Soy Protein Isolate Supplementation Favorably Regulates the Fermentation Characteristics of Debaryomyces hansenii and Flavor Profile in a Sausage Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fermentation Agent
2.2. Preparation of Sterile Sausage Medium
2.3. Colony Count
2.4. Determination of Enzyme Activity
2.5. Determination of Physicochemical Properties
2.6. Free Amino Acids
2.7. Free Fatty Acids
2.8. Volatile Compound
2.9. Electronic Tongue Analysis
2.10. Data Statistics and Analysis
3. Results and Discussion
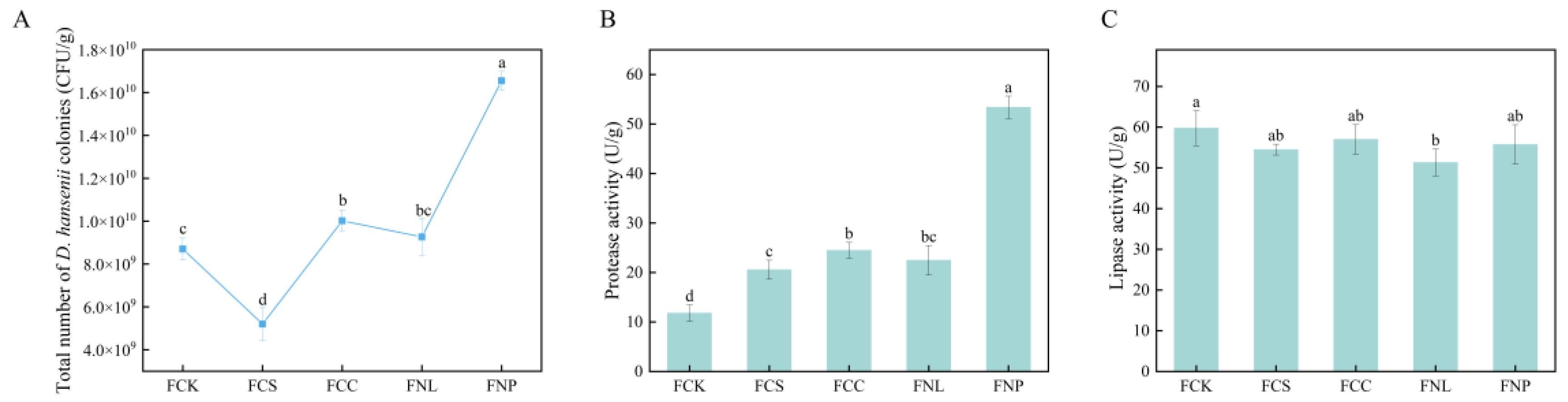
3.1. Effects of Different Carbon and Nitrogen Sources on D. hansenii Growth and Enzymatic Activities
3.2. Physicochemical Properties
3.2.1. pH, Water Activity, and Water-Holding Capacity
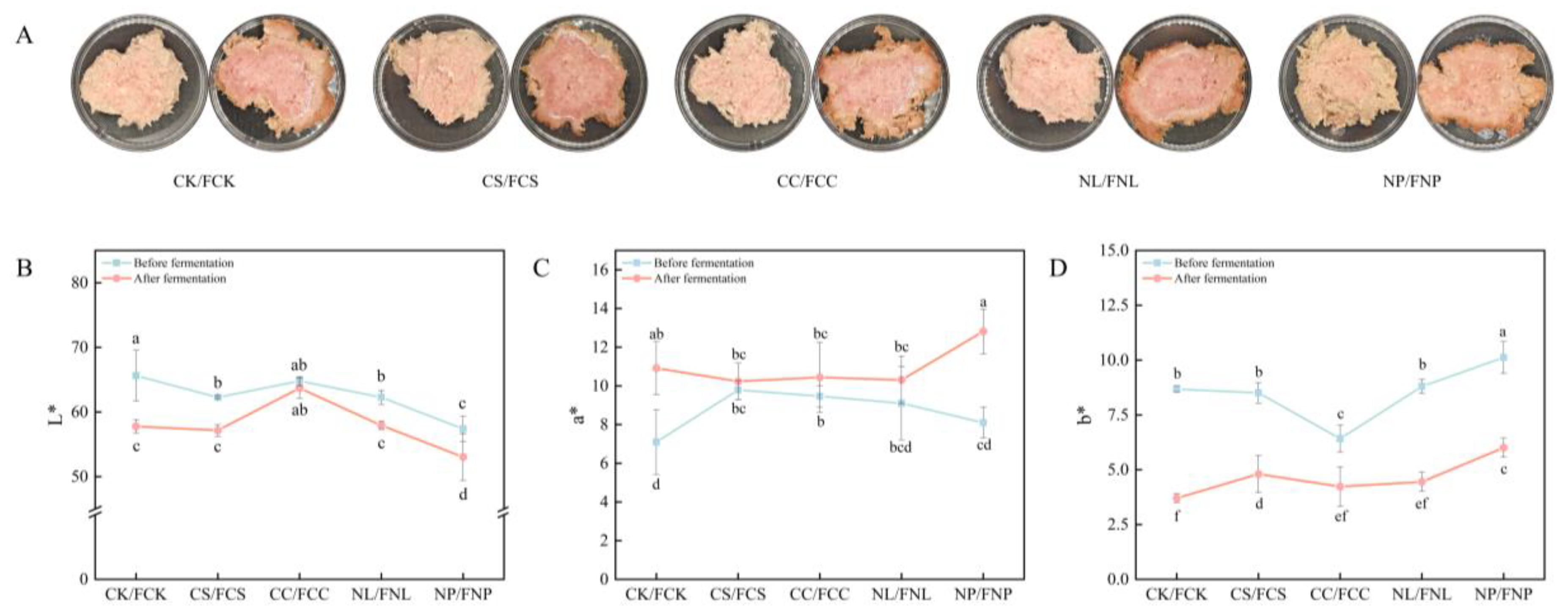
3.2.2. Color
3.3. Amino Acid Analysis
3.4. Fatty Acid Analysis
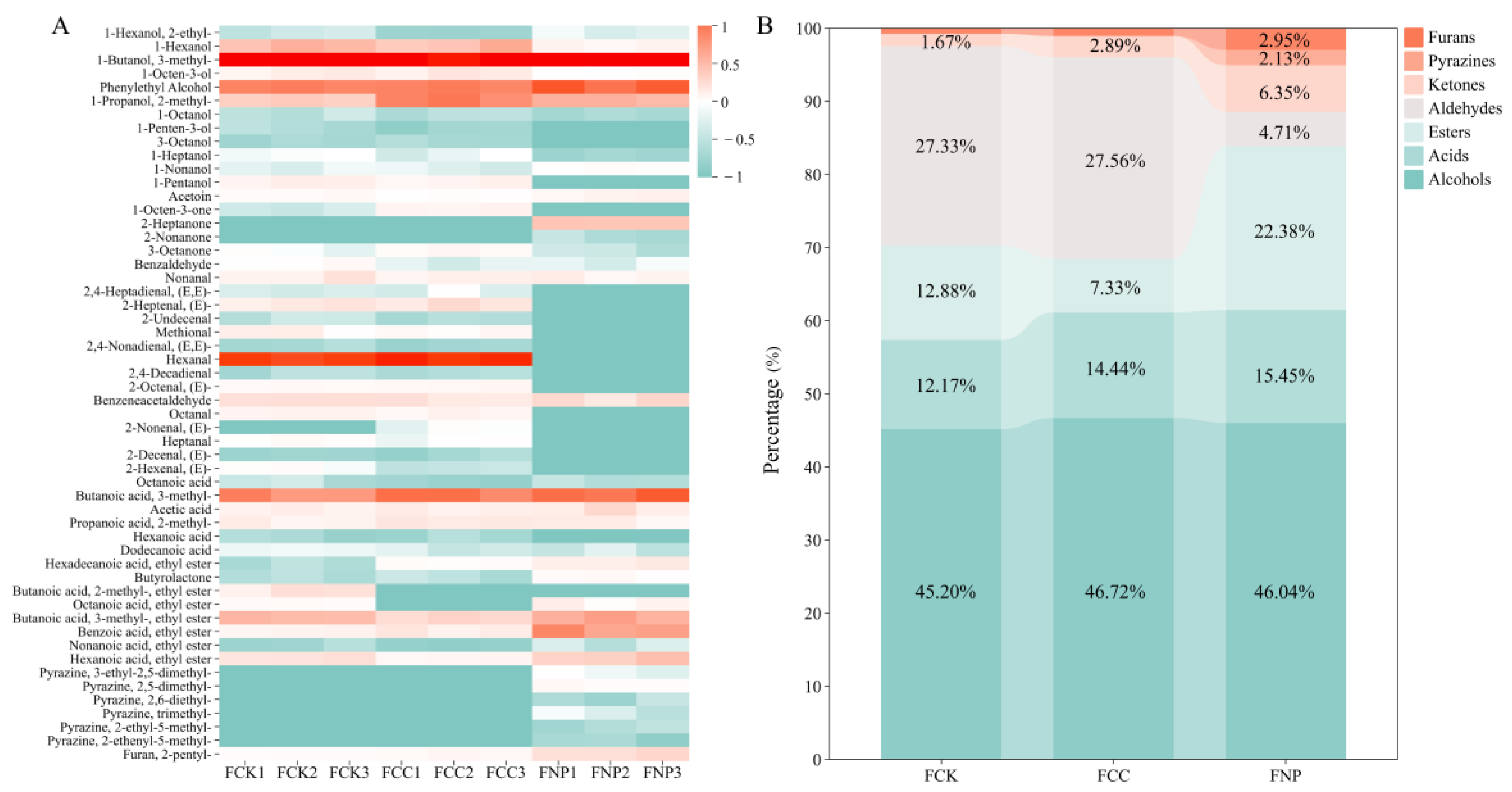
3.5. Volatile Compound Analysis
3.6. Taste Characteristics of Sausage Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, Y.; Tian, Y.; Zhu, J.; Wen, R.; Chen, Q.; Kong, B. Technological characterization and flavor-producing potential of lactic acid bacteria isolated from traditional dry fermented sausages in northeast China. Food Microbiol. 2022, 106, 104059. [Google Scholar] [CrossRef] [PubMed]
- Corral, S.; Belloch, C.; Lopez-Diez, J.J.; Flores, M. Lipolysis and aroma generation as mechanisms involved in masking boar taint in sodium reduced fermented sausages inoculated with Debaryomyces hansenii yeast. J. Sci. Food Agric. 2018, 98, 2121–2130. [Google Scholar] [CrossRef]
- Gong, X.; Chen, X.; Mi, R.; Qi, B.; Xiong, S.; Li, J.; Zhu, Q.; Wang, S. Two Debaryomyces hansenii strains as starter cultures for improving the nutritional and sensory quality of dry-cured pork belly. Food Res. Int. 2024, 183, 114227. [Google Scholar] [CrossRef] [PubMed]
- Cano-Garcia, L.; Rivera-Jimenez, S.; Belloch, C.; Flores, M. Generation of aroma compounds in a fermented sausage meat model system by Debaryomyces hansenii strains. Food Chem. 2014, 151, 364–373. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, Y.; Zhao, L.; Wang, Y.; Rao, L.; Liao, X. Pressure-resistant acclimation of lactic acid bacteria from a natural fermentation product using high pressure. Innov. Food Sci. Emerg. Technol. 2021, 69, 102660. [Google Scholar] [CrossRef]
