Toward a Comprehensive Understanding of Flavor of Sunflower Products: A Review of Confirmed and Prospective Aroma- and Taste-Active Compounds
Abstract
1. Introduction

2. Integrated Physiology of Aroma and Taste Perception

3. Characterization of Volatile Compounds in Sunflower Products and Their Potential Role in Aroma Formation
4. Mapping Non-Volatiles and Taste-Associated Metabolites in Sunflower
4.1. Macronutrients
4.1.1. Lipids
4.1.2. Proteins
4.1.3. Carbohydrates
4.2. Micronutrients
4.2.1. Minerals and Vitamins
4.2.2. Phenols
4.2.3. Other Secondary Metabolites
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, S.; Ge, Y.; Na Jom, K. A Review of Phytochemistry, Metabolite Changes, and Medicinal Uses of the Common Sunflower Seed and Sprouts (Helianthus annuus L.). Chem. Cent. J. 2017, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Singh, R. Sunflower (Helianthus annuus) Seed. In Oilseeds: Health Attributes and Food Applications; Tanwar, B., Goyal, A., Eds.; Springer: Singapore, 2021; pp. 123–143. ISBN 978-981-15-4193-3. [Google Scholar]
- Niveditha, V.R.; Sridhar, K.R.; Balasubramanian, D. Physical and Mechanical Properties of Seeds and Kernels of Canavalia of Coastal Sand Dunes. Int. Food Res. J. 2013, 20, 1547–1554. [Google Scholar]
- USDA Seeds, Sunflower Seed Kernels, Dry Roasted, with Salt Added. Available online: https://fdc.nal.usda.gov/food-details/325524/nutrients (accessed on 25 January 2025).
- Cancalon, P. Chemical Composition of Sunflower Seed Hulls. J. Am. Oil Chem. Soc. 1971, 48, 629–632. [Google Scholar] [CrossRef]
- Weisz, G.M.; Kammerer, D.R.; Carle, R. Identification and Quantification of Phenolic Compounds from Sunflower (Helianthus annuus L.) Kernels and Shells by HPLC-DAD/ESI-MSn. Food Chem. 2009, 115, 758–765. [Google Scholar] [CrossRef]
- Paja̧k, P.; Socha, R.; Gałkowska, D.; Rożnowski, J.; Fortuna, T. Phenolic Profile and Antioxidant Activity in Selected Seeds and Sprouts. Food Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef]
- Anjum, F.M.; Nadeem, M.; Khan, M.I.; Hussain, S. Nutritional and Therapeutic Potential of Sunflower Seeds: A Review. Br. Food J. 2012, 114, 544–552. [Google Scholar] [CrossRef]
- De Lamo, B.; Gómez, M. Bread Enrichment with Oilseeds. A Review. Foods 2018, 7, 191. [Google Scholar] [CrossRef]
- Sunitha, P.; Priya, N.S.; Sharonuroja, M.; Charan, P.T.S.; Naresh, S.; Srinivas, S.; Raj, P.M.S. Health Benefits of Nutritional Seeds—A Review. J. Pharmacogn. Phytochem. 2025, 14, 97–106. [Google Scholar] [CrossRef]
- Cheenam, B.; Leena, P. Effects of Sunflower Seeds on Fasting Blood Glucose in Diabetes Mellitus Type 2 Patients. J. Chem. Pharm. Res. 2016, 8, 1211–1217. [Google Scholar]
- Guo, S.; Klinkesorn, U.; Lorjaroenphon, Y.; Ge, Y.; Na Jom, K. Effects of Germinating Temperature and Time on Metabolite Profiles of Sunflower (Helianthus annuus L.) Seed. Food Sci. Nutr. 2021, 9, 2810–2822. [Google Scholar] [CrossRef]
- Puttha, R.; Venkatachalam, K.; Hanpakdeesakul, S.; Wongsa, J.; Parametthanuwat, T.; Srean, P.; Pakeechai, K.; Charoenphun, N. Exploring the Potential of Sunflowers: Agronomy, Applications, and Opportunities within Bio-Circular-Green Economy. Horticulturae 2023, 9, 1079. [Google Scholar] [CrossRef]
- Pilorgé, E. Sunflower in the Global Vegetable Oil System: Situation, Specificities and Perspectives. OCL 2020, 27, 34. [Google Scholar] [CrossRef]
- Wahyuni, A.S. Community Effects Associated with Sunflower Oil Production: Systematic Review. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2024; Volume 1379. [Google Scholar] [CrossRef]
- Pal, D. Sunflower (Helianthus annuus L.) Seeds in Health and Nutrition. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1097–1105. ISBN 9780123756886. [Google Scholar]
- Lomascolo, A.; Uzan-Boukhris, E.; Sigoillot, J.C.; Fine, F. Rapeseed and Sunflower Meal: A Review on Biotechnology Status and Challenges. Appl. Microbiol. Biotechnol. 2012, 95, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Heuzé, V.; Tran, G.; Hassoun, P.; Lessire, M.; Lebas, F. Feedipedia: Sunflower Meal. Available online: https://www.feedipedia.org/node/732 (accessed on 25 January 2025).
- Karamać, M.; Kosińska, A.; Estrella, I.; Hernández, T.; Dueñas, M. Antioxidant Activity of Phenolic Compounds Identified in Sunflower Seeds. Eur. Food Res. Technol. 2012, 235, 221–230. [Google Scholar] [CrossRef]
- Müller, A.; Wolf, D.; Gutzeit, H.O. The Black Soldier Fly, Hermetia Illucens—A Promising Source for Sustainable Production of Proteins, Lipids and Bioactive Substances. Z. Naturforschung C—Sect. C J. Biosci. 2017, 72, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Farag, M.R.; El-Hack, M.E.A.; Dhama, K. The Practical Application of Sunflower Meal in Poultry Nutrition. Adv. Anim. Vet. Sci. 2015, 3, 634–648. [Google Scholar] [CrossRef]
- Subaşı, B.G.; Casanova, F.; Capanoglu, E.; Ajalloueian, F.; Sloth, J.J.; Mohammadifar, M.A. Protein Extracts from De-Oiled Sunflower Cake: Structural, Physico-Chemical and Functional Properties after Removal of Phenolics. Food Biosci. 2020, 38, 100749. [Google Scholar] [CrossRef]
- Bisinotto, M.S.; da Silva Napoli, D.C.; Simabuco, F.M.; Bezerra, R.M.N.; Antunes, A.E.C.; Galland, F.; Pacheco, M.T.B. Sunflower and Palm Kernel Meal Present Bioaccessible Compounds after Digestion with Antioxidant Activity. Foods 2023, 12, 3283. [Google Scholar] [CrossRef]
- Such, N.; Mezőlaki, Á.; Tewelde, K.G.; Pál, L.; Horváth, B.; Poór, J.; Dublecz, K. Feeding Sunflower Meal with Pullets and Laying Hens Even at a 30% Inclusion Rate Does Not Impair the Ileal Digestibility of Most Amino Acids. Front. Vet. Sci. 2024, 11, 1347374. [Google Scholar] [CrossRef]
- Tessier, R.; Calvez, J.; Airinei, G.; Khodorova, N.; Kapel, R.; Quinsac, A.; Galet, O.; Piedcoq, J.; Benamouzig, R.; Tomé, D.; et al. The True Amino Acid Digestibility of 15N-Labelled Sunflower Biscuits Determined with Ileal Balance and Dual Isotope Methods in Healthy Humans. J. Nutr. 2022, 152, 698–706. [Google Scholar] [CrossRef]
