Alcohol or No Alcohol in Wine: Half a Century of Debate
Abstract
1. Introduction
2. Search Methodology
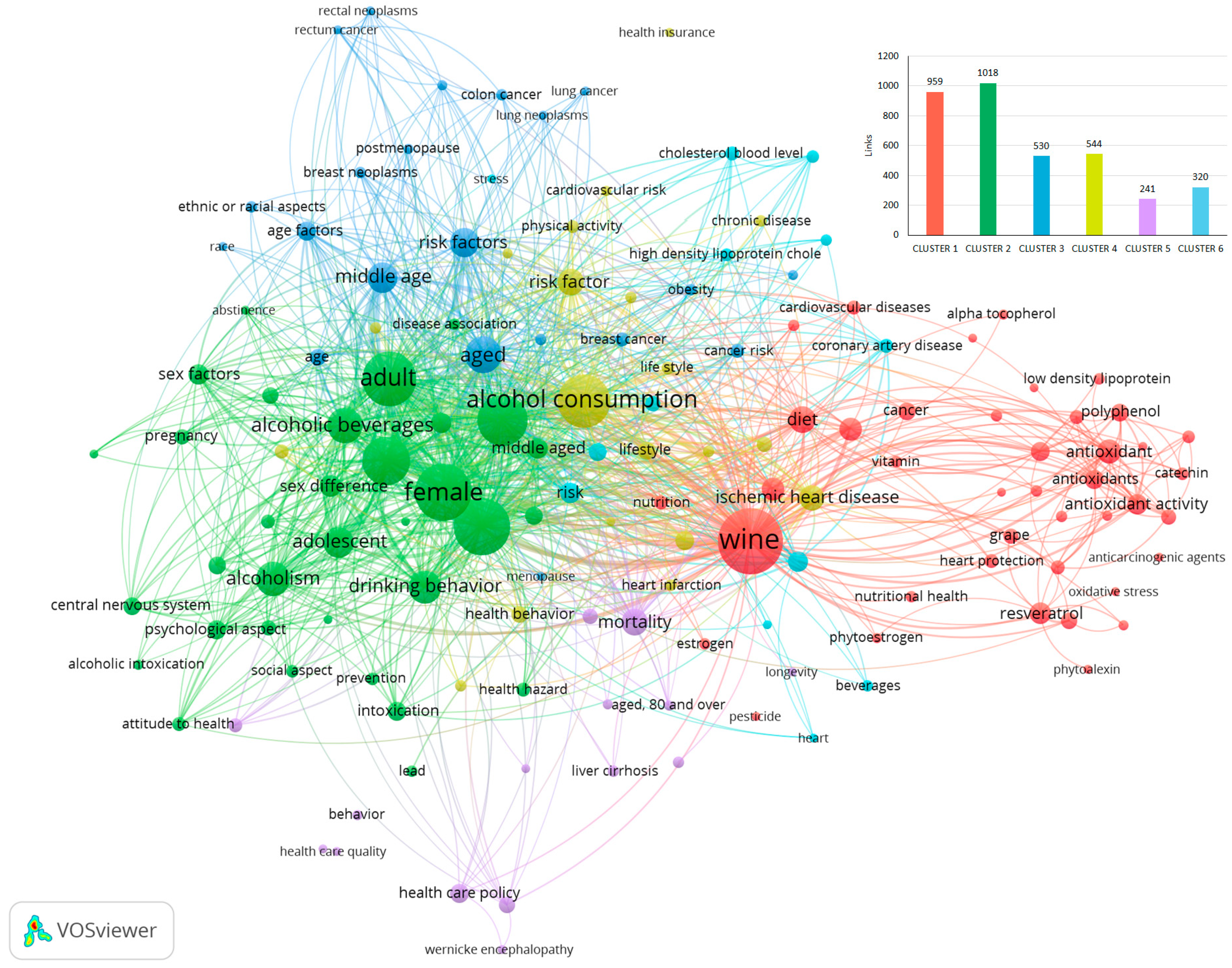
3. Bibliometric Analysis from 1970 to 1999
3.1. Wine as Health Promoter
3.2. Alcohol Abuse and Health
3.3. Wine and Its Impact on Various Diseases Related to Age and Gender
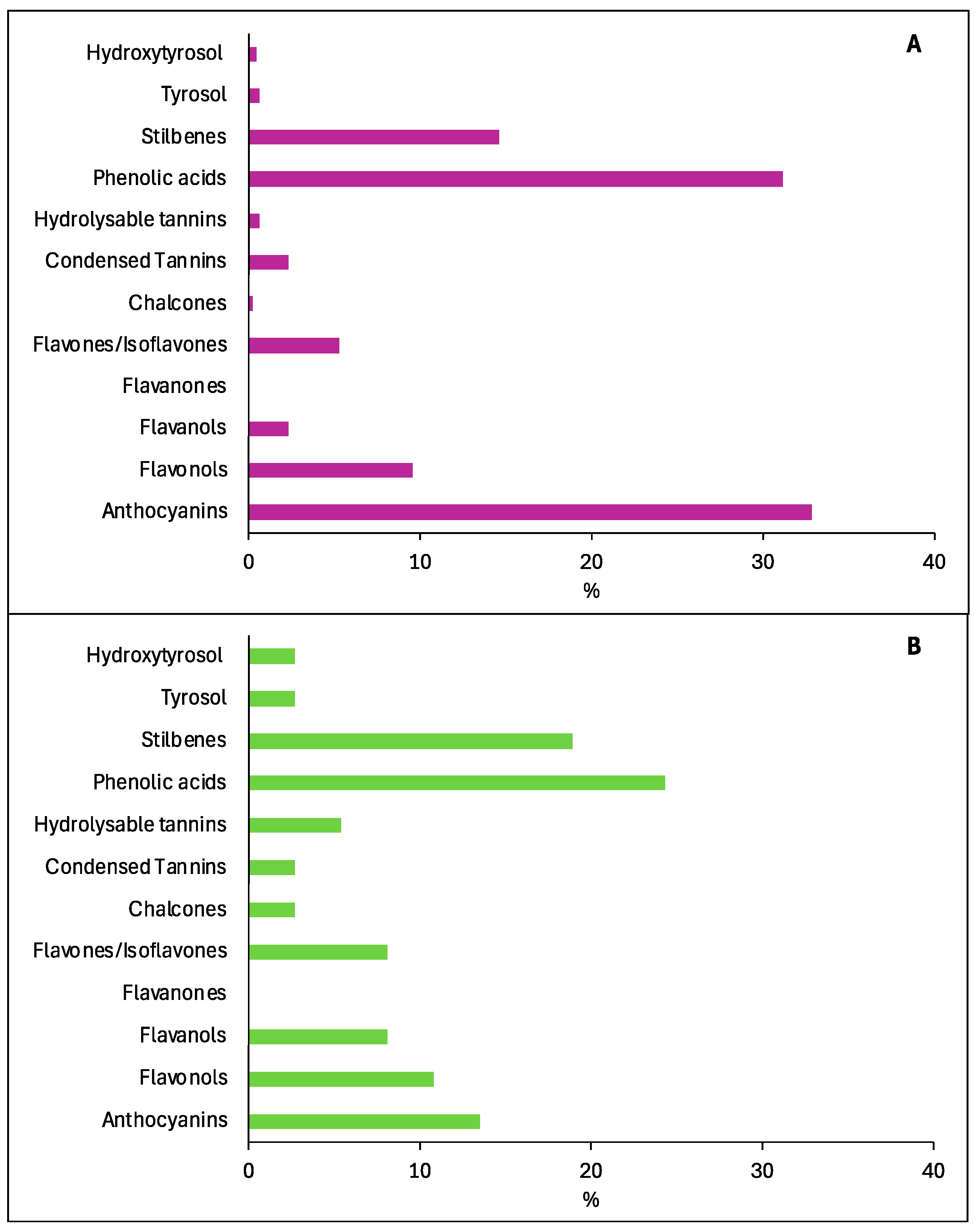
3.4. Knowledge of Wine Compounds and Assessment of Health Effects in the Years 1970–1999
| Compound | Detection in Wine | Evaluation of Health Effects |
|---|---|---|
| FLAVONOIDS | ||
| Anthocyanins | ||
| Delphinidin | [64,67,68] | [63,69,70] |
| Cyanidin | ||
| Petunidin | ||
| Peonidin | ||
| Mavidin | ||
| Vitisins | [71,72] | |
| Others | [72,73,74] | |
| Flavonols | ||
| Myricetin | [64,75] | [64,75] |
| Quercetin | [64,75] | |
| Kaempferol | [75] | [75] |
| Isorhamnetin | [75] | |
| Rutin | [75] | |
| Flavanols | ||
| Catechin | [63,64] | [63] |
| Epicatechin | [63,64] | [63] |
| Epigallocatechin | [76] | [76] |
| Epicatechin-gallate | [76] | [76] |
| Astilbin | [77] | [78] |
| Engeletin | [77] | [76] |
| Flavanones | ||
| Flavones/isoflavones | [79] | [80] |
| Chalcones | [79] | [81] |
| Condensed tannins | [79,82,83] | [52] |
| Hydrolysable tannins | ||
| Gallotannins | [84] | [52,85] |
| Ellagitannins | [62,86] | [52,85] |
| Other phenols | [87] | |
| NON-FLAVONOIDS | ||