- Olivares-Marin, I.K.; Madrigal-Perez, L.A.; Canizal-Garcia, M.; García-Almendárez, B.E.; González-Hernández, J.C.; Regalado-Gonzalez, C. Interactions between carbon and nitrogen sources depend on RIM15 and determine fermentative or respiratory growth in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2018, 102, 4535–4548. [Google Scholar] [CrossRef]
- Nair, A.; Sarma, S.J. The impact of carbon and nitrogen catabolite repression in microorganisms. Microbiol. Res. 2021, 251, 126831. [Google Scholar] [CrossRef]
- Lleixa, J.; Martin, V.; Giorello, F.; Portillo, M.C.; Carrau, F.; Beltran, G.; Mas, A. Analysis of the NCR Mechanisms in Hanseniaspora vineae and Saccharomyces cerevisiae During Winemaking. Front. Genet. 2019, 9, 747. [Google Scholar] [CrossRef] [PubMed]
- Sa Junior, P.L.S.; Silva, L.J.; Andrade, H.A.; Sucupira Maciel, M.I.; Sakugawa Shinohara, N.K.; Abreu Gloria, M.B.; Oliveira Filho, P.R.C. Optimization of mechanically separated meat washing cycles and of corn starch addition in saramunete (Pseudupeneus maculatus) sausages. J. Food Process. Preserv. 2021, 45, 16093. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, H.; Zhang, J.; Liu, S.; Tian, Y.; Cheng, T.; Guo, Z.; Wang, Z. Improve the fiber structure and texture properties of plant-based meat analogues by adjusting the ratio of soy protein isolate (SPI) to wheat gluten (WG). Food Chemistry-X 2024, 24, 101962. [Google Scholar] [CrossRef]
- Huang, P.; Shao, X.; Zhu, M.; Xu, B.; Chen, C.; Li, P. Sucrose enhances colour formation in dry sausages by up-regulating gene expression of nitric oxide synthase in Staphylococcus vitulinus. Int. J. Food Microbiol. 2020, 315, 108419. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Ma, Y.; Chen, L.; Lu, C.; Ge, Q.; Wu, M.; Xi, J.; Yu, H. Effects of the addition of leucine on flavor and quality of sausage fermented by Lactobacillus fermentum YZU-06 and Staphylococcus saprophyticus CGMCC 3475. Front. Microbiol. 2023, 13, 1118907. [Google Scholar] [CrossRef]
- Baek, J.H.; Han, D.M.; Choi, D.G.; Jeon, C.O. Unraveling the carbohydrate metabolic characteristics of Leuconostoc mesenteroides J18 through metabolite and transcriptome analyses. Food Chem. 2024, 435, 137594. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; He, W.; Dai, W.; Xie, X.; Pan, Y.; Tang, X.; Zheng, R.; Zhou, X. Quality and flavor development of solid-state fermented surimi with Actinomucor elegans: A perspective on the impacts of carbon and nitrogen sources. Food Chem. 2024, 447, 139053. [Google Scholar] [CrossRef]
