Characterization of Key Odorants During Processing of Minty-like Aroma ‘Rucheng Baimaocha’ Black Tea
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Tea Samples
2.3. Sensory Analysis
2.4. Volatile Analysis
2.5. Qualitative and Quantitative Analysis of Volatiles
2.6. OAV Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Sensory Evaluation of RCBT Samples
3.2. Identification and Quantification of Volatiles During RCBT Processing
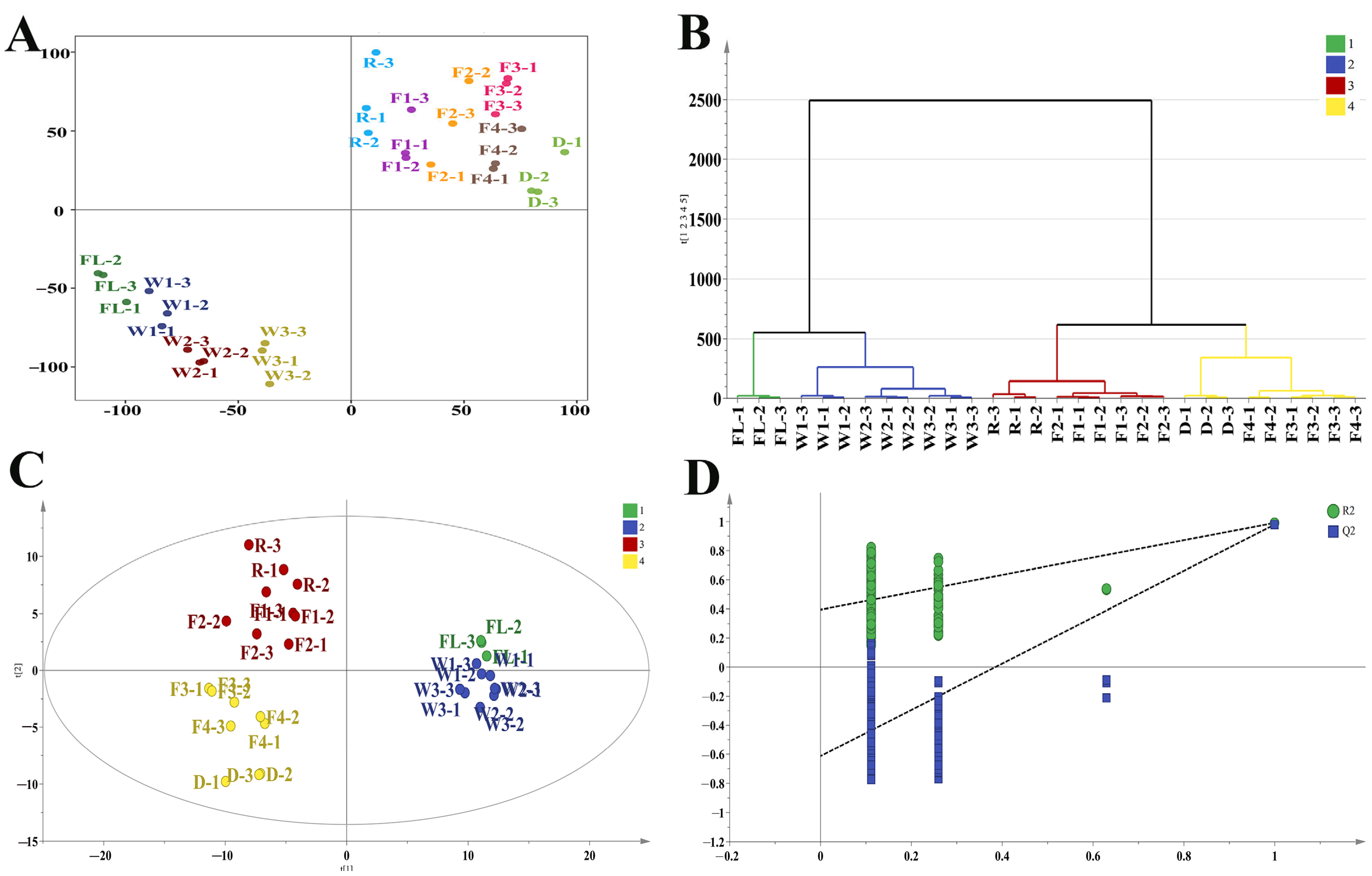
3.3. Multivariate Statistical Analysis During RCBT Processing
3.4. Key Odorants Analysis During RCBT Processing
3.5. The Change Profile of AACs During RCBT Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Hua, J.; Deng, Y.; Jiang, Y.; Qian, M.C.; Wang, J.; Li, J.; Zhang, M.; Dong, C.; Yuan, H. Aroma dynamic characteristics during the process of variable-temperature final firing of congou black tea by electronic nose and comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry. Food Res. Int. 2020, 137, 109656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ho, C.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and biological activities of processed Camellia sinensis teas: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, M.; Wang, H.; Huang, W.; Li, F.; Wang, L.; Ho, C.-T.; Zhang, Y.; Zhang, L.; Zhai, X.; et al. Decoding the specific roasty aroma Wuyi Rock tea (Camellia sinensis: Dahongpao) by the sensomics approach. J. Agric. Food Chem. 2022, 70, 10571–10583. [Google Scholar] [CrossRef]
- Su, D.; He, J.-J.; Zhou, Y.-Z.; Li, Y.-L.; Zhou, H.-J. Aroma effects of key volatile compounds in Keemun black tea at different grades: HS-SPME-GC-MS, sensory evaluation, and chemometrics. Food Chem. 2022, 373, 131587. [Google Scholar] [CrossRef]
- Ma, L.; Gao, M.; Zhang, L.; Qiao, Y.; Li, J.; Du, L.; Zhang, H.; Wang, H. Characterization of the key aroma-active compounds in high-grade dianhong tea using GC-MS and GC-O combined with sensory-directed flavor analysis. Food Chem. 2022, 378, 132058. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Yan, J.; Wang, B.; Meng, Q.; Zhang, L.; Tong, H. Identification of key odorants responsible for cooked corn-like aroma of green teas made by tea cultivar ‘Zhonghuang 1’. Food Res. Int. 2020, 136, 109355. [Google Scholar] [CrossRef]
- Ouyang, J.; Jiang, R.; Chen, H.; Liu, Q.; Yi, X.; Wen, S.; Huang, F.; Zhang, X.; Li, J.; Wen, H.; et al. Characterization of key odorants in ‘Baimaocha’ black teas from different regions. Food Chem. X 2024, 22, 101303. [Google Scholar] [CrossRef]
