Google Scholar] [CrossRef]
- Wang, R.; Yang, J.; Fan, Y.; Wang, Y.; Xu, C.; Guo, G.; Wang, Z. Microemulsification of peony (Paeonia suffruticosa Andr.) seed oil and its fatty acids: A comparative study in antioxidant and storage stability. Food Bioprod. Process. 2024, 146, 147–159. [Google Scholar] [CrossRef]
- Yu, J.; Smith, I.N.; Idris, N.; Gregory, N.; Mikiashvili, N. Oxidative Stability of Protease Treated Peanut with Reduced Allergenicity. Foods 2020, 9, 762. [Google Scholar] [CrossRef]
- Karahadian, C.; Lindsay, R.C. Evaluation of the mechanism of dilauryl thiodipropionate antioxidant activity. J. Am. Oil Chem. Soc. 1988, 65, 1159–1165. [Google Scholar] [CrossRef]
- Omar, K.A.; Shan, L.; Wang, Y.L.; Wang, X. Stabilizing flaxseed oil with individual antioxidants and their mixtures. Eur. J. Lipid Sci. Technol. 2010, 112, 1003–1011. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, Y.; Xie, Y.; Cheng, Y.; Qian, H.; Yao, W. Regeneration of tert-butylhydroquinone by tea polyphenols. Food Res. Int. 2017, 95, 1–8. [Google Scholar] [CrossRef]
- Sun, J.; Li, D.; Huyan, W.; Hong, X.; He, S.; Huo, J.; Jiang, L.; Zhang, Y. Blue honeysuckle seeds and seed oil: Composition, physicochemical properties, fatty acid profile, volatile components, and antioxidant capacity. Food Chem. X 2024, 21, 101176. [Google Scholar] [CrossRef]
- Konuskan, D.B.; Arslan, M.; Oksuz, A. Physicochemical properties of cold pressed sunflower, peanut, rapeseed, mustard and olive oils grown in the Eastern Mediterranean region. Saudi J. Biol. Sci. 2019, 26, 340–344. [Google Scholar] [CrossRef]
- Hou, J.; Zhou, X.; Yu, T.; Sop, R.T.; Ma, J.; Wang, M.; Wu, Q.; Zheng, X.; Jiang, Z. Ziziphi spinosae Semen Oil Enhance the Oxidative Stability of Soybean Oil under Frying Conditions. Eur. J. Lipid Sci. Technol. 2021, 123, 2100060. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Zu, Y.; Chen, X.; Wang, F.; Liu, F. Oxidative stability of sunflower oil supplemented with carnosic acid compared with synthetic antioxidants during accelerated storage. Food Chem. 2010, 118, 656–662. [Google Scholar] [CrossRef]
- Romano, C.S.; Abadi, K.; Repetto, V.; Vojnov, A.A.; Moreno, S. Synergistic antioxidant and antibacterial activity of rosemary plus butylated derivatives. Food Chem. 2009, 115, 456–461. [Google Scholar] [CrossRef]
