Evaluation of the Antimicrobial Effect of Bioprotective Lactic Acid Bacteria Cultures Against Listeria monocytogenes in Vacuum-Packaged Cold-Smoked Rainbow Trout (Oncorhynchus mykiss) at Different Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. In Vitro Assessments
2.2.1. Spot-on-the-Lawn Experiments
2.2.2. Experiments at Refrigeration Conditions
2.3. Challenge Test of LAB Against L. monocytogenes Cocktail in Cold-Smoked Farmed Rainbow Trout
2.3.1. Sample Preparation
2.3.2. Experimental Design
2.3.3. Inoculum Preparation
2.3.4. Rainbow Trout Fillets Inoculation
2.3.5. Microbiological, Physicochemical and Sensory Analysis
2.4. Growth Modelling
3. Results
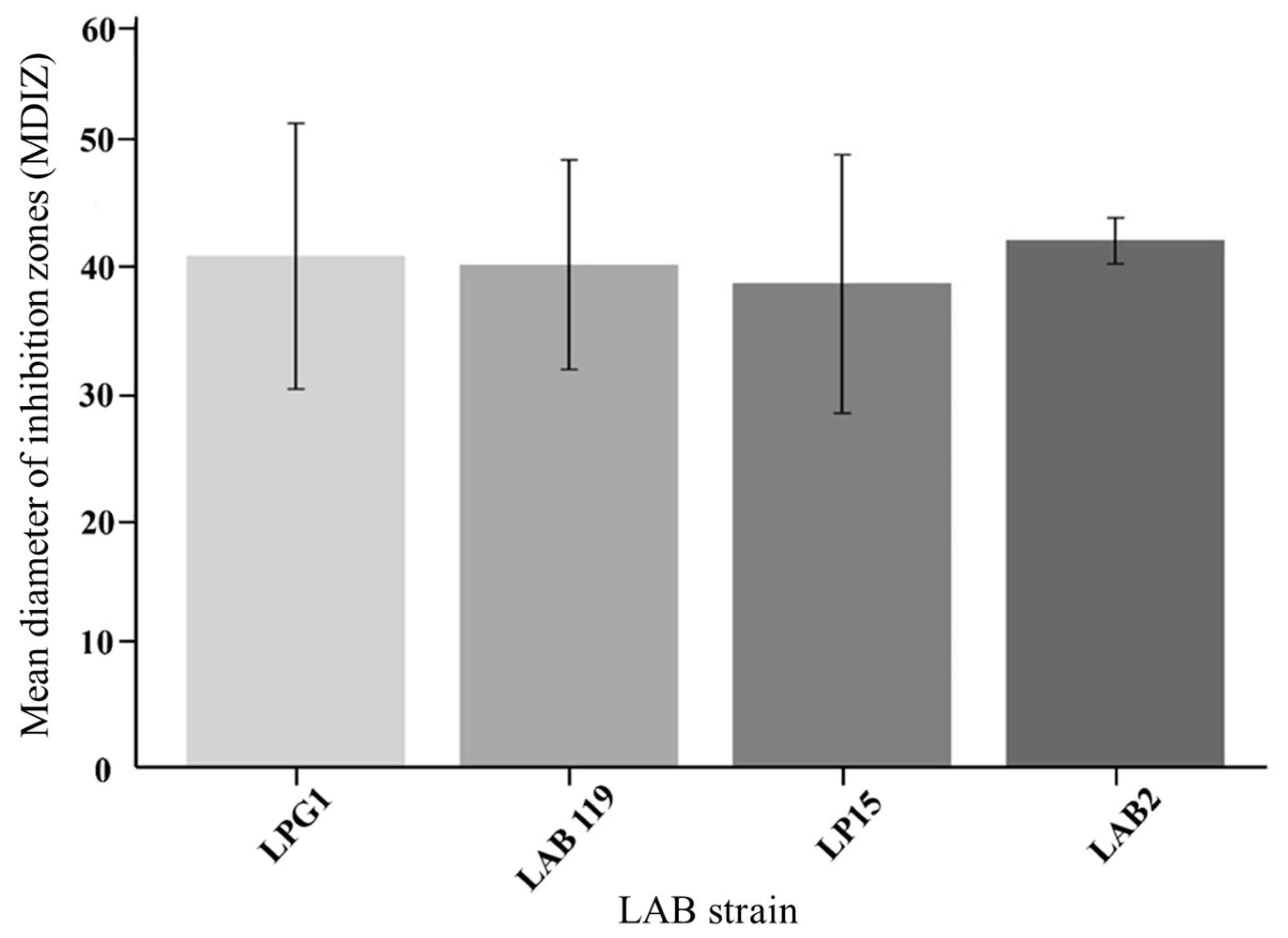
3.1. Screening of LAB Strains
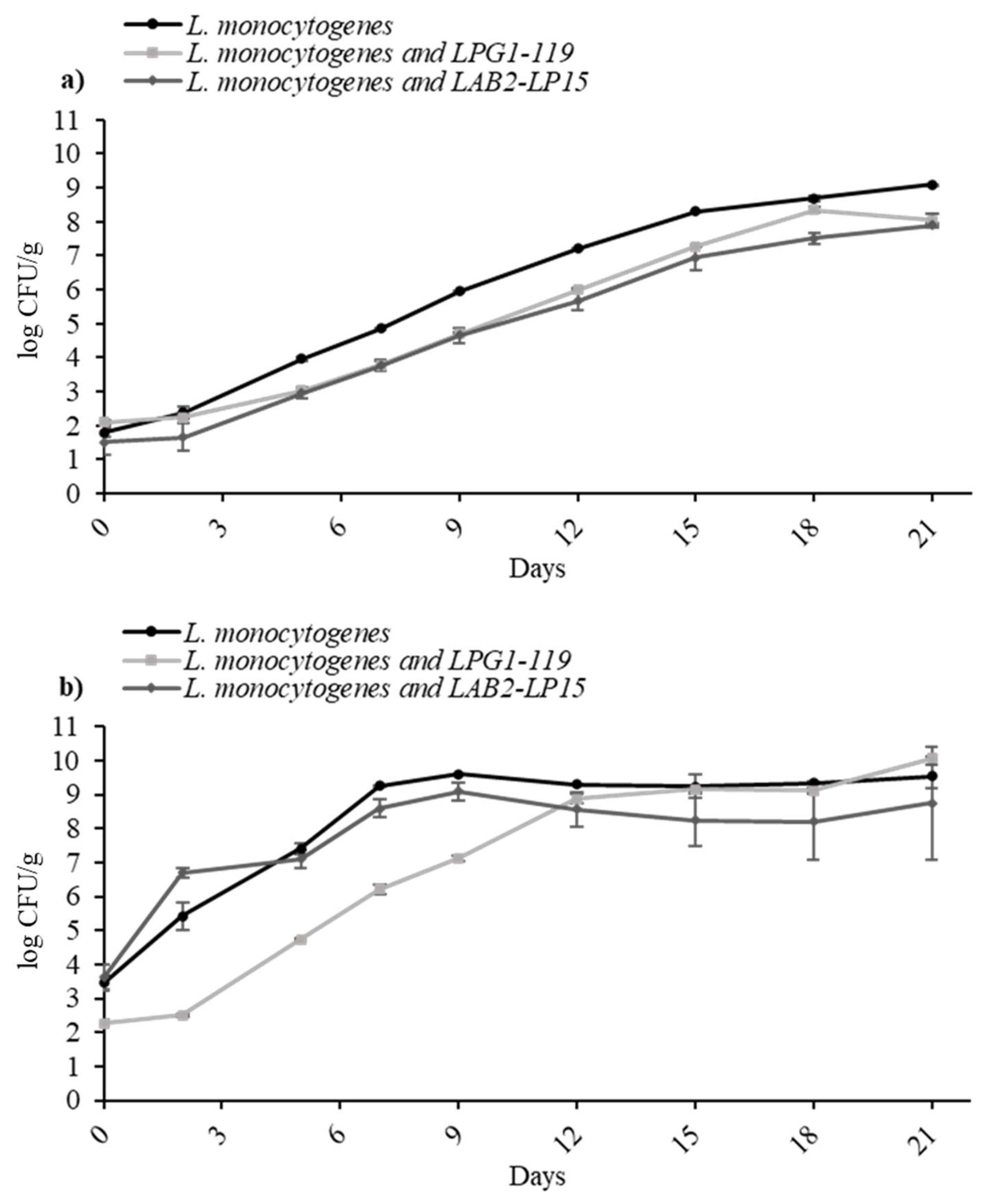
3.2. Inhibitory Effect of LAB Cultures
