Impact of Environmental Humidity on Instant Coffee Stability: Defining Moisture Thresholds for Quality Degradation and Shelf Life Prediction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Coffee Sample Preparation
2.3. Moisture of Instant Coffee Powder
2.4. Water Activity (aw)
2.5. Differential Scanning Calorimetry
2.6. Solubilization Kinetics of Instant Coffee Powder
2.7. pH of the Brew Obtained from Instant Coffee Powder
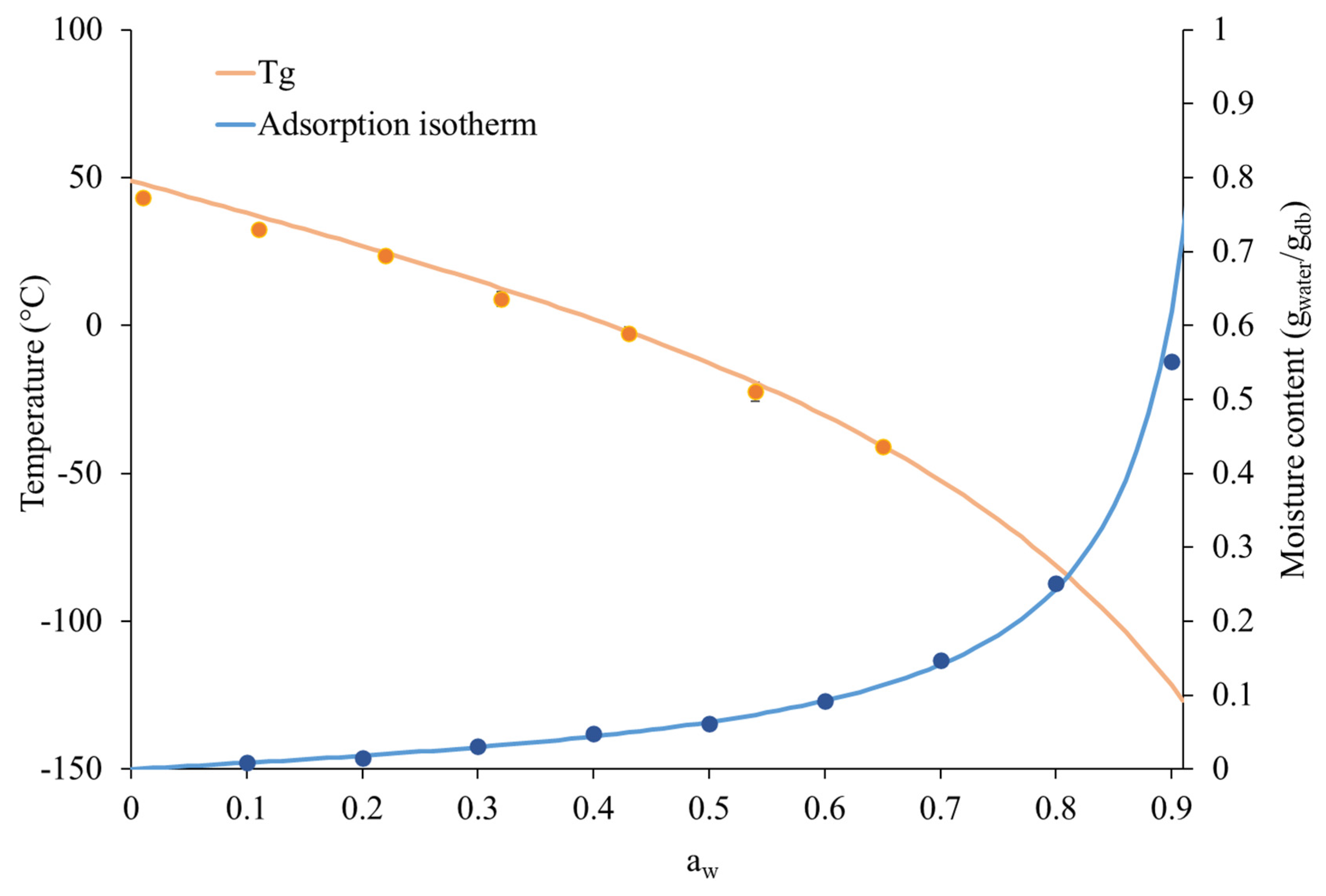
2.8. Adsorption Isotherm of Instant Coffee Powder
2.9. Color
2.10. Image Acquisition and Analysis
2.11. Statistical Analysis
3. Results and Discussion
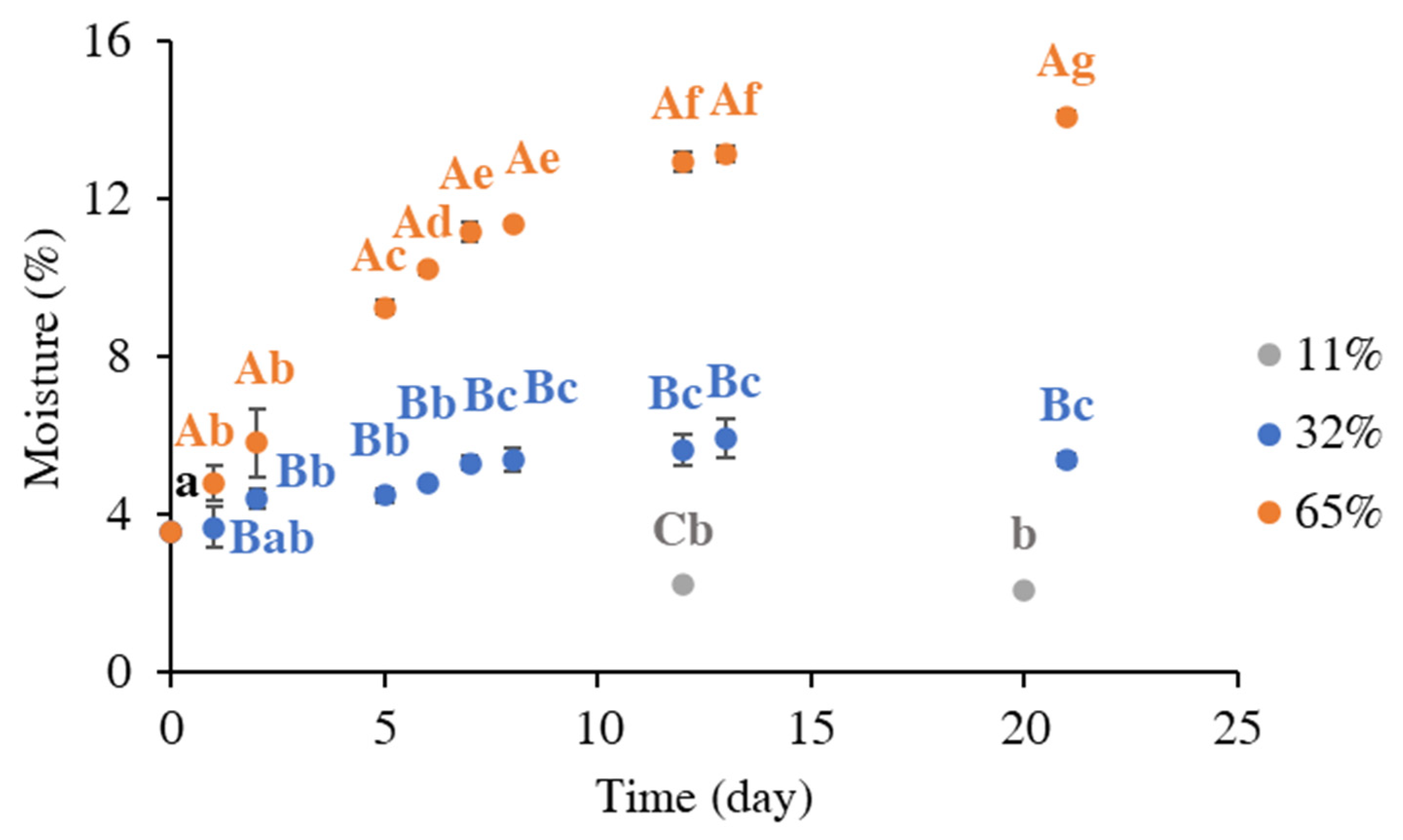