- Jin, Y.; Yao, Y.; Wu, N.; Du, H.; Xu, M.; Zhao, Y.; Luo, C.; Tu, Y. Inhibition of the liquefaction of alkali-induced egg white gel by sodium ascorbate. Food Chem. 2022, 381, 132220. [Google Scholar] [CrossRef]
- Varvara, M.; Bozzo, G.; Celano, G.; Disanto, C.; Pagliarone, C.N.; Celano, G.V. The Use of Ascorbic Acid as a Food Additive: Technical-Legal Issues. Ital. J. Food Saf. 2016, 5, 4313. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Alara, O.R.; Abayomi, O.O. Extraction, characterization and antioxidant activity of fenugreek (Trigonella-Foenum Graecum) seed oil. Mater. Sci. Energy Technol. 2019, 2, 349–355. [Google Scholar] [CrossRef]
- Alghooneh, A.; Alizadeh Behbahani, B.; Taghdir, M.; Sepandi, M.; Abbaszadeh, S. Understanding the Relationship between Microstructure and Physicochemical Properties of Ultrafiltered Feta-Type Cheese Containing Saturea bachtiarica Leaf Extract. Foods 2022, 11, 1728. [Google Scholar] [CrossRef]
- Hussein-Al-Ali, S.H.; Hussein, M.Z.; Bullo, S.; Arulselvan, P. Chlorambucil-Iron Oxide Nanoparticles as a Drug Delivery System for Leukemia Cancer Cells. Int. J. Nanomed. 2021, 16, 6205–6216. [Google Scholar] [CrossRef] [PubMed]
- Falch, E.; Velasco, J.; Aursand, M.; Andersen, M.L. Detection of radical development by ESR spectroscopy techniques for assessment of oxidative susceptibility of fish oils. Eur. Food Res. Technol. 2005, 221, 667–674. [Google Scholar] [CrossRef]
- Adiiba, S.H.; Song, C.P.; Lee, Y.Y.; Amelia; Chang, M.Y.; Chan, E.-S. Effects of water-soluble secondary antioxidants on the retention of carotene and tocols during hydrolysis of crude palm oil catalysed by Eversa® Transform 2.0 for alcohol-free production of palm phytonutrients concentrate. Ind. Crops Prod. 2024, 209, 117929. [Google Scholar] [CrossRef]



| Group Abbreviation | Fat-Soluble | Water-Soluble | |||
|---|---|---|---|---|---|
| Antioxidants | Amount (%, w/w) | Antioxidants | Amount (%, w/w) | ||
| 1 | TR | TBHQ | 0.03 | RE | 0.045 |
| 2 | TP | TBHQ | 0.03 | PA | 0.03 |
| 3 | TT | TBHQ | 0.03 | TPs | 0.03 |
| 4 | TS | TBHQ | 0.03 | SA | 0.03 |
| 5 | BR | BHT | 0.03 | RE | 0.045 |
| 6 | BP | BHT | 0.03 | PA | 0.03 |
| 7 | BT | BHT | 0.03 | TPs | 0.03 |
| 8 | BS | BHT | 0.03 | SA | 0.03 |
| 9 | DR | DLTP | 0.03 | RE | 0.045 |
| 10 | DP | DLTP | 0.03 | PA | 0.03 |
| 11 | DT | DLTP | 0.03 | TPs | 0.03 |
| 12 | DS | DLTP | 0.03 | SA | 0.03 |