| Phenolic acids | ||
| Hydroxybenzoic acids | ||
| p-hydroxybenzoic acid | [62,68,88] | [89] |
| Gallic acid | [62] | [63] |
| Vanillic acid | [62,86] | [90] |
| Gentisic acid | [91] | [92] |
| Syringic acid | [62,86] | |
| Salicylic acid | [68] | [52] |
| Protocatechuic acid | [93] | [93] |
| Hydroxycinnamic acids | ||
| Caffeic acid | [62,63,64] | [63] |
| Coumaric acid | [62,67] | [94] |
| Sinapic acid | [95] | [95] |
| Ferulic acid | [62] | [96] |
| Stilbenes | ||
| trans-resveratrol | [97,98] | [52,58,59,60,61] |
| trans-piceid | [98,99] | |
| trans-astringin | [99,100] | |
| Piceatannol | [99] | |
| Tyrosol | [68] | [101] |
| Hydroxytyrosol | [68] | [94] |
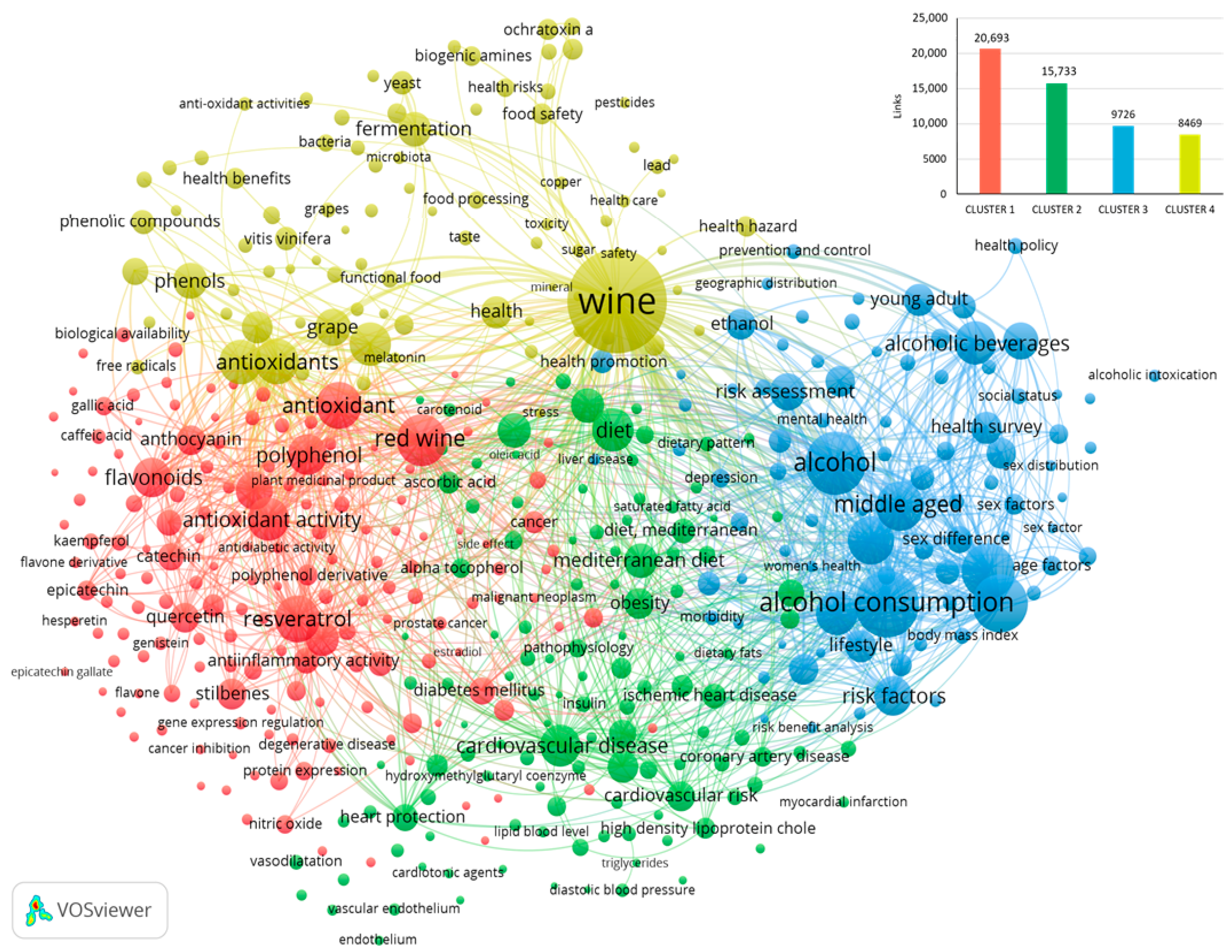
4. Bibliometric Analysis from 2000 to 2024
4.1. Awareness of the Health Implications of Wine Consumption in the Years 2000–2024
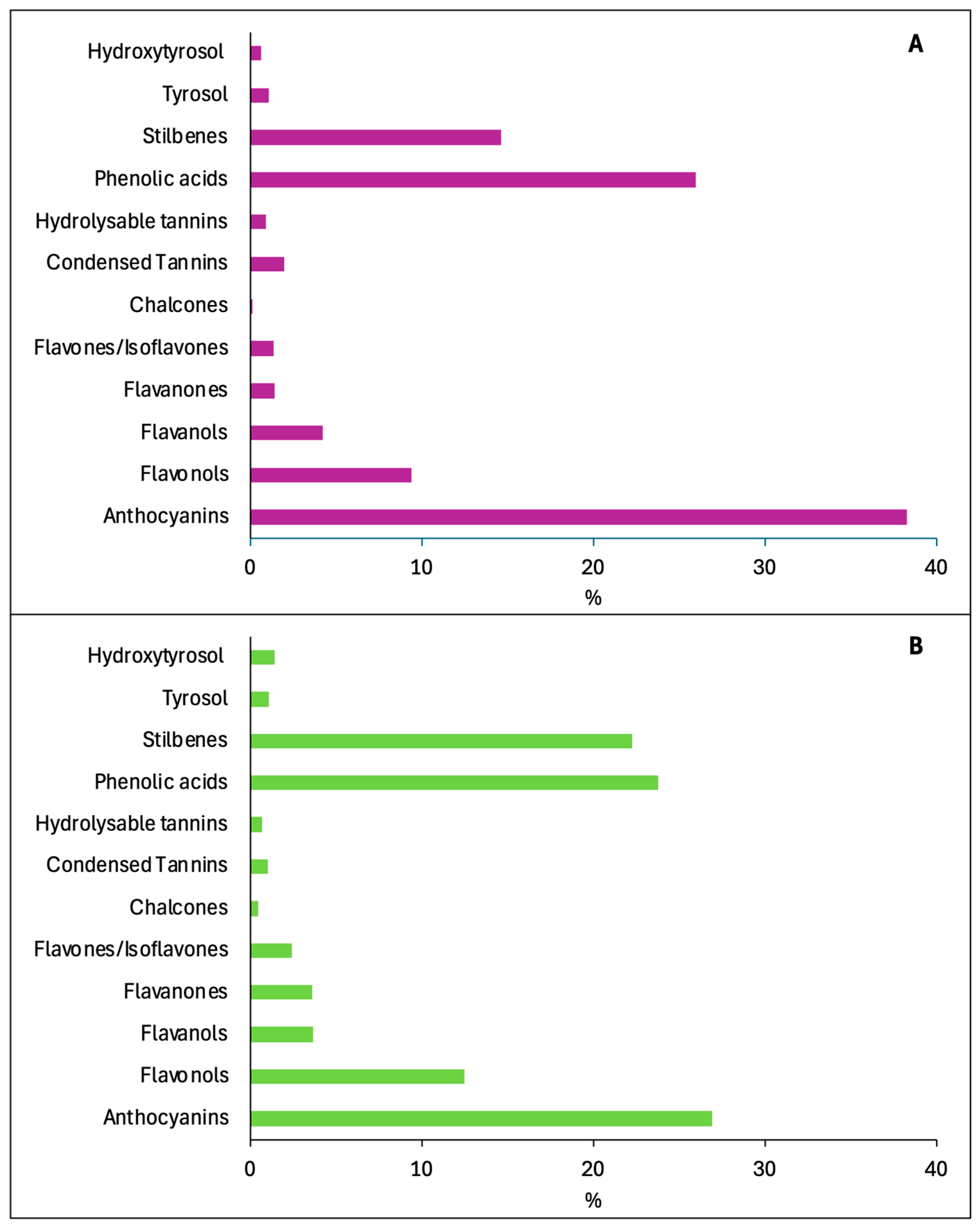
4.2. Assessment of Health Effects of New and Already Known Wine Compounds
4.2.1. Wine Anthocyanins
4.2.2. Wine Flavonols
4.2.3. Wine Flavanols
4.2.4. Wine Flavanones
4.2.5. Wine Phenolic Acids
4.2.6. Wine Stilbenes
4.2.7. Wine Hydrolysable Tannins
| Compound | Detection in Wine | Evaluation of Health Effects |
|---|---|---|
| FLAVONOIDS | ||
| Anthocyanins | ||
| Anthocyanin derivates | [199,200,201] | [123,124,125,126,127,128,129,130,131,202,203] |
