Qualitative Proteomic Profiling of Saccharomyces cerevisiae E1 Strain During Alcoholic Fermentation of Yellow Passion Fruit: A First Approximation
Abstract
1. Introduction
2. Materials and Methods
2.1. Physicochemical Characterization of the Fruit
2.2. Must Preparation
2.3. Yeast Strain and Inoculum Preparation
2.4. Alcoholic Fermentation
2.5. Proteomics
2.5.1. Sampling and Cell Concentration
2.5.2. Cell Lysis and Protein Extraction
2.5.3. Protein Precipitation and Solubilization
2.5.4. Protein Quantification
2.5.5. LC-MS/MS Analysis
2.5.6. Raw Data Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Yellow Passion Fruit Pulp
3.2. Analysis of Variables Throughout the Fermentation Process
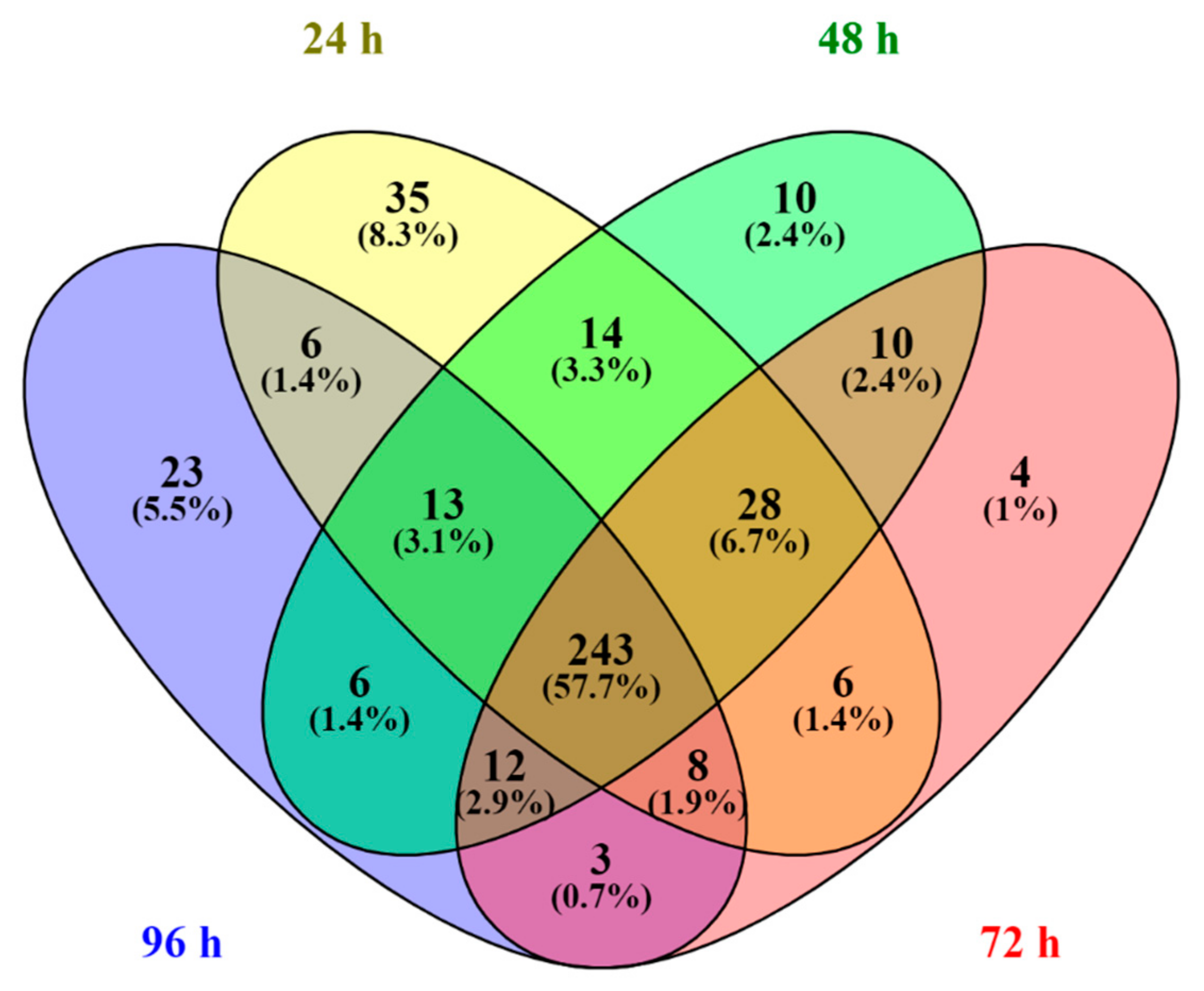
3.3. Proteomic Analysis
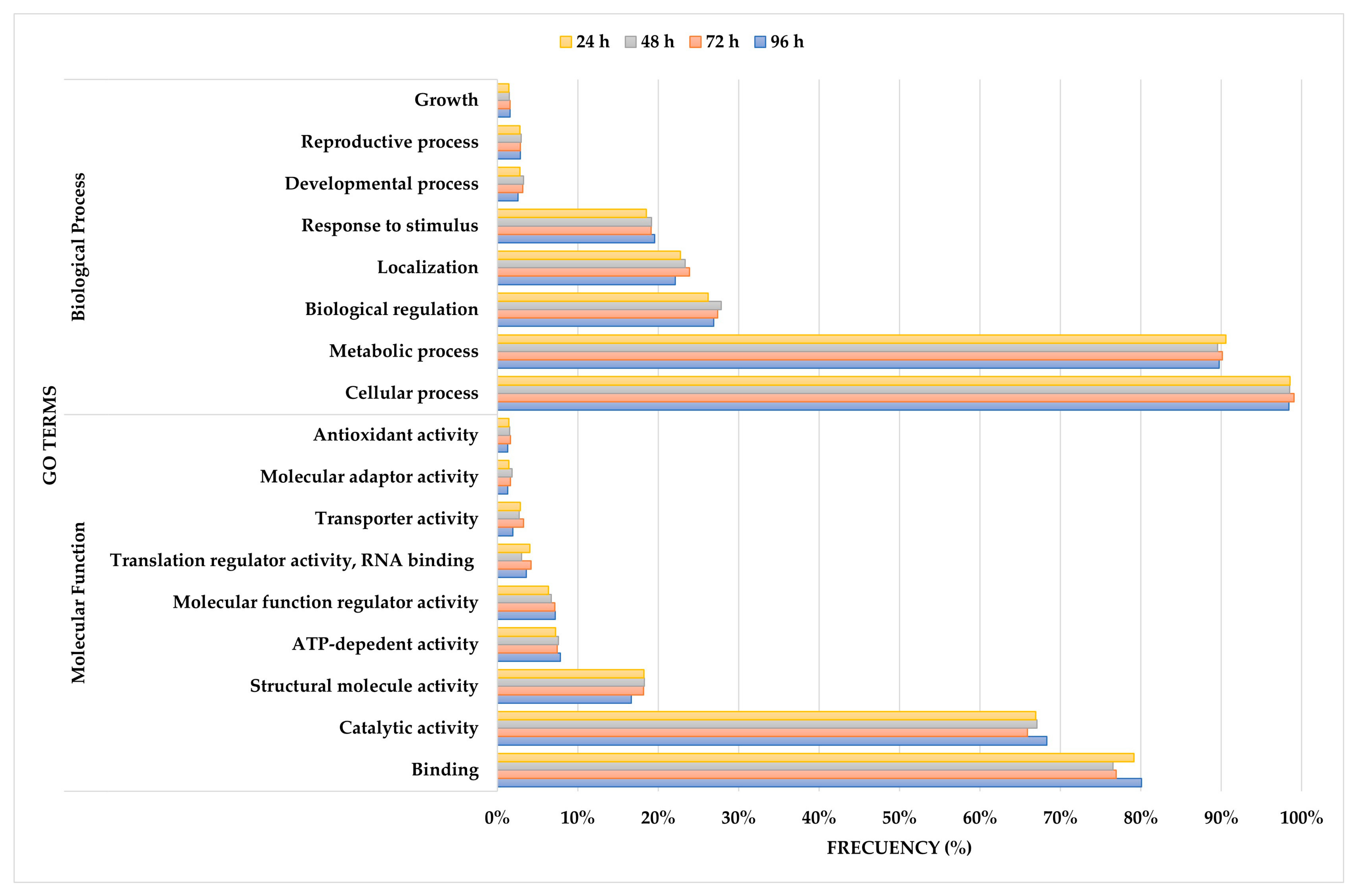
3.4. GO Term Enrichment Analysis
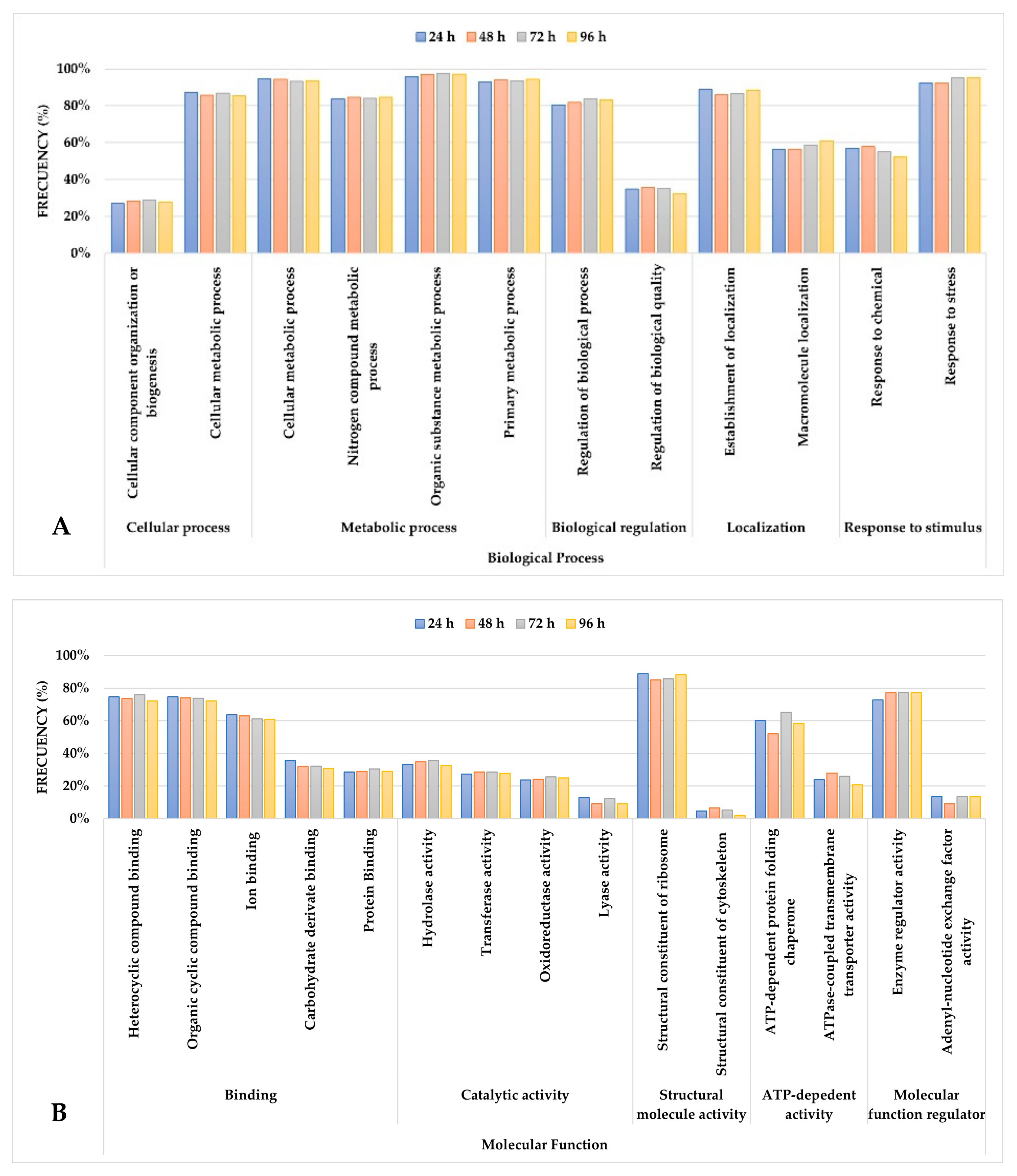
3.4.1. Biological Process Domain
3.4.2. Molecular Function Domain
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villalba, M.; Yepes, I.; Arrázola Paternina, G.S. Caracterización Fisicoquímica de Frutas de La Zona Del Sinú Para Su Agroindustrialización. Temas Agrar. 2006, 11, 15–23. [Google Scholar] [CrossRef]
- Arrázola Paternina, G.; Villalba Cadavid, M. Fruit, Vegetables and Tubers, Agroindustrialization Prospects, 1st ed.; Alpha Editores: Córdoba, Spain, 2018; ISBN 978-958-48-2408-0. [Google Scholar]
- Wijeratnam, S.W. Passion Fruit. In Encyclopedia of Food and Health; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 230–234. ISBN 9780123849533. [Google Scholar]
- He, X.; Luan, F.; Yang, Y.; Wang, Z.; Zhao, Z.; Fang, J.; Wang, M.; Zuo, M.; Li, Y. Passiflora Edulis: An Insight into Current Researches on Phytochemistry and Pharmacology. Front. Pharmacol. 2020, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, R.C.G.; Peralta, R.M.; Haminiuk, C.W.I.; Maciel, G.M.; Bracht, A.; Ferreira, I.C.F.R. The Past Decade Findings Related with Nutritional Composition, Bioactive Molecules and Biotechnological Applications of Passiflora spp. (Passion Fruit). Trends Food Sci. Technol. 2016, 58, 79–95. [Google Scholar] [CrossRef]