| 13 | PR | PG | 0.015 | RE | 0.045 |
| 14 | PP | PG | 0.015 | PA | 0.03 |
| 15 | PT | PG | 0.015 | TPs | 0.03 |
| 16 | PS | PG | 0.015 | SA | 0.03 |
| Days of Oxidation | Group | Palmitic Acid (C16:0, %) | Stearic Acid (C18:0, %) | Oleic Acid (C18:1, %) | Linoleic Acid (C18:2, %) | α-Linolenic Acid (C18:3, %) | SFAs | UFAs |
|---|---|---|---|---|---|---|---|---|
| Initial state | 6.00 ± 0.01 | 2.92 ± 0.00 | 20.66 ± 0.04 | 59.81 ± 0.04 | 10.53 ± 0.01 | 8.92 ± 0.01 | 91.00 ± 0.01 | |
| Day 4 | Control | 6.64 ± 0.05 a | 3.05 ± 0.00 d | 17.96 ± 0.01 j | 61.51 ± 0.03 f | 10.76 ± 0.00 h | 9.69 ± 0.04 a | 90.23 ± 0.04 j |
| Positive | 6.38 ± 0.01 cde | 2.62 ± 0.01 k | 17.31 ± 0.01 m | 62.05 ± 0.04 d | 11.56 ± 0.04 b | 9.01 ± 0.00 ij | 90.92 ± 0.00 ab | |
| TR | 6.25 ± 0.03 fg | 2.92 ± 0.00 g | 19.02 ± 0.01 d | 61.07 ± 0.05 i | 10.65 ± 0.02 i | 9.18 ± 0.03 fg | 90.74 ± 0.04 de | |
| TP | 6.05 ± 0.03 h | 3.06 ± 0.00 cd | 18.25 ± 0.01 g | 61.21 ± 0.02 h | 11.35 ± 0.03 e | 9.11 ± 0.03 gh | 90.81 ± 0.02 cd | |
| TT | 6.19 ± 0.01 g | 2.85 ± 0.01 i | 19.94 ± 0.02 a | 60.66 ± 0.03 k | 10.27 ± 0.00 k | 9.05 ± 0.02 hi | 90.87 ± 0.02 bc | |
| TS | 6.33 ± 0.03 ef | 2.86 ± 0.01 i | 17.03 ± 0.02 n | 62.66 ± 0.00 b | 11.05 ± 0.01 g | 9.18 ± 0.03 efg | 90.74 ± 0.03 de | |
| BR | 6.24 ± 0.04 fg | 2.89 ± 0.01 h | 18.41 ± 0.00 f | 60.82 ± 0.05 j | 11.56 ± 0.02 b | 9.13 ± 0.04 gh | 90.78 ± 0.04 cd | |
| BP | 6.27 ± 0.04 fg | 3.05 ± 0.01 d | 18.08 ± 0.03 i | 61.42 ± 0.02 fg | 11.11 ± 0.03 f | 9.32 ± 0.04 cd | 90.60 ± 0.04 gh | |
| BT | 6.17 ± 0.01 g | 3.07 ± 0.01 bc | 18.16 ± 0.00 h | 61.49 ± 0.03 f | 11.02 ± 0.01 g | 9.25 ± 0.02 def | 90.67 ± 0.02 efg | |
| BS | 6.39 ± 0.03 cde | 2.98 ± 0.00 f | 17.27 ± 0.02 m | 61.44 ± 0.02 fg | 11.84 ± 0.01 a | 9.37 ± 0.03 c | 90.55 ± 0.03 h | |
| DR | 6.48 ± 0.05 bc | 2.86 ± 0.01 i | 17.54 ± 0.02 k | 62.01 ± 0.05 d | 11.03 ± 0.02 g | 9.34 ± 0.05 cd | 90.58 ± 0.06 gh | |
| DP | 6.22 ± 0.05 g | 2.97 ± 0.01 f | 16.09 ± 0.02 o | 63.29 ± 0.03 a | 11.36 ± 0.02 de | 9.19 ± 0.05 efg | 90.73 ± 0.04 def | |
| DT | 6.02 ± 0.04 h | 3.17 ± 0.01 a | 19.14 ± 0.02 c | 60.01 ± 0.02 m | 11.58 ± 0.01 b | 9.19 ± 0.04 efg | 90.73 ± 0.04 def | |
| DS | 6.45 ± 0.01 cd | 2.65 ± 0.01 j | 18.61 ± 0.01 e | 61.76 ± 0.01 e | 10.47 ± 0.00 j | 9.10 ± 0.02 ghi | 90.83 ± 0.02 bcd | |