| Pinotin A | [204] | |
| Oxovitisins | [205] | |
| Others | [206,207,208] | |
| Flavonols | [209] | |
| Laricitrin | [136] | [210] |
| Syringetin | [135] | [137] |
| Flavanols | ||
| Flavanol monomer and dimer hexosides | [211] | [144,145] |
| Flavanol derivates | [201] | |
| Flavanones | ||
| Naringenin | [148] | [152] |
| Flavones/isoflavones | [212] | |
| Chalcones | [213] | |
| Condensed tannins | [146] | |
| Hydrolysable tannins | ||
| Ellagitannin derivates | [195] | [195] |
| Other flavonoids | [214] | |
| NON-FLAVONOIDS | ||
| Phenolic acids | ||
| Various hydroxy acids | [215] | [110] |
| Stilbenes | ||
| Hopeaphenol | [216] | [217] |
| C-Glucosides of resveratrol | [218] | [219] |
| Parthenocessin A | [220] | |
| Pallidol | [218] | [217] |
| Viniferins | [221] | [217] |
| Miyabenol C | [170] | [222] |
| Ampelopsin D | [170] | [223] |
| trans-ω-viniferin | [170] | [224] |
| cis-scirpusin A | [170] | |
| trans-scirpusin A | [170] | |
| Restrisol A | [170] | |
| Parthenostilbenin A | [170] | |
| Parthenostilbenin B | [170] | |
| (E)-miyabenol C | [170] | [217] |
| (Z)-miyabenol C | [170] | [217] |
| Other stilbenoids | [170] | |
| Other non-flavonoids | [214] |
4.3. New Insights into the Role of Wine in Human Health
5. Low-Alcohol and Dealcoholised Wine
5.1. Low-Alcohol and Dealcoholised Wine Market
5.2. Dealcoholisation Techniques: Impact on Consumer Health and Sensory Quality of Wines
5.3. Health Benefits of the Consumption of Low- and No-Alcohol Wines
5.4. Consideration of Health Implications of Low- and No-Alcohol Wine Consumption
5.4.1. Cardioprotective Benefits of Low- and No-Alcohol Wine
5.4.2. Low- and No-Alcohol Wine Stability
5.4.3. What Is Admitted in the Production of Low- and No-Alcohol Wine?
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teall, E.K. Medicine and Doctoring in Ancient Mesopotamia. Grand Val. J. Hist. 2014, 3, 2. [Google Scholar]
- Johnson, M.Y.; Hayden, S. A Brief History of Wine in Medicine. J. Emerg. Med. 2024, 66, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, P.; Stoyanov, Z.; Doncheva, D.; Trendafilova, S. Wine as a Medicine in Ancient Times. Scr. Sci. Pharm. 2018, 5, 14–21. [Google Scholar] [CrossRef]
- Dewan, A.; Nagaraja, S.K.; Yadav, S.; Bishnoi, P.; Malik, M.; Chhikara, N.; Luthra, A.; Singh, A.; Davis, C.; Poonam. Advances in Wine Processing: Current Insights, Prospects, and Technological Interventions. Food Bioprocess Technol. 2025, 18, 5058–5093. [Google Scholar] [CrossRef]
- Sahu, P.; Verma, H.K.; Bhaskar, L. Alcohol and Alcoholism Associated Neurological Disorders: Current Updates in a Global Perspective and Recent Recommendations. World J. Exp. Med. 2025, 15, 100402. [Google Scholar] [CrossRef]
- El Rayess, Y.; Nehme, N.; Azzi-Achkouty, S.; Julien, S.G. Wine Phenolic Compounds: Chemistry, Functionality and Health Benefits. Antioxidants 2024, 13, 1312. [Google Scholar] [CrossRef]
- Kumar, Y.; Ricci, A.; Parpinello, G.P.; Versari, A. Dealcoholized Wine: A Scoping Review of Volatile and Non-Volatile Profiles, Consumer Perception, and Health Benefits. Food Bioprocess Technol. 2024, 17, 3525–3545. [Google Scholar] [CrossRef]
- St Leger, A.; Cochrane, A.; Moore, F. Factors Associated with Cardiac Mortality in Developed Countries with Particular Reference to the Consumption of Wine. Lancet 1979, 313, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Klurfeld, D.M.; Kritchevsky, D. Differential Effects of Alcoholic Beverages on Experimental Atherosclerosis in Rabbits. Exp. Mol. Pathol. 1981, 34, 62–71. [Google Scholar] [CrossRef]
- Baum-Baicker, C. The Health Benefits of Moderate Alcohol Consumption: A Review of the Literature. Drug Alcohol Depend. 1985, 15, 207–227. [Google Scholar] [CrossRef]
- Middleton, E., Jr. Some Biological Properties of Plant Flavonoids. Ann. Allergy 1988, 61, 53–57. [Google Scholar] [PubMed]
- Renaud, S.d.; de Lorgeril, M. Wine, Alcohol, Platelets, and the French Paradox for Coronary Heart Disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Ferro-Luzzi, A.; Cialfa, E.; Leclercq, C.; Toti, E. The Mediterranean Diet Revisited. Focus on Fruit and Vegetables. Int. J. Food Sci. Nutr. 1994, 45, 291–300. [Google Scholar] [CrossRef]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean Diet Pyramid: A Cultural Model for Healthy Eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef]
- Kushi, L.H.; Lenart, E.B.; Willett, W.C. Health Implications of Mediterranean Diets in Light of Contemporary Knowledge. 2. Meat, Wine, Fats, and Oils. Am. J. Clin. Nutr. 1995, 61, 1416S–1427S. [Google Scholar] [CrossRef] [PubMed]
- Rimm, E.B.; Ellison, R.C. Alcohol in the Mediterranean Diet. Am. J. Clin. Nutr. 1995, 61, 1378S–1382S. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Lagiou, P. Healthy Traditional Mediterranean Diet: An Expression of Culture, History, and Lifestyle. Nutr. Rev. 1997, 55, 383–389. [Google Scholar] [CrossRef]
- Leighton, F.; Cuevas, A.; Guasch, V.; Perez, D.; Strobel, P.; San Martin, A.; Urzua, U.; Diez, M.; Foncea, R.; Castillo, O. Plasma Polyphenols and Antioxidants, Oxidative DNA Damage and Endothelial Function in a Diet and Wine Intervention Study in Humans. Drugs Under Exp. Clin. Res. 1999, 25, 133–141. [Google Scholar]
- Mills, K.C.; McCarty, D.; Ward, J.; Minuto, L.; Patzynski, J. A Residence Hall Tavern as a Collegiate Alcohol Abuse Prevention Activity. Addict. Behav. 1983, 8, 105–108. [Google Scholar] [CrossRef]
- Téllez, C. Chile: Alcoholism in a Wine-Drinking Country. Br. J. Addict. 1984, 79, 447–448. [Google Scholar] [CrossRef]
- Fitzgerald, J.; Mulford, H. Factors Related to Problem-Drinking Rates. J. Stud. Alcohol 1984, 45, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Wallace, P.; Haines, A. Use of a Questionnaire in General Practice to Increase the Recognition of Patients with Excessive Alcohol Consumption. Br. Med. J. (Clin. Res. Ed.) 1985, 290, 1949–1953. [Google Scholar] [CrossRef] [PubMed]
- Gruenewald, P.J.; Ponicki, W.R. The Relationship of Alcohol Sales to Cirrhosis Mortality. J. Stud. Alcohol 1995, 56, 635–641. [Google Scholar] [CrossRef]
- Room, R. Alcohol Control and Public Health. Annu. Rev. Public Health 1984, 5, 293–317. [Google Scholar] [CrossRef]
- Ruhm, C.J. Alcohol Policies and Highway Vehicle Fatalities. J. Health Econ. 1996, 15, 435–454. [Google Scholar] [CrossRef] [PubMed]
- Millikan, L.E. History and Epidemiology of Alcohol Use and Abuse. Clin. Dermatol. 1999, 17, 353–356. [Google Scholar] [CrossRef]
- Marmot, M.; Shipley, M.; Rose, G.; Thomas, B. Alcohol and Mortality: A U-Shaped Curve. Lancet 1981, 317, 580–583. [Google Scholar] [CrossRef]
- Kune, G.A.; Vitetta, L. Alcohol Consumption and the Etiology of Colorectal Cancer: A Review of the Scientific Evidence from 1957 to 1991. Nutr. Cancer 1992, 18, 97–111. [Google Scholar] [CrossRef]
- Seitz, H.K.; Pöschl, G.; Simanowski, U.A. Alcohol and Cancer. Recent Developments in Alcoholism: The Consequences of Alcoholism Medical Neuropsychiatric Economic Cross-Cultural; Kluver Academic/Plenum Publisher: New York, NY, USA, 2000; pp. 67–95. [Google Scholar]
- Wickramasinghe, S.; Hasan, R.; Khalpey, Z. Differences in the Serum Levels of Acetaldehyde and Cytotoxic Acetaldehyde-Albumin Complexes after the Consumption of Red and White Wine: In Vitro Effects of Flavonoids, Vitamin E, and Other Dietary Antioxidants on Cytotoxic Complexes. Alcohol. Clin. Exp. Res. 1996, 20, 799–803. [Google Scholar] [CrossRef]
- Williams, R.R.; Horm, J.W. Association of Cancer Sites with Tobacco and Alcohol Consumption and Socioeconomic Status of Patients: Interview Study from the Third National Cancer Survey. J. Natl. Cancer Inst. 1977, 58, 525–547. [Google Scholar] [CrossRef]
- Hoey, J.; MONTVERNAY, C.; LAMBERT, R. Wine and Tobacco: Risk Factors for Gastric Cancer in France. Am. J. Epidemiol. 1981, 113, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Chow, W.; Yang, G.; McLaughlin, J.K.; Gao, R.; Zheng, W.; Shu, X.; Jin, F.; Fraumeni, J.F., Jr.; Gao, Y. The Influence of Cigarette Smoking, Alcohol, and Green Tea Consumption on the Risk of Carcinoma of the Cardia and Distal Stomach in Shanghai, China. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1996, 77, 2449–2457. [Google Scholar] [CrossRef]
- Buiatti, E.; Palli, D.; Decarli, A.; Amadori, D.; Avellini, C.; Bianchi, S.; Biserni, R.; Cipriani, F.; Cocco, P.; Giacosa, A. A Case-control Study of Gastric Cancer and Diet in Italy. Int. J. Cancer 1989, 44, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Nomura, A.; Grove, J.S.; Stemmermann, G.N.; Severson, R.K. A Prospective Study of Stomach Cancer and Its Relation to Diet, Cigarettes, and Alcohol Consumption. Cancer Res. 1990, 50, 627–631. [Google Scholar]
- Sokol, R.J.; Miller, S.I.; Debanne, S.; Golden, N.; Collins, G.; Kaplan, J.; Martier, S. The Cleveland NIAAA Prospective Alcohol-in-Pregnancy Study: The First Year. Neurobehav. Toxicol. Teratol. 1981, 3, 203–209. [Google Scholar] [PubMed]
- Kuzma, J.W.; Sokol, R.J. Maternal Drinking Behavior and Decreased Intrauterine Growth. Alcohol. Clin. Exp. Res. 1982, 6, 396–402. [Google Scholar] [CrossRef]
- Sulaiman, N.D.; Florey, C.d.V.; Taylor, D.J.; Ogston, S.A. Alcohol Consumption in Dundee Primigravidas and Its Effects on Outcome of Pregnancy. Br. Med. J. (Clin. Res. Ed.) 1988, 296, 1500–1503. [Google Scholar] [CrossRef]
- Waterson, E.; Murray-Lyon, I.M. Drinking and Smoking Patterns amongst Women Attending an Antenatal Clinic—II. During Pregnancy. Alcohol Alcohol. 1989, 24, 163–173. [Google Scholar] [CrossRef]
