Volatile Flavor of Tricholoma matsutake from the Different Regions of China by Using GC×GC-TOF MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Materials and Sample Production
2.2. GC×GC-TOF MS Analysis
2.3. Statistical Analysis
3. Results
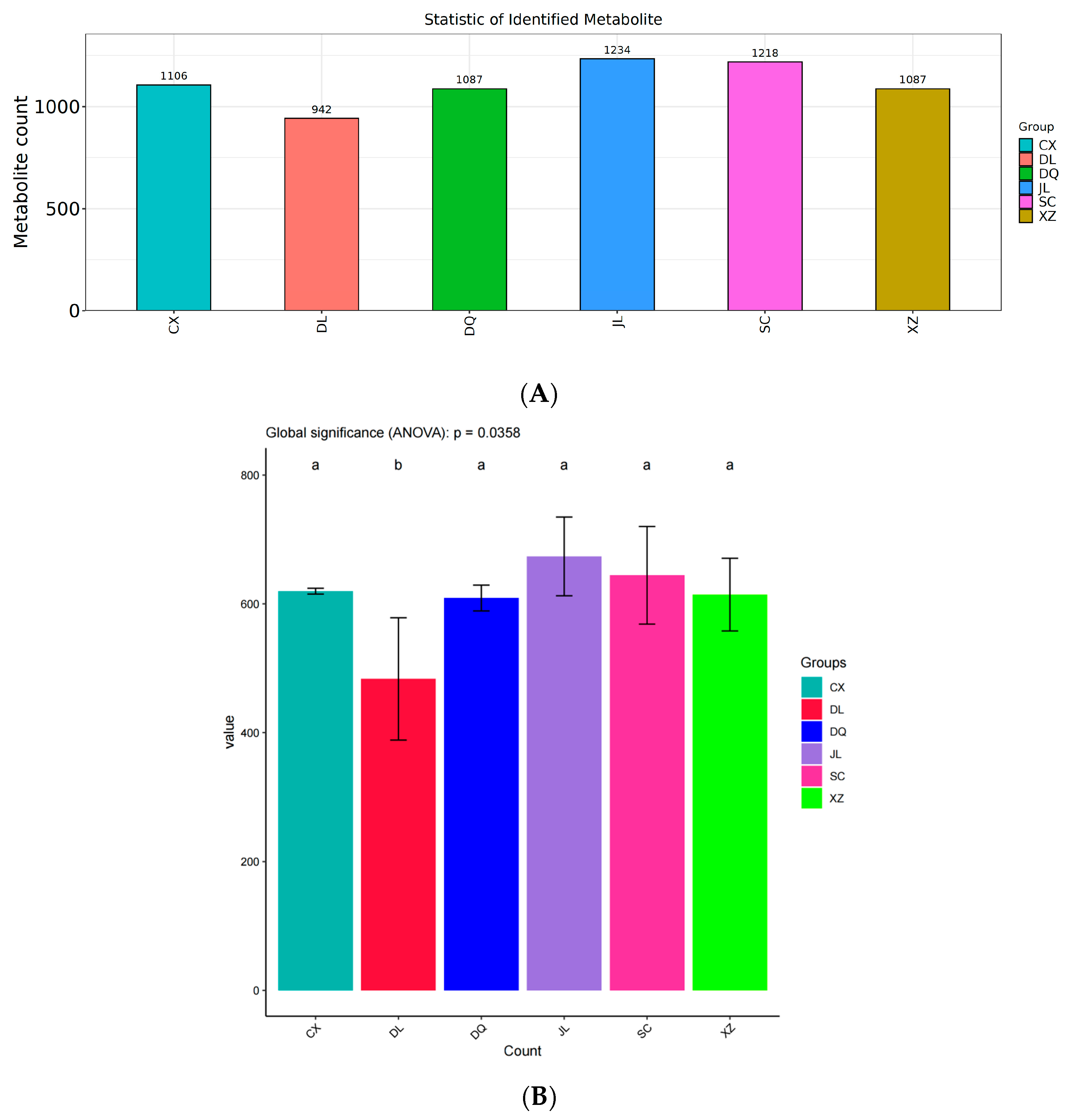
