Insights into Isolation and Purification Strategies of Egg Allergens
Abstract
1. Introduction
2. Methodology
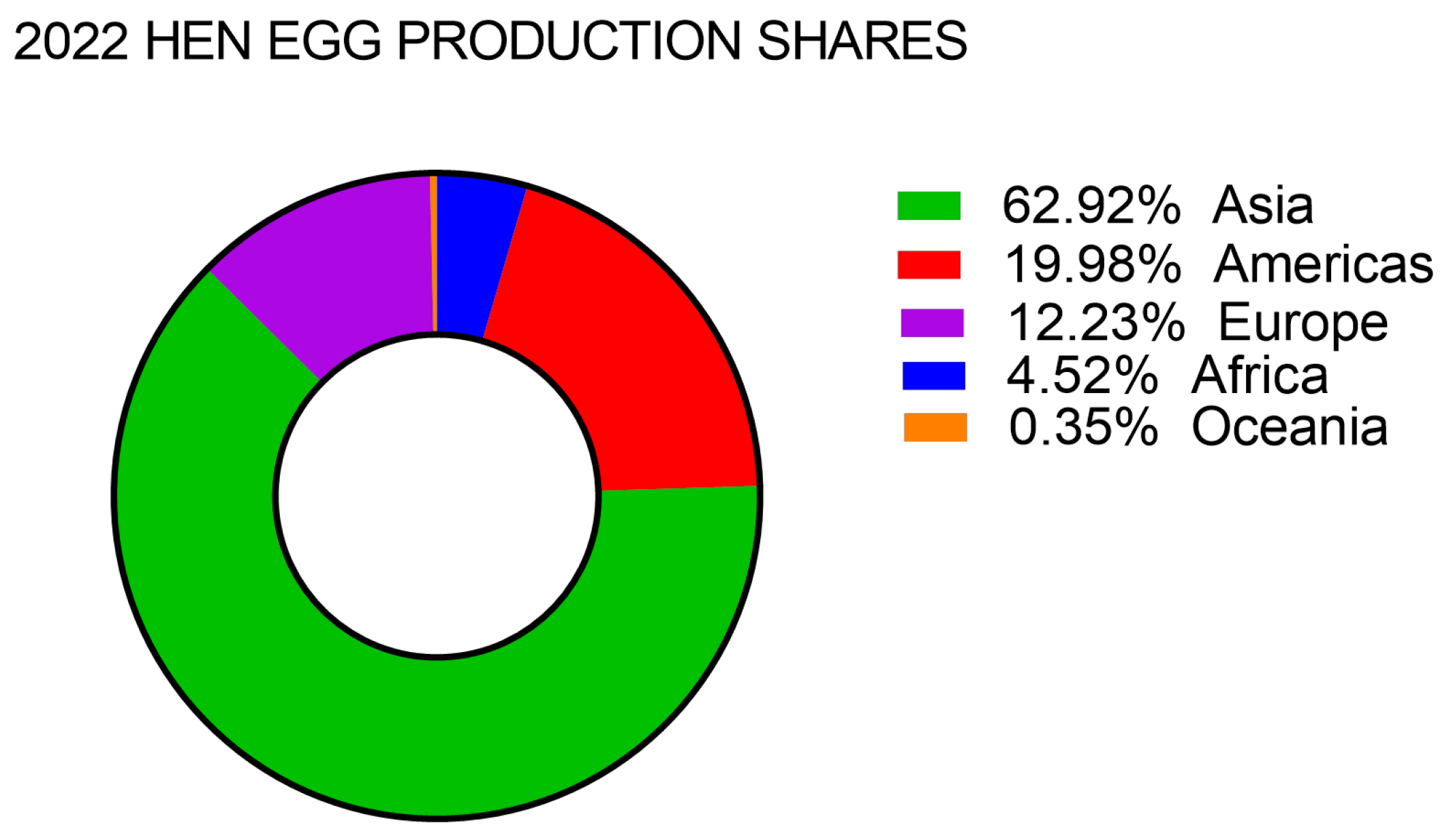
3. Egg Production and Consumption Across the Globe
4. Egg Allergy Prevalence
5. Hen Egg Allergens
6. Isolation of Hen Egg Allergens
6.1. Precipitation of Egg White Allergens
6.2. Co-Purification of Egg White Allergens
6.3. Isolation of Egg Yolk Allergens
7. Isolation of Egg Proteins from Other Avian Species
8. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Seuss-Baum, I.; Nau, F.; Guérin-Dubiard, C. The Nutritional Quality of Eggs. In Improving the Safety and Quality of Eggs and Egg Products; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; Volume 2, pp. 201–236. ISBN 9780857090720. [Google Scholar] [CrossRef]
- Walker, S.; Baum, J.I. Eggs as an Affordable Source of Nutrients for Adults and Children Living in Food-Insecure Environments. Nutr. Rev. 2022, 80, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Palosuo, K.; Karisola, P.; Savinko, T.; Fyhrquist, N.; Alenius, H.; Mäkelä, M.J. A Randomized, Open-Label Trial of Hen’s Egg Oral Immunotherapy: Efficacy and Humoral Immune Responses in 50 Children. J. Allergy Clin. Immunol. Pract. 2021, 9, 1892–1901.e1. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.L.K.; Mullins, R.J. Food Allergy: Is Prevalence Increasing? Intern. Med. J. 2017, 47, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Grimshaw, K.E.C.; Roberts, G.; Selby, A.; Reich, A.; Butiene, I.; Clausen, M.; Dubakiene, R.; Fiandor, A.; Fiocchi, A.; Grabenhenrich, L.B.; et al. Risk Factors for Hen’s Egg Allergy in Europe: EuroPrevall Birth Cohort. J. Allergy Clin. Immunol. Pract. 2020, 8, 1341–1348.e5. [Google Scholar] [CrossRef]
- Ehlers, A.M.; Otten, H.G.; Wierzba, E.; Flügge, U.; Le, T.M.; Knulst, A.C.; Suer, W. Detection of Specific IgE against Linear Epitopes from Gal d 1 Has Additional Value in Diagnosing Hen’s Egg Allergy in Adults. Clin. Exp. Allergy 2020, 50, 1415–1423. [Google Scholar] [CrossRef]
- Tsuang, A.; Chan, E.S.; Wang, J. Food-Induced Anaphylaxis in Infants: Can New Evidence Assist with Implementation of Food Allergy Prevention and Treatment? J. Allergy Clin. Immunol. Pract. 2021, 9, 57–69. [Google Scholar] [CrossRef]
- Akashi, M.; Hayashi, D.; Kajita, N.; Kinoshita, M.; Ishii, T.; Tsumura, Y.; Horimukai, K.; Yoshida, K.; Takahashi, T.; Morita, H. Recent Dramatic Increase in Patients with Food Protein–Induced Enterocolitis Syndrome (FPIES) Provoked by Hen’s Egg in Japan. J. Allergy Clin. Immunol. Pract. 2022, 10, 1110–1112.e2. [Google Scholar] [CrossRef]
- Caffarelli, C.; Giannetti, A.; Rossi, A.; Ricci, G. Egg Allergy in Children and Weaning Diet. Nutrients 2022, 14, 1540. [Google Scholar] [CrossRef]
- Meyer, R. Nutritional Disorders Resulting from Food Allergy in Children. Pediatr. Allergy Immunol. 2018, 29, 689–704. [Google Scholar] [CrossRef]
- Miyagi, Y.; Yamamoto-Hanada, K.; Ogita, H.; Kiguchi, T.; Inuzuka, Y.; Toyokuni, K.; Nishimura, K.; Irahara, M.; Ishikawa, F.; Sato, M.; et al. Avoidance of Hen’s Egg Based on IgE Levels Should Be Avoided for Children With Hen’s Egg Allergy. Front. Pediatr. 2021, 8, 583224. [Google Scholar] [CrossRef]
- Corica, D.; Aversa, T.; Caminiti, L.; Lombardo, F.; Wasniewska, M.; Pajno, G.B. Nutrition and Avoidance Diets in Children With Food Allergy. Front. Pediatr. 2020, 8, 518. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhang, L.; Zhang, K.; Zhou, P. Difference of Egg Ovalbumin Sensitization between Egg and Duck Eggs in BALB/c Mice. Eur. Food Res. Technol. 2022, 248, 1035–1048. [Google Scholar] [CrossRef]