- Byers, T.; Funch, D. Alcohol and Breast Cancer. Lancet 1982, 319, 799–800. [Google Scholar] [CrossRef]
- Lê, M.G.; Hill, C.; Kramar, A.; Flamant, R. Alcoholic Beverage Consumption and Breast Cancer in a French Case-Control Study. Am. J. Epidemiol. 1984, 120, 350–357. [Google Scholar] [CrossRef]
- Harvey, E.B.; Schairer, C.; Brinton, L.A.; Hoover, R.N.; Fraumeni, J.F., Jr. Alcohol Consumption and Breast Cancer. J. Natl. Cancer Inst. 1987, 78, 657–661. [Google Scholar] [PubMed]
- Plant, M.L. Alcohol and Breast Cancer: A Review. Int. J. Addict. 1992, 27, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Longnecker, M.P.; Newcomb, P.A.; Mittendorf, R.; Greenberg, E.R.; Clapp, R.W.; Bogdan, G.F.; Baron, J.; MacMahon, B.; Willett, W.C. Risk of Breast Cancer in Relation to Lifetime Alcohol Consumption. JNCI J. Natl. Cancer Inst. 1995, 87, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Rohan, T.E.; Jain, M.; Howe, G.R.; Miller, A.B. Alcohol Consumption and Risk of Breast Cancer: A Cohort Study. Cancer Causes Control 2000, 11, 239–247. [Google Scholar] [CrossRef]
- Wu, A.H.; Paganini-Hill, A.; Ross, R.; Henderson, B. Alcohol, Physical Activity and Other Risk Factors for Colorectal Cancer: A Prospective Study. Br. J. Cancer 1987, 55, 687–694. [Google Scholar] [CrossRef]
- Klatsky, A.L.; Armstrong, M.A.; Friedman, G.D.; Hiatt, R.A. The Relations of Alcoholic Beverage Use to Colon and Rectal Cancer. Am. J. Epidemiol. 1988, 128, 1007–1015. [Google Scholar] [CrossRef]
- Newcomb, P.A.; Storer, B.E.; Marcus, P.M. Cancer of the Large Bowel in Women in Relation to Alcohol Consumption: A Case-Control Study in Wisconsin (United States). Cancer Causes Control. 1993, 4, 405–411. [Google Scholar] [CrossRef]
- Gapstur, S.M.; Potter, J.D.; Folsom, A.R. Alcohol Consumption and Colon and Rectal Cancer in Postmenopausal Women. Int. J. Epidemiol. 1994, 23, 50–57. [Google Scholar] [CrossRef]
- Hendriks, H.F.; Veenstra, J.; Velthuis-te Wierik, E.J.; Shaafsma, G.; Kluft, C. Effect of Moderate Dose of Alcohol with Evening Meal on Fibrinolytic Factors. BMJ 1994, 308, 1003–1006. [Google Scholar] [CrossRef]
- Obisesan, T.O.; Hirsch, R.; Kosoko, O.; Carlson, L.; Parrott, M. Moderate Wine Consumption Is Associated with Decreased Odds of Developing Age-related Macular Degeneration in NHANES-1. J. Am. Geriatr. Soc. 1998, 46, 1–7. [Google Scholar] [CrossRef]
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. Wine as a Biological Fluid: History, Production, and Role in Disease Prevention. J. Clin. Lab. Anal. 1997, 11, 287–313. [Google Scholar] [CrossRef]
- Jeong, H.-S.; Chung, H.; Song, S.-H.; Kim, C.-I.; Lee, J.-G.; Kim, Y.-S. Validation and Determination of the Contents of Acetaldehyde and Formaldehyde in Foods. Toxicol. Res. 2015, 31, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Huang, W.; You, Y.; Zhan, J. Controlling Strategies of Methanol Generation in Fermented Fruit Wine: Pathways, Advances, and Applications. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70048. [Google Scholar] [CrossRef] [PubMed]
- Kelley, C.J.; Mahajan, J.; Brooks, L.C.; Neubert, L.A.; Breneman, W.; Carmack, M. Polyphenolic Acids of Lithospermum Ruderale (Boraginaceae). I. Isolation and Structure Determination of Lithospermic Acid. J. Org. Chem. 1975, 40, 1804–1815. [Google Scholar] [CrossRef]
- Petkov, V. Plants with Hypotensive, Antiatheromatous and Coronarodilatating Action. Am. J. Chin. Med. 1979, 7, 197–236. [Google Scholar] [CrossRef]
- Brun, S.; Cabanis, J.C.; Mestres, J.P. Analytical Chemistry. Experientia 1986, 42, 893–904. [Google Scholar] [CrossRef]
- Bertelli, A.; Giovannini, L.; Stradi, R.; Urien, S.; Tillement, J.; Bertelli, A. Evaluation of Kinetic Parameters of Natural Phytoalexin in Resveratrol Orally Administered in Wine to Rats. Drugs Under Exp. Clin. Res. 1998, 24, 51–55. [Google Scholar]
- Rotondo, S.; Rajtar, G.; Manarini, S.; Celardo, A.; Rotilio, D.; De Gaetano, G.; Evangelista, V.; Cerletti, C. Effect of Trans-resveratrol, a Natural Polyphenolic Compound, on Human Polymorphonuclear Leukocyte Function. Br. J. Pharmacol. 1998, 123, 1691–1699. [Google Scholar] [CrossRef]
- Pendurthi, U.R.; Williams, J.T.; Rao, L.V.M. Resveratrol, a Polyphenolic Compound Found in Wine, Inhibits Tissue Factor Expression in Vascular Cells: A Possible Mechanism for the Cardiovascular Benefits Associated with Moderate Consumption of Wine. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 419–426. [Google Scholar] [CrossRef]
- Tomera, J.F. Current Knowledge of the Health Benefits and Disadvantages of Wine Consumption. Trends Food Sci. Technol. 1999, 10, 129–138. [Google Scholar] [CrossRef]
- Buiarelli, F.; Cartoni, G.; Coccioli, F.; Levetsovitou, Z. Determination of Phenolic Acids in Wine by High-Performance Liquid Chromatography with a Microbore Column. J. Chromatogr. A 1995, 695, 229–235. [Google Scholar] [CrossRef]
- Frankel, E.N.; Waterhouse, A.L.; Teissedre, P.L. Principal Phenolic Phytochemicals in Selected California Wines and Their Antioxidant Activity in Inhibiting Oxidation of Human Low-Density Lipoproteins. J. Agric. Food Chem. 1995, 43, 890–894. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Constant, J. Alcohol, Ischemic Heart Disease, and the French Paradox. Clin. Cardiol. 1997, 20, 420–424. [Google Scholar] [CrossRef]
- Frémont, L. Biological Effects of Resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Somers, T.; Verette, E. Phenolic Composition of Natural Wine Types. In Wine Analysis; Springer: Berlin/Heidelberg, Germany, 1988; pp. 219–257. [Google Scholar]
- Macheix, J.; Sapis, J.; Fleuriet, A.; Lee, C. Phenolic Compounds and Polyphenoloxidase in Relation to Browning in Grapes and Wines. Crit. Rev. Food Sci. Nutr. 1991, 30, 441–486. [Google Scholar] [CrossRef]
- Lapidot, T.; Harel, S.; Granit, R.; Kanner, J. Bioavailability of Red Wine Anthocyanins as Detected in Human Urine. J. Agric. Food Chem. 1998, 46, 4297–4302. [Google Scholar] [CrossRef]
- Holiman, P.C.; Hertog, M.G.; Katan, M.B. Analysis and Health Effects of Flavonoids. Food Chem. 1996, 57, 43–46. [Google Scholar] [CrossRef]
- Bakker, J.; Bridle, P.; Honda, T.; Kuwano, H.; Saito, N.; Terahara, N.; Timberlake, C.F. Identification of an Anthocyanin Occurring in Some Red Wines. Phytochemistry 1997, 44, 1375–1382. [Google Scholar] [CrossRef]
- Bakker, J.; Timberlake, C.F. Isolation, Identification, and Characterization of New Color-Stable Anthocyanins Occurring in Some Red Wines. J. Agric. Food Chem. 1997, 45, 35–43. [Google Scholar] [CrossRef]
- Fulcrand, H.; dos Santos, P.-J.C.; Sarni-Manchado, P.; Cheynier, V.; Favre-Bonvin, J. Structure of New Anthocyanin-Derived Wine Pigments. J. Chem. Soc. Perkin Trans. 1996, 1, 735–739. [Google Scholar] [CrossRef]
- Fulcrand, H.; Benabdeljalil, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. A New Class of Wine Pigments Generated by Reaction between Pyruvic Acid and Grape Anthocyanins. Phytochemistry 1998, 47, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, P.; Pietta, P.; Testolin, G. Polyphenol Content and Total Antioxidant Potential of Selected Italian Wines. J. Agric. Food Chem. 1997, 45, 1152–1155. [Google Scholar] [CrossRef]
- Cheng, T.O. Antioxidants in Wine and Tea. J. R. Soc. Med. 1999, 92, 157. [Google Scholar] [CrossRef] [PubMed]
- Trousdale, E.K.; Singleton, V.L. Astilbin and Engeletin in Grapes and Wine. Phytochemistry 1983, 22, 619–620. [Google Scholar] [CrossRef]
- Igarashi, K.; Uchida, Y.; Murakami, N.; Mizutani, K.; Masuda, H. Effect of Astilbin in Tea Processed from Leaves of Engelhardtia Chrysolepis., on the Serum and Liver Lipid Concentrations and on the Erythrocyte and Liver Antioxidative Enzyme Activities of Rats. Biosci. Biotechnol. Biochem. 1996, 60, 513–515. [Google Scholar] [CrossRef]
- Brouillard, R.; George, F.; Fougerousse, A. Polyphenols Produced during Red Wine Ageing. Biofactors 1997, 6, 403–410. [Google Scholar] [CrossRef]
- Brandi, M.L. Natural and Synthetic Isoflavones in the Prevention and Treatment of Chronic Diseases. Calcif. Tissue Int. 1997, 61, S5–S8. [Google Scholar] [CrossRef]
- Hsieh, H.-K.; Lee, T.-H.; Wang, J.-P.; Wang, J.-J.; Lin, C.-N. Synthesis and Anti-Inflammatory Effect of Chalcones and Related Compounds. Pharm. Res. 1998, 15, 39–46. [Google Scholar] [CrossRef]
- Castagnino, C.; Vercauteren, J. Castavinol, a New Series of Polyphenols from Bordeaux Red Wines. Tetrahedron Lett. 1996, 37, 7739–7742. [Google Scholar] [CrossRef]
- Lea, A.G.; Bridle, P.; Timberlake, C.F.; Singleton, V.L. The Procyanidins of White Grapes and Wines. Am. J. Enol. Vitic. 1979, 30, 289–300. [Google Scholar] [CrossRef]
- Dadic, M.; Van Gheluwe, J.E.; Weaver, R.L. Thin Layer Densitometric Determination of Gallic Acid and Gallotannins in Wine and Cider. J. Assoc. Off. Anal. Chem. 1980, 63, 1–4. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Viriot, C.; Scalbert, A.; Lapierre, C.; Moutounet, M. Ellagitannins and Lignins in Aging of Spirits in Oak Barrels. J. Agric. Food Chem. 1993, 41, 1872–1879. [Google Scholar] [CrossRef]
- Shimizu, J.; Watanabe, M. Volatile Components Identified in the Phenolic Fractions of Wines from Koshu and Zenkoji Grapes. Agric. Biol. Chem. 1982, 46, 1447–1452. [Google Scholar]
- Ghiselli, A.; Nardini, M.; Baldi, A.; Scaccini, C. Antioxidant Activity of Different Phenolic Fractions Separated from an Italian Red Wine. J. Agric. Food Chem. 1998, 46, 361–367. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. Cinnamates and Hydroxybenzoates in the Diet: Antioxidant Activity Assessed Using the ABTS•+ Radical Cation. Br. Food J. 1997, 99, 57–62. [Google Scholar] [CrossRef]