- Cho, A.J.; Han, S.; Nahar, S.; Her, E.; Kang, J.G.; Ha, S.-D. Synergistic effects of ε-poly-l-lysine and lysozyme against Pseudomonas aeruginosa and Listeria monocytogenes biofilms on beef and food contact surfaces. Meat Sci. 2024, 214, 109534. [Google Scholar] [CrossRef]
- Prestes, R.C.; Silva, L.B.; Torri, A.M.P.; Kubota, E.H.; Rosa, C.S.; Roman, S.S.; Kempka, A.P.; Demiate, I.M. Sensory and physicochemical evaluation of low-fat chicken mortadella with added native and modified starches. J. Food Sci. Technol. 2015, 52, 4369–4377. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.H.; Xiong, K.X.; Wang, Y.Y.; Xiong, Z.M.; Lu, H.Y.; Peng, L.J.; Jin, W.Z.; Wang, W.; Ni, L.X.; Wang, H.B. Physical and flavor characteristics of reduced sodium sausages containing plant proteins and blends of chloride salts. Lwt-Food Sci. Technol. 2025, 215, 117268. [Google Scholar] [CrossRef]
- Xi, X.; Ke, J.; Ma, Y.; Liu, X.; Gu, X.; Wang, Y. Physiochemical and taste characteristics of traditional Chinese fermented food sufu. J. Food Process. Preserv. 2022, 46, 16845. [Google Scholar] [CrossRef]
- Lin, Y.; Yuan, D.D.; Zhang, S.J.; Wu, T.X. Differences in flavor characteristics of rounds of Sauce-based Baijiu based on GC-IMS, GC-MS, and electronic tongue. J. Food Compos. Anal. 2024, 135, 106696. [Google Scholar] [CrossRef]
- Liu, B.-Y.; Zhu, K.-X.; Guo, X.-N.; Peng, W.; Zhou, H.-M. Effect of deamidation-induced modification on umami and bitter taste of wheat gluten hydrolysates. J. Sci. Food Agric. 2017, 97, 3181–3188. [Google Scholar] [CrossRef]
- Egli, T.; Zinn, M. The concept of multiple-nutrient-limited growth of microorganisms and its application in biotechnological processes. Biotechnol. Adv. 2003, 22, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, C.; Frost, A.T.; Ramos-Moreno, L.; Krum, M.R.; Martinez, J.L. A physiological characterization in controlled bioreactors reveals a novel survival strategy for Debaryomyces hansenii at high salinity. Yeast 2021, 38, 302–315. [Google Scholar] [CrossRef]
- Gong, X.; Mi, R.; Chen, X.; Zhu, Q.; Xiong, S.; Qi, B.; Wang, S. Evaluation and selection of yeasts as potential aroma enhancers for the production of dry-cured ham. Food Sci. Hum. Wellness 2023, 12, 324–335. [Google Scholar] [CrossRef]
- Sun, Y.; Qian, Y.C.; Zhang, J.X.; Wang, Y.F.; Li, X.H.; Zhang, W.C.; Wang, L.S.; Liu, H.; Zhong, Y.H. Extracellular protease production regulated by nitrogen and carbon sources in Trichoderma reesei. J. Basic Microbiol. 2021, 61, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Zhang, W.X.; Chen, X.L. Mechanisms for Induction of Microbial Extracellular Proteases in Response to Exterior Proteins. Appl. Environ. Microbiol. 2020, 86, e01036-20. [Google Scholar] [CrossRef]