- Elliott, J.; Cross, S.; Zolkipli, Z.; Grainger-Allen, E.; Powell, C.; Abayasiriwardana, J.; Erlewyn-Lajeunesse, M. Duck Egg Tolerance in Children with Hen’s Egg Allergy. Clin. Exp. Allergy 2021, 51, 1519–1522. [Google Scholar] [CrossRef]
- Hao, M.; Yang, S.; Han, S.; Che, H. The Amino Acids Differences in Epitopes May Promote the Different Allergenicity of Ovomucoid Derived from Hen Eggs and Quail Eggs. Food Sci. Hum. Well. 2023, 12, 861–870. [Google Scholar] [CrossRef]
- Reininger, R.; Exner, H.; Kuderna, C.; Rumpold, H.; Balic, N.; Valenta, R.; Spitzauer, S. Possible Modes of Allergen-Specific Sensitization and Boosting in an Atopic Child. Int. Arch. Allergy Immunol. 2003, 130, 275–279. [Google Scholar] [CrossRef]
- Martorell, A.; Alonso, E.; Boné, J.; Echeverría, L.; López, M.C.; Martín, F.; Nevot, S.; Plaza, A.M. Position Document: IgE-Mediated Allergy to Egg Protein. Allergol. Immunopathol. 2013, 41, 320–336. [Google Scholar] [CrossRef]
- Miguel, M.; Manso, M.A.; López-Fandiño, R.; Ramos, M. Comparative Study of Egg White Proteins from Different Species by Chromatographic and Electrophoretic Methods. Eur. Food Res. Technol. 2005, 221, 542–546. [Google Scholar] [CrossRef]
- Tan, J.W.; Joshi, P. Egg Allergy: An Update. J. Paediatr. Child Health 2014, 50, 11–15. [Google Scholar] [CrossRef]
- Gavage, M.; Van Vlierberghe, K.; Van Poucke, C.; De Loose, M.; Gevaert, K.; Dieu, M.; Renard, P.; Arnould, T.; Gillard, N. Selection of Egg Peptide Biomarkers in Processed Food Products by High Resolution Mass Spectrometry. J. Chromatogr. A 2019, 1584, 115–125. [Google Scholar] [CrossRef]
- Moghtaderi, M.; Nabavizadeh, S.H.; Hosseini Teshnizi, S. The Frequency of Cross-Reactivity with Various Avian Eggs among Children with Hen’s Egg Allergy Using Skin Prick Test Results: Fewer Sensitizations with Pigeon and Goose Egg. Allergol. Immunopathol. 2020, 48, 265–269. [Google Scholar] [CrossRef]
- Maehashi, K.; Matano, M.; Irisawa, T.; Uchino, M.; Itagaki, Y.; Takano, K.; Kashiwagi, Y.; Watanabe, T. Primary Structure of Potential Allergenic Proteins in Emu (Dromaius Novaehollandiae) Egg White. J. Agric. Food Chem. 2010, 58, 12530–12536. [Google Scholar] [CrossRef] [PubMed]
- Añíbarro, B.; Seoane, F.J.; Vila, C.; Lombardero, M. Allergy to Eggs from Duck and Goose without Sensitization to Hen Egg Proteins. J. Allergy Clin. Immunol. 2000, 105, 834–836. [Google Scholar] [CrossRef]
- Caro Contreras, F.J.; Giner Muñoz, M.T.; Martin Mateos, M.A.; Plaza Martin, A.M.; Sierra Martinez, J.I.; Lombardero, M. Allergy to Quail’s Egg without Allergy to Chicken’s Egg. Case Report. Allergol. Immunopathol. 2008, 36, 234–237. [Google Scholar] [CrossRef]
- Alessandri, C.; Calvani, M.; Rosengart, L.; Madella, C. Anaphylaxis to Quail Egg. Allergy 2005, 60, 128–129. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, X.; Zheng, W.; Wang, P.; Duan, Z.; Xu, G. Dynamic Changes in Egg Quality, Heritability and Correlation of These Traits and Yolk Nutrient throughout the Entire Laying Cycle. Foods 2023, 12, 4472. [Google Scholar] [CrossRef]
- Sarantidi, E.; Ainatzoglou, A.; Papadimitriou, C.; Stamoula, E.; Maghiorou, K.; Miflidi, A.; Trichopoulou, A.; Mountzouris, K.C.; Anagnostopoulos, A.K. Egg White and Yolk Protein Atlas: New Protein Insights of a Global Landmark Food. Foods 2023, 12, 3470. [Google Scholar] [CrossRef] [PubMed]
- Samady, W.; Warren, C.; Wang, J.; Das, R.; Gupta, R.S. Egg Allergy in US Children. J. Allergy Clin. Immunol. Pract. 2020, 8, 3066–3073.e6. [Google Scholar] [CrossRef]
- Caubet, J.C.; Wang, J. Current Understanding of Egg Allergy. Pediatr. Clin. North Am. 2011, 58, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.D.; Peters, R.L.; Koplin, J.J.; Dharmage, S.C.; Gurrin, L.C.; Ponsonby, A.L.; Martino, D.J.; Neeland, M.; Tang, M.L.K.; Allen, K.J. Egg Allergen Specific IgE Diversity Predicts Resolution of Egg Allergy in the Population Cohort HealthNuts. Allergy 2019, 74, 318–326. [Google Scholar] [CrossRef]