- Aruoma, O. Nutrition and Health Aspects of Free Radicals and Antioxidants. Food Chem. Toxicol. 1994, 32, 671–683. [Google Scholar] [CrossRef]
- Betés-Saura, C.; Andrés-Lacueva, C.; Lamuela-Raventos, R.M. Phenolics in White Free Run Juices and Wines from Penedes by High-Performance Liquid Chromatography: Changes during Vinification. J. Agric. Food Chem. 1996, 44, 3040–3046. [Google Scholar] [CrossRef]
- Curto, E.V.; Kwong, C.; Hermersdörfer, H.; Glatt, H.; Santis, C.; Virador, V.; Hearing, V.J., Jr.; Dooley, T.P. Inhibitors of Mammalian Melanocyte Tyrosinase: In Vitro Comparisons of Alkyl Esters of Gentisic Acid with Other Putative Inhibitors. Biochem. Pharmacol. 1999, 57, 663–672. [Google Scholar] [CrossRef]
- Abu-Amsha, R.; Croft, K.D.; Puddey, I.B.; Proudfoot, J.M.; Beilin, L.J. Phenolic Content of Various Beverages Determines the Extent of Inhibition of Human Serum and Low-Density Lipoprotein Oxidation in Vitro: Identification and Mechanism of Action of Some Cinnamic Acid Derivatives from Red Wine. Clin. Sci. 1996, 91, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F. Natural Antioxidants: Chemistry, Health Effects, and Applications; The American Oil Chemists Society: Urbana, IL, USA, 1997; ISBN 0-935315-77-2. [Google Scholar]
- Teissedre, P.L.; Frankel, E.N.; Waterhouse, A.L.; Peleg, H.; German, J.B. Inhibition ofIn Vitrohuman LDL Oxidation by Phenolic Antioxidants from Grapes and Wines. J. Sci. Food Agric. 1996, 70, 55–61. [Google Scholar] [CrossRef]
- Chesson, A.; Provan, G.J.; Russell, W.R.; Scobbie, L.; Richardson, A.J.; Stewart, C. Hydroxycinnamic Acids in the Digestive Tract of Livestock and Humans. J. Sci. Food Agric. 1999, 79, 373–378. [Google Scholar] [CrossRef]
- Siemann, E.; Creasy, L. Concentration of the Phytoalexin Resveratrol in Wine. Am. J. Enol. Vitic. 1992, 43, 49–52. [Google Scholar] [CrossRef]
- Mattivi, F.; Reniero, F.; Korhammer, S. Isolation, Characterization, and Evolution in Red Wine Vinification of Resveratrol Monomers. J. Agric. Food Chem. 1995, 43, 1820–1823. [Google Scholar] [CrossRef]
- Ribeiro de Lima, M.T.; Waffo-Téguo, P.; Teissedre, P.L.; Pujolas, A.; Vercauteren, J.; Cabanis, J.C.; Mérillon, J.M. Determination of Stilbenes (Trans-Astringin, Cis-and Trans-Piceid, and Cis-and Trans-Resveratrol) in Portuguese Wines. J. Agric. Food Chem. 1999, 47, 2666–2670. [Google Scholar] [CrossRef]
- Mérillon, J.-M.; Fauconneau, B.; Teguo, P.W.; Barrier, L.; Vercauteren, J.; Huguet, F. Antioxidant Activity of the Stilbene Astringin, Newly Extracted from Vitis Vinifera Cell Cultures. Clin. Chem. 1997, 43, 1092–1093. [Google Scholar] [CrossRef]
- Giovannini, C.; Straface, E.; Modesti, D.; Coni, E.; Cantafora, A.; De Vincenzi, M.; Malorni, W.; Masella, R. Tyrosol, the Major Olive Oil Biophenol, Protects against Oxidized-LDL-Induced Injury in Caco-2 Cells. J. Nutr. 1999, 129, 1269–1277. [Google Scholar] [CrossRef]
- Ruf, J. Overview of Epidemiological Studies on Wine, Health and Mortality. Drugs Under Exp. Clin. Res. 2003, 29, 173–179. [Google Scholar]
- Caimi, G.; Carollo, C.; Presti, R.L. Diabetes Mellitus: Oxidative Stress and Wine. Curr. Med. Res. Opin. 2003, 19, 581–586. [Google Scholar] [CrossRef]
- Letenneur, L. Risk of Dementia and Alcohol and Wine Consumption: A Review of Recent Results. Biol. Res. 2004, 37, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Caruana, M.; Cauchi, R.; Vassallo, N. Putative Role of Red Wine Polyphenols against Brain Pathology in Alzheimer’s and Parkinson’s Disease. Front. Nutr. 2016, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Candore, G.; Caruso, C.; Jirillo, E.; Covelli, V. Polyphenols from Red Wine Modulate Immune Responsiveness: Biological and Clinical Significance. Curr. Pharm. Des. 2008, 14, 2733–2748. [Google Scholar] [CrossRef] [PubMed]
- Guilford, J.M.; Pezzuto, J.M. Wine and Health: A Review. Am. J. Enol. Vitic. 2011, 62, 471–486. [Google Scholar] [CrossRef]
- Artero, A.; Artero, A.; Tarín, J.J.; Cano, A. The Impact of Moderate Wine Consumption on Health. Maturitas 2015, 80, 3–13. [Google Scholar] [CrossRef]
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of Red Wine Consumption to Human Health Protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Beneficial Effects of Red Wine Polyphenols on Human Health: Comprehensive Review. Curr. Issues Mol. Biol. 2023, 45, 782–798. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; González-Manzano, S.; González-Paramás, A.M. Wine, Polyphenols, and Mediterranean Diets. What Else Is There to Say? Molecules 2021, 26, 5537. [Google Scholar] [CrossRef]
- Hrelia, S.; Di Renzo, L.; Bavaresco, L.; Bernardi, E.; Malaguti, M.; Giacosa, A. Moderate Wine Consumption and Health: A Narrative Review. Nutrients 2022, 15, 175. [Google Scholar] [CrossRef]
- Dobroslavska, P.; Silva, M.L.; Vicente, F.; Pereira, P. Mediterranean Dietary Pattern for Healthy and Active Aging: A Narrative Review of an Integrative and Sustainable Approach. Nutrients 2024, 16, 1725. [Google Scholar] [CrossRef]
- Kao, W.L.; Puddey, I.B.; Boland, L.L.; Watson, R.L.; Brancati, F.L. Alcohol Consumption and the Risk of Type 2 Diabetes Mellitus: Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 2001, 154, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, S.; Vaucher, E.; Aider, R.; Martin, S.; Perney, P.; Balmès, J.L.; Nalpas, B. Wine Consumption Is Not Associated with a Decreased Risk of Alcoholic Cirrhosis in Heavy Drinkers. Alcohol Alcohol. 2002, 37, 618–621. [Google Scholar] [CrossRef]
- Altieri, A.; Bosetti, C.; Gallus, S.; Franceschi, S.; Dal Maso, L.; Talamini, R.; Levi, F.; Negri, E.; Rodriguez, T.; La Vecchia, C. Wine, Beer and Spirits and Risk of Oral and Pharyngeal Cancer: A Case–Control Study from Italy and Switzerland. Oral Oncol. 2004, 40, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, R.G.; Shields, P.G. The Etiology of Alcohol-Induced Breast Cancer. Alcohol 2005, 35, 213–225. [Google Scholar] [CrossRef]
- Baer-Dubowska, W.; Bartoszek, A.; Malejka-Giganti, D. Carcinogenic and Anticarcinogenic Food Components; CRC Press: Boca Raton, FL, USA, 2005; ISBN 0-429-12041-9. [Google Scholar]
- Vasilopoulou, E.; Georga, K.; Joergensen, M.B.; Naska, A.; Trichopoulou, A. The Antioxidant Properties of Greek Foods and the Flavonoid Content of the Mediterranean Menu. Curr. Med. Chem.-Immunol. Endocr. Metab. Agents 2005, 5, 33–45. [Google Scholar] [CrossRef]
- Li, H.; Liang, X.; Guo, A.; Huang, D. Arginine Metabolism in Wine Malolactic Bacteria. Wei Sheng Wu Xue Bao = Acta Microbiol. Sin. 2006, 46, 663–667. [Google Scholar]
- Hague, T.; Petroczi, A.; Andrews, P.L.; Barker, J.; Naughton, D.P. Determination of Metal Ion Content of Beverages and Estimation of Target Hazard Quotients: A Comparative Study. Chem. Cent. J. 2008, 2, 1–9. [Google Scholar] [CrossRef]
- Ortiz-Villeda, B.; Lobos, O.; Aguilar-Zuniga, K.; Carrasco-Sánchez, V. Ochratoxins in Wines: A Review of Their Occurrence in the Last Decade, Toxicity, and Exposure Risk in Humans. Toxins 2021, 13, 478. [Google Scholar] [CrossRef] [PubMed]
- Maggiolini, M.; Recchia, A.; Bonofiglio, D.; Catalano, S.; Vivacqua, A.; Carpino, A.; Rago, V.; Rossi, R.; Andò, S. The Red Wine Phenolics Piceatannol and Myricetin Act as Agonists for Estrogen Receptor α in Human Breast Cancer Cells. J. Mol. Endocrinol. 2005, 35, 269–281. [Google Scholar] [CrossRef]
- Mazza, G. Anthocyanins and Heart Health. Ann.-Ist. Super. Di Sanita 2007, 43, 369. [Google Scholar]
- Rivero-Pérez, M.; Muniz, P.; González-Sanjosé, M. Contribution of Anthocyanin Fraction to the Antioxidant Properties of Wine. Food Chem. Toxicol. 2008, 46, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The Case for Anthocyanin Consumption to Promote Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; De Freitas, V. Wine Flavonoids in Health and Disease Prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Setford, P.C.; Jeffery, D.W.; Grbin, P.R.; Muhlack, R.A. A New Approach to Predicting the Extraction of Malvidin-3-Glucoside during Red Wine Fermentation at Industrial-Scale. Food Bioprod. Process. 2022, 131, 217–223. [Google Scholar] [CrossRef]
- Leopoldini, M.; Rondinelli, F.; Russo, N.; Toscano, M. Pyranoanthocyanins: A Theoretical Investigation on Their Antioxidant Activity. J. Agric. Food Chem. 2010, 58, 8862–8871. [Google Scholar] [CrossRef]
- Azevedo, J.; Oliveira, J.; Cruz, L.; Teixeira, N.; Brás, N.F.; De Freitas, V.; Mateus, N. Antioxidant Features of Red Wine Pyranoanthocyanins: Experimental and Theoretical Approaches. J. Agric. Food Chem. 2014, 62, 7002–7009. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. 2015, 6, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Schreiber IV, H.L.; Mazmanian, S.K. The Gut Microbiota–Brain Axis in Behaviour and Brain Disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Lujan-Barroso, L.; Slimani, N.; Romieu, I.; Touillaud, M.; Kaaks, R.; Teucher, B.; Mattiello, A.; Grioni, S. Estimation of the Intake of Anthocyanidins and Their Food Sources in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Br. J. Nutr. 2011, 106, 1090–1099. [Google Scholar] [CrossRef]
