Do Seaweeds Contribute to Nutritional Composition and Acceptance in Traditional Portuguese Recipes?
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Recipes and Ingredients
2.2. Sensory Analysis
2.2.1. Quantitative Descriptive Method—Sensory Profile
2.2.2. Affective Method—Acceptance Test (Overall Liking)
2.3. Physicochemical Analysis
2.3.1. Sample Constitution
2.3.2. Proximate Composition
2.3.3. Macroelements
2.3.4. Analytical Quality Assurance
2.4. Statistical Analysis
3. Results and Discussion
3.1. Sea Spaghetti (Himanthalia elongata) and Wakame (Undaria pinnatifida) Composition
3.2. Chemical Composition of the Recipes
3.3. Characteristics of the Recipes
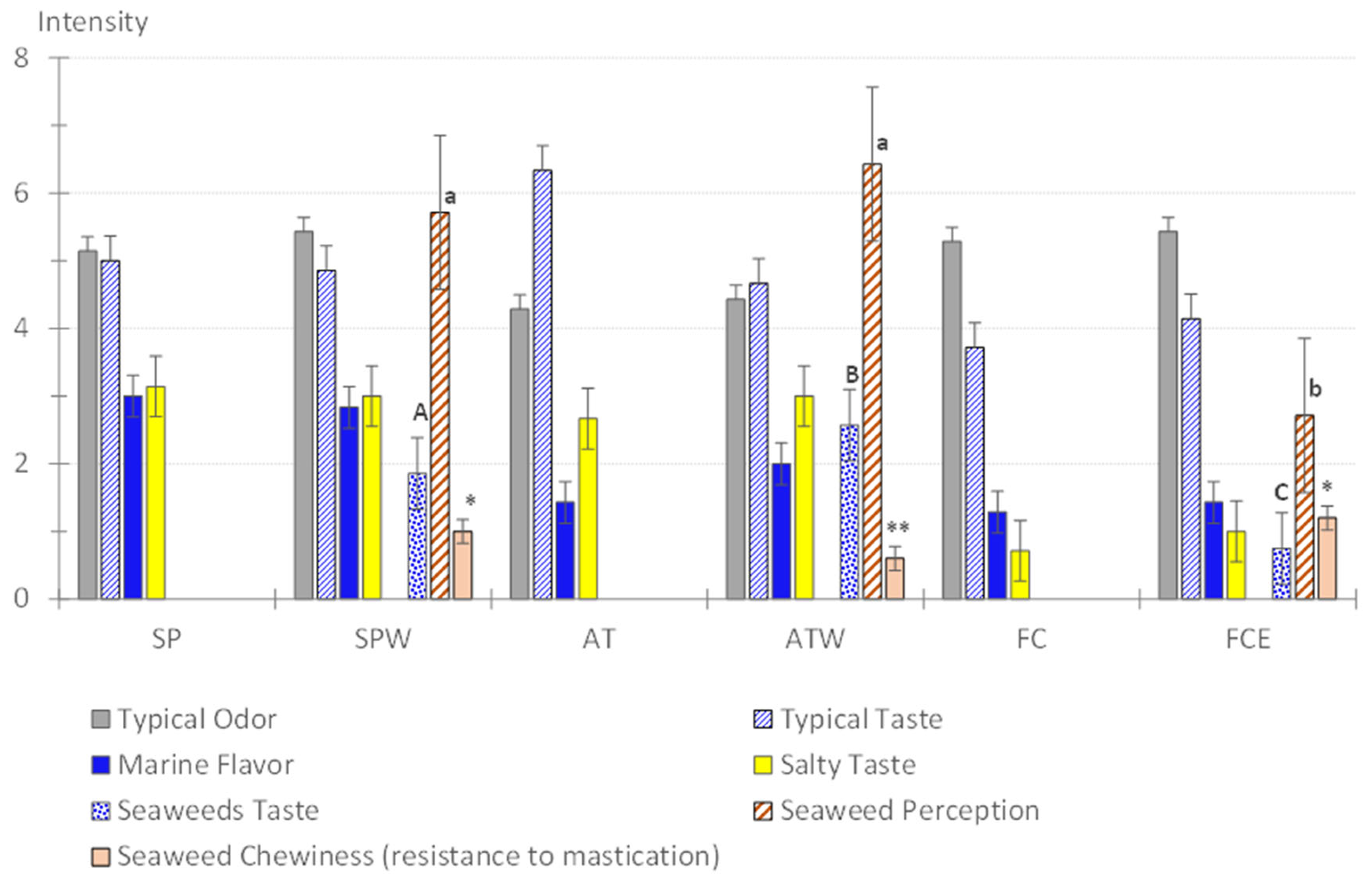
3.4. Quantitative Descriptive Sensory Analysis
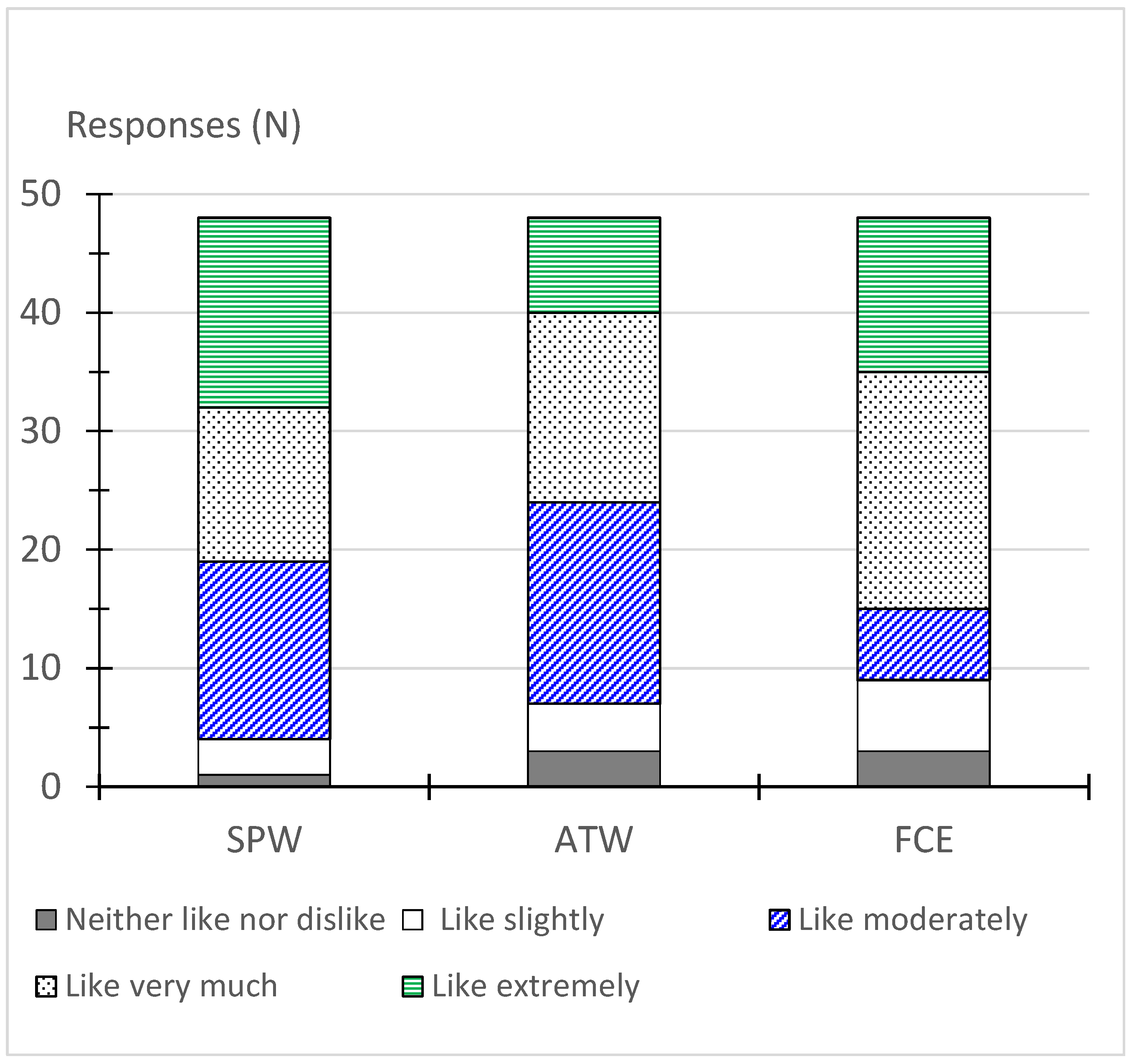
3.5. Acceptance Test
3.6. Strengths and Limitations
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, L.; Correia, F. Algas Marinhas Da Costa Portuguesa—Ecologia, Biodiversidade e Utilizações; Nota de Rodapé Editores: Coimbra, Portugal, 2015. [Google Scholar]
- Mouritsen, O.G.; Rhatigan, P.; Pérez-Lloréns, J.L. World Cuisine of Seaweeds: Science Meets Gastronomy. Int. J. Gastron. Food Sci. 2018, 14, 55–65. [Google Scholar] [CrossRef]
- Nova, P.; Martins, A.P.; Teixeira, C.; Abreu, H.; Silva, J.G.; Silva, A.M.; Freitas, A.C.; Gomes, A.M. Foods with Microalgae and Seaweeds Fostering Consumers Health: A Review on Scientific and Market Innovations. J. Appl. Phycol. 2020, 32, 1789–1802. [Google Scholar] [CrossRef]
- Mæhre, H.K.; Malde, M.K.; Eilertsen, K.; Elvevoll, E.O. Characterization of Protein, Lipid and Mineral Contents in Common Norwegian Seaweeds and Evaluation of Their Potential as Food and Feed. J. Sci. Food Agric. 2014, 94, 3281–3290. [Google Scholar] [CrossRef]
- Stévant, P.; Rebours, C.; Chapman, A. Seaweed Aquaculture in Norway: Recent Industrial Developments and Future Perspectives. Aquac. Int. 2017, 25, 1373–1390. [Google Scholar] [CrossRef]