- Akhtar, M.W.; Mirza, A.Q.; Chughtai, M.I. Lipase Induction in Mucor hiemalis. Appl. Environ. Microbiol. 1980, 40, 257–263. [Google Scholar] [CrossRef]
- Jayaram, V.B.; Cuyvers, S.; Lagrain, B.; Verstrepen, K.J.; Delcour, J.A.; Courtin, C.M. Mapping of Saccharomyces cerevisiae metabolites in fermenting wheat straight-dough reveals succinic acid as pH-determining factor. Food Chem. 2013, 136, 301–308. [Google Scholar] [CrossRef]
- Brown, J.; Grimaud, A. Proton-donating and chemistry-dependent buffering capability of amino acids for the hydrogen evolution reaction. Phys. Chem. Chem. Phys. 2023, 25, 8005–8012. [Google Scholar] [CrossRef]
- Lopez-Silva, M.; Agama-Acevedo, E.; Bello-Perez, L.A.; Alvarez-Ramirez, J. Influence of gelatinization degree and octenyl succinic anhydride esterification on the water sorption characteristics of corn starch. Carbohydr. Polym. 2021, 270, 118378. [Google Scholar] [CrossRef]
- Webb, D.; Dogan, H.; Li, Y.; Alavi, S. Physico-Chemical Properties and Texturization of Pea, Wheat and Soy Proteins Using Extrusion and Their Application in Plant-Based Meat. Foods 2023, 12, 1586. [Google Scholar] [CrossRef]
- Bellissent-Funel, M.-C.; Hassanali, A.; Havenith, M.; Henchman, R.; Pohl, P.; Sterpone, F.; van der Spoel, D.; Xu, Y.; Garcia, A.E. Water Determines the Structure and Dynamics of Proteins. Chem. Rev. 2016, 116, 7673–7697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Fang, L.; Wang, C.; Shi, L.; Chang, T.; Yang, H.; Cui, M. Effects of starches on the textural, rheological, and color properties of surimi-beef gels with microbial tranglutaminase. Meat Sci. 2013, 93, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Hoa, V.-B.; Cho, S.-H.; Seong, P.-N.; Kang, S.-M.; Kim, Y.-S.; Moon, S.-S.; Choi, Y.-M.; Kim, J.-H.; Seol, K.-H. The significant influences of pH, temperature and fatty acids on meat myoglobin oxidation: A model study. J. Food Sci. Technol. 2021, 58, 3972–3980. [Google Scholar] [CrossRef] [PubMed]
- Bedia, M.; Méndez, L.; Bañón, S. Evaluation of different starter cultures (Staphylococci plus Lactic Acid Bacteria) in semi-ripened Salami stuffed in swine gut. Meat Sci. 2011, 87, 381–386. [Google Scholar] [CrossRef]