3.1. Moisture Uptake by Instant Coffee Powder During Storage Under Different Environmental Humidities
3.2. Evolution of Quality Indicators of Instant Coffee Powder Stored Under Different Environmental Humidities
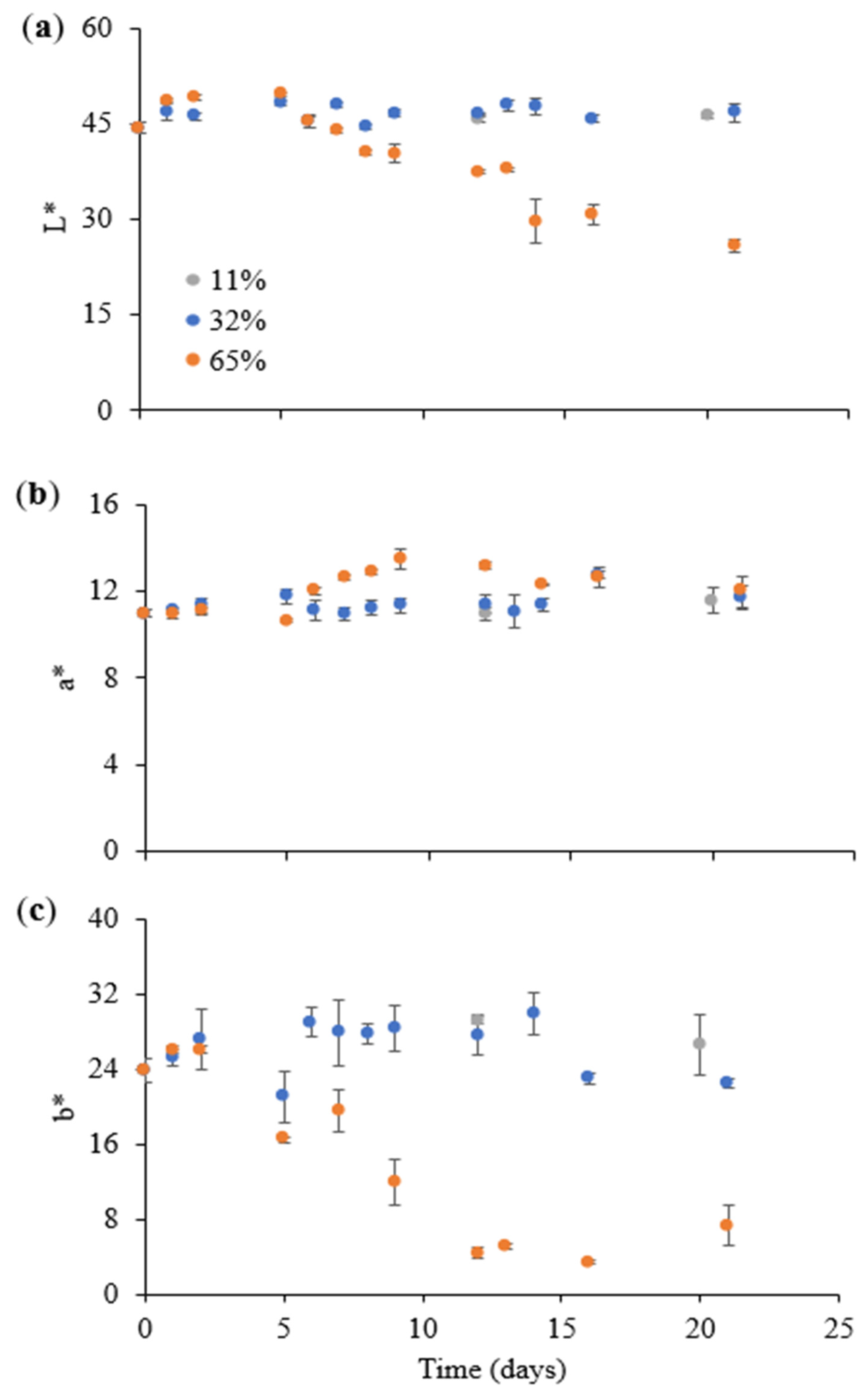
3.2.1. Effect on the Visual Appearance
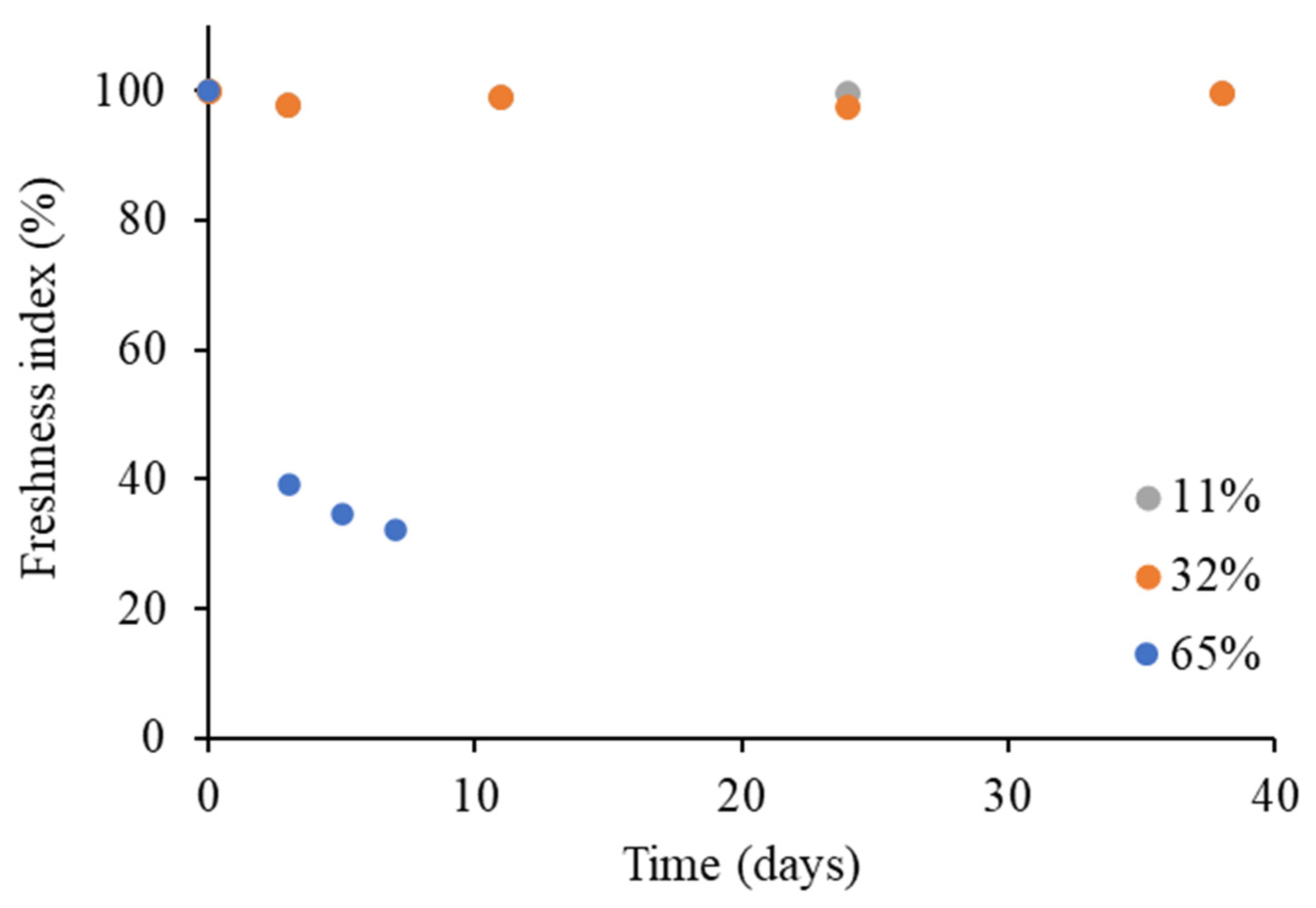
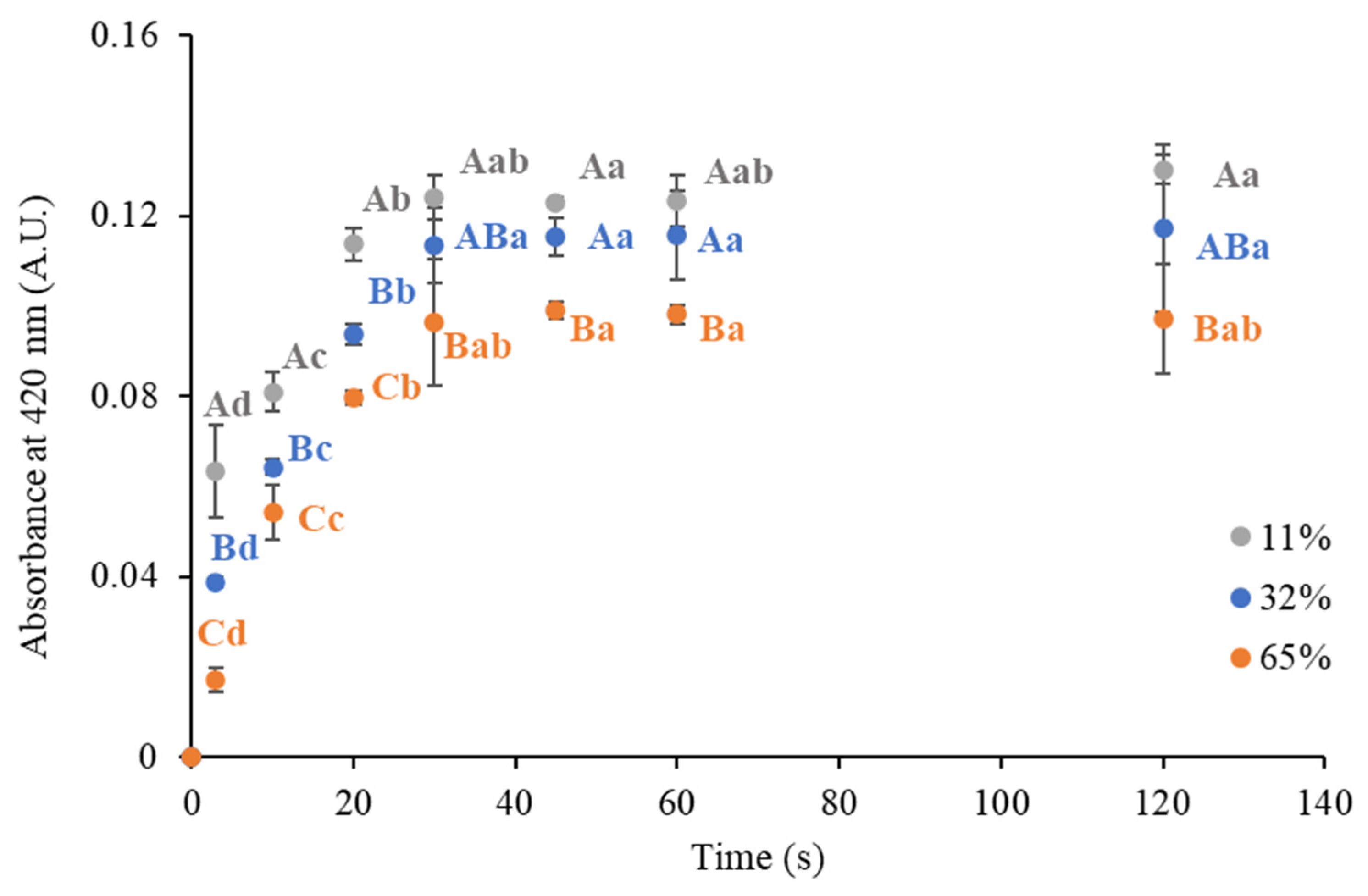