- Osborne, N.J.; Koplin, J.J.; Martin, P.E.; Gurrin, L.C.; Lowe, A.J.; Matheson, M.C.; Ponsonby, A.L.; Wake, M.; Tang, M.L.K.; Dharmage, S.C.; et al. Prevalence of Challenge-Proven IgE-Mediated Food Allergy Using Population-Based Sampling and Predetermined Challenge Criteria in Infants. J. Allergy Clin. Immunol. 2011, 127, 668–676.e2. [Google Scholar] [CrossRef]
- Xepapadaki, P.; Fiocchi, A.; Grabenhenrich, L.; Roberts, G.; Grimshaw, K.E.C.; Fiandor, A.; Larco, J.I.; Sigurdardottir, S.; Clausen, M.; Papadopoulos, N.G.; et al. Incidence and Natural History of Hen’s Egg Allergy in the First 2 Years of Life—The EuroPrevall Birth Cohort Study. Allergy 2016, 71, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Shao, M.; Liu, C.; Sun, Z.; Sha, L.; Chen, Y.; Li, J.; Wu, Y. Epidemiology of Food Allergy in Children from 31 Cities in China. Int. J. Pediatr. 2017, 44, 637–641. [Google Scholar]
- Leung, A.S.Y.; Jie, S.; Gu, Y.; Wong, G.W.K. Food Allergy in Children in China. Clin. Exp. Allergy 2024. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, H.; Liao, Y.; Zhao, H.; Chen, J. 493 The Prevalence of Food Allergy in Children under 2 Years in Three Cities in China. World Allergy Organ. J. 2012, 5, 173–174. [Google Scholar] [CrossRef]
- Leung, A.S.Y.; Tham, E.H.; Pacharn, P.; Xing, Y.; Trinh, H.K.T.; Lee, S.; Ahn, K.; Chatchatee, P.; Sato, S.; Ebisawa, M.; et al. Disparities in Pediatric Anaphylaxis Triggers and Management across Asia. Allergy 2024, 79, 1317–1328. [Google Scholar] [CrossRef]
- Allen, C.W.; Campbell, D.E.; Kemp, A.S. Egg Allergy: Are All Childhood Food Allergies the Same? J. Paediatr. Child Health 2007, 43, 214–218. [Google Scholar] [CrossRef]
- Kim, J.H. Clinical and Laboratory Predictors of Egg Allergy Resolution in Children. Allergy Asthma Immunol. Res. 2019, 11, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, A. Optimizing Patient Care in Egg Allergy Diagnosis and Treatment. J. Asthma Allergy 2021, 14, 621–628. [Google Scholar] [CrossRef]
- Leech, S.C.; Ewan, P.W.; Skypala, I.J.; Brathwaite, N.; Erlewyn- Lajeunesse, M.; Heath, S.; Ball, H.; James, P.; Murphy, K.; Clark, A.T. BSACI 2021 Guideline for the Management of Egg Allergy. Clin. Exp. Allergy 2021, 51, 1262–1278. [Google Scholar] [CrossRef]
- Nolting, A.; Hasler, S.; Probst-Mueller, E.; Schmid-Grendelmeier, P.; Lanz, J.; Guillet, C. Hen’s Egg White Allergy in Adults Leading to Strong Impairment of Quality of Life. Sci. Rep. 2024, 14, 29401. [Google Scholar] [CrossRef]
- Turner, P.J.; Conrado, A.B.; Kallis, C.; O’Rourke, E.; Haider, S.; Ullah, A.; Custovic, D.; Custovic, A.; Quint, J.K. Time Trends in the Epidemiology of Food Allergy in England: An Observational Analysis of Clinical Practice Research Datalink Data. Lancet Public Health 2024, 9, e664–e673. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.H.; Zhao, L.Y.; Wei, X.Q.; Fang, H.Y.; Li, J.X.; Wu, X.X.; Xu, X.L.; Cai, S.Y.; Gong, W.Y.; Yu, D.M. Prevalence and Influencing Factors on Food Allergy among Children Aged 0–5 Years in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2024, 45, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.W.K.; Li, J.; Ma, Y.; Leung, T.F.; Chen, Y.Z.; Zhong, N.S. Comparative Study Of Food Allergy In Urban And Rural Schoolchildren: The Europrevall-INCO Survey In China. J. Allergy Clin. Immunol. 2011, 127, AB34. [Google Scholar] [CrossRef]
- Leung, A.S.; Xing, Y.; Fernández-Rivas, M.; Wong, G.W. The Relationship Between Dietary Patterns and the Epidemiology of Food Allergy. Allergy 2025, 80, 690–702. [Google Scholar] [CrossRef]