- Ardo, Y. Flavour formation by amino acid catabolism. Biotechnol. Adv. 2006, 24, 238–242. [Google Scholar] [CrossRef]
- Zhang, W.; Du, G.; Zhou, J.; Chen, J. Regulation of Sensing, Transportation, and Catabolism of Nitrogen Sources in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2018, 82, 10-1128. [Google Scholar] [CrossRef]
- Heitman, J.; Movva, N.R.; Hall, M.N. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 1991, 253, 905–909. [Google Scholar] [CrossRef]
- Bifari, F.; Ruocco, C.; Decimo, I.; Fumagalli, G.; Valerio, A.; Nisoli, E. Amino acid supplements and metabolic health: A potential interplay between intestinal microbiota and systems control. Genes Nutr. 2017, 12, 27. [Google Scholar] [CrossRef]
- Xiao, F.; Guo, F.F. Impacts of essential amino acids on energy balance. Mol. Metab. 2022, 57, 101393. [Google Scholar] [CrossRef]
- Simpson-Lavy, K.; Kupiec, M. Carbon catabolite repression: Not only for glucose. Curr. Genet. 2019, 65, 1321–1323. [Google Scholar] [CrossRef]
- Kleetz, J.; Vasilopoulos, G.; Czolkoss, S.; Aktas, M.; Narberhaus, F. Recombinant and endogenous ways to produce methylated phospholipids in Escherichia coli. Appl. Microbiol. Biotechnol. 2021, 105, 8837–8851. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Nunez, B.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J. Nutr. Biochem. 2016, 36, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.I.; De Cara, A.; Segura, J.F.; Martí, P.; Hechavarría, T.; Calvo, L. Dietary oleuropein extract supplementation and its combination with α-tocopheryl acetate and selenium modifies the free fatty acid profile of pork and improves its stability. J. Sci. Food Agric. 2021, 101, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- Sallan, S.; Kaban, G.; Kaya, M. The effects of nitrite, sodium ascorbate and starter culture on volatile compounds of a semi-dry fermented sausage. Lwt-Food Sci. Technol. 2022, 153, 112540. [Google Scholar] [CrossRef]
- Wen, R.; Li, X.-A.; Han, G.; Chen, Q.; Kong, B. Fungal community succession and volatile compound dynamics in Harbin dry sausage during fermentation. Food Microbiol. 2021, 99, 103764. [Google Scholar] [CrossRef]
- Xu, Z.; Lin, L.; Chen, Z.; Wang, K.; Sun, J.; Zhu, T. The same genetic regulation strategy produces inconsistent effects in different Saccharomyces cerevisiae strains for 2-phenylethanol production. Appl. Microbiol. Biotechnol. 2022, 106, 4041–4052. [Google Scholar] [CrossRef]