| PR | 6.27 ± 0.01 fg | 3.01 ± 0.01 e | 18.23 ± 0.02 g | 61.36 ± 0.01 g | 11.05 ± 0.01 g | 9.28 ± 0.02 cde | 90.64 ± 0.03 fgh | |
| PP | 6.38 ± 0.05 cde | 3.09 ± 0.00 b | 19.51 ± 0.03 b | 60.21 ± 0.02 l | 10.72 ± 0.01 h | 9.47 ± 0.05 b | 90.44 ± 0.05 i | |
| PT | 6.35 ± 0.04 def | 2.94 ± 0.00 g | 16.99 ± 0.02 n | 62.24 ± 0.04 c | 11.41 ± 0.02 d | 9.28 ± 0.04 cde | 90.64 ± 0.03 fgh | |
| PS | 6.56 ± 0.02 ab | 2.98 ± 0.01 f | 17.45 ± 0.02 l | 61.44 ± 0.04 fg | 11.48 ± 0.02 c | 9.54 ± 0.02 b | 90.38 ± 0.01 i | |
| Day 7 | Control | 7.01 ± 0.02 a | 2.95 ± 0.02 c | 17.47 ± 0.01 o | 61.67 ± 0.04 b | 10.91 ± 0.01 n | 9.96 ± 0.04 a | 90.04 ± 0.04 l |
| Positive | 6.42 ± 0.04 hi | 2.81 ± 0.02 g | 19.30 ± 0.03 g | 60.18 ± 0.04 h | 11.28 ± 0.03 k | 9.23 ± 0.02 ijk | 90.77 ± 0.02 bcd | |
| TR | 6.43 ± 0.01 hi | 2.87 ± 0.01 de | 19.55 ± 0.03 e | 59.45 ± 0.03 j | 11.71 ± 0.01 ef | 9.29 ± 0.01 ghi | 90.71 ± 0.01 def | |
| TP | 6.37 ± 0.01 i | 2.80 ± 0.01 g | 19.40 ± 0.01 f | 60.40 ± 0.01 f | 11.03 ± 0.01 m | 9.17 ± 0.02 kl | 90.83 ± 0.02 ab | |
| TT | 6.46 ± 0.05 gh | 2.82 ± 0.01 fg | 21.67 ± 0.04 a | 57.84 ± 0.03 m | 11.22 ± 0.02 l | 9.27 ± 0.05 hij | 90.73 ± 0.05 cde | |
| TS | 6.25 ± 0.03 j | 2.97 ± 0.01 c | 20.69 ± 0.01 b | 57.97 ± 0.03 l | 12.13 ± 0.02 a | 9.21 ± 0.02 ijk | 90.79 ± 0.02 bcd | |
| BR | 6.53 ± 0.03 fg | 2.84 ± 0.00 efg | 19.66 ± 0.03 d | 59.77 ± 0.00 i | 11.21 ± 0.03 l | 9.37 ± 0.03 fg | 90.63 ± 0.03 fg | |
| BP | 6.63 ± 0.05 de | 2.99 ± 0.01 c | 17.45 ± 0.02 o | 61.16 ± 0.02 d | 11.77 ± 0.02 d | 9.62 ± 0.06 cde | 90.38 ± 0.06 hij | |
| BT | 6.75 ± 0.00 bc | 2.86 ± 0.01 def | 18.26 ± 0.01 l | 60.91 ± 0.01 e | 11.22 ± 0.02 l | 9.61 ± 0.01 de | 90.39 ± 0.01 hi | |
| BS | 6.76 ± 0.00 b | 2.89 ± 0.03 d | 16.80 ± 0.02 p | 61.53 ± 0.03 c | 12.02 ± 0.01 b | 9.65 ± 0.03 bcd | 90.35 ± 0.03 ijk | |
| DR | 6.67 ± 0.03 cd | 3.04 ± 0.00 b | 16.62 ± 0.01 q | 62.07 ± 0.03 a | 11.60 ± 0.01 g | 9.71 ± 0.03 bc | 90.29 ± 0.03 jk | |
| DP | 6.42 ± 0.02 hi | 2.88 ± 0.01 de | 19.05 ± 0.02 i | 60.17 ± 0.01 h | 11.48 ± 0.02 ij | 9.29 ± 0.01 ghi | 90.71 ± 0.01 def | |
| DT | 6.59 ± 0.00 def | 2.75 ± 0.01 h | 19.73 ± 0.01 c | 59.25 ± 0.04 k | 11.68 ± 0.02 f | 9.34 ± 0.02 gh | 90.66 ± 0.02 ef | |
| DS | 6.57 ± 0.03 ef | 2.62 ± 0.01 i | 18.97 ± 0.01 j | 60.28 ± 0.04 g | 11.56 ± 0.01 gh | 9.19 ± 0.04 jkl | 90.81 ± 0.04 abc | |
| PR | 6.66 ± 0.00 d | 2.89 ± 0.04 d | 18.41 ± 0.03 k | 60.15 ± 0.05 h | 11.89 ± 0.00 c | 9.55 ± 0.04 e | 90.45 ± 0.04 h | |