- Jiménez, A.M.; Sierra, C.A.; Rodríguez-Pulido, F.J.; González-Miret, M.L.; Heredia, F.J.; Osorio, C. Physicochemical Characterisation of Gulupa (Passiflora edulis Sims. fo edulis) Fruit from Colombia during the Ripening. Food Res. Int. 2011, 44, 1912–1918. [Google Scholar] [CrossRef]
- Contreras-Calderón, J.; Calderón-Jaimes, L.; Guerra-Hernández, E.; García-Villanova, B. Antioxidant Capacity, Phenolic Content and Vitamin C in Pulp, Peel and Seed from 24 Exotic Fruits from Colombia. Food Res. Int. 2011, 44, 2047–2053. [Google Scholar] [CrossRef]
- Pereira, Z.C.; dos Anjos Cruz, J.M.; Corrêa, R.F.; Sanches, E.A.; Campelo, P.H.; de Araújo Bezerra, J. Passion Fruit (Passiflora spp.) Pulp: A Review on Bioactive Properties, Health Benefits and Technological Potential. Food Res. Int. 2023, 166, 112626. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, X.; Hao, L.; Lin, Q.; Bao, Y. Effects of Different Fermentation Methods on the Quality and Microbial Diversity of Passion Fruit Wine. Fermentation 2023, 9, 439. [Google Scholar] [CrossRef]
- Albuquerque, W.; Ghezellou, P.; Seidel, L.; Burkert, J.; Will, F.; Schweiggert, R.; Spengler, B.; Zorn, H.; Gand, M. Mass Spectrometry-Based Proteomic Profiling of a Silvaner White Wine. Biomolecules 2023, 13, 650. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, Z.; Zhu, X.; Chen, B.; Zhou, L.; Liu, X.; Liu, H. Yeast Proteins: Proteomics, Extraction, Modification, Functional Characterization, and Structure: A Review. J. Agric. Food Chem. 2024, 72, 18774–18793. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Pretorius, I.S. Yeast Modulation of Wine Flavor. Adv. Appl. Microbiol. 2005, 57, 131–175. [Google Scholar] [CrossRef]