- Wang, H.; Race, E.J.; Shrikhande, A.J. Anthocyanin Transformation in Cabernet Sauvignon Wine during Aging. J. Agric. Food Chem. 2003, 51, 7989–7994. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol Profiles of Vitis Vinifera Red Grapes and Their Single-Cultivar Wines. J. Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, M.; Stompor-Gorący, M. The Spectrum of Pharmacological Actions of Syringetin and Its Natural Derivatives—A Summary Review. Nutrients 2022, 14, 5157. [Google Scholar] [CrossRef]
- Wu, B.; Song, H.-P.; Zhou, X.; Liu, X.-G.; Gao, W.; Dong, X.; Li, H.-J.; Li, P.; Yang, H. Screening of Minor Bioactive Compounds from Herbal Medicines by in Silico Docking and the Trace Peak Exposure Methods. J. Chromatogr. A 2016, 1436, 91–99. [Google Scholar] [CrossRef]
- Büchter, C.; Ackermann, D.; Honnen, S.; Arnold, N.; Havermann, S.; Koch, K.; Wätjen, W. Methylated Derivatives of Myricetin Enhance Life Span in Caenorhabditis Elegans Dependent on the Transcription Factor DAF-16. Food Funct. 2015, 6, 3383–3392. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Gao, M.; Li, H.; Han, X.; Zhang, X.; Li, Y.; Guo, D.; Liu, B. Three New Bisflavonols from the Seeds of Hovenia Dulcis Thunb. and Their Anti-RSV Activities. Fitoterapia 2020, 143, 104587. [Google Scholar] [CrossRef]
- Grewal, A.S.; Singh, S.; Sharma, N.; Grover, R. In Silico Docking Studies of Some Flavonoids against Multiple Targets of Alzheimer’s Disease. Plant Arch 2020, 20, 3271–3278. [Google Scholar]
- Ramezani, M.; Darbandi, N.; Khodagholi, F.; Hashemi, A. Myricetin Protects Hippocampal CA3 Pyramidal Neurons and Improves Learning and Memory Impairments in Rats with Alzheimer’s Disease. Neural Regen. Res. 2016, 11, 1976–1980. [Google Scholar] [CrossRef]
- Hsu, Y.; Liang, H.; Hung, C.; Kuo, P. Syringetin, a Flavonoid Derivative in Grape and Wine, Induces Human Osteoblast Differentiation through Bone Morphogenetic Protein-2/Extracellular Signal-regulated Kinase 1/2 Pathway. Mol. Nutr. Food Res. 2009, 53, 1452–1461. [Google Scholar] [CrossRef]
- Corder, R.; Mullen, W.; Khan, N.; Marks, S.; Wood, E.; Carrier, M.; Crozier, A. Red Wine Procyanidins and Vascular Health. Nature 2006, 444, 566. [Google Scholar] [CrossRef]
- Khan, N.Q.; Patel, B.; Kang, S.S.; Dhariwal, S.K.; Husain, F.; Wood, E.G.; Pothecary, M.R.; Corder, R. Regulation of Vascular Endothelial Function by Red Wine Procyanidins: Implications for Cardiovascular Health. Tetrahedron 2015, 71, 3059–3065. [Google Scholar] [CrossRef]
- Martinez-Micaelo, N.; González-Abuín, N.; Ardevol, A.; Pinent, M.; Blay, M.T. Procyanidins and Inflammation: Molecular Targets and Health Implications. Biofactors 2012, 38, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gupta, S.; Chauhan, S.; Nair, A.; Sharma, P. Astilbin: A Promising Unexplored Compound with Multidimensional Medicinal and Health Benefits. Pharmacol. Res. 2020, 158, 104894. [Google Scholar] [CrossRef] [PubMed]
- Jandera, P.; Škeříková, V.; Řehová, L.; Hájek, T.; Baldriánová, L.; Škopová, G.; Kellner, V.; Horna, A. RP-HPLC Analysis of Phenolic Compounds and Flavonoids in Beverages and Plant Extracts Using a CoulArray Detector. J. Sep. Sci. 2005, 28, 1005–1022. [Google Scholar] [CrossRef]
- Erlund, I. Review of the Flavonoids Quercetin, Hesperetin, and Naringenin. Dietary Sources, Bioactivities, Bioavailability, and Epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Mandalari, G.; Bennett, R.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C.; Gasson, M.; Narbad, A. Antimicrobial Activity of Flavonoids Extracted from Bergamot (Citrus Bergamia Risso) Peel, a Byproduct of the Essential Oil Industry. J. Appl. Microbiol. 2007, 103, 2056–2064. [Google Scholar] [CrossRef]
- Lombardi, S.J.; Pannella, G.; Coppola, F.; Vergalito, F.; Maiuro, L.; Succi, M.; Sorrentino, E.; Tremonte, P.; Coppola, R. Plant-Based Ingredients Utilized as Fat Replacers and Natural Antimicrobial Agents in Beef Burgers. Foods 2024, 13, 3229. [Google Scholar] [CrossRef]
- Felgines, C.; Texier, O.; Morand, C.; Manach, C.; Scalbert, A.; Régerat, F.; Rémésy, C. Bioavailability of the Flavanone Naringenin and Its Glycosides in Rats. Am. J. Physiol. -Gastrointest. Liver Physiol. 2000, 279, G1148–G1154. [Google Scholar] [CrossRef]
- Kim, D.-O.; Lee, C.Y. Comprehensive Study on Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Various Polyphenolics in Scavenging a Free Radical and Its Structural Relationship. Crit. Rev. Food Sci. Nutr. 2004, 44, 253–273. [Google Scholar] [CrossRef]
- Caccetta, R.A.-A.; Croft, K.D.; Beilin, L.J.; Puddey, I.B. Ingestion of Red Wine Significantly Increases Plasma Phenolic Acid Concentrations but Does Not Acutely Affect Ex Vivo Lipoprotein Oxidizability. Am. J. Clin. Nutr. 2000, 71, 67–74. [Google Scholar] [CrossRef]
- Jagan, S.; Ramakrishnan, G.; Anandakumar, P.; Kamaraj, S.; Devaki, T. Antiproliferative Potential of Gallic Acid against Diethylnitrosamine-Induced Rat Hepatocellular Carcinoma. Mol. Cell. Biochem. 2008, 319, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.E.; Kim, H.S.; Lee, C.S.; Park, D.-H.; Kim, Y.-N.; Lee, M.-J.; Lee, J.W.; Park, J.-W.; Kim, M.-S.; Ye, S.K. Caffeic Acid and Its Synthetic Derivative CADPE Suppress Tumor Angiogenesis by Blocking STAT3-Mediated VEGF Expression in Human Renal Carcinoma Cells. Carcinogenesis 2007, 28, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Ombra, M.N.; Coppola, F.; De Giulio, B.; d’Acierno, A.; Coppola, R.; Fratianni, F. Antibacterial Activity and Prebiotic Properties of Six Types of Lamiaceae Honey. Antibiotics 2024, 13, 868. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Waterhouse, A.L. Metabolites Are Key to Understanding Health Effects of Wine Polyphenolics. J. Nutr. 2009, 139, 1824S–1831S. [Google Scholar] [CrossRef]
- Mudnic, I.; Modun, D.; Rastija, V.; Vukovic, J.; Brizic, I.; Katalinic, V.; Kozina, B.; Medic-Saric, M.; Boban, M. Antioxidative and Vasodilatory Effects of Phenolic Acids in Wine. Food Chem. 2010, 119, 1205–1210. [Google Scholar] [CrossRef]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory Mechanism against Oxidative Stress of Caffeic Acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef]
- Morales, M.; Ros Barcelo, A.; Pedreno, M. Plant Stilbenes: Recent Advances in Their Chemistry and Biology. Adv. Plant Physiol. 2000, 3, 39–70. [Google Scholar]
- Wenzel, E.; Somoza, V. Metabolism and Bioavailability of Trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef]
- Szkudelski, T.; Szkudelska, K. Resveratrol and Diabetes: From Animal to Human Studies. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 1145–1154. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.; Gomez-Cabrera, M.; Vina, J. Properties of Resveratrol: In Vitro and in Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxidative Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D. Resveratrol and Cardiovascular Diseases. Nutrients 2016, 8, 250. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska, P.; Rychlicka, M.; Groborz, S.; Kruszyńska, A.; Ledesma-Amaro, R.; Rapak, A.; Gliszczyńska, A.; Lazar, Z. Studies on the Anticancer and Antioxidant Activities of Resveratrol and Long-Chain Fatty Acid Esters. Int. J. Mol. Sci. 2023, 24, 7167. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Xiong, B.; Qiu, S. Progress of Antimicrobial Mechanisms of Stilbenoids. Pharmaceutics 2024, 16, 663. [Google Scholar] [CrossRef]
- Moss, R.; Mao, Q.; Taylor, D.; Saucier, C. Investigation of Monomeric and Oligomeric Wine Stilbenoids in Red Wines by Ultra-high-performance Liquid Chromatography/Electrospray Ionization Quadrupole Time-of-flight Mass Spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 1815–1827. [Google Scholar] [CrossRef] [PubMed]
- El Khawand, T.; Courtois, A.; Valls, J.; Richard, T.; Krisa, S. A Review of Dietary Stilbenes: Sources and Bioavailability. Phytochem. Rev. 2018, 17, 1007–1029. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Andres-Lacueva, C.; Lamuela-Raventós, R.M.; Berenguer, T.; Jakszyn, P.; Martínez, C.; Sanchez, M.J.; Navarro, C.; Chirlaque, M.D.; Tormo, M.-J. Concentrations of Resveratrol and Derivatives in Foods and Estimation of Dietary Intake in a Spanish Population: European Prospective Investigation into Cancer and Nutrition (EPIC)-Spain Cohort. Br. J. Nutr. 2008, 100, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Wang, Z.-R.; Hsieh, T.-C.; Bruder, J.L.; Zou, J.-G.; Huang, Y.-Z. Mechanism of Cardioprotection by Resveratrol, a Phenolic Antioxidant Present in Red Wine. Int. J. Mol. Med. 2001, 8, 3–17. [Google Scholar] [CrossRef]
- Bianchini, F.; Vainio, H. Wine and Resveratrol: Mechanisms of Cancer Prevention? Eur. J. Cancer Prev. 2003, 12, 417–425. [Google Scholar] [CrossRef]
- Vidavalur, R.; Otani, H.; Singal, P.K.; Maulik, N. Significance of Wine and Resveratrol in Cardiovascular Disease: French Paradox Revisited. Exp. Clin. Cardiol. 2006, 11, 217. [Google Scholar] [PubMed]
- Bertelli, A.A.; Das, D.K. Grapes, Wines, Resveratrol, and Heart Health. J. Cardiovasc. Pharmacol. 2009, 54, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Mar, M.; Mateos, R.; Garcia-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive Compounds in Wine: Resveratrol, Hydroxytyrosol and Melatonin: A Review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef]
- Francioso, A.; Mastromarino, P.; Masci, A.; d’Erme, M.; Mosca, L. Chemistry, Stability and Bioavailability of Resveratrol. Med. Chem. 2014, 10, 237–245. [Google Scholar] [CrossRef]
- Diaz-Gerevini, G.T.; Repossi, G.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A.R. Beneficial Action of Resveratrol: How and Why? Nutrition 2016, 32, 174–178. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The Therapeutic Potential of Resveratrol: A Review of Clinical Trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef]