- Rosa, P.M.; Antoniassi, R.; Freitas, S.C.; Bizzo, H.R.; Zanotto, D.L.; Oliveira, M.F.; Castiglioni, V.B.R. Chemical Composition of Brazilian Sunflower Varieties. Helia 2009, 32, 145–156. [Google Scholar] [CrossRef]
- Zaky, A.A.; Hussein, A.S.; Mostafa, S.; Abd El-Aty, A.M. Impact of Sunflower Meal Protein Isolate Supplementation on Pasta Quality. Separations 2022, 9, 429. [Google Scholar] [CrossRef]
- Ertl, P.; Knaus, W.; Zollitsch, W. An Approach to Including Protein Quality When Assessing the Net Contribution of Livestock to Human Food Supply. Animal 2016, 10, 1883–1889. [Google Scholar] [CrossRef]
- Toutirais, L.; Walrand, S.; Vaysse, C. Are Oilseeds a New Alternative Protein Source for Human Nutrition? Food Funct. 2024, 15, 2366–2380. [Google Scholar] [CrossRef]
- Petrusán, J.I.; Rawel, H.; Huschek, G. Protein-Rich Vegetal Sources and Trends in Human Nutrition: A Review. Curr. Top. Pept. Protein Res. 2016, 17, 1–19. [Google Scholar]
- Pöri, P.; Lille, M.; Edelmann, M.; Aisala, H.; Santangelo, D.; Coda, R.; Sozer, N. Technological and Sensory Properties of Plant-Based Meat Analogues Containing Fermented Sunflower Protein Concentrate. Future Foods 2023, 8, 100244. [Google Scholar] [CrossRef]
- Toutirais, L.; Walrand, S.; Vaysse, C. Digestibility of Oilseed Protein Products and the Digestibility-Matrix Composition Relationship. SSRN 2025, 1–35. [Google Scholar] [CrossRef]
- Alexandrino, T.D.; Ferrari, R.A.; de Oliveira, L.M.; de Cássia, S.C.; Ormenese, R.; Pacheco, M.T.B. Fractioning of the Sunflower Flour Components: Physical, Chemical and Nutritional Evaluation of the Fractions. LWT 2017, 84, 426–432. [Google Scholar] [CrossRef]
- Öztürk, Z.; Lille, M.; Rosa-Sibakov, N.; Sozer, N. Impact of Heat Treatment and High Moisture Extrusion on the in Vitro Protein Digestibility of Sunflower and Pea Protein Ingredients. LWT 2024, 214, 117133. [Google Scholar] [CrossRef]
- Francesca, G.; Costanza, C.; Federica, N.; Laura, P. Investigating the Suitability of Sunflower Press-Cake Proteins in Formulated Sports Beverages. Food Funct. 2025, 16, 1992–2003. [Google Scholar] [CrossRef]
- Wildermuth, S.R.; Young, E.E.; Were, L.M. Chlorogenic Acid Oxidation and Its Reaction with Sunflower Proteins to Form Green-Colored Complexes. Compr. Rev. Food Sci. Food Saf. 2016, 15, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Verde, C.L.; Pacioles, C.T.; Paterson, N.; Chin, J.; Owens, C.P.; Senger, L.W. Hydrolysis of Chlorogenic Acid in Sunflower Flour Increases Consumer Acceptability of Sunflower Flour Cookies by Improving Cookie Color. J. Food Sci. 2023, 88, 3538–3550. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.F.; Thews, G.; Lang, F. Physiologie Des Menschen 2000, Volume 69.
- Mouritsen, O.G. The Science of Taste. Flavour 2015, 4, 10–11. [Google Scholar] [CrossRef]
- Zhou, Y.; O’Connell, T.F.; Ghaninia, M.; Smith, B.H.; Hong, E.J.; Sharpee, T.O. Low-Dimensional Olfactory Signatures of Fruit Ripening and Fermentation. bioRxiv 2024. [Google Scholar] [CrossRef]
- Menini, A.; Lagostena, L.; Boccaccio, A. Olfaction: From Odorant Molecules to the Olfactory Cortex. News Physiol. Sci. 2004, 19, 101–104. [Google Scholar] [CrossRef]
- Brattoli, M.; Cisternino, E.; Rosario Dambruoso, P.; de Gennaro, G.; Giungato, P.; Mazzone, A.; Palmisani, J.; Tutino, M. Gas Chromatography Analysis with Olfactometric Detection (GC-O) as a Useful Methodology for Chemical Characterization of Odorous Compounds. Sensors 2013, 13, 16759–16800. [Google Scholar] [CrossRef]
- Schieberle, P.; Hofmann, T. Mapping the Combinatorial Code of Food Flavors by Means of Molecular Sensory Science Approach. In Food Flavors: Chemical, Sensory and Technological Properties; Jelen, H., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 413–438. [Google Scholar]
- Granvogl, M.; Schieberle, P. The Sensomics Approach: A Useful Tool to Unravel the Genuine Aroma Blueprint of Foods and Aroma Changes during Food Processing. Compr. Anal. Chem. 2022, 96, 41–68. [Google Scholar] [CrossRef]
- Matheis, K.; Granvogl, M. Characterization of Key Odorants Causing a Fusty/Musty Off-Flavor in Native Cold-Pressed Rapeseed Oil by Means of the Sensomics Approach. J. Agric. Food Chem. 2016, 64, 8168–8178. [Google Scholar] [CrossRef]
- Dirndorfer, S.; Hammerl, R.; Kitajima, S.; Kitada, R.; Frank, O.; Dunkel, A.; Hofmann, T. Identification and Quantitation of Taste-Active Compounds in Dried Scallops by Combined Application of the Sensomics and a Quantitative NMR Approach. J. Agric. Food Chem. 2022, 70, 247–259. [Google Scholar] [CrossRef]
- Matheis, K.; Granvogl, M. Unraveling of the Fishy Off-Flavor in Steam-Treated Rapeseed Oil Using the Sensomics Concept. J. Agric. Food Chem. 2019, 67, 1484–1494. [Google Scholar] [CrossRef]
- Toelstede, S.; Hofmann, T. Sensomics Mapping and Identification of the Key Bitter Metabolites in Gouda Cheese. J. Agric. Food Chem. 2008, 56, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Dawid, C.; Hofmann, T. Identification of Sensory-Active Phytochemicals in Asparagus (Asparagus offIcinalis L.). J. Agric. Food Chem. 2012, 60, 11877–11888. [Google Scholar] [CrossRef]
- Hald, C.; Dawid, C.; Tressel, R.; Hofmann, T. Kaempferol 3-O-(2‴-O-Sinapoyl-β-Sophoroside) Causes the Undesired Bitter Taste of Canola/Rapeseed Protein Isolates. J. Agric. Food Chem. 2018, 67. [Google Scholar] [CrossRef]
- Biesalski, H.K. Mikronährstoffe Als Motor Der Evolution; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Yamaguchi, S.; Ninomiya, K. Umami and Food Palatability. J. Nutr. 2000, 130, 921S–926S. [Google Scholar] [CrossRef]