- Porras-Agüera, J.A.; Román-Camacho, J.J.; Moreno-García, J.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Effect of Endogenous CO2 Overpressure on the Yeast “Stressome” during the “Prise de Mousse” of Sparkling Wine. Food Microbiol. 2020, 89, 103431. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Yeast Interactions and Wine Flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jia, B.; Sun, X.; Ai, J.; Wang, L.; Wang, C.; Zhao, F.; Zhan, J.; Huang, W. Effect of Initial PH on Growth Characteristics and Fermentation Properties of Saccharomyces Cerevisiae. J. Food Sci. 2015, 80, M800–M808. [Google Scholar] [CrossRef] [PubMed]
- Parapouli, M.; Fragkos-Livanios, L.; Samiotaki, M.; Koukkou, A.I.; Perisynakis, A.; Hatziloukas, E.; Panayotou, G.; Drainas, C. Comparative Proteomic Analysis of Alcoholic Fermentation Employing a New Environmental Strain of Saccharomyces Cerevisiae. Process Biochem. 2010, 45, 1094–1102. [Google Scholar] [CrossRef]
- González-Jiménez, M.D.C.; García-Martínez, T.; Mauricio, J.C.; Sánchez-León, I.; Puig-Pujol, A.; Moreno, J.; Moreno-García, J. Comparative Study of the Proteins Involved in the Fermentation-Derived Compounds in Two Strains of Saccharomyces Cerevisiae during Sparkling Wine Second Fermentation. Microorganisms 2020, 8, 1209. [Google Scholar] [CrossRef]
- González-Jiménez, M.D.C.; García-Martínez, T.; Puig-Pujol, A.; Capdevila, F.; Moreno-García, J.; Moreno, J.; Mauricio, J.C. Biological Processes Highlighted in Saccharomyces Cerevisiae during the Sparkling Wines Elaboration. Microorganisms 2020, 8, 1216. [Google Scholar] [CrossRef]
- Romano, P.; Braschi, G.; Siesto, G.; Patrignani, F.; Lanciotti, R. Role of Yeasts on the Sensory Component of Wines. Foods 2022, 11, 1921. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.S.; Hatziloukas, E. Saccharomyces Cerevisiae and Its Industrial Applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef]
- Peng, C.; Andersen, B.; Arshid, S.; Larsen, M.R.; Albergaria, H.; Lametsch, R.; Arneborg, N. Proteomics Insights into the Responses of Saccharomyces Cerevisiae during Mixed-Culture Alcoholic Fermentation with Lachancea Thermotolerans. FEMS Microbiol. Ecol. 2019, 95, fiz126. [Google Scholar] [CrossRef]