- Pastor, R.F.; Restani, P.; Di Lorenzo, C.; Orgiu, F.; Teissedre, P.-L.; Stockley, C.; Ruf, J.C.; Quini, C.I.; Garcìa Tejedor, N.; Gargantini, R. Resveratrol, Human Health and Winemaking Perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 1237–1255. [Google Scholar] [CrossRef]
- Banez, M.J.; Geluz, M.I.; Chandra, A.; Hamdan, T.; Biswas, O.S.; Bryan, N.S.; Von Schwarz, E.R. A Systemic Review on the Antioxidant and Anti-Inflammatory Effects of Resveratrol, Curcumin, and Dietary Nitric Oxide Supplementation on Human Cardiovascular Health. Nutr. Res. 2020, 78, 11–26. [Google Scholar] [CrossRef]
- Haunschild, R.; Marx, W. On Health Effects of Resveratrol in Wine. Int. J. Environ. Res. Public Health 2022, 19, 3110. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Qasem, R.J. The Estrogenic Activity of Resveratrol: A Comprehensive Review of in Vitro and in Vivo Evidence and the Potential for Endocrine Disruption. Crit. Rev. Toxicol. 2020, 50, 439–462. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Bavaresco, L.; Lucini, L.; Busconi, M.; Flamini, R.; De Rosso, M. Wine Resveratrol: From the Ground Up. Nutrients 2016, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Khanbabaee, K.; Van Ree, T. Tannins: Classification and Definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar]
- Jourdes, M.; Michel, J.; Saucier, C.; Quideau, S.; Teissedre, P.-L. Identification, Amounts, and Kinetics of Extraction of C-Glucosidic Ellagitannins during Wine Aging in Oak Barrels or in Stainless Steel Tanks with Oak Chips. Anal. Bioanal. Chem. 2011, 401, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Versari, A.; Du Toit, W.; Parpinello, G.P. Oenological Tannins: A Review. Aust. J. Grape Wine Res. 2013, 19, 1–10. [Google Scholar] [CrossRef]
- Canas, S. Phenolic Composition and Related Properties of Aged Wine Spirits: Influence of Barrel Characteristics. A Review. Beverages 2017, 3, 55. [Google Scholar] [CrossRef]
- Laqui-Estaña, J.; López-Solís, R.; Peña-Neira, Á.; Medel-Marabolí, M.; Obreque-Slier, E. Wines in Contact with Oak Wood: The Impact of the Variety (Carménère and Cabernet Sauvignon), Format (Barrels, Chips and Staves), and Aging Time on the Phenolic Composition. J. Sci. Food Agric. 2019, 99, 436–448. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T. Ellagitannins as Active Constituents of Medicinal Plants. Planta Medica 1989, 55, 117–122. [Google Scholar] [CrossRef]
- Clifford, M.N.; Scalbert, A. Ellagitannins–Nature, Occurrence and Dietary Burden. J. Sci. Food Agric. 2000, 80, 1118–1125. [Google Scholar] [CrossRef]
- Quideau, S.; Jourdes, M.; Lefeuvre, D.; Montaudon, D.; Saucier, C.; Glories, Y.; Pardon, P.; Pourquier, P. The Chemistry of Wine Polyphenolic C-glycosidic Ellagitannins Targeting Human Topoisomerase II. Chem.–A Eur. J. 2005, 11, 6503–6513. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Ito, H. Tannins of Constant Structure in Medicinal and Food Plants—Hydrolyzable Tannins and Polyphenols Related to Tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef]
- Landete, J. Ellagitannins, Ellagic Acid and Their Derived Metabolites: A Review about Source, Metabolism, Functions and Health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Antony, P.J.; Ceasar, S.A.; Vasconcelos, A.B.S.; Montalvão, M.M.; Farias de Franca, M.N.; Resende, A.d.S.; Sharanya, C.S.; Liu, Y.; Hariharan, G. Health Functions and Related Molecular Mechanisms of Ellagitannin-Derived Urolithins. Crit. Rev. Food Sci. Nutr. 2024, 64, 280–310. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Asenstorfer, R.E. Screening for Potential Pigments Derived from Anthocyanins in Red Wine Using Nanoelectrospray Tandem Mass Spectrometry. J. Agric. Food Chem. 2002, 50, 756–761. [Google Scholar] [CrossRef]
- Mateus, N.; Silva, A.M.; Rivas-Gonzalo, J.C.; Santos-Buelga, C.; De Freitas, V. A New Class of Blue Anthocyanin-Derived Pigments Isolated from Red Wines. J. Agric. Food Chem. 2003, 51, 1919–1923. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-San José, M.L. Evolution of Flavanols, Anthocyanins, and Their Derivatives during the Aging of Red Wines Elaborated from Grapes Harvested at Different Stages of Ripening. J. Agric. Food Chem. 2004, 52, 1181–1189. [Google Scholar] [CrossRef]
- Lee, E.-R.; Kang, G.-H.; Cho, S.-G. Effect of Flavonoids on Human Health: Old Subjects but New Challenges. Recent Pat. Biotechnol. 2007, 1, 139–150. [Google Scholar] [CrossRef]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, Ș.C.; Răchișan, A.L.; Negrean, V.; Perné, M.-G.; Donca, V.I.; Alexescu, T.-G.; Para, I. The Effects of Flavonoids in Cardiovascular Diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Schwarz, M.; Jerz, G.; Winterhalter, P. Isolation and Structure of Pinotin A, a New Anthocyanin Derivative from Pinotage Wine. VITIS-J. Grapevine Res. 2015, 42, 105. [Google Scholar]
- He, J.; Oliveira, J.; Silva, A.M.; Mateus, N.; De Freitas, V. Oxovitisins: A New Class of Neutral Pyranone-Anthocyanin Derivatives in Red Wines. J. Agric. Food Chem. 2010, 58, 8814–8819. [Google Scholar] [CrossRef] [PubMed]
- Remy, S.; Fulcrand, H.; Labarbe, B.; Cheynier, V.; Moutounet, M. First Confirmation in Red Wine of Products Resulting from Direct Anthocyanin–Tannin Reactions. J. Sci. Food Agric. 2000, 80, 745–751. [Google Scholar] [CrossRef]
- De Freitas, V.; Mateus, N. Formation of Pyranoanthocyanins in Red Wines: A New and Diverse Class of Anthocyanin Derivatives. Anal. Bioanal. Chem. 2011, 401, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-K.; Lan, Y.-B.; Huang, Y.; Zhao, X.; Duan, C.-Q. Targeted Metabolomics of Anthocyanin Derivatives during Prolonged Wine Aging: Evolution, Color Contribution and Aging Prediction. Food Chem 2021, 339, 127795. [Google Scholar] [CrossRef]
- Wang, L.; Tu, Y.-C.; Lian, T.-W.; Hung, J.-T.; Yen, J.-H.; Wu, M.-J. Distinctive Antioxidant and Antiinflammatory Effects of Flavonols. J. Agric. Food Chem. 2006, 54, 9798–9804. [Google Scholar] [CrossRef]
- Lee, S.; Choi, Y.J.; Huo, C.; Alishir, A.; Kang, K.S.; Park, I.-H.; Jang, T.; Kim, K.H. Laricitrin 3-Rutinoside from Ginkgo Biloba Fruits Prevents Damage in TNF-α-Stimulated Normal Human Dermal Fibroblasts. Antioxidants 2023, 12, 1432. [Google Scholar] [CrossRef]
- Zerbib, M.; Mazauric, J.-P.; Meudec, E.; Le Guernevé, C.; Lepak, A.; Nidetzky, B.; Cheynier, V.; Terrier, N.; Saucier, C. New Flavanol O-Glycosides in Grape and Wine. Food Chem. 2018, 266, 441–448. [Google Scholar] [CrossRef]
- Najmanová, I.; Vopršalová, M.; Saso, L.; Mladěnka, P. The Pharmacokinetics of Flavanones. Crit. Rev. Food Sci. Nutr. 2020, 60, 3155–3171. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Chamkhi, I.; El Omari, N.; Balahbib, A.; Sharifi-Rad, J.; Bouyahya, A.; Akram, M.; Iqbal, M.; Docea, A.O. Pharmacological Properties of Chalcones: A Review of Preclinical Including Molecular Mechanisms and Clinical Evidence. Front. Pharmacol. 2021, 11, 592654. [Google Scholar] [CrossRef]
- Monagas, M.; Bartolomé, B.; Gómez-Cordovés, C. Updated Knowledge about the Presence of Phenolic Compounds in Wine. Crit. Rev. Food Sci. Nutr. 2005, 45, 85–118. [Google Scholar] [CrossRef]
- Gracia-Moreno, E.; Lopez, R.; Ferreira, V. Quantitative Determination of Five Hydroxy Acids, Precursors of Relevant Wine Aroma Compounds in Wine and Other Alcoholic Beverages. Anal. Bioanal. Chem. 2015, 407, 7925–7934. [Google Scholar] [CrossRef] [PubMed]
- Guebailia, H.A.; Chira, K.; Richard, T.; Mabrouk, T.; Furiga, A.; Vitrac, X.; Monti, J.-P.; Delaunay, J.-C.; Mérillon, J.-M. Hopeaphenol: The First Resveratrol Tetramer in Wines from North Africa. J. Agric. Food Chem. 2006, 54, 9559–9564. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.-Q.; Di, J.-M.; Luo, Y.; Cheng, K.-J.; Wei, X.; Shi, Z. Resveratrol Oligomers for the Prevention and Treatment of Cancers. Oxidative Med. Cell. Longev. 2014, 2014, 765832. [Google Scholar] [CrossRef] [PubMed]
- Baderschneider, B.; Winterhalter, P. Isolation and Characterization of Novel Stilbene Derivatives from Riesling Wine. J. Agric. Food Chem. 2000, 48, 2681–2686. [Google Scholar] [CrossRef]
- Duta-Bratu, C.-G.; Nitulescu, G.M.; Mihai, D.P.; Olaru, O.T. Resveratrol and Other Natural Oligomeric Stilbenoid Compounds and Their Therapeutic Applications. Plants 2023, 12, 2935. [Google Scholar] [CrossRef]
- Vitrac, X.; Castagnino, C.; Waffo-Téguo, P.; Delaunay, J.-C.; Vercauteren, J.; Monti, J.-P.; Deffieux, G.; Mérillon, J.-M. Polyphenols Newly Extracted in Red Wine from Southwestern France by Centrifugal Partition Chromatography. J. Agric. Food Chem. 2001, 49, 5934–5938. [Google Scholar] [CrossRef]
- Vitrac, X.; Bornet, A.; Vanderlinde, R.; Valls, J.; Richard, T.; Delaunay, J.-C.; Mérillon, J.-M.; Teissédre, P.-L. Determination of Stilbenes (δ-Viniferin, Trans-Astringin, Trans-Piceid, Cis-and Trans-Resveratrol, ε-Viniferin) in Brazilian Wines. J. Agric. Food Chem. 2005, 53, 5664–5669. [Google Scholar] [CrossRef]
- Sáez, V.; Pastene, E.; Vergara, C.; Mardones, C.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; Gómez, M.V.; Theoduloz, C.; Riquelme, S.; von Baer, D. Oligostilbenoids in Vitis Vinifera L. Pinot Noir Grape Cane Extract: Isolation, Characterization, in Vitro Antioxidant Capacity and Anti-Proliferative Effect on Cancer Cells. Food Chem. 2018, 265, 101–110. [Google Scholar] [CrossRef]