3.3. Mono-Culture and Co-Culture Modelling
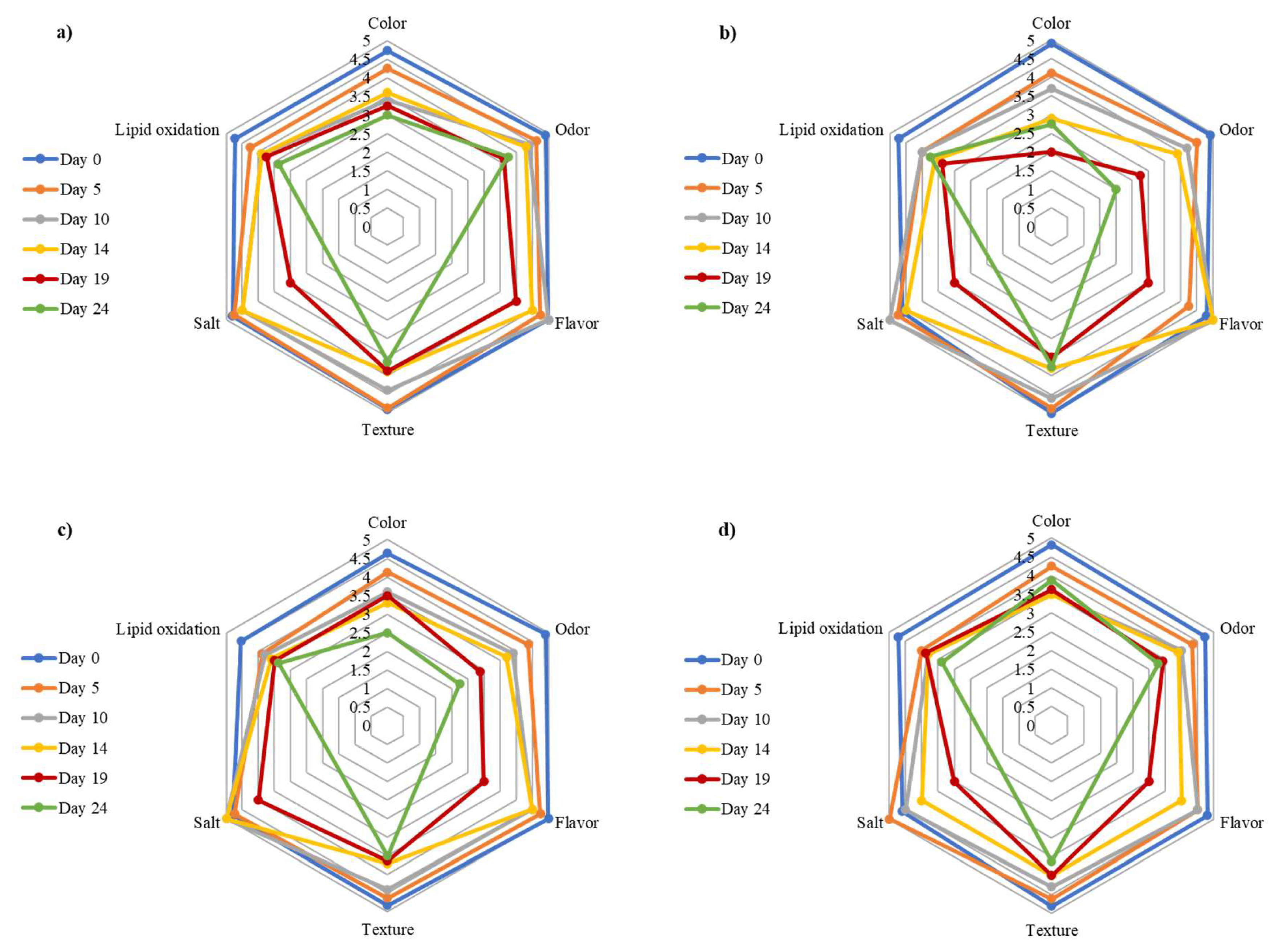
3.4. Sensory Evaluation
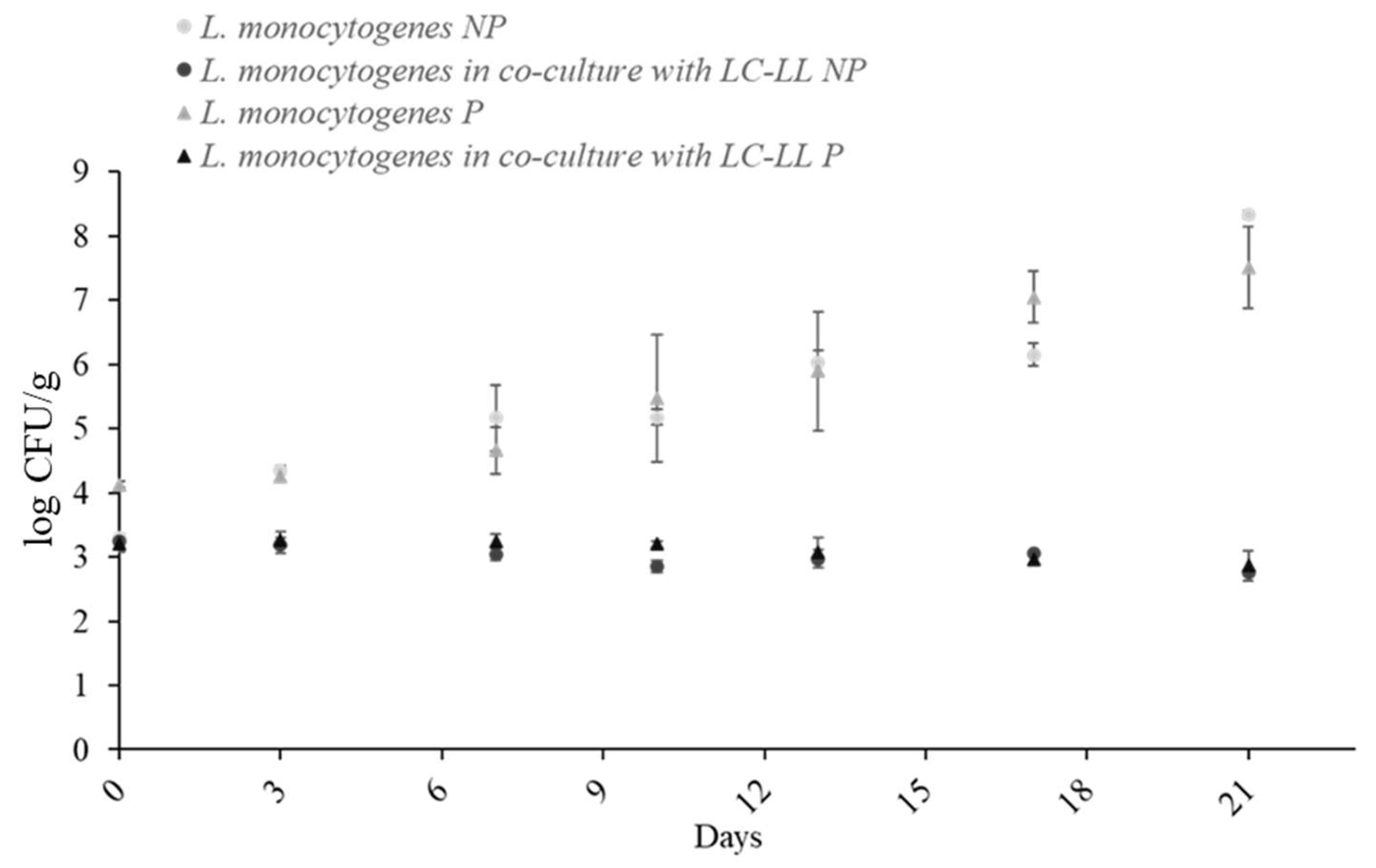
3.5. Effect of Packaging
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| RTE | Ready-to-Eat |
| LAB | Lactic Acid Bacteria |
| CFU | Colony Forming Unit |
| NaCl | Sodium Chloride |
| IL-8 | Interleukin 8 |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| MRS | de Man, Rogosa and Sharpe |
| LC-LL | Leuconostoc carnosum and Lactococcus lactis subsp. lactis |
| RCF | Relative Centrifugal Force |
| CSIC | Consejo Superior de Investigaciones Científicas |
| CECT | Spanish Type Culture Collection |
| BCCM | Belgian Coordinated Collections of Microorganisms |
| ANSES | French Agency for Food, Environmental and Occupational Health & Safety |
| TSB | Tryptone Soya Broth |
| PA | Polyamide |