- Blikra, M.J.; Altintzoglou, T.; Løvdal, T.; Rognså, G.; Skipnes, D.; Skåra, T.; Sivertsvik, M.; Noriega Fernández, E. Seaweed Products for the Future: Using Current Tools to Develop a Sustainable Food Industry. Trends Food Sci. Technol. 2021, 118, 765–776. [Google Scholar] [CrossRef]
- Wang, H.-M.D.; Li, X.-C.; Lee, D.-J.; Chang, J.-S. Potential Biomedical Applications of Marine Algae. Bioresour. Technol. 2017, 244, 1407–1415. [Google Scholar] [CrossRef]
- Blikra, M.J.; Henjum, S.; Aakre, I. Iodine from Brown Algae in Human Nutrition, with an Emphasis on Bioaccessibility, Bioavailability, Chemistry, and Effects of Processing: A Systematic Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1517–1536. [Google Scholar] [CrossRef]
- Din, N.A.S.; Mohd Alayudin, A.S.; Sofian-Seng, N.-S.; Rahman, H.A.; Mohd Razali, N.S.; Lim, S.J.; Wan Mustapha, W.A. Brown Algae as Functional Food Source of Fucoxanthin: A Review. Foods 2022, 11, 2235. [Google Scholar] [CrossRef]
- Rocha, C.P.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweeds as Valuable Sources of Essential Fatty Acids for Human Nutrition. Int. J. Environ. Res. Public Health 2021, 18, 4968. [Google Scholar] [CrossRef]
- Pereira, L. As Algas Na Alimentação. Rev. Ciência Elementar 2021, 9, 6. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Demarco, M.; Oliveira de Moraes, J.; Matos, Â.P.; Derner, R.B.; de Farias Neves, F.; Tribuzi, G. Digestibility, Bioaccessibility and Bioactivity of Compounds from Algae. Trends Food Sci. Technol. 2022, 121, 114–128. [Google Scholar] [CrossRef]
- Nielsen, C.W.; Rustad, T.; Holdt, S.L. Vitamin C from Seaweed: A Review Assessing Seaweed as Contributor to Daily Intake. Foods 2021, 10, 198. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhou, J.; Tavares, J.; Pinto, C.A.; Saraiva, J.A.; Prieto, M.A.; Cao, H.; Xiao, J.; Simal-Gandara, J.; Barba, F.J. Applications of Algae to Obtain Healthier Meat Products: A Critical Review on Nutrients, Acceptability and Quality. Crit. Rev. Food Sci. Nutr. 2023, 63, 8357–8374. [Google Scholar] [CrossRef]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of Interest as Food Source: Biochemical Composition and Digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Machado, L.; Carvalho, G.; Pereira, R.N. Effects of Innovative Processing Methods on Microalgae Cell Wall: Prospects towards Digestibility of Protein-Rich Biomass. Biomass 2022, 2, 80–102. [Google Scholar] [CrossRef]