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The Golden Egg: Nutritional Value, Bioactivities, and Emerging Benefits for Human Health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Borres, M.P.; Maruyama, N.; Sato, S.; Ebisawa, M. Recent Advances in Component Resolved Diagnosis in Food Allergy. Allergol. Int. 2016, 65, 378–387. [Google Scholar] [CrossRef]
- The World Health Organization and International Union of Immunological Societies (WHO/IUIS) Allergen Nomenclature Sub-Committee Allergen Database. Available online: https://www.allergen.org/ (accessed on 13 March 2025).
- Gazme, B.; Rezaei, K.; Udenigwe, C.C. Epitope Mapping and the Effects of Various Factors on the Immunoreactivity of Main Allergens in Egg White. Food Funct. 2022, 13, 38–51. [Google Scholar] [CrossRef]
- De Silva, C.; Dhanapala, P.; King, S.; Doran, T.; Tang, M.; Suphioglu, C. Immunological Comparison of Native and Recombinant Hen’s Egg Yolk Allergen, Chicken Serum Albumin (Gal d 5), Produced in Kluveromy Ceslactis. Nutrients 2018, 10, 757. [Google Scholar] [CrossRef]
- D’Urbano, L.E.; Pellegrino, K.; Artesani, M.C.; Donnanno, S.; Luciano, R.; Riccardi, C.; Tozzi, A.E.; Ravà, L.; De Benedetti, F.; Cavagni, G. Performance of a Component-Based Allergen-Microarray in the Diagnosis of Cow’s Milk and Hen’s Egg Allergy. Clin. Exp. Allergy 2010, 40, 1561–1570. [Google Scholar] [CrossRef]
- Amo, A.; Rodríguez-Pérez, R.; Blanco, J.; Villota, J.; Juste, S.; Moneo, I.; Caballero, M.L. Gal d 6 Is the Second Allergen Characterized from Egg Yolk. J. Agric. Food Chem. 2010, 58, 7453–7457. [Google Scholar] [CrossRef]
- Kiyota, K.; Yoshimitsu, M.; Uchida, K.; Kajimura, K. Development of a Liquid Chromatography-Tandem Mass Spectrometry Method for Simultaneous Quantification of Hen’s Egg White Allergens Gal d 1–4 in Fresh and Processed Eggs. Food Chem. 2021, 345, 128022. [Google Scholar] [CrossRef]
- Monji, H.; Zand, H.; Ghorbani, A.; Pourvali, K. The Effects of Ovalbumin on Proliferation, Migration, and Stemness Properties of Chemoresistant SW480 Colon Cancer Cells. Nutr. Cancer 2022, 74, 3714–3722. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Z.; Xue, C. The Past and Future of Ovotransferrin: Physicochemical Properties, Assembly and Applications. Trends Food Sci. Technol. 2021, 116, 47–62. [Google Scholar] [CrossRef]
- Hemmer, W.; Klug, C.; Swoboda, I. Update on the Bird-Egg Syndrome and Genuine Poultry Meat Allergy. Allergo. J. Int. 2016, 25, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Quirce, S.; Marañón, F.; Umpiérrez, A.; Heras, M.D.L.; Fernández-Caldas, E.; Sastre, J. Chicken Serum Albumin (Gal d 5*) Is a Partially Heat-Labile Inhalant and Food Allergen Implicated in the Bird-Egg Syndrome. Allergy 2001, 56, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Tankrathok, A.; Daduang, S.; Patramanon, R.; Araki, T.; Thammasirirak, S. Purification Process for the Preparation and Characterizations of Hen Egg White Ovalbumin, Lysozyme, Ovotransferrin, and Ovomucoid. Prep. Biochem. Biotechnol. 2009, 39, 380–399. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. Separation of Ovotransferrin and Ovomucoid from Chicken Egg White. Poult. Sci. 2014, 93, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. Sequential Separation of Lysozyme, Ovomucin, Ovotransferrin, and Ovalbumin from Egg White. Poult. Sci. 2014, 93, 1001–1009. [Google Scholar] [CrossRef]
- Roy, I.; Rao, M.V.S.; Gupta, M.N. Purification of Lysozyme From Hen Egg White 55 An Integrated Process for Purification of Lysozyme, Ovalbumin, and Ovomucoid From Hen Egg White. Appl. Biochem. Biotechnol. 2003, 111, 55–63. [Google Scholar] [CrossRef]