- Ouyang, J.; Jiang, R.; An, H.; Ou, X.; Wang, J.; Xie, H.; Fu, W.; Zhang, J.; Chen, H.; Liu, Q.; et al. Decoding the specific minty-like aroma of ‘Rucheng Baimaocha’ (Camellia Pubescens) black tea. Food Chem. X 2025, 26, 102253. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, Y.; Liu, Y.; Liu, Y.; Dong, C.; Lin, Z.; Teng, J. Black tea aroma formation during the fermentation period. Food Chem. 2022, 374, 131640. [Google Scholar] [CrossRef]
- Wu, H.; Huang, W.; Chen, Z.; Chen, Z.; Shi, J.; Kong, Q.; Sun, S.; Jiang, X.; Chen, D.; Yan, S. GC–MS-Based metabolomic study reveals dynamic changes of chemical compositions during black tea processing. Food Res. Int. 2019, 120, 330–338. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Y.; Li, M.; Wang, Y.; Zhang, L.; Wan, X.; Yang, X. Tea aroma formation from six model manufacturing processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Q.; Liu, D.; Yang, L.; Hu, W.; Kuang, L.; Huang, Y.; Teng, J.; Liu, Y. Multi-omics and enzyme activity analysis of flavour substances formation: Major metabolic pathways alteration during congou black tea processing. Food Chem. 2023, 403, 134263. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, Y.; Zhang, T.; Tang, X.; Wang, X.; Li, C. Review on aroma change during black tea processing. J. Tea Sci. 2018, 38, 9–19. [Google Scholar]
- Aaqil, M.; Kamil, M.; Kamal, A.; Nawaz, T.; Peng, C.; Alaraidh, I.A.; Al-Amri, S.S.; Okla, M.K.; Hou, Y.; Fahad, S.; et al. Metabolomics reveals a differential attitude in phytochemical profile of black tea (Camellia sinensis Var. assamica) during processing. Food Chem. X 2024, 24, 101899. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Li, W.-X.; Zhang, Y.-H.; Yan, H.; Guo, L.Y.; Zhang, Y.; Lv, H.P.; Zhou, L.H.; Lin, Z.; Wu, W.L.; et al. Insight into volatile metabolites and key odorants in black teas processed from Jianghua Kucha tea germplasm (Camellia sinensis var. assamica cv. Jianghua). Food Chem. 2025, 464, 141794. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Q.; Xie, J.; Deng, Y.; Zhu, J.; Xie, Z.; Yuan, H.; Jiang, Y. Uncovering the dynamic alterations of volatile components in sweet and floral aroma black tea during processing. Foods 2024, 13, 728. [Google Scholar] [CrossRef]
- Zhong, X.G.; Huang, H.S.; Li, N.; Su, B.W. Effect of withering and fermentation technology on the quality of ‘Rucheng Baimaocha’ processed black tea. Food Ferment. Ind. 2022, 48, 137–144. [Google Scholar]
- Miao, Y.C.; Tan, J.H.; Liu, W.; Zhou, L.H.; Fan, J.J.; Liu, Y.R.; Li, Z.G. Effects of shaking and fermentation on the quality of summer black tea of Rucheng Baimaocha. Food Res. Dev. 2022, 43, 31–39. [Google Scholar]