3.1. Statistical Identification of VOCs in Six T. matsutake Samples from Different Regions Using GC×GC-ToF MS
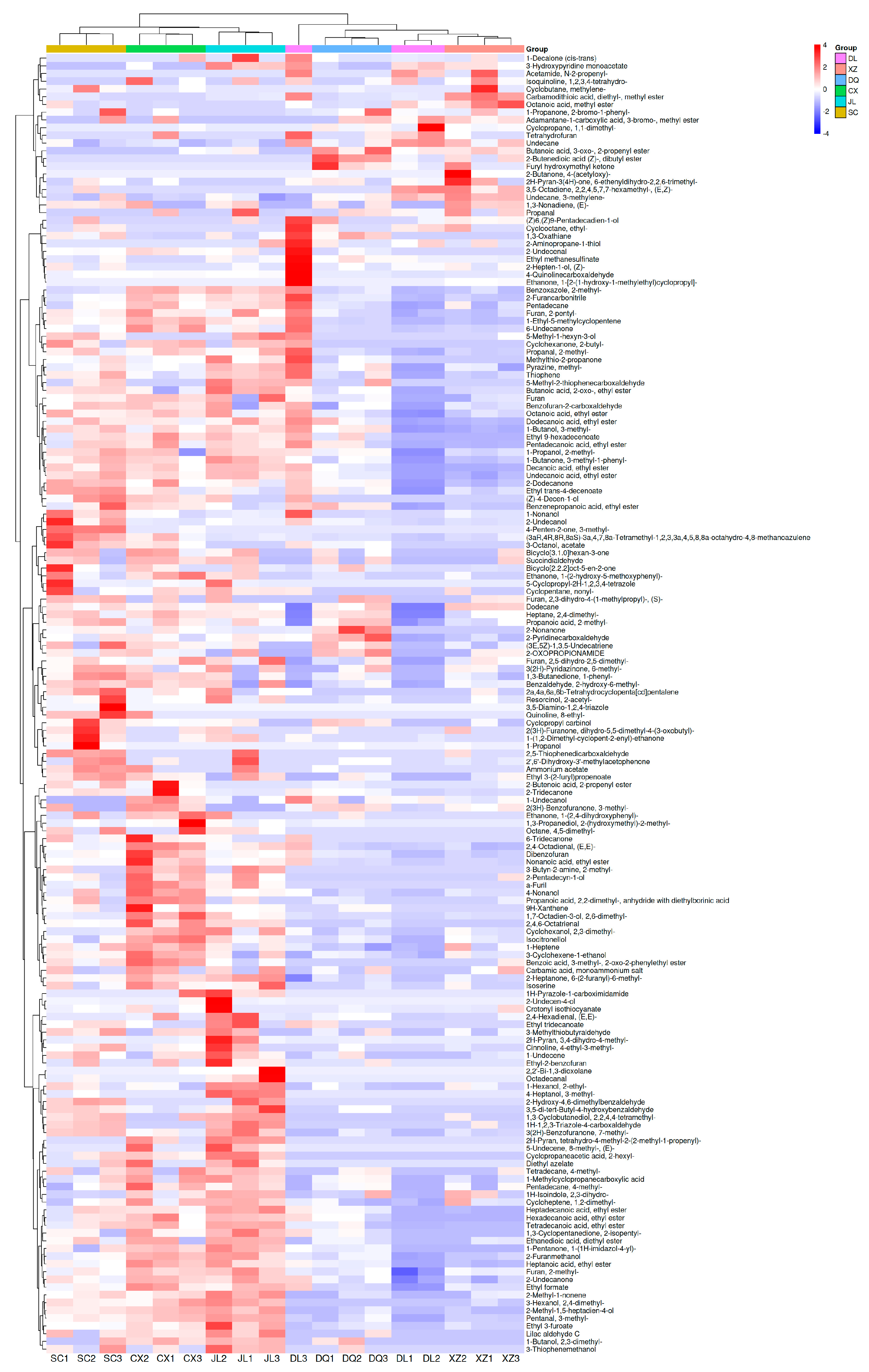
3.2. Analysis of VOCs Obtained by GC×GC-ToF MS
3.3. PCA and PLS-DA Results of Six T. matsutake Samples in Different Regions