| PE | Polyethylene |
| EVOH | Ethylene Vinyl Alcohol |
| MAP | Modified Atmosphere Packaging |
| QIM | Quality Index Method |
| µmax | Maximum Specific Growth Rate |
| N0 | Initial Cell Concentration |
| Nmax | Maximum Population Density |
| EU | European Union |
| EFSA | European Food Safety Authority |
| CTAQUA | Andalusian Aquaculture Technological Centre |
| CO2 | Carbon Dioxide |
References
- Chen, J.; Jayachandran, M.; Bai, W.; Xu, B. A critical review on the health benefits of fish consumption and its bioactive constituents. Food Chem. 2022, 369, 130874. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2022; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Tirloni, E.; Centorotola, G.; Pomilio, F.; Torresi, M.; Bernardi, C.; Stella, S. Listeria monocytogenes in ready-to-eat (RTE) delicatessen foods: Prevalence, genomic characterization of isolates and growth potential. Int. J. Food Microbiol. 2024, 410, 110515. [Google Scholar] [CrossRef]
- da Silva, E.P.; De Martinis, E.C.P. Current knowledge and perspectives on biofilm formation: The case of Listeria monocytogenes. Appl. Microbiol. Biotechnol. 2013, 97, 957–968. [Google Scholar] [CrossRef]
- EFSA. The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar] [CrossRef]
- EFSA. Prolonged multi-country cluster of Listeria monocytogenes ST155 infections linked to ready-to-eat fish products. EFSA Support. Publ. 2023, 20, 8538E. [Google Scholar] [CrossRef]
- AESAN: Alerta por Presencia de Listeria monocytogenes en Salmón Ahumado Procedente de España (Ref ES2024/306). Available online: https://www.aesan.gob.es/AECOSAN/web/seguridad_alimentaria/alertas_alimentarias/2024_37.htm (accessed on 29 May 2025).