- Kumar, R.; Hegde, A.S.; Sharma, K.; Parmar, P.; Srivatsan, V. Microalgae as a Sustainable Source of Edible Proteins and Bioactive Peptides—Current Trends and Future Prospects. Food Res. Int. 2022, 157, 111338. [Google Scholar] [CrossRef]
- Gullón, P.; Astray, G.; Gullón, B.; Franco, D.; Campagnol, P.C.B.; Lorenzo, J.M. Inclusion of Seaweeds as Healthy Approach to Formulate New Low-Salt Meat Products. Curr. Opin. Food Sci. 2021, 40, 20–25. [Google Scholar] [CrossRef]
- Rajauria, G.; Cornish, L.; Ometto, F.; Msuya, F.E.; Villa, R. Identification and Selection of Algae for Food, Feed, and Fuel Applications. In Seaweed Sustainability Food and Non-Food Applications; Tiwari, B.K., Troy, B.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 315–345. [Google Scholar]
- Choi, Y.-S.; Kum, J.-S.; Jeon, K.-H.; Park, J.-D.; Choi, H.-W.; Hwang, K.-E.; Jeong, T.-J.; Kim, Y.-B.; Kim, C.-J. Effects of Edible Seaweed on Physicochemical and Sensory Characteristics of Reduced-Salt Frankfurters. Korean J. Food Sci. Anim. Resour. 2015, 35, 748–756. [Google Scholar] [CrossRef]
- Vilar, E.G.; Ouyang, H.; O’Sullivan, M.G.; Kerry, J.P.; Hamill, R.M.; O’Grady, M.N.; Mohammed, H.O.; Kilcawley, K.N. Effect of Salt Reduction and Inclusion of 1% Edible Seaweeds on the Chemical, Sensory and Volatile Component Profile of Reformulated Frankfurters. Meat Sci. 2020, 161, 108001. [Google Scholar] [CrossRef]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and Benefits of Consuming Edible Seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [PubMed]
- Taboada, M.C.; Millán, R.; Miguez, M.I. Nutritional Value of the Marine Algae Wakame (Undaria pinnatifida) and Nori (Porphyra purpurea) as Food Supplements. J. Appl. Phycol. 2013, 25, 1271–1276. [Google Scholar] [CrossRef]
- Klnc, B.; Cirik, S.; Turan, G.; Tekogul, H.; Koru, E. Seaweeds for Food and Industrial Applications. In Food Industry; InTech: Houston, TX, USA, 2013. [Google Scholar]