- Ong, J.S.; Hwang, D.L.D.; Zhong, V.W.; An, J.; Gharahkhani, P.; Breslin, P.A.S.; Wright, M.J.; Lawlor, D.A.; Whitfield, J.; MacGregor, S.; et al. Understanding the Role of Bitter Taste Perception in Coffee, Tea and Alcohol Consumption through Mendelian Randomization. Sci. Rep. 2018, 8, 16414. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Parra, P.A.; De Anda-Lobo, I.C.; Viejo, C.G.; Villarreal-Lara, R.; Clorio-Carillo, J.A.; Marín-Obispo, L.M.; Obispo-Fortunato, D.J.; Escobedo-Avellaneda, Z.; Fuentes, S.; Pérez-Carillo, E.; et al. Consumer Insights into the At-Home Liking of Commercial Beers: Integrating Nonvolatile and Volatile Flavor Chemometrics. Food Sci. Nutr. 2024, 12, 4063–4075. [Google Scholar] [CrossRef] [PubMed]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The Molecular Receptive Ranges of Human TAS2R Bitter Taste Receptors. Chem. Senses 2009, 35, 157–170. [Google Scholar] [CrossRef]
- Roura, E.; Aldayyani, A.; Thavaraj, P.; Prakash, S.; Greenway, D.; Thomas, W.G.; Meyerhof, W.; Roudnitzky, N.; Foster, S.R. Variability in Human Bitter Taste Sensitivity to Chemically Diverse Compounds Can Be Accounted for by Differential TAS2R Activation. Chem. Senses 2015, 40, 427–435. [Google Scholar] [CrossRef]
- Keast, R.S.J.; Roper, J. A Complex Relationship among Chemical Concentration, Detection Threshold, and Suprathreshold Intensity of Bitter Compounds. Chem. Senses 2007, 32, 245–253. [Google Scholar] [CrossRef]
- Dietrich, A.M. The Sense of Smell: Contributions of Orthonasal and Retronasal Perception Applied to Metallic Flavor of Drinking Water. J. Water Supply Res. Technol.-AQUA 2009, 58, 562–570. [Google Scholar] [CrossRef]
- Bachmanov, A.; Bosak, N.; Lin, C.; Matsumoto, I.; Ohmoto, M.; Reed, D.; Nelson, T. Genetics of Taste Receptors. Curr. Pharm. Des. 2014, 20, 2669–2683. [Google Scholar] [CrossRef]
- Gravina, S.A.; Yep, G.L.; Khan, M. Human Biology of Taste. Ann. Saudi Med. 2013, 33, 217–222. [Google Scholar] [CrossRef]
- Risso, D.; Tofanelli, S.; Morini, G.; Luiselli, D.; Drayna, D. Genetic Variation in Taste Receptor Pseudogenes Provides Evidence for a Dynamic Role in Human Evolution. BMC Evol. Biol. 2014, 14, 198. [Google Scholar] [CrossRef]
- Ki, S.Y.; Jeong, Y.T. Taste Receptors beyond Taste Buds. Int. J. Mol. Sci. 2022, 23, 9677. [Google Scholar] [CrossRef] [PubMed]
- Roper, S.D.; Chaudhari, N. Taste Buds: Cells, Signals and Synapses. Nat. Rev. Neurosci. 2017, 18, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Rhyu, M.R.; Lyall, V. Interaction of Taste-Active Nutrients with Taste Receptors. Curr. Opin. Physiol. 2021, 20, 64–69. [Google Scholar] [CrossRef]
- Li, X.; Staszewski, L.; Xu, H.; Durick, K.; Zoller, M.; Adler, E. Human Receptors for Sweet and Umami Taste. Proc. Natl. Acad. Sci. USA 2002, 99, 4692–4696. [Google Scholar] [CrossRef]
- Chaudhari, N.; Roper, S.D. The Cell Biology of Taste. J. Cell Biol. 2010, 190, 285–296. [Google Scholar] [CrossRef]
- Born, S.; Levit, A.; Niv, M.Y.; Meyerhof, W.; Behrens, M. The Human Bitter Taste Receptor TAS2R10 Is Tailored to Accommodate Numerous Diverse Ligands. J. Neurosci. 2013, 33, 201–213. [Google Scholar] [CrossRef]
- Meyerhof, W.; Born, S.; Brockhoff, A.; Behrens, M. Molecular Biology of Mammalian Bitter Taste Receptors. A Review. Flavour. Fragr. J. 2011, 26, 260–268. [Google Scholar] [CrossRef]
- Meyerhof, W.; Behrens, M.; Brockhoff, A.; Bufe, B.; Kuhn, C. Human Bitter Taste Perception. Chem. Senses 2005, 30, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Keast, R.S.J.; Breslin, P.A.S. An Overview of Binary Taste-Taste Interactions. Food Qual. Prefer. 2003, 14, 111–124. [Google Scholar] [CrossRef]
- Scharbert, S.; Holzmann, N.; Hofmann, T. Identification of the Astringent Taste Compounds in Black Tea Infusions by Combining Instrumental Analysis and Human Bioresponse. J. Agric. Food Chem. 2004, 52, 3498–3508. [Google Scholar] [CrossRef]
- Fricker, A. Lebensmittel—Mit Allen Sinnen Prüfen! Springer: Berlin/Heidelberg, Germany, 1984; ISBN 9783642823251. [Google Scholar]
- Degenhardt, A.G.; Hofmann, T. Bitter-Tasting and Kokumi-Enhancing Molecules in Thermally Processed Avocado (Persea americana Mill.). J. Agric. Food Chem. 2010, 58, 12906–12915. [Google Scholar] [CrossRef]
- Singldinger, B.; Dunkel, A.; Hofmann, T. The Cyclic Diarylheptanoid Asadanin as the Main Contributor to the Bitter Off-Taste in Hazelnuts (Corylus avellana L.). J. Agric. Food Chem. 2017, 65, 1677–1683. [Google Scholar] [CrossRef]
- Rotzoll, N.; Dunkel, A.; Hofmann, T. Quantitative Studies, Taste Reconstitution, and Omission Experiments on the Key Taste Compounds in Morel Mushrooms (Morchella deliciosa Fr.). J. Agric. Food Chem. 2006, 54, 2705–2711. [Google Scholar] [CrossRef]
- Jünger, M.; Mittermeier-Kleßinger, V.K.; Farrenkopf, A.; Dunkel, A.; Stark, T.; Fröhlich, S.; Somoza, V.; Dawid, C.; Hofmann, T. Sensoproteomic Discovery of Taste-Modulating Peptides and Taste Re-Engineering of Soy Sauce. J. Agric. Food Chem. 2022, 70, 6503–6518. [Google Scholar] [CrossRef]
- Scharbert, S.; Hofmann, T. Molecular Definition of Black Tea Taste by Means of Quantitative Studies, Taste Reconstitution, and Omission Experiments. J. Agric. Food Chem. 2005, 53, 5377–5384. [Google Scholar] [CrossRef]
- Dunkel, A.; Köster, J.; Hofmann, T. Molecular and Sensory Characterization of γ-Glutamyl Peptides as Key Contributors to the Kokumi Taste of Edible Beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2007, 55, 6712–6719. [Google Scholar] [CrossRef]
- Yin, W.; Shi, R.; Li, S.; Ma, X.; Wang, X.; Wang, A. Changes in Key Aroma-Active Compounds and Sensory Characteristics of Sunflower Oils Induced by Seed Roasting. J. Food Sci. 2022, 87, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Laemont, J.; Barringer, S. Effect of PH, Reducing Sugars, and Protein on Roasted Sunflower Seed Aroma Volatiles. Foods 2023, 12, 4155. [Google Scholar] [CrossRef]
- Guo, S.; Na Jom, K.; Ge, Y. Influence of Roasting Condition on Flavor Profile of Sunflower Seeds: A Flavoromics Approach. Sci. Rep. 2019, 9, 11295. [Google Scholar] [CrossRef]
- Guo, S.; Lan, W.; Chen, X.; Ge, Y. Exploring the Metabolic and Flavoromic Variations of Germinated Sunflower Seed during Roasting Conditions. Food Mater. Res. 2024, 4, e012. [Google Scholar] [CrossRef]