- Ghaly, A.E.; Dave, D.; Budge, S.; Brooks, M.S. Fish Spoilage Mechanisms and Preservation Techniques: Review. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef]
- Fagerlund, A.; Langsrud, S.; Møretrø, T. Microbial diversity and ecology of biofilms in food industry environments associated with Listeria monocytogenes persistence. Curr. Opin. Food Sci. 2021, 37, 171–178. [Google Scholar] [CrossRef]
- Mazaheri, T.; Cervantes-Huamán, B.R.H.; Bermúdez-Capdevila, M.; Ripolles-Avila, C.; Rodríguez-Jerez, J.J. Listeria monocytogenes Biofilms in the Food Industry: Is the Current Hygiene Program Sufficient to Combat the Persistence of the Pathogen? Microorganisms 2021, 9, 181. [Google Scholar] [CrossRef]
- Nie, X.; Zhang, R.; Cheng, L.; Zhu, W.; Li, S.; Chen, X. Mechanisms underlying the deterioration of fish quality after harvest and methods of preservation. Food Control. 2022, 135, 108805. [Google Scholar] [CrossRef]
- Vignolo, G.; Castellano, P.; Fadda, S. Bioprotective Cultures. In Handbook of Fermented Meat and Poultry, 2nd ed.; John Wiley and Sons Ltd.: Chichester, UK, 2014; pp. 129–137. [Google Scholar] [CrossRef]
- Said, L.B.; Gaudreau, H.; Dallaire, L.; Tessier, M.; Fliss, I. Bioprotective Culture: A New Generation of Food Additives for the Preservation of Food Quality and Safety. Ind. Biotechnol. 2019, 15, 138–147. [Google Scholar] [CrossRef]
- Hashimoto, K.; Bari, M.L.; Inatsu, Y.; Kawamoto, S.; Shima, J. Biopreservation of Kamaboko (Steamed Surimi) Using Piscicolin KH1 Produced by Carnobacterium maltalomaticum KH1. Jpn. J. Food Microbiol. 2011, 28, 193–200. [Google Scholar] [CrossRef][Green Version]
- Delcarlo, S.B.; Parada, R.; Schelegueda, L.I.; Vallejo, M.; Marguet, E.R.; Campos, C.A. From the isolation of bacteriocinogenic LAB strains to the application for fish paste biopreservation. LWT 2019, 110, 239–246. [Google Scholar] [CrossRef]
- Bolívar, A.; Costa, J.C.C.P.; Posada-Izquierdo, G.D.; Bover-Cid, S.; Zurera, G.; Pérez-Rodríguez, F. Quantifying the bioprotective effect of Lactobacillus sakei CTC494 against Listeria monocytogenes on vacuum packaged hot-smoked sea bream. Food Microbiol. 2021, 94, 103649. [Google Scholar] [CrossRef]
- Techeu, U.D.F.; Kaktcham, P.M.; Momo, H.K.; Kouam, E.M.F.; Piame, L.T.; Ngouenam, R.J.; Ngoufack, F.Z. Isolation, Characterization, and Effect on Biofilm Formation of Bacteriocin Produced by Lactococcus lactis F01 Isolated from Cyprinus carpio and Application for Biopreservation of Fish Sausage. BioMed Res. Int. 2022, 2022, 8437926. [Google Scholar] [CrossRef]
- Daliri, E.B.M.; Lee, B.H. New perspectives on probiotics in health and disease. Food Sci. Hum. Wellness 2015, 4, 56–65. [Google Scholar] [CrossRef]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health benefits of Lactic Acid Bacteria (LAB) fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef]