- Gupta, S.; Abu-Ghannam, N. Bioactive Potential and Possible Health Effects of Edible Brown Seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef]
- Domínguez, H. Functional Ingredients from Algae for Foods and Nutraceuticals, 1st ed.; Woodhead Publishing Limited: Sawston, UK, 2013. [Google Scholar]
- Katt, F.; Meixner, O. A Systematic Review of Drivers Influencing Consumer Willingness to Pay for Organic Food. Trends Food Sci. Technol. 2020, 100, 374–388. [Google Scholar] [CrossRef]
- Figueroa, V.; Farfán, M.; Aguilera, J.M. Seaweeds as Novel Foods and Source of Culinary Flavors. Food Rev. Int. 2023, 39, 1–26. [Google Scholar] [CrossRef]
- Sáa, C.F. As Verduras Do Oceano: Algas Do Atlântico, Alimento e Saúde, 1st ed.; Algamar: Vilamoura, Portugal, 2002; Volume 1. [Google Scholar]
- Oliveira, H.; Muniz, J.A.; Bandarra, N.M.; Castanheira, I.; Coelho, I.R.; Delgado, I.; Gonçalves, S.; Lourenço, H.M.; Motta, C.; Duarte, M.P.; et al. Effects of Industrial Boiling on the Nutritional Profile of Common Octopus (Octopus Vulgaris). Foods 2019, 8, 411. [Google Scholar] [CrossRef]
- ISO International Standard 8589; Sensory Analysis—General Guidance for the Design of Test Rooms. International Organization for Standardization: Genève, Germany, 2007.
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques; Taylor & Francis: Boca Raton, FL, USA, 2007; ISBN 9780849338397. [Google Scholar]
- Foley, M.; Beckley, J.; Ashman, H.; Moskowitz, H.R.H.R.; Hollis, A.; Moskowitz, H.R.H.R. The Mind-Set of Teens towards Food Communications Revealed by Conjoint Measurement and Multi-Food Databases. Appetite 2009, 52, 554–560. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- FAO (Ed.). Food Energy—Methods of Analysis and Conversion Factors, 1st ed.; FAO: Rome, Italy, 2003; Volume 1. [Google Scholar]
- Jorhem, L.; Afthan, G.; Cumont, G.; Dypdahl, H.P.; Gadd, K.; Havre, G.N.; Julshamn, K.; Kåverud, K.; Lind, B.; Loimaranta, J.; et al. Determination of Metals in Foods by Atomic Absorption Spectrometry after Dry Ashing: NMKL1 Collaborative Study. J. AOAC Int. 2000, 83, 1204–1211. [Google Scholar] [CrossRef]