- Dunkel, A.; Steinhaus, M.; Kotthoff, M.; Nowak, B.; Krautwurst, D.; Schieberle, P.; Hofmann, T. Nature’s Chemical Signatures in Human Olfaction: A Foodborne Perspective for Future Biotechnology. Angew. Chem. Int. Ed. 2014, 53, 7124–7143. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Shen, X.; Chen, J.; Yang, T.; Hou, R. Determination of Volatile Compounds of Chinese Traditional Aromatic Sunflower Seeds (Helianthus annulus L.). Int. J. Food Eng. 2015, 11, 85–95. [Google Scholar] [CrossRef]
- Bocci, F.; Frega, N. Analysis of the Volatile Fraction from Sunflower Oil Extracted under Pressure. JAOCS J. Am. Oil Chem. Soc. 1996, 73, 713–716. [Google Scholar] [CrossRef]
- Bendini, A.; Barbieri, S.; Valli, E.; Buchecker, K.; Canavari, M.; Toschi, T.G. Quality Evaluation of Cold Pressed Sunflower Oils by Sensory and Chemical Analysis. Eur. J. Lipid Sci. Technol. 2011, 113, 1375–1384. [Google Scholar] [CrossRef]
- Yin, W.; Shi, R.; Li, K.; Wang, X.; Wang, A.; Zhao, Y.; Zhai, Z. Effect of Microwave Pretreatment of Sunflower Kernels on the Aroma-Active Composition, Sensory Quality, Lipid Oxidation, Tocopherols, Heterocyclic Amines and Polycyclic Aromatic Hydrocarbons of Sunflower Oil. LWT 2022, 17, 114077. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, H.; Chang, X.; Li, X.; Zhang, Y.; Zhu, B.; Wang, X. Investigation of Aroma Characteristics of Seven Chinese Commercial Sunflower Seed Oils Using a Combination of Descriptive Analysis, GC-Quadrupole-MS, and GC-Orbitrap-MS. Food Chem. X 2023, 18, 100690. [Google Scholar] [CrossRef]
- Li, W.; Wang, Q.; Huan, H.; Wu, G.; Jin, Q.; Zhang, Y.; Wang, X. Characterization of Volatile Compounds and Odorants in Different Sichuan Pepper Varieties in Tallow Hotpot. Foods 2025, 14, 627. [Google Scholar] [CrossRef] [PubMed]
- Galisteo, A.; Pérez Rodríguez, Á.; González, A.; Barrero, A.F.; Quílez del Moral, J.F. Terpenoid Diversity in Sunflower (Helianthus annuus L.) and Their Potential in Crop Protection. Phytochem. Rev. 2023, 23, 583–623. [Google Scholar] [CrossRef]
- Lattanzio, V. Phenolic Compounds: Introduction. In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1543–1580. ISBN 9783642221446. [Google Scholar]
- Robertson, J.A.; Lyon, B.G.; Morrison, W.H.; Miller, J.F. Sensory and Chemical Evaluation of Stored Oil-Roasted, High Oleic Nonoil Sunflower Kernels. J. Am. Oil Chem. Soc. 1988, 65, 985–989. [Google Scholar] [CrossRef]
- Rayman Ergün, A. Investigation of Conventional and Microwave Combined Roasting Effect on the Physicochemical, Textural and Sensory Properties of Sunflower Seed. Gıda 2024, 49, 891–902. [Google Scholar] [CrossRef]
- Muttagi, G.C.; Joshi, N.; Shadakshari, Y.G.; Chandru, R. Storage Stability of Value Added Products from Sunflower Kernels. J. Food Sci. Technol. 2014, 51, 1806–1816. [Google Scholar] [CrossRef][Green Version]
- Mosayebi, M.; Kashaninejad, M.; Najafian, L. Optimizing Physiochemical and Sensory Properties of Infrared-Hot Air Roasted Sunflower Kernels Using Response Surface Methodology. J. Food Qual. 2018, 2018, 4186050. [Google Scholar] [CrossRef]
- Chernova, A.; Gubaev, R.; Mazin, P.; Goryunova, S.; Demurin, Y.; Gorlova, L.; Vanushkina, A.; Mair, W.; Anikanov, N.; Martynova, E.; et al. UPLC–MS Triglyceride Profiling in Sunflower and Rapeseed Seeds. Biomolecules 2019, 9, 9. [Google Scholar] [CrossRef]
- Fernández-Moya, V.; Martínez-Force, E.; Garcés, R. Identification of Triacylglycerol Species from High-Saturated Sunflower (Helianthus annuus) Mutants. J. Agric. Food Chem. 2000, 48, 764–769. [Google Scholar] [CrossRef]
- Gotor, A.A.; Rhazi, L. Effects of Refining Process on Sunflower Oil Minor Components: A Review. OCL—Oilseeds Fats Crops Lipids 2016, 23, D207. [Google Scholar] [CrossRef]
- Romano, R.; Filosa, G.; Pizzolongo, F.; Durazzo, A.; Lucarini, M.; Severino, P.; Souto, E.B.; Santini, A. Oxidative Stability of High Oleic Sunflower Oil during Deep-Frying Process of Purple Potato Purple Majesty. Heliyon 2021, 7, e06294. [Google Scholar] [CrossRef]
- Buzgău, G.; Petruț, G.; Bîrsan, P.; Muste, S. The Usage of High Oleic Sunflower Oil in Production of “ Tortilla Chips ” Type Product. J. Agroaliment. Process. Technol. 2022, 28, 199–202. [Google Scholar]
- Álvarez Graña, S.; Abarquero, D.; Claro, J.; Combarros-Fuertes, P.; Fresno, J.M.; Tornadijo, M.E. Behaviour of Sunflower (Helianthus annuus L.) Oil and High Oleic Sunflower Oil during the Frying of Churros. Food Chem. Adv. 2025, 6, 100899. [Google Scholar] [CrossRef]
- Günther-Jordanland, K.; Dawid, C.; Hofmann, T. Quantitation and Taste Contribution of Sensory Active Molecules in Oat (Avena sativa L.). J. Agric. Food Chem. 2020, 68, 10097–10108. [Google Scholar] [CrossRef] [PubMed]
- Thepthanee, C.; Li, H.; Wei, H.; Prakitchaiwattana, C.; Siriamornpun, S. Effect of Soaking, Germination, and Roasting on Phenolic Composition, Antioxidant Activities, and Fatty Acid Profile of Sunflower (Helianthus annuus L.) Seeds. Horticulturae 2024, 10, 387. [Google Scholar] [CrossRef]
- Guo, S.; Jom, K.N.; Ge, Y. Effects of Storage Temperature and Time on Metabolic and Flavouromic Profiles of Roasted Germinated Sunflower Seeds. J. Food Nutr. Res. 2020, 59, 219–232. [Google Scholar]
- De Leonardis, A.; Macciola, V.; Di Rocco, A. Oxidative Stabilization of Cold-Pressed Sunflower Oil Using Phenolic Compounds of the Same Seeds. J. Sci. Food Agric. 2003, 83, 523–528. [Google Scholar] [CrossRef]
- Grompone, M.A. Sunflower and High-Oleic Sunflower Oils. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; Wiley: Chichester, UK, 2020; pp. 1–54. ISBN 047167849X. [Google Scholar]
- Ruiz-Méndez, M.-V.; Velasco, J.; Lastrucci, A.S.; Márquez-Ruiz, G. Lipid Quality Changes in French Fries, Chicken Croquettes, and Chicken Nuggets Fried with High-Linoleic and High-Oleic Sunflower Oils in Domestic Deep Fryers. Foods 2024, 13, 2419. [Google Scholar] [CrossRef]
- Mittermeier-Kleßinger, V.K.; Hofmann, T.; Dawid, C. Mitigating Off-Flavors of Plant-Based Proteins. J. Agric. Food Chem. 2021, 69, 9202–9207. [Google Scholar] [CrossRef]