3.2.2. Effect on Rehydration Performance
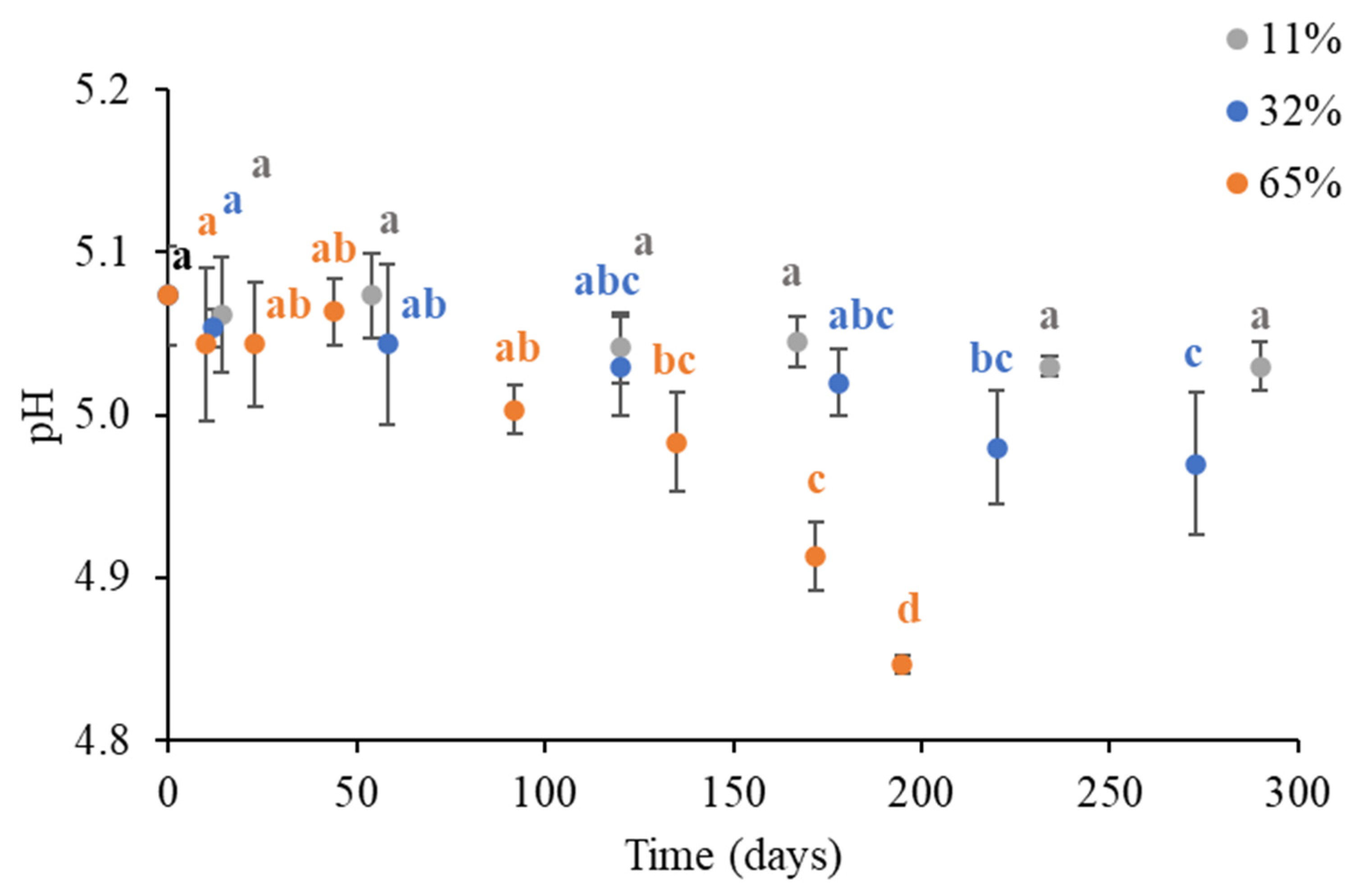
3.3. Changes in the pH of Brews Obtained from Instant Coffee Powder Stored Under Different Environmental Humidities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samoggia, A.; Riedel, B. Consumers’ perceptions of coffee health benefits and motives for coffee consumption and purchasing. Nutrients 2019, 3, 653. [Google Scholar] [CrossRef] [PubMed]
- FSSAI. Food Safety and Standard (Food Products Standards and Food Additives) Regulations. 2022. Available online: https://www.fssai.gov.in/upload/uploadfiles/files/Compendium_Food_Additives_Regulations_20_12_2022 (accessed on 27 March 2025).