- Zhong, N.; Zhao, X.; Yu, P.; Huang, H.; Zheng, H. Analysis on the quality characteristics of congou black tea produced from new strains of Rucheng Baimaocha. J. Tea Commun. 2023, 50, 352–363. [Google Scholar]
- Kang, S.; Yan, H.; Zhu, Y.; Liu, X.; Lv, H.-P.; Zhang, Y.; Dai, W.-D.; Guo, L.; Tan, J.-F.; Peng, Q.-H.; et al. Identification and quantification of key odorants in the world’s four most famous black teas. Food Res. Int. 2019, 121, 73–83. [Google Scholar] [CrossRef]
- Zhai, X.; Hu, Y.; Pei, Z.; Yu, J.; Li, M.; Zhang, L.; Ho, C.-T.; Zhang, Y.; Wan, X. Insights into the key odorants in large-leaf yellow tea (Camellia sinensis) by application of the sensomics approach. J. Agric. Food Chem. 2023, 71, 690–699. [Google Scholar] [CrossRef] [PubMed]
- GB/T 23776; Methodology for Sensory Evaluation of Tea. Standards Press of China: Beijing, China, 2018.
- Ouyang, J.; Jiang, R.; Xu, H.; Wen, S.; Liu, C.; Liu, Y.; Chen, H.; Zhai, Y.; Xie, H.; Chen, J.; et al. Insights into the flavor profiles of different grades of Huangpu black tea using sensory histology techniques and metabolomics. Food Chem. X 2024, 23, 101600. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, G.; Xie, H.; Zhang, J.; Huang, J.; Liu, Z.; Wang, C. Characterization of the key differential aroma compounds in five dark teas from different geographical regions integrating GC–MS, ROAV and chemometrics approaches. Food Res. Int. 2024, 194, 114928. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, L.; Granvogl, M.; Ho, C.; Wan, X. Flavor of tea (Camellia sinensis): A review on odorants and analytical techniques. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3867–3909. [Google Scholar] [CrossRef]
- Gong, L.J.; Bo, J.H.; Zhang, T.T.; Sun, H.Y.; Chen, Y.Q.; Pei, R.Y.; Xiao, L.Z. Dynamic changes of aroma components and related enzyme activities during Huangjincha black tea processing. Food Ferment. Ind. 2022, 48, 204–209. [Google Scholar]
- Fang, J.T. Analysis on Metabolic Chemical Composition of Keemun Black Tea in Processing. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2016. [Google Scholar]
- Wei, Y.; Zhang, J.; Li, T.; Zhao, M.; Song, Z.; Wang, Y.; Ning, J. GC–MS, GC–O, and sensomics analysis reveals the key odorants underlying the improvement of yellow tea aroma after optimized yellowing. Food Chem. 2024, 431, 137139. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Su, H.; Ma, J.; Zheng, J.; He, W.; Wu, C.; Hou, Z.; Zhao, R.; Zhou, Q. Widely targeted volatileomics analysis reveals the typical aroma formation of Xinyang black tea during fermentation. Food Res. Int. 2022, 164, 112387. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.T.; Zheng, X.; Li, S. Tea aroma formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, Y.; Li, C.; Li, X.; Li, K.; Yang, W.; Liang, Y.; Lu, J.; Zhang, L.; Lu, P.; et al. Metabolomics and quantitative descriptive analysis reveal the relationship between metabolites and taste attributes of flowers in two types of albino tea cultivars. LWT 2024, 199, 116074. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, H.; Rothenberg, D.O.; Zhang, K.; Lin, Y.; Jin, K.; Li, J.; Zhou, H.; Su, H.; Ji, R.; et al. Multiomics correlation analysis on the mechanism of flavor substance formation during the processing of “Huanong 53” Black Tea. LWT 2024, 214, 117086. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Tang, J.; Huang, R.; Luo, W.; Tuo, Y.; Liao, N.; Zhuang, D.; Lin, J.; Zhang, Y.; et al. Study on dynamic changes in characteristic volatile compounds uncovers aroma development of hainan dayezhong (Camellia sinensis var. assamica) black tea. Food Chem. 2025, 477, 143578. [Google Scholar] [CrossRef] [PubMed]


| Odorants | Thresholds | Attributes | Pathway | FL | W1 | W2 | W3 | R | F1 | F2 | F3 | F4 | D |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hexanal | 4.5 | Fat, sweet | FADVs | 65.88 ± 6.50 fg | 58.38 ± 10.69 g | 64.68 ± 3.84 fg | 77.39 ± 9.58 de | 81.73 ± 6.87 de | 75.85 ± 4.77 ef | 90.77 ± 6.58 cd | 117.13 ± 5.71 b | 102.96 ± 8.10 c | 151.03 ± 12.77 a |
| (Z)-4-Heptenal | 0.06 | Baked potatoes | 69.40 ± 16.96 cde | 100.25 ± 32.72 abcde | 71.55 ± 28.85 bcde | 60.44 ± 26.98 e | 127.11 ± 25.48 a | 107.18 ± 12.80 abcd | 111.91 ± 7.91 abc | 119.38 ± 12.13 a | 114.00 ± 35.05 ab | 65.65 ± 15.93 de | |
| Heptanal | 10 | Citrus, fat, green | 7.08 ± 1.49 bc | 6.91 ± 0.52 bc | 4.40 ± 0.35 f | 2.81 ± 0.47 g | 7.66 ± 0.72 ab | 4.93 ± 0.27 ef | 6.01 ± 0.15 cd | 8.58 ± 0.13 a | 6.71 ± 0.30 bcd | 5.77 ± 0.27 de | |
| Octanal | 0.7 | Fat, green, nut | 18.70 ± 5.63 a | 7.97 ± 1.66 def | 5.84 ± 0.53 ef | 3.97 ± 2.91 f | 9.86 ± 1.83 cde | 7.49 ± 0.81 def | 7.23 ± 2.18 def | 13.37 ± 1.40 bc | 11.24 ± 1.39 cd | 15.96 ± 1.64 ab | |
| (E,E)-2,4-Heptadienal | 60 | Green fruity | 1.71 ± 0.25 c | 2.54 ± 0.32 c | 2.10 ± 0.07 c | 1.89 ± 0.28 c | 2.53 ± 0.36 c | 2.26 ± 0.20 c | 2.33 ± 0.22 c | 2.30 ± 0.09 a | 2.10 ± 0.45 a | 2.31 ± 0.22 b | |
| Nonanal | 1 | Floral, fruity | 85.35 ± 4.74 c | 70.99 ± 7.97 d | 49.57 ± 4.01 e | 44.40 ± 8.82 e | 87.47 ± 10.88 c | 71.32 ± 2.28 d | 90.84 ± 10.44 bc | 179.68 ± 10.53 a | 91.41 ± 8.39 bc | 103.72 ± 2.97 b | |
| (E,Z)-2,6-Nonadienal | 0.0045 | Fresh, cucumber | / | / | / | 57.59 ± 1.64 e | 3774.45 ± 508.59 d | 3785.8 ± 245.93 d | 4635.61 ± 811.72 c | 6084.96 ± 160.27 b | 5768.37 ± 334.72 b | 7239.38 ± 461.05 a | |