- Nishimura, T.; Kato, H. Taste of Free Amino Acids and Peptides. Food Rev. Int. 1988, 4, 175–194. [Google Scholar] [CrossRef]
- Delompré, T.; Guichard, E.; Briand, L.; Salles, C. Taste Perception of Nutrients Found in Nutritional Supplements: A Review. Nutrients 2019, 11, 2050. [Google Scholar] [CrossRef]
- Bachmanov, A.A.; Bosak, N.P.; Glendinning, J.I.; Inoue, M.; Li, X.; Manita, S.; McCaughey, S.A.; Murata, Y.; Reed, D.R.; Tordoff, M.G.; et al. Genetics of Amino Acid Taste and Appetite. Adv. Nutr. 2016, 7, 806S–822S. [Google Scholar] [CrossRef] [PubMed]
- Solms, J. Taste of Amino Acids, Peptides, and Proteins. J. Agric. Food Chem. 1969, 17, 686–688. [Google Scholar] [CrossRef]
- Tanase, R.; Senda, R.; Matsunaga, Y.; Narukawa, M. Taste Characteristics of Various Amino Acid Derivatives. J. Nutr. Sci. Vitaminol. 2022, 68, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.J.; Schieber, A.; Gänzle, M.G. Formation of Taste-Active Amino Acids, Amino Acid Derivatives and Peptides in Food Fermentations—A Review. Food Res. Int. 2016, 89, 39–47. [Google Scholar] [CrossRef]
- Chen, H.; Cui, H.; Zhang, M.; Hayat, K.; Yu, J.; Xia, S.; Zhai, Y.; Zhang, X. Improving the Flavor and Oxidation Resistance of Processed Sunflower Seeds with Maillard Peptides. Food Bioproc Technol. 2019, 12, 809–819. [Google Scholar] [CrossRef]
- Ivanova, P.; Chalova, V.; Koleva, L.; Pishtiyski, I. Amino Acid Composition and Solubility of Proteins Isolated from Sunflower Meal Produced in Bulgaria. Int. Food Res. J. 2013, 20, 2995–3000. [Google Scholar]
- Petraru, A.; Ursachi, F.; Amariei, S. Nutritional Characteristics Assessment of Sunflower Seeds, Oil and Cake. Perspective of Using Sunflower Oilcakes as a Functional Ingredient. Plants 2021, 10, 2487. [Google Scholar] [CrossRef]
- Ordóñez, C.; Benítez, C.; González, J.L. Amino Acid Production from a Sunflower Wholemeal Protein Concentrate. Bioresour. Technol. 2008, 99, 4749–4754. [Google Scholar] [CrossRef]
- Akande, K.E. Proximate and Amino Acid Analyses of Full-Fat Sunflower (Helianthus annuus L.) Seed Meal. Singap. J. Sci. Res. 2011, 1, 179–183. [Google Scholar] [CrossRef]
- Hillmann, H.; Hofmann, T. Quantitation of Key Tastants and Re-Engineering the Taste of Parmesan Cheese. J. Agric. Food Chem. 2016, 64, 1794–1805. [Google Scholar] [CrossRef]
- Bao, X.; Ma, S.; Fu, Y.; Wu, J.; Zhang, M. Sensory and Structural Characterization of Umami Peptides Derived from Sunflower Seed. CYTA-J. Food 2020, 18, 485–492. [Google Scholar] [CrossRef]
- Eric, K.; Raymond, L.V.; Huang, M.; Cheserek, M.J.; Hayat, K.; Savio, N.D.; Amédée, M.; Zhang, X. Sensory Attributes and Antioxidant Capacity of Maillard Reaction Products Derived from Xylose, Cysteine and Sunflower Protein Hydrolysate Model System. Food Res. Int. 2013, 54, 1437–1447. [Google Scholar] [CrossRef]
- Karangwa, E.; Zhang, X.; Murekatete, N.; Masamba, K.; Raymond, L.V.; Shabbar, A.; Zhang, Y.; Duhoranimana, E.; Muhoza, B.; Song, S. Effect of Substrate Type on Sensory Characteristics and Antioxidant Capacity of Sunflower Maillard Reaction Products. Eur. Food Res. Technol. 2015, 240, 939–960. [Google Scholar] [CrossRef]
- Muttagi, G.C.; Joshi, N. Physico-Chemical Composition of Selected Sunflower Seed Cultivars. Int. J. Chem. Stud. 2020, 8, 2095–2100. [Google Scholar] [CrossRef]
- Nadeem, M.; Anjum, F.M.; Arshad, M.U.; Hussain, S. Chemical Characteristics and Antioxidant Activity of Different Sunflower Hybrids and Their Utilization in Bread. Afr. J. Food Sci. 2010, 4, 618–626. [Google Scholar]
- USDA Seeds, Sunflower Seed, Kernel, Raw. Available online: https://fdc.nal.usda.gov/ (accessed on 25 January 2025).
- Sabir, M.A.; Sosulski, F.W.; Hamon, N.W. Sunflower Carbohydrates. J. Agric. Food Chem. 1975, 23, 16–19. [Google Scholar] [CrossRef]
- Stark, T.; Bareuther, S.; Hofmann, T. Molecular Definition of the Taste of Roasted Cocoa Nibs (Theobroma cacao) by Means of Quantitative Studies and Sensory Experiments. J. Agric. Food Chem. 2006, 54, 5530–5539. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Dackis, C. Taste of Nutrients: Amino Acids, Vitamins, and Fatty Acids. Percept. Psychophys. 1975, 17, 140–146. [Google Scholar] [CrossRef]
- Delompré, T.; Belloir, C.; Martin, C.; Salles, C.; Briand, L. Detection of Bitterness in Vitamins Is Mediated by the Activation of Bitter Taste Receptors. Nutrients 2022, 14, 4141. [Google Scholar] [CrossRef]
- Bickel-Sandkötter, S. Nutzpflanzen Und Ihre Inhaltsstoffe; Quelle & Meyer: Wiebelsheim, Germany, 2001; Volume 1, pp. 1–481. [Google Scholar]
- Ghisoni, S.; Chiodelli, G.; Rocchetti, G.; Kane, D.; Lucini, L. UHPLC-ESI-QTOF-MS Screening of Lignans and Other Phenolics in Dry Seeds for Human Consumption. J. Funct. Foods 2017, 34, 229–236. [Google Scholar] [CrossRef]
- Aliani, M.; Eskin, N.A.M. Bitterness: Perception, Chemistry and Food Processing; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 9781118590294. [Google Scholar]
- Ozdal, T.; Yalcinkaya, İ.E.; Toydemir, G.; Capanoglu, E. Polyphenol-Protein Interactions and Changes in Functional Properties and Digestibility. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 566–577. [Google Scholar] [CrossRef]
- Pedrosa, M.M.; Muzquiz, M.; García-Vallejo, C.; Burbano, C.; Cuadrado, C.; Ayet, G.; Robredo, L.M. Determination of Caffeic and Chlorogenic Acids and Their Derivatives in Different Sunflower Seeds. J. Sci. Food Agric. 2000, 80, 459–464. [Google Scholar] [CrossRef]
- Lule, S.U.; Xia, W. Food Phenolics, Pros and Cons: A Review. Food Rev. Int. 2005, 21, 367–388. [Google Scholar] [CrossRef]
- Žilic, S.; Maksimovic Dragišic, J.; Maksimovic, V.; Maksimovic, M.; Basic, Z.; Crevar, M.; Stankovic, G. The Content of Antioxidants in Sunflower Seed and Kernel. Helia 2010, 33, 75–84. [Google Scholar] [CrossRef]
- Bohm, B.A.; Stuessy, T.F. Flavonoids of the Sunflower Family (Asteraceae); Springer: Vienna, Austria, 2001; Volume 50, ISBN 3800135388.