- Albergaria, H.; Arneborg, N. Dominance of Saccharomyces Cerevisiae in Alcoholic Fermentation Processes: Role of Physiological Fitness and Microbial Interactions. Appl. Microbiol. Biotechnol. 2016, 100, 2035–2046. [Google Scholar] [CrossRef]
- Zannini, E.; Lynch, K.M.; Nyhan, L.; Sahin, A.W.; O’ Riordan, P.; Luk, D.; Arendt, E.K. Influence of Substrate on the Fermentation Characteristics and Culture-Dependent Microbial Composition of Water Kefir. Fermentation 2022, 9, 28. [Google Scholar] [CrossRef]
- Seguinot, P.; Ortiz-Julien, A.; Camarasa, C. Impact of Nutrient Availability on the Fermentation and Production of Aroma Compounds Under Sequential Inoculation with M. pulcherrima and S. cerevisiae. Front. Microbiol. 2020, 11, 518930. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulou, M.; Goulioti, E.; Troianou, V.; Toumpeki, C.; Paramithiotis, S.; Gosselin, Y.; Dorignac, E.; Papadopoulos, G.; Kotseridis, Y. Effect of Saccharomyces Cerevisiae and Saccharomyces Pastorianus Co-Inoculation on Alcoholic Fermentation Behavior and Aromatic Profile of Sauvignon Blanc Wine. Fermentation 2022, 8, 539. [Google Scholar] [CrossRef]
- He, L.; Yan, Y.; Wu, M.; Ke, L. Advances in the Quality Improvement of Fruit Wines: A Review. Horticulturae 2024, 10, 93. [Google Scholar] [CrossRef]
- Thuy, C.X.; Pham, V.T.; Nguyen, T.T.N.H.; Nguyen, T.T.N.; Ton, N.T.A.; Tuu, T.T.; Vu, N.D. Effect of Fermentation Conditions (Dilution Ratio, Medium PH, Total Soluble Solids, and Saccharomyces cerevisiae Yeast Ratio) on the Ability to Ferment Cider from Tamarillo (Solanum betaceum) Fruit. J. Food Process. Preserv. 2024, 2024, 8841207. [Google Scholar] [CrossRef]
- González-Jiménez, M.d.C.; Moreno-García, J.; García-Martínez, T.; Moreno, J.J.; Puig-Pujol, A.; Capdevilla, F.; Mauricio, J.C. Differential Analysis of Proteins Involved in Ester Metabolism in Two Saccharomyces Cerevisiae Strains during the Second Fermentation in Sparkling Wine Elaboration. Microorganisms 2020, 8, 403. [Google Scholar] [CrossRef]