| (E)-2-Nonenal | 0.08 | Fresh, cucumber-like | / | / | / | 6.22 ± 1.47 e | 78.30 ± 11.54 d | 76.07 ± 3.20 d | 166.46 ± 27.93 c | 190.09 ± 10.98 ab | 181.79 ± 4.38 bc | 202.17 ± 7.78 a | |
| 2,4-Nonadienal | 0.1 | Fat, green | 8.78 ± 2.27 f | 12.39 ± 1.65 f | 18.66 ± 0.87 de | 24.08 ± 2.78 bc | 17.31 ± 1.57 e | 21.72 ± 1.62 cde | 21.00 ± 4.66 cde | 22.92 ± 1.58 bce | 26.78 ± 3.32 b | 38.66 ± 1.62 a | |
| (E,Z)-2,4-Decadienal | 0.04 | Fat, flower, fried | 1.42 ± 0.24 e | 3.76 ± 0.72 c | 2.45 ± 0.26 d | 3.75 ± 0.43 c | 5.99 ± 0.59 ab | 5.23 ± 0.35 b | 5.78 ± 0.73 ab | 6.37 ± 0.50 a | 3.96 ± 0.46 c | 3.84 ± 0.63 c | |
| 2,4-Decadienal | 0.3 | Fat, sweet | 0.37 ± 0.03 d | 0.90 ± 0.11 bc | 0.84 ± 0.08 c | 1.36 ± 0.16 a | 1.34 ± 0.17 a | 1.04 ± 0.10 b | 0.98 ± 0.05 bc | 1.03 ± 0.05 b | 0.82 ± 0.08 c | 1.47 ± 0.08 a | |
| (Z)-3-Hexen-1-ol | 70 | Grass, green, herb | 6.29 ± 1.07 e | 12.87 ± 1.33 d | 11.77 ± 0.60 d | 13.42 ± 1.46 d | 25.85 ± 2.46 a | 22.53 ± 0.98 b | 18.79 ± 1.29 c | 16.98 ± 0.29 c | 11.56 ± 0.49 d | 5.68 ± 0.29 e | |
| (E)-2-Hexen-1-ol | 100 | Fat, fruity, geranium | 0.31 ± 0.05 g | 1.49 ± 0.16 f | 1.16 ± 0.09 fg | 2.58 ± 0.27 e | 12.14 ± 1.25 a | 11.48 ± 0.36 ab | 10.80 ± 0.97 b | 10.73 ± 0.35 b | 9.68 ± 0.85 c | 3.86 ± 0.25 d | |
| 2-Heptanol | 70 | Coconut, fried | 1.23 ± 0.34 d | 1.24 ± 0.10 d | 1.16 ± 0.19 d | 1.93 ± 0.36 c | 3.53 ± 0.13 a | 3.55 ± 0.26 a | 3.40 ± 0.42 a | 2.69 ± 0.17 b | 1.81 ± 0.12 c | 1.08 ± 0.03 d | |
| 1-Heptanol | 5.6 | Green, putrid, wood | 0.37 ± 0.05 cd | 1.56 ± 0.22 a | 0.93 ± 0.40 b | 0.11 ± 0.06 d | 0.52 ± 0.11 c | 0.54 ± 0.17 c | 0.44 ± 0.07 c | 0.51 ± 0.05 c | 0.26 ± 0.09 cd | 0.07 ± 0.03 d | |
| 1-Octen-3-ol | 1 | Earth, mushroom, | 49.97 ± 2.94 d | 36.18 ± 5.45 e | 32.66 ± 2.17 e | 34.11 ± 4.64 e | 79.19 ± 7.10 a | 72.43 ± 3.14 ab | 64.59 ± 5.69 bc | 75.76 ± 10.69 a | 56.89 ± 6.08 cd | 61.39 ± 6.11 c | |
| (Z)-Jasmone | 1.9 | Floral, like jasmine | 160.88 ± 12.61 a | 95.25 ± 12.21 c | 70.53 ± 3.15 d | 65.44 ± 7.34 d | 118.34 ± 10.18 b | 93.71 ± 5.86 c | 98.86 ± 14.58 c | 106.27 ± 3.76 bc | 90.77 ± 4.48 c | 89.49 ± 7.53 c | |
| Neral | 100 | Citrus, fruit, lemon | VTs | 0.24 ± 0.03 e | 0.22 ± 0.01 e | 0.18 ± 0.01 e | 0.28 ± 0.03 e | 1.35 ± 0.12 d | 1.43 ± 0.09 d | 1.63 ± 0.19 c | 2.11 ± 0.08 a | 1.90 ± 0.05 b | 1.36 ± 0.07 d |
| Citral | 1 | Fruity, like lemon | 10.14 ± 1.48 e | 8.91 ± 0.83 e | 6.82 ± 0.74 e | 12.69 ± 1.62 e | 75.27 ± 6.98 d | 79.51 ± 5.42 d | 91.21 ± 11.00 c | 119.39 ± 4.76 a | 107.18 ± 3.14 b | 75.74 ± 4.35 d | |