- Kou, X.; Fan, J.; Chen, N. Potential Molecular Targets of Ampelopsin in Prevention and Treatment of Cancers. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2017, 17, 1610–1616. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Milton-Laskibar, I.; Eseberri, I.; Beaumont, P.; Courtois, A.; Krisa, S.; Portillo, M.P. Beneficial Effects of ε-Viniferin on Obesity and Related Health Alterations. Nutrients 2023, 15, 928. [Google Scholar] [CrossRef]
- Schroeter, H.; Heiss, C.; Spencer, J.P.; Keen, C.L.; Lupton, J.R.; Schmitz, H.H. Recommending Flavanols and Procyanidins for Cardiovascular Health: Current Knowledge and Future Needs. Mol. Asp. Med. 2010, 31, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A. Should We Remove Wine from the Mediterranean Diet?: A Narrative Review. Am. J. Clin. Nutr. 2024, 119, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Opie, L.H.; Lecour, S. The Red Wine Hypothesis: From Concepts to Protective Signalling Molecules. Eur. Heart J. 2007, 28, 1683–1693. [Google Scholar] [CrossRef]
- Booyse, F.M.; Pan, W.; Grenett, H.E.; Parks, D.A.; Darley-Usmar, V.M.; Bradley, K.M.; Tabengwa, E.M. Mechanism by Which Alcohol and Wine Polyphenols Affect Coronary Heart Disease Risk. Ann. Epidemiol. 2007, 17, S24–S31. [Google Scholar] [CrossRef]
- Walzem, R. Wine and Health: State of Proofs and Research Needs. Inflammopharmacology 2008, 16, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Förstermann, U. Red Wine and Cardiovascular Health. Circ. Res. 2012, 111, 959–961. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Arranz, S.; Lamuela-Raventos, R.M.; Estruch, R. Effects of Wine, Alcohol and Polyphenols on Cardiovascular Disease Risk Factors: Evidences from Human Studies. Alcohol Alcohol. 2013, 48, 270–277. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Choleva, M.; Antonopoulou, S.; Demopoulos, C.A. Wine and Its Metabolic Effects. A Comprehensive Review of Clinical Trials. Metabolism 2018, 83, 102–119. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2019; ISBN 92-4-156563-2. [Google Scholar]
- Minzer, S.; Estruch, R.; Casas, R. Wine Intake in the Framework of a Mediterranean Diet and Chronic Non-Communicable Diseases: A Short Literature Review of the Last 5 Years. Molecules 2020, 25, 5045. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- Karaulli, J.; Xhaferaj, N.; Coppola, F.; Testa, B.; Letizia, F.; Kyçyk, O.; Kongoli, R.; Ruci, M.; Lamçe, F.; Sulaj, K.; et al. Bioprospecting of Metschnikowia Pulcherrima Strains, Isolated from a Vineyard Ecosystem, as Novel Starter Cultures for Craft Beer Production. Fermentation 2024, 10, 513. [Google Scholar] [CrossRef]
- Sam, F.E.; Ma, T.-Z.; Salifu, R.; Wang, J.; Jiang, Y.-M.; Zhang, B.; Han, S.-Y. Techniques for Dealcoholization of Wines: Their Impact on Wine Phenolic Composition, Volatile Composition, and Sensory Characteristics. Foods 2021, 10, 2498. [Google Scholar] [CrossRef] [PubMed]
- Silva, P. Low-Alcohol and Nonalcoholic Wines: From Production to Cardiovascular Health, along with Their Economic Effects. Beverages 2024, 10, 49. [Google Scholar] [CrossRef]
- Testa, B.; Coppola, F.; Succi, M.; Iorizzo, M. Biotechnological Strategies for Ethanol Reduction in Wine. Fermentation 2025, 11, 159. [Google Scholar] [CrossRef]
- Waehning, N.; Wells, V.K. Product, Individual and Environmental Factors Impacting the Consumption of No and Low Alcoholic Drinks: A Systematic Review and Future Research Agenda. Food Qual. Prefer. 2024, 117, 105163. [Google Scholar] [CrossRef]
- Loose, S.M.; del Rey, R. State of the International Wine Markets in 2023. The Wine Market at a Crossroads: Temporary or Structural Challenges? Wine Econ. Policy 2024, 13, 3–14. [Google Scholar] [CrossRef]
- World Health Organization. The SAFER Initiative: A World Free from Alcohol Related Harm. Available online: https://www.who.int/initiatives/SAFER#:~:Text=The%20SAFER%20initiative%20A%20world%20free%20from%20alcohol,international%20partners%2C%20launched%20the%20SAFER%20initiative%20 (accessed on 10 April 2025).
- Movendi International 70th World Congress. Available online: https://movendi.ngo/event/movendi-international-70th-world-congress/ (accessed on 24 April 2025).
- Testa, B.; Coppola, F.; Letizia, F.; Albanese, G.; Karaulli, J.; Ruci, M.; Pistillo, M.; Germinara, G.S.; Messia, M.C.; Succi, M.; et al. Versatility of Saccharomyces Cerevisiae 41CM in the Brewery Sector: Use as a Starter for “Ale” and “Lager” Craft Beer Production. Processes 2022, 10, 2495. [Google Scholar] [CrossRef]
- Zamora, F. Dealcoholised Wines and Low-Alcohol Wines. In Wine Safety, Consumer Preference, and Human Health; Springer International Publishing: Cham, Switzerland, 2016; pp. 163–182. [Google Scholar]
- Akhtar, W.; Ceci, A.T.; Longo, E.; Marconi, M.A.; Lonardi, F.; Boselli, E. Dealcoholized Wine: Techniques, Sensory Impacts, Stability, and Perspectives of a Growing Industry. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70171. [Google Scholar] [CrossRef] [PubMed]
- Boban, M.; Stockley, C.; Teissedre, P.-L.; Restani, P.; Fradera, U.; Stein-Hammer, C.; Ruf, J.-C. Drinking Pattern of Wine and Effects on Human Health: Why Should We Drink Moderately and with Meals? Food Funct. 2016, 7, 2937–2942. [Google Scholar] [CrossRef]
- Fiore, M.; Alaimo, L.S.; Chkhartishvil, N. The Amazing Bond among Wine Consumption, Health and Hedonistic Well-Being. Br. Food J. 2020, 122, 2707–2723. [Google Scholar] [CrossRef]
- Stockwell, T.; Zhao, J.; Panwar, S.; Roemer, A.; Naimi, T.; Chikritzhs, T. Do “Moderate” Drinkers Have Reduced Mortality Risk? A Systematic Review and Meta-Analysis of Alcohol Consumption and All-Cause Mortality. J. Stud. Alcohol Drugs 2016, 77, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.G.; Fullman, N.; Hawley, C.; Arian, N.; Zimsen, S.R.; Tymeson, H.D.; Venkateswaran, V.; Tapp, A.D.; Forouzanfar, M.H.; Salama, J.S. Alcohol Use and Burden for 195 Countries and Territories, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar] [CrossRef] [PubMed]
- Global Status Report on Alcohol and Health and Treatment of Substance Use Disorders. Available online: https://www.who.int/publications/i/item/9789240096745 (accessed on 24 April 2025).
- Costanzo, S.; de Gaetano, G.; Di Castelnuovo, A.; Djoussé, L.; Poli, A.; van Velden, D.P. Moderate Alcohol Consumption and Lower Total Mortality Risk: Justified Doubts or Established Facts? Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Maulik, N.; Das, D.K. Cardioprotection with Alcohol: Role of Both Alcohol and Polyphenolic Antioxidants. Ann. New York Acad. Sci. 2002, 957, 122–135. [Google Scholar] [CrossRef]
- Boban, N.; Tonkic, M.; Budimir, D.; Modun, D.; Sutlovic, D.; Punda-Polic, V.; Boban, M. Antimicrobial Effects of Wine: Separating the Role of Polyphenols, pH, Ethanol, and Other Wine Components. J. Food Sci. 2010, 75, M322–M326. [Google Scholar] [CrossRef]
- Liberale, L.; Bonaventura, A.; Montecucco, F.; Dallegri, F.; Carbone, F. Impact of Red Wine Consumption on Cardiovascular Health. Curr. Med. Chem. 2019, 26, 3542–3566. [Google Scholar] [CrossRef]
- Iriti, M.; Varoni, E.M. Cardioprotective Effects of Moderate Red Wine Consumption: Polyphenols vs. Ethanol. J. Appl. Biomed. 2014, 12, 193–202. [Google Scholar] [CrossRef]
- Haseeb, S.; Alexander, B.; Baranchuk, A. Wine and Cardiovascular Health: A Comprehensive Review. Circulation 2017, 136, 1434–1448. [Google Scholar] [CrossRef]
- Lombardo, M.; Feraco, A.; Camajani, E.; Caprio, M.; Armani, A. Health Effects of Red Wine Consumption: A Narrative Review of an Issue That Still Deserves Debate. Nutrients 2023, 15, 1921. [Google Scholar] [CrossRef]
- Sánchez-Rubio, M.; Guerrouj, K.; Taboada-Rodríguez, A.; López-Gómez, A.; Marín-Iniesta, F. Control of Native Spoilage Yeast on Dealcoholized Red Wine by Preservatives Alone and in Binary Mixtures. J. Food Sci. 2017, 82, 2128–2133. [Google Scholar] [CrossRef]
- Delegated Regulation—2019/934—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg_del/2019/934/oj/eng (accessed on 24 April 2025).
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS). Scientific Opinion on the Re-evaluation of Sulfur Dioxide (E 220), Sodium Sulfite (E 221), Sodium Bisulfite (E 222), Sodium Metabisulfite (E 223), Potassium Metabisulfite (E 224), Calcium Sulfite (E 226), Calcium Bisulfite (E 227) and Potassium Bisulfite (E 228) as Food Additives. EFSA J. 2016, 14, 4438. [Google Scholar]
- Testa, B.; Coppola, F.; Iorizzo, M.; Di Renzo, M.; Coppola, R.; Succi, M. Preliminary Characterisation of Metschnikowia Pulcherrima to Be Used as a Starter Culture in Red Winemaking. Beverages 2024, 10, 88. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Succi, M.; Coppola, F.; Testa, B.; Pellegrini, M.; Iorizzo, M. Alcohol or No Alcohol in Wine: Half a Century of Debate. Foods 2025, 14, 1854. https://doi.org/10.3390/foods14111854
Succi M, Coppola F, Testa B, Pellegrini M, Iorizzo M. Alcohol or No Alcohol in Wine: Half a Century of Debate. Foods. 2025; 14(11):1854. https://doi.org/10.3390/foods14111854
Chicago/Turabian StyleSucci, Mariantonietta, Francesca Coppola, Bruno Testa, Michela Pellegrini, and Massimo Iorizzo. 2025. "Alcohol or No Alcohol in Wine: Half a Century of Debate" Foods 14, no. 11: 1854. https://doi.org/10.3390/foods14111854
APA StyleSucci, M., Coppola, F., Testa, B., Pellegrini, M., & Iorizzo, M. (2025). Alcohol or No Alcohol in Wine: Half a Century of Debate. Foods, 14(11), 1854. https://doi.org/10.3390/foods14111854