- Román-Camacho, J.J.; Mauricio, J.C.; Sánchez-León, I.; Santos-Dueñas, I.M.; Fuentes-Almagro, C.A.; Amil-Ruiz, F.; García-Martínez, T.; García-García, I. Implementation of a Novel Method for Processing Proteins from Acetic Acid Bacteria via Liquid Chromatography Coupled with Tandem Mass Spectrometry. Molecules 2024, 29, 2548. [Google Scholar] [CrossRef]
- Liu, S.; Kerr, E.D.; Pegg, C.L.; Schulz, B.L. Proteomics and Glycoproteomics of Beer and Wine. Proteomics 2022, 22, 2100329. [Google Scholar] [CrossRef]
- Li, H.; Byrne, K.; Galiamov, R.; Mendoza-Porras, O.; Bose, U.; Howitt, C.A.; Colgrave, M.L. Using LC-MS to Examine the Fermented Food Products Vinegar and Soy Sauce for the Presence of Gluten. Food Chem. 2018, 254, 302–308. [Google Scholar] [CrossRef]
- Goldman, A.R.; Beer, L.A.; Tang, H.Y.; Hembach, P.; Zayas-Bazan, D.; Speicher, D.W. Proteome Analysis Using Gel-LC-MS/MS. Curr. Protoc. Protein Sci. 2019, 96, e93. [Google Scholar] [CrossRef]
- Hart, R.S.; Jolly, N.P.; Ndimba, B.K. Characterisation of Hybrid Yeasts for the Production of Varietal Sauvignon Blanc Wine—A Review. J. Microbiol. Methods 2019, 165, 105699. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, Y.; Zhu, Y.; Kortesniemi, M.; Zhu, B.; Li, H. Aromatic Characteristics of Passion Fruit Wines Measured by E-Nose, GC-Quadrupole MS, GC-Orbitrap-MS and Sensory Evaluation. Foods 2022, 11, 3789. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vázquez, C.; Román-Camacho, J.J.; Consuegra-Rivera, R.; Santos-Dueñas, I.M.; García-García, I.; García-Martínez, T.; Mauricio, J.C. Exploring Microbial Diversity and Functionality in Verdejo Wine Vinegar Fermentation through LC-MS/MS Analysis. LWT 2024, 213, 117054. [Google Scholar] [CrossRef]
- Official Methods of Analysis of AOAC INTERNATIONAL|Oxford Academic. Available online: https://academic.oup.com/officialmethodsofanalysis-aoac (accessed on 11 November 2024).