| Linalool | 6 | Floral, fruity | 243.34 ± 21.25 a | 203.48 ± 27.22 b | 165.88 ± 8.01 c | 157.93 ± 18.15 c | 244.32 ± 21.88 a | 209.66 ± 11.10 b | 197.97 ± 17.48 b | 205.16 ± 3.34 b | 168.53 ± 7.17 c | 161.75 ± 11.95 c | |
| Geraniol | 7.5 | Floral, lemon peel | 46.33 ± 1.71 b | 53.14 ± 3.20 b | 39.83 ± 3.07 b | 43.47 ± 4.65 b | 96.75 ± 10.34 a | 91.18 ± 6.17 a | 93.56 ± 8.37 a | 96.21 ± 16.70 a | 91.99 ± 1.02 a | 95.09 ± 9.20 a | |
| Methyl geranate | 21.44 | Fruity, fresh | 2.49 ± 0.21 f | 1.41 ± 0.08 g | 1.05 ± 0.09 g | 1.67 ± 0.22 g | 5.24 ± 0.49 e | 5.94 ± 0.12 d | 6.89 ± 0.68 c | 8.09 ± 0.32 a | 7.56 ± 0.31 ab | 6.96 ± 0.58 bc | |
| Benzeneacetaldehyde | 4 | Fruity, floral, honey | AADVs | / | / | / | / | 75.32 ± 11.86 e | 111.31 ± 6.13 d | 164.66 ± 16.05 c | 199.74 ± 4.37 b | 165.54 ± 9.20 c | 234.36 ± 17.79 a |
| Phenylethyl Alcohol | 1000 | Floral, fruit, honey | 1.64 ± 0.25 a | 1.48 ± 0.11 abc | 1.26 ± 0.10 cd | 1.06 ± 0.11 d | 1.68 ± 0.14 a | 1.38 ± 0.07 bc | 1.49 ± 0.16 ab | 1.70 ± 0.05 a | 1.53 ± 0.06 ab | 1.52 ± 0.07 ab | |
| Methyl salicylate | 40 | Fresh, mint | 7.07 ± 0.64 c | 8.04 ± 0.60 c | 7.77 ± 0.56 c | 7.75 ± 0.85 c | 17.27 ± 1.30 a | 15.9 ± 0.75 a | 15.98 ± 1.72 a | 16.17 ± 0.39 a | 14.33 ± 0.52 b | 12.97 ± 0.67 b | |
| Damascenone | 0.002 | Floral, fruit, honey | CDVs | / | / | / | / | / | / | / | 697.39 ± 162.84 a | / | 343.21 ± 66.50 b |
| α-Ionone | 0.4 | Floral, fruit, | 7.58 ± 0.70 bcd | 10.03 ± 0.77 a | 8.37 ± 1.14 bc | 7.77 ± 0.87 bc | 7.07 ± 0.57 cd | 6.18 ± 0.33 d | 7.15 ± 1.24 cd | 8.92 ± 0.70 ab | 7.72 ± 0.80 bcd | 7.75 ± 0.76 bcd | |
| (E)-β-Ionone | 0.007 | Floral, sweet | 2938.01 ± 174.56 e | 4520.28 ± 435.7 ab | 3952.33 ± 590.46 bc | 3225.32 ± 354.67 cde | 3272.72 ± 323.27 cde | 3043.48 ± 164.9 de | 3809.23 ± 691.13 bcd | 4763.08 ± 219.17 a | 4289.59 ± 344.18 ab | 4453.36 ± 519.56 ab |
| Compounds | Odor Description | FL | W1 | W2 | W3 | R | F1 | F2 | F3 | F4 | D |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hexanal | Fresh, cut grass | 2.00 ± 0.00 a | 2.33 ± 0.47 a | 2.67 ± 0.47 a | 2.67 ± 0.47 a | 2.67 ± 0.47 a | 2.67 ± 0.47 a | 2.67 ± 0.47 a | 2.83 ± 0.62 a | 2.33 ± 0.47 a | 2.33 ± 0.62 a |
| (Z)-3-Hexen-1-ol | Grass, green | 1.00 ± 0.00 a | 1.33 ± 0.47 a | 1.33 ± 0.47 a | 1.67 ± 0.47 a | 1.67 ± 0.47 a | 1.33 ± 0.47 a | 1.33 ± 0.47 a | 1.33 ± 0.47 a | 1.33 ± 0.47 a | 1.00 ± 0.00 a |
| (Z)-4-Heptenal | Baked potatoes | 1.67 ± 0.47 ab | 1.83 ± 0.62 ab | 1.83 ± 0.62 ab | 1.33 ± 0.47 b | 2.33 ± 0.47 ab | 2.67 ± 0.62 a | 2.17 ± 0.47 ab | 2.17 ± 0.24 ab | 2.67 ± 0.47 a | 1.67 ± 0.47 ab |
| (E,E)-2,4-Hexadienal | Fat, green | / | / | / | / | / | 1.33 ± 0.47 b | 1.67 ± 0.47 ab | 2.00 ± 0.00 a | 2.33 ± 0.47 a | 2.33 ± 0.47 a |