- Jacobsen, B.; Hoffmann-Sommergruber, K.; Have, T.T.; Foss, N.; Briza, P.; Oberhuber, C.; Radauer, C.; Alessandri, S.; Knulst, A.C.; Fernandez-Rivas, M.; et al. The Panel of Egg Allergens, Gal d 1-Gal d 5: Their Improved Purification and Characterization. Mol. Nutr. Food Res. 2008, 52, S176–S185. [Google Scholar] [CrossRef]
- Geng, F.; Xie, Y.; Wang, J.; Li, S.; Jin, Y.; Ma, M. Large-Scale Purification of Ovalbumin Using Polyethylene Glycol Precipitation and Isoelectric Precipitation. Poult. Sci. 2019, 98, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Guérin-Dubiard, C.; Pasco, M.; Hietanen, A.; Quiros Del Bosque, A.; Nau, F.; Croguennec, T. Hen Egg White Fractionation by Ion-Exchange Chromatography. J. Chromatogr. A 2005, 1090, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Omana, D.A.; Wu, J. A New Method of Separating Ovomucin from Egg White. J. Agric. Food Chem. 2009, 57, 3596–3603. [Google Scholar] [CrossRef] [PubMed]
- Omana, D.A.; Wang, J.; Wu, J. Co-Extraction of Egg White Proteins Using Ion-Exchange Chromatography from Ovomucin-Removed Egg Whites. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 1771–1776. [Google Scholar] [CrossRef]
- Brand, J.; Dachmann, E.; Pichler, M.; Lotz, S.; Kulozik, U. A Novel Approach for Lysozyme and Ovotransferrin Fractionation from Egg White by Radial Flow Membrane Adsorption Chromatography: Impact of Product and Process Variables. Sep. Purif. Technol. 2016, 161, 44–52. [Google Scholar] [CrossRef]
- Ji, S.; Ahn, D.U.; Zhao, Y.; Li, K.; Li, S.; Huang, X. An Easy and Rapid Separation Method for Five Major Proteins from Egg White: Successive Extraction and MALDI-TOF-MS Identification. Food Chem. 2020, 315, 126207. [Google Scholar] [CrossRef]
- Geng, F.; Huang, Q.; Wu, X.; Ren, G.; Shan, Y.; Jin, G.; Ma, M. Co-Purification of Chicken Egg White Proteins Using Polyethylene Glycol Precipitation and Anion-Exchange Chromatography. Sep. Purif. Technol. 2012, 96, 75–80. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ham, J.S.; Ahn, D.U. Separation of Ovotransferrin from Chicken Egg White without Using Organic Solvents. Poult. Sci. 2013, 92, 1091–1097. [Google Scholar] [CrossRef]
- Ko, K.Y.; Ahn, D.U. An Economic and Simple Purification Procedure for the Large-Scale Production of Ovotransferrin from Egg White. Poult. Sci. 2008, 87, 1441–1450. [Google Scholar] [CrossRef]
- Jiang, X.; Mu, H.; Hsieh, Y.H.P.; Rao, Q. Isolation and Characterization of Chicken Serum Albumin (Hen Egg Alpha-Livetin, Gal d 5). Foods 2022, 11, 1637. [Google Scholar] [CrossRef]
- Burley, R.W.; Vadehra, D.V. Chromatographic Separation of the Soluble Proteins of Hen’s Egg Yolk: An Analytical and Preparative Study. Anal. Biochem. 1979, 94, 53–59. [Google Scholar] [CrossRef] [PubMed]
- De Silva, C.; Dhanapala, P.; Doran, T.; Tang, M.L.K.; Suphioglu, C. Molecular and Immunological Analysis of Hen’s Egg Yolk Allergens with a Focus on YGP42 (Gal d 6). Mol. Immunol. 2016, 71, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Dhanapala, P.; Doran, T.; Tang, M.L.K.; Suphioglu, C. Production and Immunological Analysis of IgE Reactive Recombinant Egg White Allergens Expressed in Escherichia coli. Mol. Immunol. 2015, 65, 104–112. [Google Scholar] [CrossRef]
- Rupa, P.; Mine, Y. Immunological Comparison of Native and Recombinant Egg Allergen, Ovalbumin, Expressed in Escherichia coli. Biotechnol. Lett. 2003, 25, 1917–1924. [Google Scholar] [CrossRef]
- Zhernov, Y.; Curin, M.; Khaitov, M.; Karaulov, A.; Valenta, R. Recombinant Allergens for Immunotherapy: State of the Art. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Samaraweera, H.; Zhang, W.G.; Lee, E.J.; Ahn, D.U. Egg Yolk Phosvitin and Functional Phosphopeptides-Review. J. Food Sci. 2011, 76, R143–R150. [Google Scholar] [CrossRef]