- Benítez-Cabello, A.; Torres-Maravilla, E.; Bermúdez-Humarán, L.; Langella, P.; Martín, R.; Jiménez-Díaz, R.; Jiménez-Díaz, F.N. Probiotic Properties of Lactobacillus Strains Isolated from Table Olive Biofilms. Probiotics Antimicrob. Proteins 2020, 12, 1071–1082. [Google Scholar] [CrossRef]
- Bolívar, A.; Achou, C.G.; Tarlak, F.; Cantalejo, M.J.; Costa, J.C.C.P.; Pérez-Rodríguez, F. Modeling the Growth of Six Listeria monocytogenes Strains in Smoked Salmon Pâté. Foods 2023, 12, 1123. [Google Scholar] [CrossRef]
- Garre, A.; Koomen, J.; den Besten, H.M.W.; Zwietering, M.H. Modeling Population Growth in R with the biogrowth Package. J. Stat. Softw. 2023, 107, 1–51. [Google Scholar] [CrossRef]
- Freitas, J.; Vaz-Pires, P.; Câmara, J.S. Quality Index Method for fish quality control: Understanding the applications, the appointed limits and the upcoming trends. Trends Food Sci. Technol. 2021, 111, 333–345. [Google Scholar] [CrossRef]
- Milillo, S.R.; Story, R.S.; Pak, D.; O’Bryan, C.A.; Crandall, P.G.; Ricke, S.C. Antimicrobial properties of three lactic acid bacterial cultures and their cell free supernatants against Listeria monocytogenes. J. Environ. Sci. Health 2013, 48, 63–68. [Google Scholar] [CrossRef]
- Sinclair, P.; Rolon, M.L.; Feng, J.; Padín-López, A.F.; LaBorde, L.; Kovac, J. Ability of Two Strains of Lactic Acid Bacteria To Inhibit Listeria monocytogenes by Spot Inoculation and in an Environmental Microbiome Context. Microbiol. Spectr. 2022, 10, e01018-22. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Parente, E.; Moles, M.; Ricciardi, A. Leucocin F10, a bacteriocin from Leuconostoc carnosum. Int. J. Food Microbiol. 1996, 33, 231–243. [Google Scholar] [CrossRef]
- Balay, D.R.; Dangeti, R.V.; Kaur, K.; McMullen, L.M. Purification of leucocin A for use on wieners to inhibit Listeria monocytogenes in the presence of spoilage organisms. Int. J. Food Microbiol. 2017, 255, 25–31. [Google Scholar] [CrossRef]
- Balay, D.R.; Gänzle, M.G.; McMullen, L.M. The effect of carbohydrates and bacteriocins on the growth kinetics and resistance of Listeria monocytogenes. Front. Microbiol. 2018, 9, 347. [Google Scholar] [CrossRef]
- Kilinç, B.; Çakli, Ş. Growth of Listeria monocytogenes as affected by thermal treatments of rainbow trout fillets prepared with liquid smoke. Turk. J. Fish. Aquat. Sci. 2012, 12, 13. [Google Scholar] [CrossRef]
- Bolívar, A.; Tarlak, F.; Costa, J.C.C.P.; Cejudo-Gómez, M.; Bover-Cid, S.; Zurera, G.; Pérez-Rodríguez, F. A new expanded modelling approach for investigating the bioprotective capacity of Latilactobacillus sakei CTC494 against Listeria monocytogenes in ready-to-eat fish products. Food Res. Int. 2021, 147, 110545. [Google Scholar] [CrossRef]
- Shin, J.H.; Kang, D.H.; Rasco, B. Effect of different packaging methods and storage temperatures on the growth of Listeria monocytogenes in raw and hot smoked rainbow trout (Oncorhynchus mykiss). J. Aquat. Food Prod. Technol. 2008, 17, 137–155. [Google Scholar] [CrossRef]