- Borém, F.M.; de Abreu, G.F.; de Carvalho Alves, A.P.; dos Santos, C.M.; Teixeira, D.E. Volatile compounds indicating latent damage to sensory attributes in coffee stored in permeable and hermetic packaging. Food Packag. Shelf Life 2021, 29, 100705. [Google Scholar] [CrossRef]
- Ishwarya, S.P.; Anandharamakrishnan, C. Spray-Freeze-Drying approach for soluble coffee processing and its effect on quality characteristics. J. Food Eng. 2015, 149, 171–180. [Google Scholar] [CrossRef]
- Alves, R.M.V.; Milanez, C.R.; Padula, M. Alternative packaging for instant coffee. Food Sci. Technol. 2000, 20, 204–211. [Google Scholar]
- Jannah, S.; Rahayu, E.; Yanti, R.; Suroto, D.; Wikandari, R. Study of viability, storage stability, and shelf life of probiotic instant coffee lactiplantibacillus plantarum subsp. Plantarum dad-13 in vacuum and nonvacuum packaging at different storage temperatures. Int. J. Food Sci. 2022, 2022, 1663772. [Google Scholar] [CrossRef]
- Manzocco, L.; Calligaris, S.; Anese, M.; Nicoli, M.C. The stability and shelf life of coffee products. In The Stability and Shelf Life of Food; Subramaniam, P., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; p. 398. [Google Scholar]
- Robertson, G.L. Food Packaging: Principles and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Ruan, R.; Choi, Y.J.; Chung, M.S. Caking in food powders. Food Sci. Biotechnol. 2007, 16, 329–336. [Google Scholar]
- Anese, A.; Manzocco, L.; Maltini, E. Effect of coffee physical structure on volatile release. Eur. Food Res. Technol. 2005, 221, 434–438. [Google Scholar] [CrossRef]
- Chuy, L.E.; Labuza, T.P. Caking and stickiness of dairy-based food powders as related to glass transition. J. Food Sci. 1994, 59, 43–46. [Google Scholar] [CrossRef]
- Clarke, R.J.; Macrae, R. Coffee—Chemistry; Elsevier Applied Science Publications: Amsterdam, The Netherlands, 1985. [Google Scholar]
- Ginz, M.; Balzer, H.H.; Bradbury, A.G.W.; Maier, H.G. Formation of aliphatic acids by carbohydrate degradation during roasting of coffee. Eur. Food Res. Technol. 2000, 211, 404–410. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Savonitti, O. Storage and Packaging. In Espresso Coffee. The Science of Quality; Illy, A., Viani, R., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2005. [Google Scholar]
- Santanatoglia, A.; Angeloni, S.; Fiorito, M.; Fioretti, L.; Ricciutelli, M.; Sagratini, G.; Vittori, S.; Alessandroni, L.; Caprioli, G. Development of new analytical methods for the quantification of organic acids, chlorogenic acids and caffeine in espresso coffee by using solid-phase extraction (SPE) and high-performance liquid chromatography-diode array detector (HPLC-DAD). J. Food Compos. Anal. 2023, 125, 105732. [Google Scholar] [CrossRef]
- Sivetz, M.; Foote, H.E. Coffee Processing Technology; Avi Publishing Company: Westport, CT, USA, 1963. [Google Scholar]
- Thomas, E.; Puget, S.; Valentin, D.; Songer, P. Sensory Evaluation—Profiling and Preferences. In The Craft and Science of Coffee; Folmer, B., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 419–456. [Google Scholar]
- Manzocco, L.; Nicoli, M.C. Modeling the effect of water activity and storage temperature on chemical stability of coffee brews. J. Agric. Food Chem. 2007, 55, 6521–6526. [Google Scholar] [CrossRef] [PubMed]
- Türker, D.; Göksel Saraç, M.; Dogan, M. The Influence of Anti-Caking Agents on Powder Flow Properties of Ready-to-Drink Coffee During Storage. Harran Tarım Gıda Bilim. Derg. 2024, 28, 258–266. [Google Scholar] [CrossRef]
- European Bioplastics. Bioplastic market development update. In Bioplastic Market Development Update 2024; European Bioplastics: Berlin, Germany, 2024. [Google Scholar]
- European Union. Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the reduction of the impact of certain plastic products on the environment. Off. J. Eur. Union 2019, 155, 1–19. [Google Scholar]