- Walsh, B.; Barnett, D.; Burley, R.; Elliott, C.; Hill, D.; Howden, M. New Allergens from Hen’s Egg White and Egg Yolk: In Vitro Study of Ovomucin, Apovitellenin I and VI, and Phosvitin. Int. Arch. Allergy Immunol. 1988, 87, 81–86. [Google Scholar] [CrossRef]
- Huang, L.; Shao, Y.; Li, X.; Li, H.; Liu, Y.; Zhu, G. Designing Multi-Epitope Diagnosis of Gal d 5 and Gal d 6 Based on Immunoinformatics Approaches Against Egg Yolk Allergy. Int. J. Pept. Res. Ther. 2021, 27, 1577–1588. [Google Scholar] [CrossRef]
- Walsh, B.J.; Hill, D.J.; Macoun, P.; Cairns, D.; Howden, M.E.H. Detection of Four Distinct Groups of Hen Egg Allergens Binding IgE in the Sera of Children with Egg Allergy. Allergol. Immunopathol. 2005, 33, 183–191. [Google Scholar] [CrossRef]
- Mecham, D.K.; Phosvitin, O.H.S. the Principal Phosphoprotein of Egg Yolk. J. Am. Chem. Soc. 1949, 71, 3670–3679. [Google Scholar] [CrossRef]
- Ko, K.Y.; Nam, K.C.; Jo, C.; Lee, E.J.; Ahn, D.U. A Simple and Efficient Method for Preparing Partially Purified Phosvitin from Egg Yolk Using Ethanol and Salts. Poult. Sci. 2011, 90, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.H.; Benjakul, S. Trypsin Inhibitor from Duck Albumen: Purification and Characterization. J. Food Biochem. 2019, 43, e12841. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Khoo, K.H.; Chen, H.C.; Johnson, J.R.; Lee, Y.C. Isolation and Characterization of Major Glycoproteins of Pigeon Egg White: Ubiquitous Presence of Unique N-Glycans Containing Galα1-4Gal. J. Biol. Chem. 2001, 276, 23221–23229. [Google Scholar] [CrossRef]
- Liu, Q.; Lin, S.; Sun, N. How Does Food Matrix Components Affect Food Allergies, Food Allergens and the Detection of Food Allergens? A Systematic Review. Trends Food Sci. Technol. 2022, 127, 280–290. [Google Scholar] [CrossRef]
- Tuppo, L.; Giangrieco, I.; Tamburrini, M.; Alessandri, C.; Mari, A.; Ciardiello, M.A. Detection of Allergenic Proteins in Foodstuffs: Advantages of the Innovative Multiplex Allergen Microarray-Based Immunoassay Compared to Conventional Methods. Foods 2022, 11, 878. [Google Scholar] [CrossRef]
- Hildebrandt, S.; Steinhart, H.; Paschke, A. Comparison of Different Extraction Solutions for the Analysis of Allergens in Hen’s Egg. Food Chem. 2008, 108, 1088–1093. [Google Scholar] [CrossRef]
- Ma, X.; Liang, R.; Yang, X.; Gou, J.; Li, Y.; Lozano-Ojalvo, D. Simultaneous Separation of the Four Major Allergens of Hen Egg White. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1152, 122231. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, X.; Tang, Q.; Ma, M.; Jin, Y.; Sheng, L. Functional Properties and Extraction Techniques of Chicken Egg White Proteins. Foods 2022, 11, 2434. [Google Scholar] [CrossRef]
- Wingfield, P. Protein Precipitation Using Ammonium Sulfate. Curr. Protoc. Protein Sci. 1998, 13, A-3F. [Google Scholar] [CrossRef]
- Mattos, C. Proteins in Organic Solvents. Curr. Opin. Struct. Biol. 2001, 11, 761–764. [Google Scholar] [CrossRef]
- Lei, B.; Wu, J. Purification of Egg Yolk Phosvitin by Anion Exchange Chromatography. J. Chromatogr. A 2012, 1223, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wang, L.; Wang, X.; Wu, S.; Li, D.; Liu, C.; Feng, Z. Ultrasonic Thermal-Assisted Extraction of Phosvitin from Egg Yolk and Evaluation of Its Properties. Polymers 2019, 11, 1353. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Du, T.; Guo, J.; Lv, W.; Adhikari, B.; Xu, J. Extraction and Characterization of Lysozyme from Salted Duck Egg White. Foods 2022, 11, 3567. [Google Scholar] [CrossRef] [PubMed]
- Jiaqi, W.; Yanjun, C. Research Progress on the Allergic Mechanism, Molecular Properties, and Immune Cross-Reactivity of the Egg Allergen Gal d 5. Front. Nutr. 2023, 10, 1205671. [Google Scholar] [CrossRef]