| PP | 6.46 ± 0.02 gh | 2.98 ± 0.00 c | 19.17 ± 0.04 h | 59.42 ± 0.04 j | 11.97 ± 0.01 d | 9.44 ± 0.02 f | 90.56 ± 0.02 g | |
| PT | 6.83 ± 0.04 b | 2.81 ± 0.00 fg | 17.64 ± 0.02 n | 61.20 ± 0.03 d | 11.52 ± 0.01 hi | 9.64 ± 0.04 bcde | 90.36 ± 0.04 hijk | |
| PS | 6.60 ± 0.03 def | 3.11 ± 0.01 a | 17.71 ± 0.03 m | 61.11 ± 0.01 d | 11.47 ± 0.02 j | 9.71 ± 0.03 b | 90.29 ± 0.03 k | |
| Day 9 | Control | 7.31 ± 0.02 a | 2.84 ± 0.00 de | 17.23 ± 0.01 h | 60.16 ± 0.02 f | 12.36 ± 0.01 abc | 10.16 ± 0.02 a | 89.76 ± 0.02 g |
| Positive | 6.72 ± 0.02 ij | 2.97 ± 0.01 a | 19.08 ± 0.05 b | 58.97 ± 0.09 j | 12.18 ± 0.01 de | 9.68 ± 0.03 defg | 90.23 ± 0.03 abcd | |
| TR | 6.76 ± 0.03 hij | 2.93 ± 0.02 bc | 16.35 ± 0.04 l | 61.89 ± 0.10 a | 11.99 ± 0.19 fg | 9.68 ± 0.05 defg | 90.23 ± 0.05 abcd | |
| TP | 6.97 ± 0.08 def | 2.70 ± 0.01 i | 16.87 ± 0.03 j | 60.94 ± 0.10 d | 12.44 ± 0.02 ab | 9.67 ± 0.07 defg | 90.25 ± 0.07 abcd | |
| TT | 6.69 ± 0.02 j | 2.92 ± 0.00 bc | 18.44 ± 0.04 d | 59.88 ± 0.07 g | 11.98 ± 0.03 fg | 9.62 ± 0.02 efg | 90.29 ± 0.03 abc | |
| TS | 6.91 ± 0.02 efg | 2.82 ± 0.01 e | 18.48 ± 0.00 d | 59.34 ± 0.04 hi | 12.37 ± 0.02 abc | 9.73 ± 0.03 def | 90.18 ± 0.03 bcd | |
| BR | 6.90 ± 0.04 efg | 2.76 ± 0.01 fg | 16.20 ± 0.04 m | 61.59 ± 0.05 b | 12.46 ± 0.03 a | 9.66 ± 0.04 defg | 90.25 ± 0.05 abcd | |
| BP | 7.01 ± 0.03 cde | 2.71 ± 0.01 hi | 16.96 ± 0.03 j | 60.95 ± 0.05 d | 12.30 ± 0.02 bcd | 9.71 ± 0.04 def | 90.21 ± 0.04 bcd | |
| BT | 7.11 ± 0.03 bc | 2.90 ± 0.01 c | 16.52 ± 0.01 k | 61.44 ± 0.04 bc | 11.94 ± 0.01 fg | 10.01 ± 0.03 b | 89.90 ± 0.03 f | |
| BS | 7.01 ± 0.04 cde | 2.75 ± 0.00 fg | 19.20 ± 0.02 a | 59.32 ± 0.02 i | 11.63 ± 0.02 h | 9.77 ± 0.04 cd | 90.15 ± 0.04 de | |
| DR | 6.80 ± 0.03 ghij | 2.95 ± 0.01 ab | 17.54 ± 0.00 f | 61.37 ± 0.02 c | 11.26 ± 0.04 i | 9.75 ± 0.04 de | 90.17 ± 0.03 cd | |
| DP | 7.15 ± 0.06 b | 2.84 ± 0.02 de | 17.24 ± 0.02 h | 60.33 ± 0.03 ef | 12.36 ± 0.03 abc | 9.99 ± 0.05 b | 89.93 ± 0.05 f | |
| DT | 6.84 ± 0.06 ghi | 2.78 ± 0.00 f | 17.42 ± 0.01 g | 61.59 ± 0.04 b | 11.29 ± 0.03 i | 9.62 ± 0.06 fg | 90.30 ± 0.06 ab | |
| DS | 6.86 ± 0.03 fgh | 2.70 ± 0.01 hi | 18.80 ± 0.01 c | 59.50 ± 0.04 h | 12.05 ± 0.02 ef | 9.57 ± 0.03 g | 90.35 ± 0.03 a | |