- Folino, A.; Karageorgiou, A.; Calabrò, P.S.; Komilis, D. Biodegradation of wasted bioplastics in natural and industrial environments: A review. Sustainability 2020, 12, 6030. [Google Scholar] [CrossRef]
- Gamboni, J.E.; Bonfiglio, G.V.; Slavutsky, A.M.; Bertuzzi, M.A. Evaluation of edible films as single-serve pouches for a sustainable packaging system. Food Chem. Adv. 2023, 3, 100547. [Google Scholar] [CrossRef]
- Mujtaba, M.; Lipponen, J.; Ojanen, M.; Puttonen, S.; Vaittinen, H. Trends and challenges in the development of bio-based barrier coating materials for paper/cardboard food packaging: A review. Sci. Total Environ. 2022, 851, 158328. [Google Scholar] [CrossRef]
- Alves, R.M.V.; Bordin, M.R. Shelf-life prediction of instant coffee by mathematic method. Food Sci. Technol. 1998, 18, 19–24. [Google Scholar] [CrossRef]
- Alongi, M.; Sillani, S.; Lagazio, C.; Manzocco, L. Effect of expiry date communication on acceptability and waste of fresh-cut lettuce during storage at different temperatures. Food Res. Int. 2019, 116, 1121–1125. [Google Scholar] [CrossRef]
- AOAC. AOAC method n° 926.08. In Official Methods of Analysis of the AOAC International, 15th ed.; Horwitz, G.W., Latimer, W., Eds.; AOAC: Arlington, VA, USA, 2005. [Google Scholar]
- Baiano, A.; Tamagnone, P.; Marchitelli, V.; Del Nobile, A. Quality decay kinetics of semi-preserved sauce as affected by packaging. J. Food Sci. 2005, 70, 92–97. [Google Scholar] [CrossRef]
- Gordon, M.; Taylor, J.S. Ideal copolymers and the second-order transitions of synthetic rubbers in non-crystalline copolymers. J. Appl. Chem. 1952, 2, 493–500. [Google Scholar] [CrossRef]
- Anese, M.; Manzocco, L.; Nicoli, M.C. Modeling the secondary shelf life of ground roasted coffee. J. Agric. Food Chem. 2006, 54, 5571–5576. [Google Scholar] [CrossRef] [PubMed]
- Iaccheri, E.; Ragni, L.; Cevoli, C.; Romani, S.; Dalla Rosa, M.; Rocculi, P. Glass transition of green and roasted coffee investigated by calorimetric and dielectric techniques. Food Chem. 2019, 301, 125187. [Google Scholar] [CrossRef] [PubMed]
- Manzocco, L.; Rumignani, A.; Lagazio, C. Use of images in shelf life assessment of fruit salad. J. Food Sci. 2012, 77, 258–262. [Google Scholar] [CrossRef]
- Baty, F.; Ritz, C.; Charles, S.; Brutsche, M.; Flandrois, J.-P.; Delignette-Muller, M.-L. A toolbox for nonlinear regression in R: The package nlstools. J. Stat. Softw. 2015, 66, 1–21. [Google Scholar] [CrossRef]
- Burmester, K.; Pietsch, A.; Eggers, R. A basic investigation on instant coffee production by vacuum belt drying. Procedia Food Sci. 2011, 1, 1344–1352. [Google Scholar] [CrossRef]
- Rahman, M.S. Glass Transition and State Diagram of Foods. In Handbook of Food Preservation; Rahman, M.S., Ed.; CRC Press: Boca Raton, FL, USA, 2009; p. 335. [Google Scholar]
- Ayyad, Z. Quality Control of Virgin Olive Oils with Regard to Different Storage and Shipment Conditions. Ph.D. Thesis, University of Bologna, Bologna, Italy, 2015. [Google Scholar]
- Roudaut, G.; Debeaufort, F. Moisture loss, gain and migration in foods and its impact on food quality. In Chemical Deterioration and Physical Instability of Food and Beverages; Skibsted, L.H., Risbo, J., Andersen, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 143–185. [Google Scholar]
- Ong, X.Y.; Taylor, S.E.; Ramaioli, M. Rehydration of food powders: Interplay between physical properties and process conditions. Powder Technol. 2020, 371, 142–153. [Google Scholar] [CrossRef]
- Juarez-Enriquez, E.; Olivas, G.; Zamudio-Flores, P.; Perez-Vega, S.; Salmeron, I.; Ortega-Rivas, E.; Sepulveda, D.R. A review on the influence of water on food powder flowability. J. Food Process Eng. 2022, 45, e14031. [Google Scholar] [CrossRef]
- Jung, H.; Lee, Y.; Yoon, W. Effect of moisture content on the grinding process and powder properties in food: A review. Processes 2018, 6, 69. [Google Scholar] [CrossRef]