- Rioux, L.E.; Beaulieu, L.; Turgeon, S.L. Seaweeds: A Traditional Ingredients for New Gastronomic Sensation. Food Hydrocoll. 2017, 68, 255–265. [Google Scholar] [CrossRef]
- Mohammed, H.O.; O’grady, M.N.; O’sullivan, M.G.; Hamill, R.M.; Kilcawley, K.N.; Kerry, J.P. An Assessment of Selected Nutritional, Bioactive, Thermal and Technological Properties of Brown and Red Irish Seaweed Species. Foods 2021, 10, 2784. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.; Neuhäuser-Berthold, M.; et al. Dietary Reference Values for Potassium. EFSA J. 2016, 14, e04592. [Google Scholar] [CrossRef]
- Nadeeshani, H.; Hassouna, A.; Lu, J. Proteins Extracted from Seaweed Undaria Pinnatifida and Their Potential Uses as Foods and Nutraceuticals. Crit. Rev. Food Sci. Nutr. 2022, 62, 6187–6203. [Google Scholar] [CrossRef] [PubMed]
- Pires, C.; Sapatinha, M.; Mendes, R.; Bandarra, N.M.; Gonçalves, A. Dehydration, Rehydration and Thermal Treatment: Effect on Bioactive Compounds of Red Seaweeds Porphyra Umbilicalis and Porphyra Linearis. Marine Drugs 2024, 22, 166. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Pelaez, C.; et al. Dietary Reference Values for Sodium. EFSA J. 2019, 17, e05778. [Google Scholar] [CrossRef]
- Milinovic, J.; Fernando, A.L.; Campos, B.; Leite, B.; Mata, P.; Diniz, M.; Sardinha, J.; Noronha, J.P. Nutritional Benefits of Edible Macroalgae from the Central Portuguese Coast: Inclusion of Low-Calorie ‘Sea Vegetables’ in Human Diet. Int. J. Environ. Sci. Nat. Resour. 2021, 28, 556250. [Google Scholar] [CrossRef]
- Cox, S.; Abu-Ghannam, N.; Gupta, S. Effect of Processing Conditions on Phytochemical Constituents of Edible Irish Seaweed Himanthalia Elongata. J. Food Process Preserv. 2012, 36, 348–363. [Google Scholar] [CrossRef]
- Cox, S.; Gupta, S.; Abu-Ghannam, N. Effect of Different Rehydration Temperatures on the Moisture, Content of Phenolic Compounds, Antioxidant Capacity and Textural Properties of Edible Irish Brown Seaweed. LWT 2012, 47, 300–307. [Google Scholar] [CrossRef]