| 2010 | 2020 | Percent Increase from 2010 to 2020 | ||
|---|---|---|---|---|
| Region | g/Capita/Day | |||
| World | 21.56 | 26.09 | 21.01% | |
| Africa | Northern Africa | 12.21 | 13.84 | 13.35% |
| Southern Africa | 13.06 | 16.1 | 23.28% | |
| Eastern Africa | 2.91 | 3.03 | 4.12% | |
| Middle Africa | 1.01 | 1.07 | 5.94% | |
| Western Africa | 6.45 | 5.67 | −12.09% | |
| Americas | Northern America | 37.92 | 43.4 | 14.45% |
| Central America | 42.94 | 48.3 | 12.48% | |
| Caribbean | 14.77 | 19.97 | 35.21% | |
| South America | 22.46 | 31.8 | 41.59% | |
| Asia | Central Asia | 13.4 | 21.68 | 61.79% |
| Eastern Asia | 41.13 | 48.97 | 19.06% | |
| Southern Asia | 6.67 | 11.04 | 65.52% | |
| Southeastern Asia | 12.17 | 26.68 | 119.23% | |
| Western Asia | 15.7 | 20.69 | 31.78% | |
| Europe | Eastern Europe | 38.29 | 39.88 | 4.15% |
| Northern Europe | 26.12 | 28.01 | 7.24% | |
| Southern Europe | 30.4 | 31.6 | 3.95% | |
| Western Europe | 33.46 | 37.59 | 12.34% | |
| Oceania | Australia and New Zealand | 18.45 | 21.96 | 19.02% |
| Melanesia | 2.98 | 3.12 | 4.70% | |
| Micronesia | 2.89 | 11.78 | 307.61% | |
| Polynesia | 18.46 | 19.41 | 5.15% | |
| Protein | Apparent Molecular Weight (kDa) | Measured Isoelectric Point (pI) | Egg White (EW) or Yolk (EY) | Characteristics | References |
|---|---|---|---|---|---|
| HEN | |||||
| Ovomucoid (OVM) Gal d 1 (WHO/IUIS) P01005 (UniProt) | 28–37 | 3.7–4.5 | EW | Glycoprotein, trypsin inhibitor, high heat, acid, and enzymatic hydrolysis stability. | [18,53,58,59,60,61,62] |
| Ovalbumin (OVA) Gal d 2 (WHO/IUIS) P01012 (UniProt) | 41–46 | 4.5–4.8 | EW | Globular phosphoglycoprotein, the major EW protein, a heat-labile, serin protease inhibitor. | [18,27,53,54,55,58,59,60,61,62,63,64,65,66,67,68,69] |
| Ovotransferrin (OVT) Gal d 3 (WHO/IUIS) P02789 (UniProt) | 75–79 | 6.0–7.2 | EW | Glycoprotein, transports iron, has antimicrobial properties, and is heat labile. | [18,53,58,59,60,62,63,64,65,66,67,70,71] |
| Lysozyme (LYS) Gal d 4 (WHO/IUIS) P00698 (UniProt) | 14.3 | 10.7 | EW | Antimicrobial (hydrolyzes bacterial cell walls) and heat labile. | [18,53,58,59,60,62,64,65,66,67] |
| α-livetin (LIV) Gal d 5 (WHO/IUIS) P19121 (UniProt) | 65–70 | 4.6–4.8 | EY | Highly prevalent in EY, similar to chicken serum albumin in bird tissues, and is heat labile. | [56,57,62,72,73,74] |
| Yolk glycoprotein 42 (YGP42) Gal d 6 (WHO/IUIS) | 35, 42 | 5.88 theoretical | EY | Cleaved from the primary translation product of vitellogenin-1 (UniProt: P87498), heat stable. | [53,74,75,76,77,78,79,80] |
| Phosvitin (PSV) | 35–45 | 4 | EY | Phosphoprotein, heat stable. IgE binding detected, minor clinical significance. | [73,80,81,82,83] |
| DUCK | |||||
| OVA | 40–48 | EW | Similar to hen egg OVA. | [23] | |
| Trypsin inhibitor (TI) | 43 | EW | Serine protease inhibitor, stable within 40–60 °C at pH of 7–9. | [84] | |
| LYS | 14 | >10 | EW | Thermostable between 30 and 60 °C at pH of 4–7. | [78] |
| PIGEON | |||||
| OVM | 45 | EW | [85] | ||
| OVA | 49–53 | EW | [85] | ||
| OVT | 76 | EW | [85] |
| Hen Egg | Other Eggs | Details | References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Egg White | Egg Yolk | Duck Egg White | Pigeon Egg White | ||||||||||
| OVM | OVA | OVT | LYS | LIV | YGP42 | PSV | TI | LYS | OVA | OVT | OVM | ||
| ✓ | (1) PEG, (2) pH 4.5 precipitation. Yield: 46.4%, purity: >95.1%. | [63] | |||||||||||
| ✓ | AS/citric acid precipitation. Yield and purity: ≥83%. | [70] | |||||||||||
| ✓ | Ethanol precipitation. Yield: 99%, purity: >80%. | [71] | |||||||||||
| ✓ | ✓ | (1) Ethanol, (2) AS/citric acid precipitation. (3) Heating (for OVM). Yield: OVM > 96%, OVT > 92%. Purity: OVM and OVT > 88%. | [59] | ||||||||||