- Matheis, K.; Granvogl, M.; Schieberle, P. Quantitation and Enantiomeric Ratios of Aroma Compounds Formed by an Ehrlich Degradation of L-Isoleucine in Fermented Foods. J. Agric. Food Chem. 2016, 64, 646–652. [Google Scholar] [CrossRef]
- Hiltunen, J.K.; Mursula, A.M.; Rottensteiner, H.; Wierenga, R.K.; Kastaniotis, A.J.; Gurvitz, A. The biochemistry of peroxisomal beta-oxidation in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2003, 27, 35–64. [Google Scholar] [CrossRef]
- Huang, L.; Jia, S.; Wu, R.; Chen, Y.; Ding, S.; Dai, C.; He, R. The structure, antioxidant and antibacterial properties of thiol-modified soy protein isolate induced by allicin. Food Chem. 2022, 396, 133713. [Google Scholar] [CrossRef]
- Dominguez, R.; Purrinos, L.; Perez-Santaescolastica, C.; Pateiro, M.; Barba, F.J.; Tomasevic, I.; Bastianello Campagnol, P.C.; Lorenzo, J.M. Characterization of Volatile Compounds of Dry-Cured Meat Products Using HS-SPME-GC/MS Technique. Food Anal. Methods 2019, 12, 1263–1284. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Bogaki, T. Mechanisms of production and control of acetate esters in yeasts. J. Biosci. Bioeng. 2023, 136, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Yuzawa, S.; Mirsiaghi, M.; Jocic, R.; Fujii, T.; Masson, F.; Benites, V.T.; Baidoo, E.E.K.; Sundstrom, E.; Tanjore, D.; Pray, T.R.; et al. Short-chain ketone production by engineered polyketide synthases in Streptomyces albus. Nat. Commun. 2018, 9, 4569. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.; Moncunill, D.; Montero, R.; Javier Lopez-Diez, J.; Belloch, C. Screening of Debaryomyces hansenii Strains for Flavor Production under a Reduced Concentration of Nitrifying Preservatives Used in Meat Products. J. Agric. Food Chem. 2017, 65, 3900–3909. [Google Scholar] [CrossRef] [PubMed]
- Corral, S.; Leitner, E.; Siegmund, B.; Flores, M. Determination of sulfur and nitrogen compounds during the processing of dry fermented sausages and their relation to amino acid generation. Food Chem. 2016, 190, 657–664. [Google Scholar] [CrossRef]


| Free Amino Acids | FCK | FCC | FNP |
|---|---|---|---|
| mg/100 g | |||
| Asp | 3.30 ± 0.70 b | 2.40 ± 0.61 b | 6.47 ± 0.40 a |
| Glu | 20.85 ± 1.34 b | 22.63 ± 2.20 b | 34.03 ± 1.72 a |
| Umami FAAs | 24.30 ± 2.26 b | 25.03 ± 2.07 b | 40.50 ± 1.42 a |
| Thr * | 7.93 ± 0.67 b | 7.50 ± 0.75 b | 12.53 ± 0.71 a |
| Ser | 17.20 ± 0.71 b | 16.43 ± 1.80 b | 33.53 ± 0.72 a |
| Gly | 6.75 ± 0.35 b | 7.03 ± 0.61 b | 11.47 ± 1.20 a |
| Ala | 16.43 ± 0.81 b | 15.03 ± 1.87 b | 25.77 ± 2.89 a |