- Costa, J.C.C.P.; Bover-Cid, S.; Bolívar, A.; Zurera, G.; Pérez-Rodríguez, F. Modelling the interaction of the sakacin-producing Lactobacillus sakei CTC494 and Listeria monocytogenes in filleted gilthead sea bream (Sparus aurata) under modified atmosphere packaging at isothermal and non-isothermal conditions. Int. J. Food Microbiol. 2019, 297, 72–84. [Google Scholar] [CrossRef]
- Sauer, J.-D.; Herskovits, A.A.; O’Riordan, M.X.D. Metabolism of the gram-positive bacterial pathogen Listeria monocytogenes. In Gram-Positive Pathogens; ASM Press: Washington, DC, USA, 2019; pp. 864–872. [Google Scholar] [CrossRef]
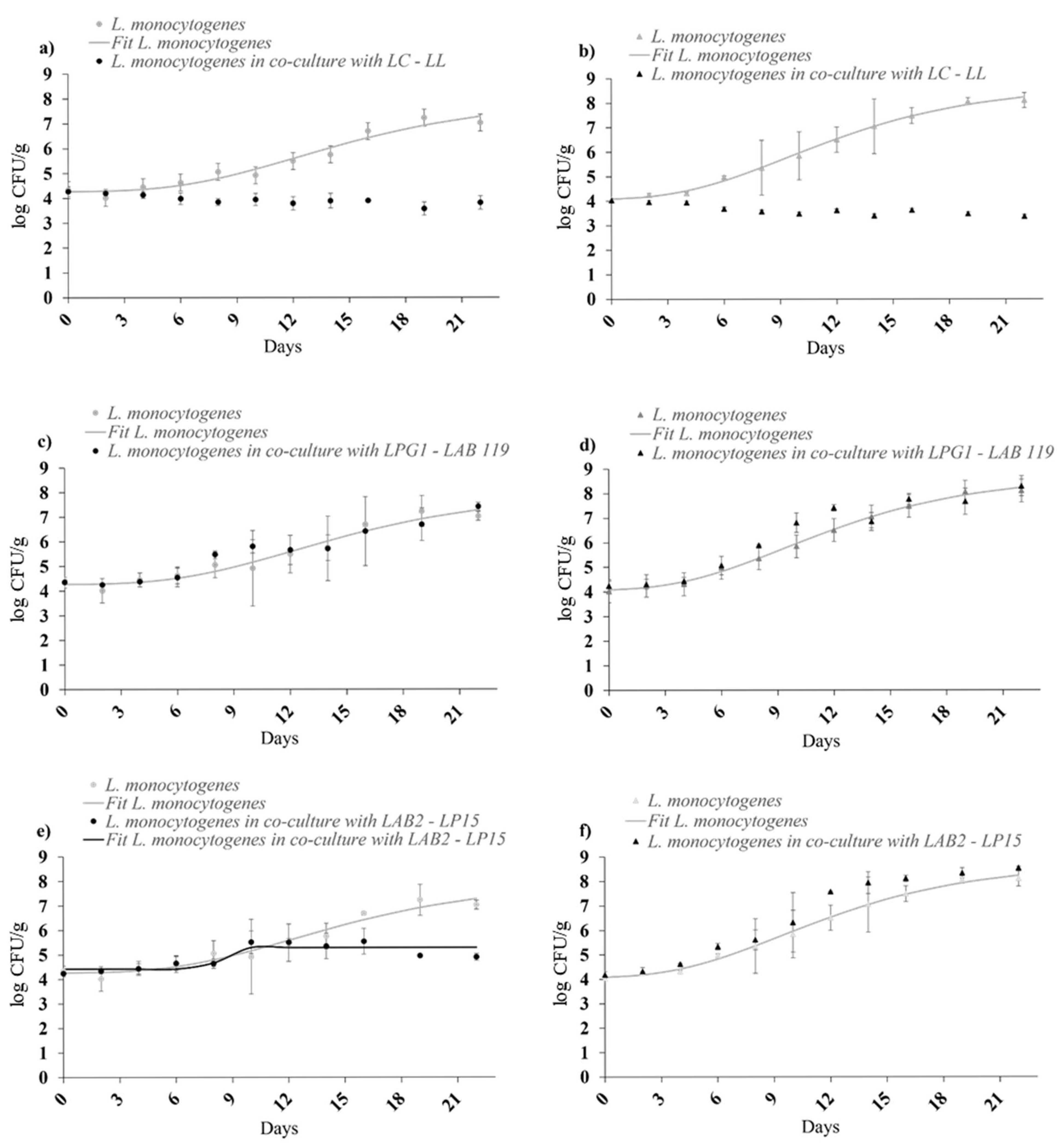
| Culture | 5 °C | 8 °C | ||||||
|---|---|---|---|---|---|---|---|---|
| λ (h) | µmax (1/h) | log N0 | log Nmax | λ (h) | µmax (1/h) | log N0 | log Nmax | |
| L. monocytogenes | 11.5 ± 12.7 A | 0.019 ± 0.001 A | 1.78 ± 0.16 AB | 8.88 ± 0.12 A | 9.5 ± 13.5 A | 0.033 ± 0.003 A | 3.45 ± 0.22 A | 9.42 ± 0.09 A |
| L. monocytogenes in co-culture with LPG1-LAB119 | 78.0 ± 10.7 B | 0.018 ± 0.001 A | 2.13 ± 0.11 B | 8.23 ± 0.12 B | 31.7 ± 23.7 A | 0.027 ± 0.003 A | 2.22 ± 0.39 B | 9.44 ± 0.23 A |
| L. monocytogenes in co-culture with LAB2-LP15 | 33.7 ± 12.5 A | 0.017 ± 0.001 A | 1.44± 0.13 A | 7.80 ± 0.12 C | 12.4 ± 11.0 A | 0.017 ± 0.008 A | 3.63 ± 0.47 A | 8.58 ± 0.22 B |
| Culture | Static 5 °C | Dynamic 4–20 °C | ||||||
|---|---|---|---|---|---|---|---|---|
| λ (h) | µmax (1/h) | log N0 | log Nmax | λ (h) | µmax (1/h) | log N0 | log Nmax | |
| L. monocytogenes | 154.93 ± 30.49 A | 0.0097 ± 0.0012 A | 4.30 ± 0.16 A | 7.18 ± 0.27 A | 94.32 ± 26.13 | 0.0125 ± 0.0012 | 4.68 ± 0.238 | 8.37 ± 0.205 |
| L. monocytogenes in co-culture with LAB2-LP15 | 137.56 ± 49.78 A | 0.0094 ± 0.0072 A | 4.36 ± 0.14 A | 5.29 ± 0.12 B | NE * | NE | NE | NE |
| Culture | Day | Color | Odor | Texture | Oxidation | Flavor | Salt |
|---|---|---|---|---|---|---|---|
| No inoculated (control) | 0 | 4.73 ± 0.47 Aa | 4.91 ± 0.30 Aa | 4.91 ± 0.30 Aa | 4.73 ± 0.47 Aa | 4.89 ± 0.00 Aa | 4.70 ± 0.45 Aa |
| 5 | 4.25 ± 0.71 Aab | 4.63 ± 0.52 Aa | 4.88 ± 0.35 Aa | 4.25 ± 0.46 Aab | 4.75 ± 0.50 Aa | 4.20 ± 0.50 Aa | |
| 10 | 3.60 ± 0.70 Abc | 4.40 ± 0.70 Aab | 4.40 ± 0.70 Aab | 3.90 ± 0.57 Abc | NE * | NE | |
| 14 | 3.40 ± 0.70 Abc | 4.30 ± 0.48 Aabc | 3.90 ± 0.74 Ab | 3.90 ± 0.57 Abc | NE | NE | |
| 19 | 3.25 ± 0.71 Ac | 3.75 ± 0.52 Abc | 3.88 ± 0.83 Ab | 3.75 ± 0.71 Abc | NE | NE | |
| 24 | 3.00 ± 0.53 ABc | 3.63 ± 0.46 Ac | 3.63 ± 1.06 Ab | 3.38 ± 0.52 Ac | NE | NE | |
| Leuconostoc carnosum and Lactococcus lactis subsp. lactis (LC-LL) | 0 | 4.91 ± 0.30 Aa | 4.91 ± 0.30 Aa | 5.00 ± 0.00 Aa | 4.73 ± 0.47 Aa | 4.56 ± 0.44 Aa | 4.60 ± 0.55 Aa |
| 5 | 4.13 ± 0.83 Aab | 4.50 ± 0.76 Aa | 4.18 ± 0.35 Aab | 4.00 ± 0.00 Aab | 5.00 ± 1.50 Aa | 4.50 ± 0.50 Aa | |
| 10 | 3.70 ± 0.95 Abc | 4.20 ± 0.63 Aa | 3.55 ± 0.52 Aab | 4.00 ± 0.47 Aab | NE | NE | |
| 14 | 2.90 ± 0.88 Abc | 3.90 ± 0.99 Aab | 3.45 ± 1.03 Aab | 3.75 ± 0.70 Ab | NE | NE | |