- Bayomy, H.M. Effects of Culinary Treatments on the Physicochemical Properties of Ulva Lactuca Collected from Tabuk Coast of Red Sea in Saudi Arabia. Saudi J. Biol. Sci. 2022, 29, 2355–2362. [Google Scholar] [CrossRef]

| Recipe | Ingredients | % of Each Ingredient | |
|---|---|---|---|
| Traditional Recipe | Modified Recipe | ||
| Octopus Salad | Cooked octopus (boiled, without salt; white onion, bay leaves and garlic) | SP 63.42 | SPW 55.25 |
| Onion (purple) | 10.68 | 9.21 | |
| Green pepper | 8.55 | 7.37 | |
| Freshly ground pepper | 0.11 | 0.09 | |
| Fresh coriander | 0.53 | 0.46 | |
| Olive oil | 10.68 | 9.21 | |
| White wine vinegar | 5.34 | 4.60 | |
| Salt | - | - | |
| Wakame (U. pinnatifida)- Rehydrated | - | 13.81 | |
| Monkfish Rice with Prawns | Monkfish (cubes) | AT 27.76 | ATW 26.54 |
| Prawn tails | 9.25 | 8.85 | |
| Onion | 6.94 | 6.63 | |
| Garlic | 0.46 | 0.44 | |
| Freshly ground pepper | 0.05 | 0.04 | |
| White whine | 4.63 | 4.42 | |
| Olive oil | 2.31 | 2.21 | |
| Carolino rice | 11.57 | 11.06 | |
| Tomato | 2.31 | 2.21 | |
| Seafood broth (olive oil prawn heads, monkfish spines, salt, onion, water) | 34.71 | 33.17 | |
| Salt | 0.18 | - | |
| Wakame (U. pinnatifida)-rehydrated | - | 4.42 | |
| Stewed cuttlefish with white beans and clams | Cuttlefish (cubes) | FC 20.70 | FCE 20.04 |
| Cooked white beans (boiled, without salt) | 27.61 | 26.72 | |
| Clam kernel | 6.90 | 6.68 | |
| Onion | 2.76 | 2.67 | |
| Garlic | 0.17 | 0.17 | |
| Freshly ground pepper | 0.07 | 0.07 | |
| White bean cooking water | 20.70 | 20.04 | |
| White wine | 3.45 | 3.34 | |
| Parsley | 0.17 | 0.17 | |
| Carrot | 5.18 | 5.01 | |
| Olive oil | 1.73 | 1.67 | |
| Tomato | 6.90 | 6.68 | |
| Bay leaves | 0.03 | 0.03 | |
| Cumin powder | 0.03 | 0.03 | |
| Tomato paste | 3.45 | 3.34 | |
| Salt | 0.14 | - | |
| Sea spaghetti (H. elongata) rehydrated—pieces | - | 3.34 | |
| Component | Method | LOQ | Proficiency Test or CRM | Certified Value | Present Work |
|---|---|---|---|---|---|
| Proximates (g/100 g) | |||||
| Moisture | Drying | 0.07 | FAPAS 25234 a | 65.6 ± 0.5 b | 65.7 ± 0.3 c |