| Pro | 9.50 ± 0.72 c | 12.13 ± 0.76 b | 14.70 ± 1.04 a |
| Sweet FAAs | 59.30 ± 1.32 b | 58.13 ± 1.52 b | 98.00 ± 0.66 a |
| Val * | 19.30 ± 0.85 b | 20.33 ± 1.44 b | 30.23 ± 1.06 a |
| Met * | 8.37 ± 0.91 a | 8.80 ± 1.00 a | 8.37 ± 0.50 a |
| Ile * | 14.83 ± 0.31 b | 14.60 ± 1.47 b | 23.17 ± 1.21 a |
| Leu * | 19.80 ± 1.25 b | 20.37 ± 0.71 b | 37.17 ± 1.29 a |
| Tyr | 15.80 ± 0.50 b | 16.33 ± 0.99 b | 25.37 ± 2.83 a |
| Phe * | 24.03 ± 1.34 b | 25.23 ± 1.92 b | 34.73 ± 1.36 a |
| His | 6.77 ± 0.49 a | 7.33 ± 0.85 a | 9.67 ± 2.31 a |
| Lys * | 25.20 ± 2.56 b | 27.03 ± 1.42 b | 40.53 ± 1.04 a |
| Arg | 10.87 ± 1.86 b | 10.07 ± 1.53 b | 17.43 ± 0.61 a |
| Bitter FAAs | 145.57 ± 1.12 b | 150.10 ± 3.72 b | 226.67 ± 9.18 a |
| Cys | 25.07 ± 0.06 a | 25.10 ± 0.56 a | 26.47 ± 1.10 a |
| Total FAAs | 255.50 ± 3.91 b | 258.37 ± 20.59 b | 391.63 ± 10.72 a |
| Free Fatty Acids | FCK | FCC | FNP |
|---|---|---|---|
| mg/100 g | |||
| Butyric Acid | 0.21 ± 0.08 a | 0.08 ± 0.01 b | 0.07 ± 0.01 b |
| Caproic acid | 0.04 ± 0.02 a | 0.02 ± 0.02 ab | 0.01 ± 0.01 b |
| Caprylic Acid | 0.17 ± 0.02 a | 0.10 ± 0.06 a | 0.11 ± 0.01 a |
| Capric Acid | 2.39 ± 0.23 a | 1.73 ± 0.79 a | 1.87 ± 0.09 a |
| Undecylic acid | 0.08 ± 0.01 a | 0.06 ± 0.03 a | 0.07 ± 0.01 a |
| Lauric Acid | 3.17 ± 0.28 a | 2.28 ± 1.03 a | 2.47 ± 0.11 a |
| Tridecylic acid | 0.07 ± 0.01 a | 0.05 ± 0.02 a | 0.05 ± 0.01 a |
| Myristic Acid | 42.36 ± 3.33 a | 29.69 ± 1.28 b | 32.11 ± 1.03 b |
| Pentadecanoic Acid | 1.19 ± 0.09 a | 0.80 ± 0.35 a | 0.85 ± 0.02 a |
| Palmitic Acid | 459.87 ± 16.92 a | 327.85 ± 7.89 b | 345.56 ± 10.99 b |
| Heptadecanoic Acid | 6.06 ± 0.60 a | 4.01 ± 1.85 a | 4.39 ± 0.26 a |
| Stearic Acid | 256.87 ± 14.02 a | 175.67 ± 4.62 b | 187.31 ± 5.16 b |
| Arachidic Acid | 5.98 ± 0.52 a | 3.75 ± 0.36 b | 4.33 ± 0.21 b |
| Heneicosanoic acid | 0.13 ± 0.02 a | 0.07 ± 0.03 b | 0.09 ± 0.01 b |
| Behenic Acid | 0.39 ± 0.04 a | 0.27 ± 0.07 b | 0.32 ± 0.01 ab |
| Tricosanoic acid | 0.08 ± 0.01 a | 0.05 ± 0.01 b | 0.06 ± 0.01 b |
| Lignoceric Acid | 0.22 ± 0.04 a | 0.14 ± 0.02 a | 0.19 ± 0.09 a |
| Myristoleic Acid | 0.50 ± 0.03 a | 0.35 ± 0.20 a | 0.35 ± 0.05 a |
| Palmitoleic Acid | 61.95 ± 4.86 a | 44.27 ± 2.91 b | 48.74 ± 2.17 b |
| Margaroleic Acid | 4.97 ± 1.96 a | 3.22 ± 1.64 a | 3.26 ± 0.09 a |
| Oleic Acid (trans) | 3.88 ± 0.31 a | 2.59 ± 1.23 a | 2.85 ± 0.12 a |
| Oleic Acid (cis) | 533.37 ± 22.61 a | 376.65 ± 6.25 b | 403.13 ± 13.20 b |