| ✓ | ✓ | Two-step membrane cation exchange/Sartobind S nano. Yield: LYS 99%, OVT 97%. Purity: LYS 96%, OVT 84%. | [67] | ||||||||||
| ✓ | ✓ | ✓ | (1) LYS—Cation exchange/StreamlineTM SP, affinity chromatography/Cibacron Blue F3GA. (2) OVA: TCA precipitation, OVM: ethanol precipitation. Yield: OVM 94%, OVA 98%, LYS 77%. | [61] | |||||||||
| ✓ | ✓ | ✓ | (1) Mucin removal. (2) LYS, OVT: cation exchange/S Ceramic Hyper DF. (3) OVA: anion exchange/Q Sepharose FF. Yield: LYS 100%, OVT 78%, OVA 50%. Purity: LYS 95%, OVT 89%, OVA 91%. | [64] | |||||||||
| ✓ | ✓ | ✓ | (1) Mucin extraction. (2) OVA, OVT: anion exchange/Q Sepharose FF. (3) OVT, LYS: cation exchange/SP Sepharose FF. Purities ranged from 47 to 80%, average yield 71%. | [65,66] | |||||||||
| ✓ | ✓ | ✓ | (1) LYS: cation exchange/FPC3500. (2) OVA, OVT: AS/citric acid precipitation. Yield: OVA > 98%, OVT and LYS > 82%. Purity > 90%. | [60] | |||||||||
| ✓ | ✓ | ✓ | (1) Mucin removal via PEG precipitation. (2) OVA, OVT, LYS—Anion exchange/Q Sepharose FF. Yield: OVA 53.17%, LYS 30.10%, OVT 77.75%. Purity: OVA 88.16%, LYS 94.55%, OVT 96.45%. | [69] | |||||||||
| ✓ | ✓ | ✓ | ✓ | (1) Mucin removal. (2) OVA, LYS: anion exchange/Q Sepharose FF. (3) OVM, OVT: cation exchange/Toyopearl CM-650 M. (4) OVM: ethanol/TCA precipitation. Yield: LYS 55%, OVA 54%, OVT 21%, OVM 21%. Purity: LYS 87%, OVA 70%, OVT 80%, OVM 90%. | [58] | ||||||||
| ✓ | ✓ | ✓ | ✓ | (1) Mucin removal. (2) OVM, OVA, OVT, LYS: Cation exchange/CM-Sepharose. Yield: OVM 60%, OVA 52.1%, OVT 29.6%, LYS 90.2%. | [89] | ||||||||
| ✓ | (1) Granula removal. (2) Anion exchange/DEAE-Sepharose CL 6B. (3) Anion exchange/Q Sepharose FF. (4) Gel filtration/Superdex 75. Yield 10%, purity > 98% | [62] | |||||||||||
| ✓ | RP-HPLC/ACE 5 C4-300, good quality for immunoassays. | [53] | |||||||||||
| ✓ | His-tagged recombinant protein. Affinity chromatography/NI-NTA. IgE reactive. | [74] | |||||||||||
| ✓ | (1) Ethanol delipidation. (2) Salt-based isolation (NaCl or AS). Yield 72% (AS) and 97% (NaCl). | [83] | |||||||||||
| ✓ | (1) Solvent-free delipidation. (2) Anion exchange chromatography/Q Sepharose FF. Yield 35.4%, purity 92.6%. | [93] | |||||||||||
| ✓ | (1) Heating, 80°, 15 min. (2) Ultrasonic processing 600 W, 15 min. Purity 80%. | [94] | |||||||||||
| ✓ | (1) AS precipitation. (2) Affinity chromatography/Trypsin-CNBr-activated Sepharose 4B. Yield 0.6%, 111.8-fold increase in purity. | [84] | |||||||||||
| ✓ | (1) Isoelectric point precipitation (pH 6.8). (2) Cation exchange/D152 resin. Yield 0.36%. | [95] | |||||||||||
| ✓ | ✓ | ✓ | RP-HPLC/C4 column. Suitable for mass spectrometry. | [85] | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sibincic, N.; Prodic, I.; Apostolovic, D.; Wai, C.Y.Y.; Leung, A.S.Y.; Stojadinovic, M. Insights into Isolation and Purification Strategies of Egg Allergens. Foods 2025, 14, 1944. https://doi.org/10.3390/foods14111944
Sibincic N, Prodic I, Apostolovic D, Wai CYY, Leung ASY, Stojadinovic M. Insights into Isolation and Purification Strategies of Egg Allergens. Foods. 2025; 14(11):1944. https://doi.org/10.3390/foods14111944
Chicago/Turabian StyleSibincic, Nikolina, Ivana Prodic, Danijela Apostolovic, Christine Y. Y. Wai, Agnes S. Y. Leung, and Marija Stojadinovic. 2025. "Insights into Isolation and Purification Strategies of Egg Allergens" Foods 14, no. 11: 1944. https://doi.org/10.3390/foods14111944
APA StyleSibincic, N., Prodic, I., Apostolovic, D., Wai, C. Y. Y., Leung, A. S. Y., & Stojadinovic, M. (2025). Insights into Isolation and Purification Strategies of Egg Allergens. Foods, 14(11), 1944. https://doi.org/10.3390/foods14111944