| Ash | Incineration | 0.18 | FAPAS 25234 a | 3.82 ± 0.12 b | 3.81 ± 0.19 c |
| Fat | Soxhlet | 0.3 | FAPAS 25234 a | 13.6 ± 0.5 b | 13.5 ± 0.8 c |
| Nitrogen | Combustion | 0.002 | FAPAS 25234 a | 2.59 ± 0.05 b | 2.60 ± 0.02 d |
| Macroelements (mg/kg) | |||||
| Potassium | FAAS | 0.07 | SQID-1 e | 4660 ± 1420 c | 5181 ± 202 d |
| Sodium | FAAS | 0.30 | SQID-1 e | 14,600 ± 1600 c | 13,565 ± 189 d |
| Seaweeds | Moisture g/100 g | Lipid g/100 g | Ash g/100 g | Protein g/100 g | Carbohydrate g/100 g * | Potassium mg/100 g | Sodium mg/100 g |
|---|---|---|---|---|---|---|---|
| U. pinnatifida (wakame) | 11.5 ± 0.1 a | 2.3 ± 0.0 a | 35.07 ± 0.39 a | 13.9 ± 0.4 a | 37.2 ± 0.7 a | 5589.3 ± 1376.0 a | 6383.2 ± 1119.9 a |
| H. elongata (sea spaghetti) | 12.1 ± 0.0 b | 1.6 ± 0.1 b | 30.84 ± 0.06 b | 10.7 ± 0.00 b | 44.7 ± 0.2 b | 7089.6 ± 929.7 a | 5530.6 ± 510.6 a |
| Recipes | Moisture g/100 g | Lipid g/100 g | Ash g/100 g | Protein g/100 g | Carbohydrate g/100 g * | Potassium mg/100 g | Sodium mg/100 g |
|---|---|---|---|---|---|---|---|
| SP | 77.6 ± 0.1 a | 6.0 ± 0.2 a | 2.09 ± 0.02 a | 13.7 ± 0.04 a | 0.7 ± 0.2 a | 66.9 ± 5.3 a | 240.7 ± 24.4 a |
| SPW | 77.7 ± 0.3 a | 6.0 ± 0.3 a | 2.58 ± 0.03 a | 12.2 ± 0.2 a | 1.5 ± 0.4 b | 157.0 ± 26.5 b | 252.4 ± 32.7 a |
| AT | 73.4 ± 0.5 a | 3.5 ± 0.1 a | 0.97 ± 0.01 a | 9.0 ± 0.2 a | 13.1 ± 0.5 a | 176.1 ± 4.9 a | 120.6 ± 6.8 a |
| ATW | 74.7 ± 0.3 a | 4.2 ± 0.2 a | 1.11 ± 0.01 a | 8.7 ± 0.1 a | 11.3 ± 0.3 b | 219.7 ± 21.4 b | 112.5 ± 11.4 a |
| FC | 73.5 ± 0.1 a | 3.5 ± 0.2 a | 1.38 ± 0.02 a | 11.1 ± 0.7 a | 10.4 ± 0.9 a | 227.3 ± 11.2 a | 181.6 ± 20.2 a |
| FCE | 74.4 ± 0.1 a | 4.1 ± 0.8 a | 1.58 ± 0.01 a | 9.0 ± 0.6 a | 10.9 ± 0.4 a | 255.8 ± 34.8 a | 173.4 ± 18.7 a |
| Population | AI | NC (%) of Recipes 2 | |||||
|---|---|---|---|---|---|---|---|
| Groups | (mg/Day) 1 | SP | SPW | AT | ATW | FC | FCE |
| 7–10 years | 1800 | 7.4 ± 0.6 a | 17.4 ± 2.9 b | 29.3 ± 0.8 a | 36.6 ± 3.6 b | 37.9 ± 1.9 a | 42.6 ± 5.8 a |
| 11–14 years | 2700 | 5.0 ± 0.4 a | 11.6 ± 2.0 b | 19.6 ± 0.5 a | 24.4 ± 2.4 b | 25.3 ± 1.2 a | 28.4 ± 3.9 a |