| 1-Octen-3-ol | Mushroom-like | 2.33 ± 0.47 a | 1.83 ± 0.24 a | 2.00 ± 0.00 a | 2.00 ± 0.82 a | 2.67 ± 0.47 a | 2.33 ± 0.47 a | 1.67 ± 0.47 a | 2.67 ± 0.47 a | 2.17 ± 0.24 a | 2.33 ± 0.47 a |
| 6-Methyl-5-hepten-2-one | Mushroom, pepper | 1.67 ± 0.47 b | 1.67 ± 0.47 b | 1.67 ± 0.47 b | 1.67 ± 0.47 b | 2.33 ± 0.47 ab | 2.33 ± 0.47 ab | 1.83 ± 0.24 b | 2.17 ± 0.24 ab | 2.00 ± 0.00 ab | 3.00 ± 0.82 a |
| Benzeneacetaldehyde | Floral, honey | 1.33 ± 0.47 c | 1.67 ± 0.47 bc | 2.00 ± 0.82 abc | 2.00 ± 0.82 abc | 2.00 ± 0.82 abc | 2.33 ± 0.47 abc | 2.33 ± 0.47 abc | 2.83 ± 0.62 ab | 2.50 ± 0.41 abc | 3.17 ± 0.62 a |
| Linalool oxide II | Roasted, sweet | 1.67 ± 0.47 a | 1.67 ± 0.47 a | 1.67 ± 0.47 a | 1.67 ± 0.47 a | 1.33 ± 0.47 a | 1.67 ± 0.47 a | 1.50 ± 0.41 a | 1.67 ± 0.47 a | 1.67 ± 0.47 a | 2.00 ± 0.41 a |
| Methyl benzoate | Fresh, fruity | 1.67 ± 0.47 a | 1.67 ± 0.47 a | 2.00 ± 0.82 a | 2.33 ± 0.47 a | 2.00 ± 0.82 a | 2.33 ± 0.47 a | 2.33 ± 0.47 a | 1.67 ± 0.47 a | 2.00 ± 0.00 a | 2.00 ± 0.00 a |
| Linalool | Floral, sweet | 3.83 ± 0.24 a | 3.67 ± 0.47 a | 3.67 ± 0.47 a | 3.33 ± 0.47 a | 3.67 ± 0.47 a | 3.67 ± 0.47 a | 3.67 ± 0.47 a | 3.67 ± 0.47 a | 4.17 ± 0.24 a | 4.17 ± 0.24 a |
| Phenylethyl Alcohol | Floral, fruit, honey | 1.33 ± 0.47 a | 1.33 ± 0.47 a | 1.33 ± 0.47 a | 1.33 ± 0.47 a | 1.67 ± 0.47 a | 1.33 ± 0.47 a | 1.33 ± 0.47 a | 1.17 ± 0.24 a | 1.17 ± 0.24 a | 1.33 ± 0.47 a |
| (2E,6Z)-Nona-2,6-dienal | Fresh, cucumber | 1.67 ± 0.47 b | 2.33 ± 0.47 b | 2.33 ± 0.47 b | 2.33 ± 0.47 b | 3.33 ± 0.47 a | 3.33 ± 0.47 a | 3.33 ± 0.47 a | 4.00 ± 0.00 a | 3.50 ± 0.41 a | 4.17 ± 0.24 a |
| (2E)-Non-2-enal | Fresh, cucumber-like | 1.00 ± 0.00 d | 1.00 ± 0.00 d | 1.33 ± 0.47 cd | 1.67 ± 0.47 cd | 2.00 ± 0.00 bc | 2.00 ± 0.00 bc | 2.67 ± 0.47 ab | 2.67 ± 0.47 ab | 2.50 ± 0.41 ab | 2.83 ± 0.24 a |
| Benzyl acetate | Fruity, fresh | / | / | / | / | 1.00 ± 0.00 a | 1.33 ± 0.47 a | 1.33 ± 0.47 a | 1.33 ± 0.47 a | 1.67 ± 0.47 a | 1.33 ± 0.47 a |
| Terpinen-4-ol | Fresh, woody | 2.33 ± 0.47 a | 2.33 ± 0.47 a | 3.00 ± 0.00 a | 3.00 ± 0.00 a | 3.00 ± 0.00 a | 3.00 ± 0.00 a | 2.67 ± 0.47 a | 3.00 ± 0.00 a | 3.00 ± 0.00 a | 2.50 ± 0.41 a |
| Methyl salicylate | Fresh, mint | / | 1.33 ± 0.47 b | 1.83 ± 0.24 ab | 1.83 ± 0.24 ab | 2.17 ± 0.24 a | 2.33 ± 0.47 a | 2.00 ± 0.00 ab | 1.67 ± 0.47 | 2.17 ± 0.24 a | 1.67 ± 0.47 ab |
| 2,4-Nonadienal | Deep-fried, fat | 1.00 ± 0.00 a | 1.83 ± 0.24 a | 1.50 ± 0.41 a | 1.83 ± 0.24 a | 1.33 ± 0.47 a | 1.67 ± 0.47 a | 1.33 ± 0.47 a | 1.33 ± 0.47 a | 1.33 ± 0.47 a | 1.67 ± 0.47 a |