- Santos, R.T.S.; Biasoto, A.C.T.; Rybka, A.C.P.; Castro, C.D.P.C.; Aidar, S.T.; Borges, G.S.C.; Silva, F.L.H. Physicochemical Characterization, Bioactive Compounds, in Vitro Antioxidant Activity, Sensory Profile and Consumer Acceptability of Fermented Alcoholic Beverage Obtained from Caatinga Passion Fruit (Passiflora cincinnata Mast.). LWT 2021, 148, 111714. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Q.; Hu, X.; Wu, W.; Zhang, Y.; Liu, S.; Li, C. Evaluation of Different Saccharomyces Cerevisiae Strains on the Profile of Volatile Compounds in Pineapple Wine. J. Food Sci. Technol. 2018, 55, 4119–4130. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Cui, M.Y.; Fu, Y.; Wang, X.H.; Yang, Q.; Zhu, Y.; Yang, X.H.; Bi, H.J.; Gao, X.L. Aroma Enhancement of Blueberry Wine by Postharvest Partial Dehydration of Blueberries. Food Chem. 2023, 426, 136593. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, M.; Ouyang, Y.; Zhao, X.; Ju, Y.; Fang, Y. Comparative Study of Aromatic Compounds in Fruit Wines from Raspberry, Strawberry, and Mulberry in Central Shaanxi Area. Food Nutr. Res. 2015, 59, 29290. [Google Scholar] [CrossRef]
- Liu, G.; Wei, P.; Tang, Y.; Pang, Y.; Sun, J.; Li, J.; Rao, C.; Wu, C.; He, X.; Li, L.; et al. Evaluation of Bioactive Compounds and Bioactivities in Plum (Prunus salicina Lindl.) Wine. Front. Nutr. 2021, 8, 766415. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.; Ren, T.; Wang, J.; Niu, C.; Zheng, F.; Li, Q. Effect of Saccharomyces Cerevisiae and Non-Saccharomyces Strains on Alcoholic Fermentation Behavior and Aroma Profile of Yellow-Fleshed Peach Wine. LWT 2022, 155, 112993. [Google Scholar] [CrossRef]
- Sood, S.; Singhal, R.; Bhat, S.; Kumar, A. Inoculum Preparation. Compr. Biotechnol. 2011, 2, 230–243. [Google Scholar] [CrossRef]
- Cañete-Rodríguez, A.M.; Santos-Dueñas, I.M.; Jiménez-Hornero, J.E.; Torija-Martínez, M.J.; Mas, A.; García-García, I. Revalorization of Strawberry Surpluses by Bio-Transforming Its Glucose Content into Gluconic Acid. Food Bioprod. Process. 2016, 99, 188–196. [Google Scholar] [CrossRef]
- Cañete-Rodríguez, A.M.; Santos-Dueñas, I.M.; Jiménez-Hornero, J.E.; Torija-Martínez, M.J.; Mas, A.; García-García, I. An Approach for Estimating the Maximum Specific Growth Rate of Gluconobacter Japonicus in Strawberry Purée without Cell Concentration Data. Biochem. Eng. J. 2016, 105, 314–320. [Google Scholar] [CrossRef]
- Cañete-Rodríguez, A.M.; Santos-Dueñas, I.M.; Torija-Martínez, M.J.; Mas, A.; Jiménez-Hornero, J.E.; García-García, I. Preparation of a Pure Inoculum of Acetic Acid Bacteria for the Selective Conversion of Glucose in Strawberry Purée into Gluconic Acid. Food Bioprod. Process. 2015, 96, 35–42. [Google Scholar] [CrossRef]
- Assay Kits—Enzyme Assays—Enzymatic Assay Kits|Megazyme. Available online: https://www.megazyme.com/view-all-products/assay-kits?p=1 (accessed on 11 November 2024).
- Román-Camacho, J.J.; Mauricio, J.C.; Santos-Dueñas, I.M.; García-Martínez, T.; García-García, I. Functional Metaproteomic Analysis of Alcohol Vinegar Microbiota during an Acetification Process: A Quantitative Proteomic Approach. Food Microbiol. 2021, 98, 103799. [Google Scholar] [CrossRef]
- Román-Camacho, J.J.; Santos-Dueñas, I.M.; García-García, I.; Moreno-García, J.; García-Martínez, T.; Mauricio, J.C. Metaproteomics of Microbiota Involved in Submerged Culture Production of Alcohol Wine Vinegar: A First Approach. Int. J. Food Microbiol. 2020, 333, 108797. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Román-Camacho, J.J.; García-García, I.; Santos-Dueñas, I.M.; García-Martínez, T.; Mauricio, J.C. Latest Trends in Industrial Vinegar Production and the Role of Acetic Acid Bacteria: Classification, Metabolism, and Applications—A Comprehensive Review. Foods 2023, 12, 3705. [Google Scholar] [CrossRef]
- Román-Camacho, J.J.; Mauricio, J.C.; Santos-Dueñas, I.M.; García-Martínez, T.; García-García, I. Unraveling the Role of Acetic Acid Bacteria Comparing Two Acetification Profiles from Natural Raw Materials: A Quantitative Approach in Komagataeibacter europaeus. Front. Microbiol. 2022, 13, 840119. [Google Scholar] [CrossRef]