- Dabrowsk, K.J.; Sosulski, F.W. Composition of Free and Hydrolyzable Phenolic Acids in Defatted Flours of Ten Oilseeds. J. Agric. Food Chem. 1984, 32, 128–130. [Google Scholar] [CrossRef]
- García, J.M.; Prieto, L.J.; Guevara, A.; Malagon, D.; Osorio, C. Chemical Studies of Yellow Tamarillo (Solanum Betaceum Cav.) Fruit Flavor by Using a Molecular Sensory Approach. Molecules 2016, 21, 1729. [Google Scholar] [CrossRef]
- Hofmann, T.; Hufnagel, J.C. Quantitative Reconstruction of the Nonvolatile Sensometabolome of a Red Wine. J. Agric. Food Chem. 2008, 56, 9190–9199. [Google Scholar]
- Siger, A.; Nogala-Kalucka, M.; Lampart-Szczapa, E. The Content and Antioxidant Activity of Phenolic Compounds in Cold-Pressed Plant Oils. J. Food Lipids 2008, 15, 137–149. [Google Scholar] [CrossRef]
- González-Pérez, S.; Vereijken, J.M. Sunflower Proteins: Overview of Their Physicochemical, Structural and Functional Properties. J. Sci. Food Agric. 2007, 87, 2173–2191. [Google Scholar] [CrossRef]
- Gök, S.B.; Erdoğdu, Y.; Göçmen, E.; Salbaş, B.; Erdem, T. Evaluation of the Phenolic Content and Antioxidant Activity of Sunflower Seeds under Deficit Irrigation Conditions. Riv. Ital. Delle Sostanze Grasse 2022, 99, 285–294. [Google Scholar]
- Özcan, M.M.; Yılmaz, F.G.; Uslu, N.; Kulluk, D.A.; Dursun, N.; Yılmaz, H. Determination of Bioactive Compounds, Phenolic Contents, Fatty Acid and Biogenic Element Profiles of the Seeds of Sunflower (Helianthus annuus L.) Genotypes. Food Humanit. 2024, 2, 100222. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. An Overview of the Phenolics of Canola and Rapeseed: Chemical, Sensory and Nutritional Significance. J. Am. Oil Chem. Soc. 1992, 69, 917–924. [Google Scholar] [CrossRef]
- Huang, C.J.; Zayas, J.F. Phenolic Acid Contributions to Taste Characteristics of Corn Germ Protein Flour Products. J. Food Sci. 1991, 56, 1308–1310. [Google Scholar] [CrossRef]
- George, K.O.; Moseti, K.O.; Wanyoko, J.K.; Kinyanjui, T.; Wachira, F.N. Quantitation of the Total Catechin Content in Oils Extracted from Seeds of Selected Tea (Camellia sinensis (L) O. Kuntze, Theaceae) Clones by RP-HPLC. Am. J. Plant Sci. 2015, 6, 1080–1089. [Google Scholar] [CrossRef]
- Thompson, L.U.; Boucher, B.A.; Liu, Z.; Cotterchio, M.; Kreiger, N. Phytoestrogen Content of Foods Consumed in Canada, Including Isoflavones, Lignans, and Coumestan. Nutr. Cancer 2006, 54, 184–201. [Google Scholar] [CrossRef]
- Roland, W.S.U.; Vincken, J.P.; Gouka, R.J.; Van Buren, L.; Gruppen, H.; Smit, G. Soy Isoflavones and Other Isoflavonoids Activate the Human Bitter Taste Receptors HTAS2R14 and HTAS2R39. J. Agric. Food Chem. 2011, 59, 11764–11771. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo-Diago, A.; Dizy, M.; Fernández-Zurbano, P. Contribution of Low Molecular Weight Phenols to Bitter Taste and Mouthfeel Properties in Red Wines. Food Chem. 2014, 154, 187–198. [Google Scholar] [CrossRef]
- Li, J.; Yao, Y.; Wang, J.; Hua, J.; Wang, J.; Yang, Y.; Dong, C.; Zhou, Q.; Jiang, Y.; Deng, Y.; et al. Rutin, γ-Aminobutyric Acid, Gallic Acid, and Caffeine Negatively Affect the Sweet-Mellow Taste of Congou Black Tea Infusions. Molecules 2019, 24, 4221. [Google Scholar] [CrossRef]
- Kuhnle, G.G.C.; Dell’Aquila, C.; Aspinall, S.M.; Runswick, S.A.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen Content of Beverages, Nuts, Seeds, and Oils. J. Agric. Food Chem. 2008, 56, 7311–7315. [Google Scholar] [CrossRef]
- Tham, D.M.; Gardner, C.D.; Haskell, W.L. Potential Health Benefits of Dietary Phytoestrogens: A Review of the Clinical, Epidemiological, and Mechanistic Evidence. J. Clin. Endocrinol. Metab. 1998, 83, 2223–2235. [Google Scholar] [CrossRef]
- Winstel, D.; Marchal, A. Lignans in Spirits: Chemical Diversity, Quantification, and Sensory Impact of (±)-Lyoniresinol. Molecules 2019, 24, 117. [Google Scholar] [CrossRef] [PubMed]
- Spring, O. Sesquiterpene Lactones in Sunflower Oil. LWT 2021, 142, 111047. [Google Scholar] [CrossRef]
- Rade, D.; Mokrovčak, Ž.; Štrucelj, D.; Škevin, D.; Nederal, S. The Effect of Processing Conditions on the Nontriacylglycerol Constituents of Sunflower Oil. Acta Aliment. 2004, 33, 7–18. [Google Scholar] [CrossRef]
- Amakura, Y.; Yoshimura, M.; Yamakami, S.; Yoshida, T. Isolation of Phenolic Constituents and Characterization of Antioxidant Markers from Sunflower (Helianthus annuus) Seed Extract. Phytochem. Lett. 2013, 6, 302–305. [Google Scholar] [CrossRef]
- Jan, A.U.; Hadi, F.; Zeb, A.; Islam, Z. Identification and Quantification of Phenolic Compounds through Reversed Phase HPLC-DAD Method in Sunflower Seeds under Various Treatments of Potassium Nitrate, Zinc Sulphate and Gibberellic Acid. J. Food Meas. Charact. 2018, 12, 269–277. [Google Scholar] [CrossRef]
- Robichaud, J.L.; Noble, A.C. Astringency and Bitterness of Selected Phenolics in Wine. J. Sci. Food Agric. 1990, 53, 343–353. [Google Scholar] [CrossRef]
- International Food Information Council (IFIC). 2020 Food and Health Survey; International Food Information Council: Washington, DC, USA, 2020. [Google Scholar]
- FAO Expert Consultation. Dietary Protein Quality Evaluation in Human Nutrition, 92nd ed.; Report of an FAQ Expert Consultation; FAO Expert Consultation: Rome, Italy, 2013; Volume 92. [Google Scholar]
- Hadidi, M.; Aghababaei, F.; McClements, D.J. Sunflower Meal/Cake as a Sustainable Protein Source for Global Food Demand: Towards a Zero-Hunger World. Food Hydrocoll. 2024, 147, 109329. [Google Scholar] [CrossRef]
- Blicharz-Kania, A.; Pecyna, A.; Zdybel, B.; Andrejko, D.; Marczuk, A. Sunflower Seed Cake as a Source of Nutrients in Gluten-Free Bread. Sci. Rep. 2023, 13, 10864. [Google Scholar] [CrossRef]
- Singh, R.; Sá, A.G.A.; Sharma, S.; Nadimi, M.; Paliwal, J.; House, J.D.; Koksel, F. Effects of Feed Moisture Content on the Physical and Nutritional Quality Attributes of Sunflower Meal-Based High-Moisture Meat Analogues. Food Bioprocess Technol. 2024, 17, 1897–1913. [Google Scholar] [CrossRef]
- Baurina, A.V.; Baurin, D.V.; Shakir, I.V.; Panfilov, V.I. Use of Sunflower Protein in Snack Bars. Chem. Eng. Trans. 2021, 87, 1–6. [Google Scholar] [CrossRef]
- Shahbandeh, M. Worldwide Oilseed Production in 2022/2023, by Type (in Million Metric Tons). Available online: https://www.statista.com/statistics/267271/worldwide-oilseed-production-since-2008/ (accessed on 6 July 2024).