| PR | 6.99 ± 0.05 cdef | 2.78 ± 0.01 f | 17.12 ± 0.04 i | 60.81 ± 0.03 d | 12.23 ± 0.01 cd | 9.76 ± 0.04 cd | 90.15 ± 0.04 de | |
| PP | 7.07 ± 0.01 bcd | 2.86 ± 0.01 d | 17.81 ± 0.06 e | 60.22 ± 0.08 ef | 11.96 ± 0.03 fg | 9.93 ± 0.01 b | 89.98 ± 0.01 f | |
| PT | 7.18 ± 0.03 ab | 2.74 ± 0.02 gh | 17.25 ± 0.01 h | 60.36 ± 0.03 e | 12.38 ± 0.03 abc | 9.92 ± 0.03 b | 89.99 ± 0.03 f | |
| PS | 7.06 ± 0.01 bcd | 2.83 ± 0.02 de | 17.29 ± 0.01 h | 60.80 ± 0.02 d | 11.94 ± 0.02 fg | 9.89 ± 0.01 bc | 90.03 ± 0.01 ef |
| Groups | Oxidation Decomposition Temperature/(°C) | 5% Oxidation Decomposition Temperature/(°C) | 10% Oxidation Decomposition Temperature/(°C) |
|---|---|---|---|
| Initial State | 355.54 | 332.15 | 355.04 |
| Control | 308.78 | 294.69 | 319.80 |
| Positive | 346.12 | 326.90 | 349.14 |
| TR | 341.98 | 320.97 | 345.45 |
| TP | 345.17 | 325.25 | 349.92 |
| TT | 353.47 | 327.76 | 353.06 |
| TS | 343.09 | 321.05 | 345.05 |
| BR | 352.51 | 328.26 | 351.78 |
| BP | 341.97 | 298.08 | 326.95 |
| BT | 329.53 | 302.34 | 329.61 |
| BS | 324.26 | 309.96 | 331.97 |
| DR | 326.33 | 299.54 | 324.93 |
| DP | 336.87 | 316.10 | 338.38 |
| DT | 344.05 | 322.50 | 346.33 |
| DS | 354.58 | 329.42 | 353.38 |
| PR | 327.45 | 309.56 | 335.05 |
| PP | 338.94 | 307.74 | 332.22 |
| PT | 329.53 | 313.74 | 337.54 |
| PS | 318.03 | 303.84 | 326.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, Y.; Wang, R.; Wen, H.; Xie, J. Effects of Water-Soluble and Fat-Soluble Antioxidant Combinations in Oil-in-Water Emulsions on the Oxidative Stability of Walnut Kernels. Foods 2025, 14, 1967. https://doi.org/10.3390/foods14111967
Jing Y, Wang R, Wen H, Xie J. Effects of Water-Soluble and Fat-Soluble Antioxidant Combinations in Oil-in-Water Emulsions on the Oxidative Stability of Walnut Kernels. Foods. 2025; 14(11):1967. https://doi.org/10.3390/foods14111967
Chicago/Turabian StyleJing, Ying, Rongrong Wang, Huiliang Wen, and Jianhua Xie. 2025. "Effects of Water-Soluble and Fat-Soluble Antioxidant Combinations in Oil-in-Water Emulsions on the Oxidative Stability of Walnut Kernels" Foods 14, no. 11: 1967. https://doi.org/10.3390/foods14111967
APA StyleJing, Y., Wang, R., Wen, H., & Xie, J. (2025). Effects of Water-Soluble and Fat-Soluble Antioxidant Combinations in Oil-in-Water Emulsions on the Oxidative Stability of Walnut Kernels. Foods, 14(11), 1967. https://doi.org/10.3390/foods14111967