- Suhag, R.; Kellil, A.; Razem, M. Factors influencing food powder flowability. Powders 2024, 3, 65–76. [Google Scholar] [CrossRef]
- Benković, M.; Jurinjak Tusek, A. Regression models for description of roasted ground coffee powder color change during secondary shelf-life as related to storage conditions and packaging material. Beverages 2018, 4, 16. [Google Scholar] [CrossRef]
- Howat, G.R.; Smith, J.A.B.; Waite, R.; Wright, N.C. Factors affecting the solubility of milk powders. IV. The influence of speed and duration of stirring on solubility, with a description of a rapid method for solubility determinations. J. Dairy Res. 1939, 10, 498–514. [Google Scholar] [CrossRef]
- Cincotta, F.; Tripodi, G.; Merlino, M.; Verzera, A.; Condurso, C. Variety and shelf-life of coffee packaged in capsules. LWT-Food Sci. Technol. 2020, 118, 108718. [Google Scholar] [CrossRef]
- Lopriore, M.; Alongi, M.; Calligaris, S.; Manzocco, L.; Ravaioli, G.; Nucci, A.; Nicoli, M.C. Moisture uptake during storage of coffee packed into compostable capsules decreases the quality of coffee brew. Food Packag. Shelf Life 2024, 46, 101403. [Google Scholar] [CrossRef]
- Strocchi, G.; Bagnulo, E.; Ravaioli, G.; Pellegrino, G.; Bicchi, C.; Liberto, E. Evaluation of the behaviour of phenols and alkaloids in samples of roasted and ground coffee stored in different types of packaging: Implications for quality and shelf life. Food Res. Int. 2023, 174, 113548. [Google Scholar]
- Yeager, S.E.; Batali, M.E.; Guinard, J.X.; Ristenpart, W.D. Acids in Coffee: A Review of Sensory Measurements and Meta-Analysis of Chemical Composition. Crit. Rev. Food Sci. Nutr. 2023, 63, 1010–1036. [Google Scholar] [CrossRef]
- Maier, H.G.; Engelhardt, U.H. Determination of non volatile acids in coffee: Comparison of capillary isotachophoresis and capillary gas chromatography. Fresenius’ Z. Für Anal. Chem. 1985, 320, 169–174. [Google Scholar]
- Maier, H.G. Kaffee. Mol. Nutr. Food Res. 1982, 26, 407–411. [Google Scholar]
| ERH (%) | k (Day−1) | RMSE | ||
|---|---|---|---|---|
| 11 | 3.5 ± 0.1 | 2.8 ± 0.5 | 0.08 ± 0.12 | 1.21 |
| 32 | 3.5 ± 0.2 | 5.8 ± 0.2 | 0.18 ± 0.06 | 0.24 |
| 65 | 3.3 ± 0.2 | 14.7 ± 0.3 | 0.15 ± 0.01 | 0.22 |
| ERH (%) | k (s−1) | RMSE | ||
|---|---|---|---|---|
| 11 | 0.008 ± 0.009 | 0.124 ± 0.005 | 0.13 ± 0.03 | 0.008 |
| 32 | 0.005 ± 0.005 | 0.118 ± 0.003 | 0.08 ± 0.01 | 0.004 |
| 65 | 0.002 ± 0.002 | 0.101 ± 0.002 | 0.07 ± 0.01 | 0.003 |
| ERH (%) | RMSE | ||
|---|---|---|---|
| 11 | 5.07 ± 0.01 | 0.38 ± 0.97 | 0.021 |
| 32 | 5.07 ± 0.01 | 0.72 ± 0.11 | 0.024 |
| 65 | 5.08 ± 0.01 | 2.11 ± 0.21 | 0.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopriore, M.; Alongi, M.; Valentino, M.; Anese, M.; Nicoli, M.C. Impact of Environmental Humidity on Instant Coffee Stability: Defining Moisture Thresholds for Quality Degradation and Shelf Life Prediction. Foods 2025, 14, 1826. https://doi.org/10.3390/foods14101826
Lopriore M, Alongi M, Valentino M, Anese M, Nicoli MC. Impact of Environmental Humidity on Instant Coffee Stability: Defining Moisture Thresholds for Quality Degradation and Shelf Life Prediction. Foods. 2025; 14(10):1826. https://doi.org/10.3390/foods14101826
Chicago/Turabian StyleLopriore, Marco, Marilisa Alongi, Marika Valentino, Monica Anese, and Maria Cristina Nicoli. 2025. "Impact of Environmental Humidity on Instant Coffee Stability: Defining Moisture Thresholds for Quality Degradation and Shelf Life Prediction" Foods 14, no. 10: 1826. https://doi.org/10.3390/foods14101826
APA StyleLopriore, M., Alongi, M., Valentino, M., Anese, M., & Nicoli, M. C. (2025). Impact of Environmental Humidity on Instant Coffee Stability: Defining Moisture Thresholds for Quality Degradation and Shelf Life Prediction. Foods, 14(10), 1826. https://doi.org/10.3390/foods14101826