- Bateman, A.; Martin, M.J.; Orchard, S.; Magrane, M.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Bye-A-Jee, H.; Cukura, A.; et al. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef]
- Ramaiya, S.D.; Bujang, J.S.; Zakaria, M.H.; King, W.S.; Sahrir, M.A.S. Sugars, Ascorbic Acid, Total Phenolic Content and Total Antioxidant Activity in Passion Fruit (Passiflora) Cultivars. J. Sci. Food Agric. 2013, 93, 1198–1205. [Google Scholar] [CrossRef]
- Santos, T.B.; de Araujo, F.P.; Neto, A.F.; de Freitas, S.T.; de Souza Araújo, J.; de Oliveira Vilar, S.B.; Araújo, A.J.B.; Lima, M.S. Phytochemical Compounds and Antioxidant Activity of the Pulp of Two Brazilian Passion Fruit Species: Passiflora cincinnata Mast. and Passiflora edulis Sims. Int. J. Fruit Sci. 2021, 21, 255–269. [Google Scholar] [CrossRef]
- Vicente, J.; Baran, Y.; Navascués, E.; Santos, A.; Calderón, F.; Marquina, D.; Rauhut, D.; Benito, S. Biological Management of Acidity in Wine Industry: A Review. Int. J. Food Microbiol. 2022, 375, 109726. [Google Scholar] [CrossRef]
- Zang, X.; Du, Q.; Jiang, J.; Liang, Y.y.; Ye, D.; Liu, Y. Impact of Combined Grape Maturity and Selected Saccharomyces cerevisiae on Flavor Profiles of Young ‘Cabernet Sauvignon’ Wines. Food Chem. X 2025, 25, 102066. [Google Scholar] [CrossRef] [PubMed]
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef]
- Cheng, J.S.; Qiao, B.; Yuan, Y.J. Comparative Proteome Analysis of Robust Saccharomyces Cerevisiae Insights into Industrial Continuous and Batch Fermentation. Appl. Microbiol. Biotechnol. 2008, 81, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Soares Rodrigues, C.I.; den Ridder, M.; Pabst, M.; Gombert, A.K.; Wahl, S.A. Comparative Proteome Analysis of Different Saccharomyces Cerevisiae Strains during Growth on Sucrose and Glucose. Sci. Rep. 2023, 13, 2126. [Google Scholar] [CrossRef]
- Moreno-García, J.; García-Martínez, T.; Millán, M.C.; Mauricio, J.C.; Moreno, J. Proteins Involved in Wine Aroma Compounds Metabolism by a Saccharomyces Cerevisiae Flor-Velum Yeast Strain Grown in Two Conditions. Food Microbiol. 2015, 51, 1–9. [Google Scholar] [CrossRef]
- Liang, Z.; Hao, J.; Ye, J.; Zhang, B.; Li, X.; Liao, X.; Wei, J.; Wei, D. Optimization of Fermentation Technology for Compound Fruit Wine of Red Pitaya and Passion Fruit. China Brew. 2023, 42, 221–227. [Google Scholar] [CrossRef]
- Xia, J.; Sánchez, B.J.; Chen, Y.; Campbell, K.; Kasvandik, S.; Nielsen, J. Proteome Allocations Change Linearly with the Specific Growth Rate of Saccharomyces Cerevisiae under Glucose Limitation. Nat. Commun. 2022, 13, 2819. [Google Scholar] [CrossRef]
- Valadi, H.; Valadi, Å.; Ansell, R.; Gustafsson, L.; Adler, L.; Norbeck, J.; Blomberg, A. NADH-Reductive Stress in Saccharomyces Cerevisiae Induces the Expression of the Minor Isoform of Glyceraldehyde-3-Phosphate Dehydrogenase (TDH1). Curr. Genet. 2004, 45, 90–95. [Google Scholar] [CrossRef]
- Chen, Y.; Nielsen, J. Flux Control through Protein Phosphorylation in Yeast. FEMS Yeast Res. 2016, 16, fow096. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S.J.; James, D.E.; Mann, M. Protein Phosphorylation: A Major Switch Mechanism for Metabolic Regulation. Trends Endocrinol. Metab. 2015, 26, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Vlastaridis, P.; Papakyriakou, A.; Chaliotis, A.; Stratikos, E.; Oliver, S.G.; Amoutzias, G.D. The Pivotal Role of Protein Phosphorylation in the Control of Yeast Central Metabolism. G3 Genes Genomes Genet. 2017, 7, 1239–1249. [Google Scholar] [CrossRef]
- Wiederhold, E.; Veenhoff, L.M.; Poolman, B.; Slotboom, D.J. Proteomics of Saccharomyces Cerevisiae Organelles. Mol. Cell. Proteom. 2010, 9, 431–445. [Google Scholar] [CrossRef]
- van Voorst, F.; Houghton-Larson, J.; Jønson, L.; Kielland-Brandt, M.C.; Brandt, A. Genome-Wide Identification of Genes Required for Growth of Saccharomyces Cerevisiae under Ethanol Stress. Yeast 2006, 23, 351–359. [Google Scholar] [CrossRef]