| 15–17 years | 3500 | 3.8 ± 0.3 a | 9.0 ± 1.5 b | 15.1 ± 0.4 a | 18.8 ± 1.8 b | 19.5 ± 1.0 a | 21.9 ± 3.0 a |
| ≥18 years 3 | 3500 | 3.8 ± 0.3 a | 9.0 ± 1.5 b | 15.1 ± 0.4 a | 18.8 ± 1.8 b | 19.5 ± 1.0 a | 21.9 ± 3.0 a |
| Lactating women | 4000 | 3.3 ± 0.3 a | 7.8 ± 1.3 b | 13.2 ± 0.4 a | 16.5 ± 1.6 b | 17.0 ± 0.8 a | 19.2 ± 2.6 a |
| Population | AI | NC (%) of Recipes 2 | |||||
|---|---|---|---|---|---|---|---|
| Groups | (g/Day) 1 | SP | SPW | AT | ATW | FC | FCE |
| 7–10 years | 1.7 | 25.7 ± 7.1 a | 29.7 ± 3.8 a | 21.3 ± 1.2 a | 18.3 ± 4.4 a | 32.1 ± 3.6 a | 30.6 ± 3.3 a |
| 11–17 years | 2.0 | 21.8 ± 6.0 a | 25.2 ± 3.3 a | 18.1 ± 1.0 a | 15.5 ± 3.7 a | 27.2 ± 3.0 a | 26.0 ± 2.8 a |
| ≥18 years 3 | 2.0 | 21.8 ± 6.0 a | 25.2 ± 3.3 a | 18.1 ± 1.0 a | 15.5 ± 3.7 a | 27.2 ± 3.0 a | 26.0 ± 2.8 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, M.L.; Pires, A.; Gonçalves, A.; Pires, C.; Lourenço, H.M.; Saraiva, A.; Zandonadi, R.P.; Ramos, F.; Raposo, A. Do Seaweeds Contribute to Nutritional Composition and Acceptance in Traditional Portuguese Recipes? Foods 2025, 14, 1947. https://doi.org/10.3390/foods14111947
Mendes ML, Pires A, Gonçalves A, Pires C, Lourenço HM, Saraiva A, Zandonadi RP, Ramos F, Raposo A. Do Seaweeds Contribute to Nutritional Composition and Acceptance in Traditional Portuguese Recipes? Foods. 2025; 14(11):1947. https://doi.org/10.3390/foods14111947
Chicago/Turabian StyleMendes, Maria Lassalete, António Pires, Amparo Gonçalves, Carla Pires, Helena Maria Lourenço, Ariana Saraiva, Renata Puppin Zandonadi, Fernando Ramos, and António Raposo. 2025. "Do Seaweeds Contribute to Nutritional Composition and Acceptance in Traditional Portuguese Recipes?" Foods 14, no. 11: 1947. https://doi.org/10.3390/foods14111947
APA StyleMendes, M. L., Pires, A., Gonçalves, A., Pires, C., Lourenço, H. M., Saraiva, A., Zandonadi, R. P., Ramos, F., & Raposo, A. (2025). Do Seaweeds Contribute to Nutritional Composition and Acceptance in Traditional Portuguese Recipes? Foods, 14(11), 1947. https://doi.org/10.3390/foods14111947