- Gläser, P.; Dawid, C.; Meister, S.; Bader-Mittermaier, S.; Schott, M.; Eisner, P.; Hofmann, T. Molecularization of Bitter Off-Taste Compounds in Pea-Protein Isolates (Pisum sativum L.). J. Agric. Food Chem. 2020, 68, 10374–10387. [Google Scholar] [CrossRef] [PubMed]
- Reineccius, G.A. Flavor Interactions with Proteins. Curr. Opin. Food Sci. 2022, 47, 100884. [Google Scholar] [CrossRef]
- Guichard, E. Interactions between Flavor Compounds and Food Ingredients and Their Influence on Flavor Perception. Food Rev. Int. 2002, 18, 49–70. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; Lang, Y.; Huang, M.; Hu, S.; Liu, H.; Sun, B.; Long, Y.; Wu, J.; Dong, W. Interactions between Food Matrices and Odorants: A Review. Food Chem. 2025, 466, 142086. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, W.; Quek, S.Y.; Zhao, L. Flavor–Food Ingredient Interactions in Fortified or Reformulated Novel Food: Binding Behaviors, Manipulation Strategies, Sensory Impacts, and Future Trends in Delicious and Healthy Food Design. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4004–4029. [Google Scholar] [CrossRef] [PubMed]
- Brannan, G.D.; Setser, C.S.; Kemp, K.E. Interaction of Astringency and Taste Characteristics. J. Sens. Stud. 2001, 16, 179–197. [Google Scholar] [CrossRef]
- Han, H.; Zhang, Z.; Yang, Z.; Blank, I.; Zhong, F.; Wang, B.; Wang, Y.; Zeng, H. A Comparative Study to Determine the Key Aroma Components of Yogurt Aroma Types Based on Sensomics and Flavoromics. Food Chem. 2024, 460, 140618. [Google Scholar] [CrossRef]

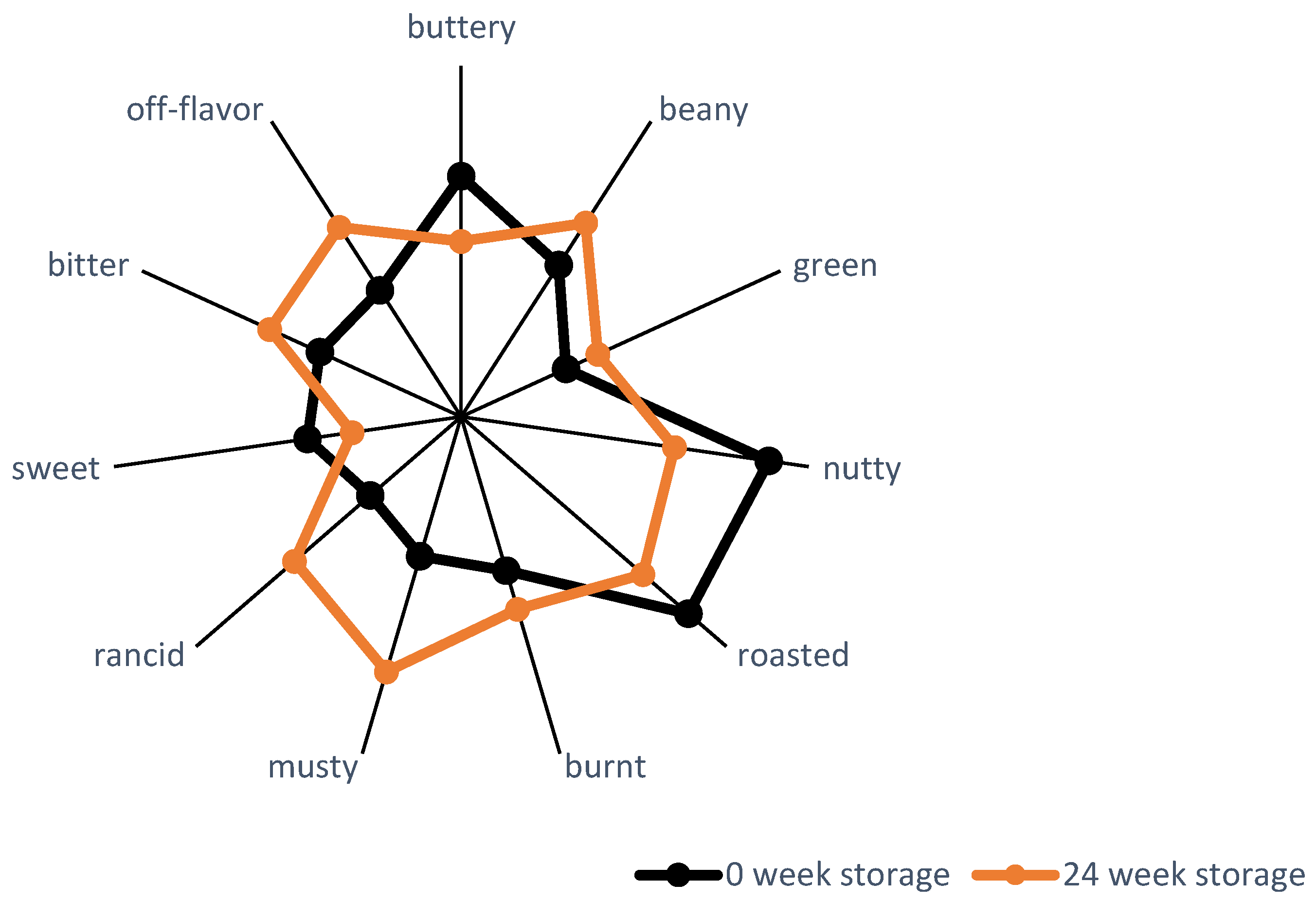
| Parameter | Content (g/100 g Kernels) |
|---|---|
| Moisture | 4.73 |
| Protein | 20.8 |
| Fat | 51.5 |
| Carbohydrates | 20.0 |
| Fiber | 8.6 |
| Ash | 3.02 |
| Compound(s) | Reported Concentrations (µg/kg) | Odor Threshold (µg/kg) | Odor Description | Method a,b,d,e | |
|---|---|---|---|---|---|
| Raw and roasted seeds | α-pinene | 7570 f, 21,850 r | 6 a,e | pine | HS-SPME-GC-MS, multivariate statistical analysis |
| β-pinene | 760 f, 3000 r | 140 a | woody, pine-like | ||
| octane | 2670 f | / | / | ||
| furfural | 950 f, 8180 r | 3000 e | almond, sweet | ||
| hexanal | 1350 f, 8490 r | 479 e | green, fatty | ||
| γ-butyrolactone | 1150 f, 3190 r | 1000 e | creamy | ||
| 2-methylbutanal | 540 f, 2110 r | 23 e | malty, almond | ||
| 2,5-dimeththylpyrazine | 200 f, 10,190 r | 800 e | roasty, cocoa | ||
| 2,3-dimethylpyrazine | 550–1300 r | 100 e | nut, peanut, cocoa, | ||
| 2-ethyl-3- methylpyrazine | 140 f 3890 r | 20 e | nutty, cereal like | ||
| 2-ethyl-3,5-dimethylpyrazine | 2090 r | 7.5 e | nutty | ||
| Cold-pressed oil | α-pinene | 11,145 a–94,890 b | 6 a | woody, pine-like | Dynamic HS-GC-MS; SPME-GC-MS, QDA; Molecular sensory science/Sensomics |
| β-pinene | 4068 a | 140 a | woody, pine-like | Dynamic HS-GC-MS; Molecular sensory science | |
| sabinene | / | 980 c | woody, citrus-like | Dynamic HS-GC-MS | |
| limonene | / | 2100 c | lemon, citrus | ||
| hexanal | 541 a | 73 a | green, grassy | Dynamic HS-GC-MS; Molecular sensory science/Sensomics | |
| 3-methyl-1-butanol | 200–480 b | 100 b | nutty, fruity | SPME-GC-MS, QDA | |
| linalool | 56 a | 6 a | citrus, fruity | Molecular sensory science/Sensomics (HS-SPME GC-O-MS, SAFE, AEDA, OAV, GC-O, recombination model) | |
| octanal | 125 a | 56 a | fruity, green | ||
| α-phellandrene | 36 a | 40 a | citrus, sweet, peel | ||
| (E)-2-octenal | 69 a | 61 a | fatty, floral | ||
| Roasted oil | 2-methylbutanal | 6726 a | 34 a | roasted, malty | Molecular sensory science/Sensomics (HS-SPME GC-O-MS, SAFE, AEDA, OAV, GC-O, recombination model) |
| 3-methylbutanal | 714 a | 15 a | fatty, almond | ||
| 2,6-dimethylpyrazine | 2329 a | 20 a | nutty, roasted, coffee | ||
| 2,5-dimethylpyrazine | 12,228 a | 200 a | nutty, potato | ||
| 2,3-dimethylpyrazine | 238 a | 8 a | nutty, popcorn | ||
| 2,3-dimethyl-5-ethylpyrazine | 213 a | 100 a | roasted, nutty, sweet | ||
| 2,3-pentanedione | 1456 a | 50 a | buttery, sweet, spicy | ||
| 2-pentylfuran | 1332 a | 130 a | buttery, caramel | ||
| 1-pentanol | 693 a | 470 a | bread-like, sweet |
| Name | Content (µmol/kg) R1 | Taste | Taste Threshold (µmol/kg) R2 | DoT |