- Sahana, G.R.; Balasubramanian, B.; Joseph, K.S.; Pappuswamy, M.; Liu, W.C.; Meyyazhagan, A.; Kamyab, H.; Chelliapan, S.; Joseph, B.V. A Review on Ethanol Tolerance Mechanisms in Yeast: Current Knowledge in Biotechnological Applications and Future Directions. Process Biochem. 2024, 138, 1–13. [Google Scholar] [CrossRef]
- Molina, A.M.; Swiegers, J.H.; Varela, C.; Pretorius, I.S.; Agosin, E. Influence of Wine Fermentation Temperature on the Synthesis of Yeast-Derived Volatile Aroma Compounds. Appl. Microbiol. Biotechnol. 2007, 77, 675–687. [Google Scholar] [CrossRef]
- Matallana, E.; Aranda, A. Biotechnological Impact of Stress Response on Wine Yeast. Lett. Appl. Microbiol. 2017, 64, 103–110. [Google Scholar] [CrossRef]
- Moreno-García, J.; Ogawa, M.; Joseph, C.M.L.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Comparative Analysis of Intracellular Metabolites, Proteins and Their Molecular Functions in a Flor Yeast Strain under Two Enological Conditions. World J. Microbiol. Biotechnol. 2019, 35, 6. [Google Scholar] [CrossRef]

| Variable | Value | |
|---|---|---|
| Mean ± SD | Density (g/mL) | 1.05 ± 0.01 |
| pH | 3.1 ± 0.1 | |
| Soluble solids (°Bx) | 14.5 ± 0.1 | |
| Titratable acidity (% w/v citric acid) | 3.9 ± 0.1 |
| Variable | 2 h | 24 h | 48 h | 72 h | 96 h | |
|---|---|---|---|---|---|---|
| Mean ± SD | Sucrose (g/L) | 130.1 ± 0.4 | 99.2 ± 9.1 | 54.9 ± 4.8 | 32.7 ± 2.4 | 17.7 ± 3.4 |
| Glucose (g/L) | 7.3 ± 0.5 | 5.4 ± 0.7 | 2.9 ± 0.7 | 3.0 ± 0.3 | 2.9 ± 0.5 | |
| Fructose (g/L) | 7.5 ± 0.7 | 5.1 ± 0.6 | 3.9 ± 0.5 | 3.9 ± 0.3 | 3.9 ± 0.4 | |
| Soluble solids (°Bx) | 14.8 ± 0.1 | 12.4 ± 0.7 | 9.5 ± 0.5 | 7.9 ± 0.5 | 6.8 ± 0.4 | |
| Ethanol (% v/v) | 0.1 ± 0.1 | 2.1 ± 0.1 | 3.3 ± 0.4 | 7.1 ± 0.4 | 8.5 ± 0.4 | |
| Cell counts (107 cel/mL) | 3.9 ± 1.1 | 10.5 ± 1.3 | 10.4 ± 0.8 | 11.1 ± 0.6 | 11.2 ± 0.6 |
| Metabolic Pathway | Key Proteins | Function |
|---|---|---|
| Glycolysis | Glyceraldehyde-3-phosphate dehydrogenase (tdh3) | Catalyzes the conversion of glyceraldehyde 3-phosphate to 1,3-bisphosphoglycerate, generating NADH |
| Pyruvate kinase (pk1) | Converts phosphoenolpyruvate to pyruvate, releasing ATP | |
| Ethanol Fermentation | Pyruvate decarboxylase (pdc1) | Decarboxylates pyruvate to acetaldehyde |
| Alcohol dehydrogenase (adh1) | Reduces acetaldehyde to ethanol using NADH, regenerating NAD+ for glycolysis, reduces aldehydes to higher alcohols | |
| Synthesis of Aroma and Flavor Compounds | Branched-chain amino acid transaminase (bat1) | Converts branched-chain amino acids (BCAAs) to keto-acids, precursors for higher alcohols |
| Acetolactate synthase (ilv2) | Initiates the biosynthesis of valine and isoleucine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Consuegra-Rivera, R.; Román-Camacho, J.J.; Santos-Dueñas, I.M.; García-Martínez, T.; Mauricio, J.C.; García-García, I. Qualitative Proteomic Profiling of Saccharomyces cerevisiae E1 Strain During Alcoholic Fermentation of Yellow Passion Fruit: A First Approximation. Foods 2025, 14, 1856. https://doi.org/10.3390/foods14111856
Consuegra-Rivera R, Román-Camacho JJ, Santos-Dueñas IM, García-Martínez T, Mauricio JC, García-García I. Qualitative Proteomic Profiling of Saccharomyces cerevisiae E1 Strain During Alcoholic Fermentation of Yellow Passion Fruit: A First Approximation. Foods. 2025; 14(11):1856. https://doi.org/10.3390/foods14111856
Chicago/Turabian StyleConsuegra-Rivera, Roger, Juan J. Román-Camacho, Inés M. Santos-Dueñas, Teresa García-Martínez, Juan Carlos Mauricio, and Isidoro García-García. 2025. "Qualitative Proteomic Profiling of Saccharomyces cerevisiae E1 Strain During Alcoholic Fermentation of Yellow Passion Fruit: A First Approximation" Foods 14, no. 11: 1856. https://doi.org/10.3390/foods14111856
APA StyleConsuegra-Rivera, R., Román-Camacho, J. J., Santos-Dueñas, I. M., García-Martínez, T., Mauricio, J. C., & García-García, I. (2025). Qualitative Proteomic Profiling of Saccharomyces cerevisiae E1 Strain During Alcoholic Fermentation of Yellow Passion Fruit: A First Approximation. Foods, 14(11), 1856. https://doi.org/10.3390/foods14111856