| Neral | Fruity, lemon | 1.33 ± 0.47 b | 1.33 ± 0.47 b | 1.33 ± 0.47 b | 2.00 ± 0.00 ab | 2.67 ± 0.47 a | 2.00 ± 0.00 ab | 2.33 ± 0.47 a | 2.67 ± 0.47 a | 2.33 ± 0.47 a | 2.50 ± 0.41 a |
| Geraniol | Floral, like rose | 2.50 ± 0.41 b | 2.83 ± 0.62 ab | 3.17 ± 0.24 ab | 3.17 ± 0.24 ab | 3.50 ± 0.41 ab | 3.50 ± 0.41 ab | 3.67 ± 0.47 a | 3.50 ± 0.41 ab | 3.33 ± 0.47 ab | 3.67 ± 0.47 a |
| Citral | Fruity, like lemon | 1.33 ± 0.47 c | 1.50 ± 0.41 bc | 1.50 ± 0.41 bc | 2.00 ± 0.00 abc | 2.17 ± 0.24 ab | 2.17 ± 0.24 ab | 2.17 ± 0.24 ab | 2.17 ± 0.24 ab | 2.50 ± 0.41 a | 2.17 ± 0.24 ab |
| Ethyl nonanoate | Fresh, fruity | / | / | 0.67 ± 0.47 a | 0.67 ± 0.47 a | 0.67 ± 0.47 a | 0.33 ± 0.47 a | 0.33 ± 0.47 a | 0.33 ± 0.47 a | 0.67 ± 0.47 a | 0.33 ± 0.47 a |
| Methyl geranate | Fruit, fresh | 1.17 ± 0.24 b | 1.33 ± 0.47 ab | 1.33 ± 0.47 ab | 1.33 ± 0.47 ab | 1.17 ± 0.24 b | 1.17 ± 0.24 b | 1.17 ± 0.24 b | 2.00 ± 0.00 a | 1.50 ± 0.41 ab | 1.83 ± 0.24 ab |
| β-Damascenone | Sweet, honey | / | / | / | / | / | / | / | 2.33 ± 0.47 a | 1.67 ± 0.47 b | 2.33 ± 0.47 a |
| (Z)-Jasmone | Floral, sweet | 1.83 ± 0.24 a | 1.33 ± 0.47 ab | 1.33 ± 0.47 ab | 1.33 ± 0.47 ab | 1.00 ± 0.00 b | 1.17 ± 0.24 ab | 1.17 ± 0.24 ab | 1.00 ± 0.00 b | 1.33 ± 0.47 ab | 1.00 ± 0.00 b |
| (E)-β-Ionone | Floral, sweet | 2.33 ± 0.47 a | 2.67 ± 0.47 a | 2.33 ± 0.47 a | 2.50 ± 0.41 a | 2.33 ± 0.47 a | 2.33 ± 0.47 a | 2.67 ± 0.47 a | 3.33 ± 0.47 a | 3.00 ± 0.00 a | 3.00 ± 0.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, J.; Jiang, R.; Liu, Q.; Chen, H.; Yi, X.; Yang, Y.; Huang, F.; Li, J.; Wen, H.; Xiong, L.; et al. Characterization of Key Odorants During Processing of Minty-like Aroma ‘Rucheng Baimaocha’ Black Tea. Foods 2025, 14, 1941. https://doi.org/10.3390/foods14111941
Ouyang J, Jiang R, Liu Q, Chen H, Yi X, Yang Y, Huang F, Li J, Wen H, Xiong L, et al. Characterization of Key Odorants During Processing of Minty-like Aroma ‘Rucheng Baimaocha’ Black Tea. Foods. 2025; 14(11):1941. https://doi.org/10.3390/foods14111941
Chicago/Turabian StyleOuyang, Jian, Ronggang Jiang, Qi Liu, Hongyu Chen, Xiaoqin Yi, Yuzi Yang, Fangfang Huang, Juan Li, Haitao Wen, Ligui Xiong, and et al. 2025. "Characterization of Key Odorants During Processing of Minty-like Aroma ‘Rucheng Baimaocha’ Black Tea" Foods 14, no. 11: 1941. https://doi.org/10.3390/foods14111941
APA StyleOuyang, J., Jiang, R., Liu, Q., Chen, H., Yi, X., Yang, Y., Huang, F., Li, J., Wen, H., Xiong, L., Huang, J., & Liu, Z. (2025). Characterization of Key Odorants During Processing of Minty-like Aroma ‘Rucheng Baimaocha’ Black Tea. Foods, 14(11), 1941. https://doi.org/10.3390/foods14111941