| 19 | 2.75 ± 1.20 Bbc | 2.75 ± 0.88 Abc | 2.73 ± 1.07 Ab | 3.60 ± 1.06 Ab | NE | NE | |
| 24 | 2.00 ± 0.89 ABd | 2.67 ± 1.16 ABc | 2.55 ± 1.16 Ab | 3.75 ± 0.46 Ab | NE | NE | |
| Lactobacillus pentosus (LPG1-LAB119) | 0 | 4.64 ± 0.67 Aa | 4.91 ± 0.30 Aa | 4.82 ± 0.40 Aa | 4.55 ± 0.69 Aa | 4.89 ± 0.00 Aa | 4.60 ± 0.45 Aa |
| 5 | 4.13 ± 0.64 Aab | 4.38 ± 0.74 Aab | 4.00 ± 0.52 Aab | 3.88 ± 0.35 Aab | 4.50 ± 0.50 Aa | 4.33 ± 0.50 Aa | |
| 10 | 3.60 ± 0.97 Aab | 3.90 ± 0.74 Aabc | 3.36 ± 0.70 Aab | 3.80 ± 0.79 Aab | NE | NE | |
| 14 | 3.50 ± 0.48 Abc | 3.70 ± 0.95 Abc | 3.36 ± 0.95 Aab | 3.60 ± 0.70 Ab | NE | NE | |
| 19 | 3.30 ± 0.76 Abc | 2.89 ± 0.93 Acd | 2.64 ± 1.06 Ab | 3.50 ± 0.76 Ab | NE | NE | |
| 24 | 2.50 ± 0.76 Bc | 2.25 ± 0.82 Bd | 2.55 ± 1.31 Ab | 3.39 ± 0.52 Ab | NE | NE | |
| Lactobacillus pentosus and Lactobacillus plantarum (LAB2-LP15) | 0 | 4.82 ± 0.40 Aa | 4.73 ± 0.47 Aa | 4.82 ± 0.40 Aa | 4.73 ± 0.47 Aa | 4.67 ± 0.45 Aa | 4.27 ± 0.55 Aa |
| 5 | 4.25 ± 0.46 Aab | 4.33 ± 0.87 Aab | 4.63 ± 0.52 Aa | 4.00 ± 0.58 Aab | 4.25 ± 1.00 Aa | 4.50 ± 0.00 Aa | |
| 10 | 3.88 ± 0.53 Aab | 4.00 ± 0.81 Aab | 4.30 ± 0.48 Aab | 3.88 ± 0.79 Aab | NE | NE | |
| 14 | 3.63 ± 0.71 Ab | 3.90 ± 0.74 Aab | 4.00 ± 0.47 Aab | 3.80 ± 0.63 Ab | NE | NE | |
| 19 | 3.50 ± 0.92 Ab | 3.43 ± 0.79 Ab | 4.00 ± 0.76 Aab | 3.80 ± 0.64 Ab | NE | NE | |
| 24 | 3.50 ± 0.99 Ab | 3.29 ± 0.76 ABb | 3.63 ± 1.06 Ab | 3.38 ± 0.52 Ab | NE | NE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Martín, J.; Serrano-Heredia, S.M.; Possas, A.; Valero, A.; Carrasco, E. Evaluation of the Antimicrobial Effect of Bioprotective Lactic Acid Bacteria Cultures Against Listeria monocytogenes in Vacuum-Packaged Cold-Smoked Rainbow Trout (Oncorhynchus mykiss) at Different Temperatures. Foods 2025, 14, 1951. https://doi.org/10.3390/foods14111951
Sánchez-Martín J, Serrano-Heredia SM, Possas A, Valero A, Carrasco E. Evaluation of the Antimicrobial Effect of Bioprotective Lactic Acid Bacteria Cultures Against Listeria monocytogenes in Vacuum-Packaged Cold-Smoked Rainbow Trout (Oncorhynchus mykiss) at Different Temperatures. Foods. 2025; 14(11):1951. https://doi.org/10.3390/foods14111951
Chicago/Turabian StyleSánchez-Martín, Javier, Salud María Serrano-Heredia, Arícia Possas, Antonio Valero, and Elena Carrasco. 2025. "Evaluation of the Antimicrobial Effect of Bioprotective Lactic Acid Bacteria Cultures Against Listeria monocytogenes in Vacuum-Packaged Cold-Smoked Rainbow Trout (Oncorhynchus mykiss) at Different Temperatures" Foods 14, no. 11: 1951. https://doi.org/10.3390/foods14111951
APA StyleSánchez-Martín, J., Serrano-Heredia, S. M., Possas, A., Valero, A., & Carrasco, E. (2025). Evaluation of the Antimicrobial Effect of Bioprotective Lactic Acid Bacteria Cultures Against Listeria monocytogenes in Vacuum-Packaged Cold-Smoked Rainbow Trout (Oncorhynchus mykiss) at Different Temperatures. Foods, 14(11), 1951. https://doi.org/10.3390/foods14111951