|---|---|---|---|---|
| α-Linolenic acid | ~539–1077 | Scratchy, bitter | ~189 a, ~277 b | ~1.9–3.9 |
| Linoleic acid | ~17,508–26,422 | Scratchy, bitter | ~270 a, ~1810 b | ~9.7–14.6 |
| Oleic acid | ~9983–29,631 | Scratchy, bitter | ~203 a, ~2180 b | ~4.6–13.6 |
| Palmitic acid | ~2067–3003 | Scratchy, bitter | ~1002 a, ~1546 b | ~1.3–1.9 |
| Stearic acid | ~949–1582 | Scratchy, bitter | ~645 a, ~726 b | ~1.3–2.2 |
| Name | Content (µmol/kg) R1 | Taste | Taste Threshold (µmol/kg) R2 | DoT |
|---|---|---|---|---|
| Alanine | ~2110.4 | Sweet | ~12,000 | <1 |
| Arginine | / | Bitter | ~75,000 | |
| Aspartic acid | ~255.53 | Umami | ~600 | <1 |
| Glutamic acid | ~1229.56 | Umami | ~1100 | 1.1 |
| Glycine | ~1810.93 | Sweet | ~25,000 | <1 |
| Histidine | / | Bitter | ~45,000 | |
| Isoleucine | / | Bitter | ~10,000 | |
| Leucine | ~383.84 | Bitter | ~11,000 | <1 |
| Lysine | / | Bitter | ~80,000 | |
| Methionine | / | Sweet | ~5000 | |
| Phenylalanine | / | Bitter | ~45,000 | |
| Proline | / | Sweet | ~25,000 | |
| Serine | / | Sweet | ~25,000 | |
| Threonine | / | Sweet | ~35,000 | |
| Tyrosine | / | Bitter | ~4000 | |
| Valine | / | Bitter | ~30,000 |
| Name | Content (µmol/kg) R1 | Taste | Taste Threshold (µmol/kg) R2 | DoT |
|---|---|---|---|---|
| Calcium | ~19,461–28,942 | Bitter, astringent | ~6200 | ~3.1–4.7 |
| Magnesium | ~124,254 | Bitter, astringent | ~6400 | ~19.4 |
| Potassium | ~168,030 | Salty | ~13,000 | ~12.9 |
| Sodium | <~1087 | Salty | ~3900 | <1 |
| Name | Content (µmol/kg) R1 | Taste | Taste Threshold (µmol/kg) R2 | DoT |
|---|---|---|---|---|
| Caffeic acid | ~142–1482 a | Astringent | ~72 | ~2–20 |
| p-Coumaric acid | ~15.2 b | Astringent | ~139 | <1 |
| Ferulic acid | ~87–639 | Astringent | ~67 | ~1.2–9.5 |
| Gallic acid | ~65.8 b | Astringent | ~292 | <1 |
| p-Hydroxybenzoic acid | / | Astringent | ~665 | |
| Protocatechuic acid | ~329.6 b | Astringent | ~206 | ~1.6 |
| Rosmarinic acid | ~233–391 | Bitter | ~102.6 | ~2–3.8 |
| Sinapic acid | ~69.6 b | Astringent | ~693 | <1 |
| Syringic acid | / | Astringent | ~263 | |
| Vanillic acid | / | Astringent | ~315 |
| Name | Content (µmol/kg) R1 | Taste | Taste Threshold (µmol/kg) R2 | DoT |
|---|---|---|---|---|
| Apigenin | ~11 | Bitter | / | |
| Catechin | / | Bitter, astringent | ~800, 410 | |
| Coumestrol | ~0.004 a | Bitter | ~250” | <1 |
| Daidzein | ~0.094 a | Bitter | ~500” | <1 |
| Epicatechin | / | Bitter, astingent | ~1000, 930 | |
| Epicatechin gallate | / | Astringent | ~260 | |
| Epigallocatechin | / | Astringent | ~520 | |
| Epigallocatechin gallate | / | Astringent | ~190 | |
| Formononetin | ~0.026 a | Bitter | ~500” | <1 |
| Genistein | ~0.074 a | Bitter | ~4–8” | <1 |
| Glycetein | ~0.018 a | Bitter | ~500” | <1 |
| Kaempferol | ~1.75 | Bitter, astringent | ~69.87 | <1 |
| Luteolin | / | Bitter | / | |
| Myricetin | ~131.9–194.8 | Bitter, astringent | ~31.42 | ~4.2–6.2 |
| Quercetin | ~19 b | Bitter, astringent | ~33.09 | <1 |
| Rutin | / | Astringent | ~0.00115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huseynli, L.; Walser, C.; Blumenthaler, L.; Vene, K.; Dawid, C. Toward a Comprehensive Understanding of Flavor of Sunflower Products: A Review of Confirmed and Prospective Aroma- and Taste-Active Compounds. Foods 2025, 14, 1940. https://doi.org/10.3390/foods14111940
Huseynli L, Walser C, Blumenthaler L, Vene K, Dawid C. Toward a Comprehensive Understanding of Flavor of Sunflower Products: A Review of Confirmed and Prospective Aroma- and Taste-Active Compounds. Foods. 2025; 14(11):1940. https://doi.org/10.3390/foods14111940
Chicago/Turabian StyleHuseynli, Lachinkhanim, Christoph Walser, Luise Blumenthaler, Kristel Vene, and Corinna Dawid. 2025. "Toward a Comprehensive Understanding of Flavor of Sunflower Products: A Review of Confirmed and Prospective Aroma- and Taste-Active Compounds" Foods 14, no. 11: 1940. https://doi.org/10.3390/foods14111940
APA StyleHuseynli, L., Walser, C., Blumenthaler, L., Vene, K., & Dawid, C. (2025). Toward a Comprehensive Understanding of Flavor of Sunflower Products: A Review of Confirmed and Prospective Aroma- and Taste-Active Compounds. Foods, 14(11), 1940. https://doi.org/10.3390/foods14111940