| Gadoleic Acid | 25.23 ± 2.33 a | 16.18 ± 1.27 b | 17.63 ± 0.95 b |
| Erucic Acid | 1.52 ± 0.29 a | 1.37 ± 0.14 a | 1.36 ± 0.02 a |
| Nervonic Acid | 0.41 ± 0.05 a | 0.34 ± 0.14 ab | 0.23 ± 0.03 b |
| Linoleic Acid | 299.71 ± 7.21 a | 216.81 ± 7.01 c | 238.00 ± 9.29 b |
| Linoleic Acid (trans) | 0.16 ± 0.01 a | 0.11 ± 0.02 b | 0.16 ± 0.01 a |
| γ-Linolenic Acid | 1.16 ± 0.10 a | 0.79 ± 0.37 a | 0.88 ± 0.07 a |
| Alpha-Linolenic Acid | 21.50 ± 2.19 a | 15.06 ± 0.94 b | 16.89 ± 0.92 b |
| Eicosadienoic Acid | 20.38 ± 1.35 a | 13.04 ± 2.45 b | 14.47 ± 0.61 b |
| Dihomo-gamma-Linolenic Acid | 2.69 ± 0.19 a | 1.82 ± 0.68 b | 2.09 ± 0.03 ab |
| Erucic acid | 1.52 ± 0.29 a | 1.37 ± 0.14 a | 1.36 ± 0.02 a |
| Eicosatrienoic Acid | 3.63 ± 0.38 a | 2.13 ± 0.85 b | 2.44 ± 0.28 b |
| Arachidonic Acid | 9.93 ± 0.21 a | 8.04 ± 1.27 b | 8.81 ± 0.15 ab |
| Eicosapentaenoic Acid | 0.26 ± 0.02 a | 0.18 ± 0.02 b | 0.19 ± 0.01 b |
| Docosadienoic Acid | 0.29 ± 0.04 a | 0.18 ± 0.08 b | 0.19 ± 0.01 ab |
| SFAs | 779.29 ± 34.73 a | 546.63 ± 15.60 b | 579.88 ± 17.63 b |
| MUFAs | 631.83 ± 29.89 a | 444.96 ± 10.67 b | 477.55 ± 16.33 b |
| PUFAs | 361.23 ± 10.92 a | 259.54 ± 12.69 c | 285.49 ± 10.64 b |
| UFAs | 993.06 ± 40.61 a | 704.50 ± 23.07 b | 763.04 ± 26.89 b |
| UFAs/SFAs | 1.27 ± 0.01 b | 1.29 ± 0.02 ab | 1.32 ± 0.01 a |
| Total FFAs | 1772.35 ± 75.34 a | 1251.13 ± 36.90 b | 1342.92 ± 44.36 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, W.; Zhu, Q.; Wan, J. Soy Protein Isolate Supplementation Favorably Regulates the Fermentation Characteristics of Debaryomyces hansenii and Flavor Profile in a Sausage Model. Foods 2025, 14, 1840. https://doi.org/10.3390/foods14111840
Duan W, Zhu Q, Wan J. Soy Protein Isolate Supplementation Favorably Regulates the Fermentation Characteristics of Debaryomyces hansenii and Flavor Profile in a Sausage Model. Foods. 2025; 14(11):1840. https://doi.org/10.3390/foods14111840
Chicago/Turabian StyleDuan, Wenwen, Qiujin Zhu, and Jing Wan. 2025. "Soy Protein Isolate Supplementation Favorably Regulates the Fermentation Characteristics of Debaryomyces hansenii and Flavor Profile in a Sausage Model" Foods 14, no. 11: 1840. https://doi.org/10.3390/foods14111840
APA StyleDuan, W., Zhu, Q., & Wan, J. (2025). Soy Protein Isolate Supplementation Favorably Regulates the Fermentation Characteristics of Debaryomyces hansenii and Flavor Profile in a Sausage Model. Foods, 14(11), 1840. https://doi.org/10.3390/foods14111840