3.4. Different Flavor Substances of Six T. matsutake Samples in Different Regions
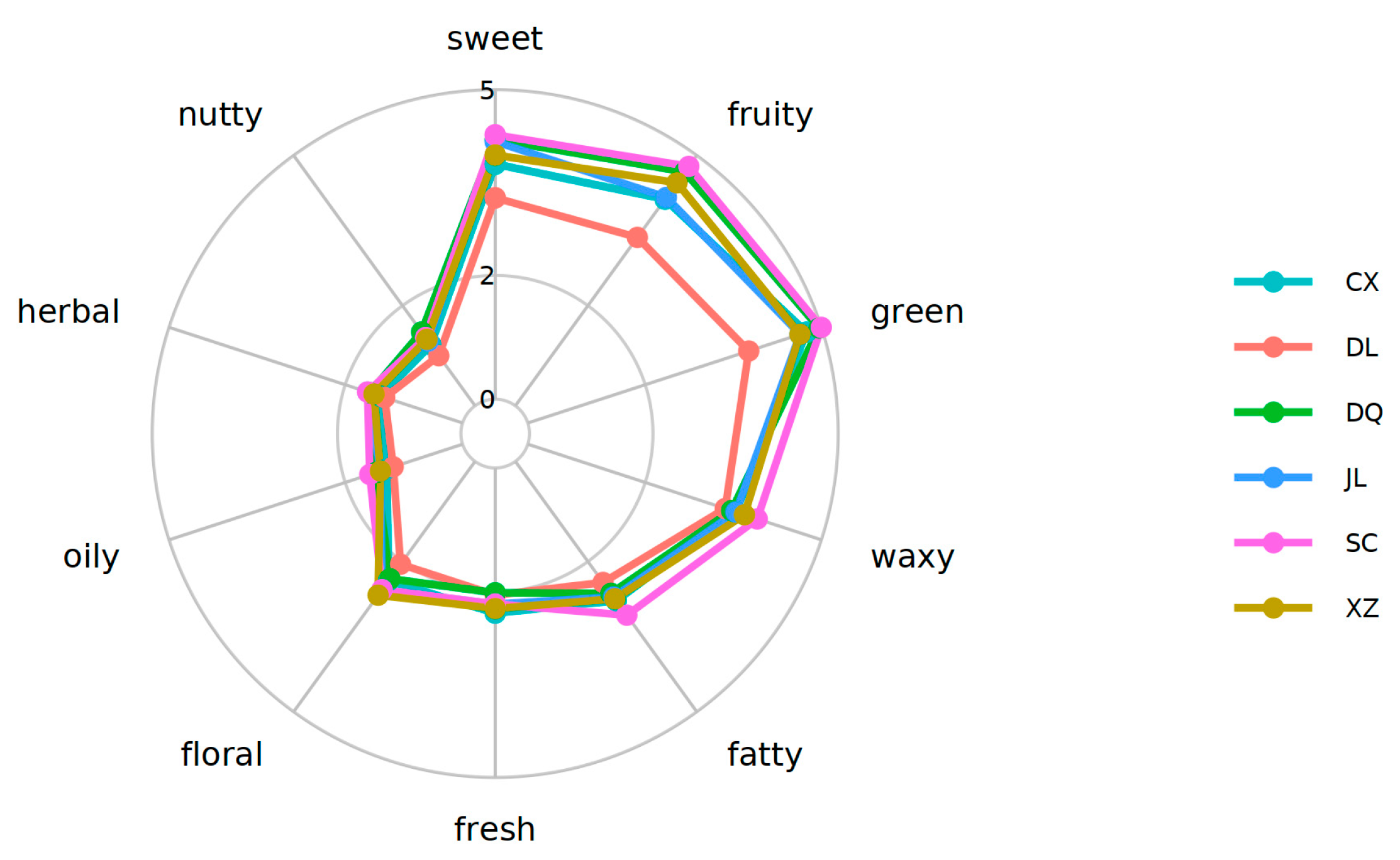
3.5. Analysis of Sensory Flavor Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Y.; Chen, D.; Dong, Y.; Ju, H.; Wu, C.; Lin, S. Characteristic Volatiles Fingerprints and Changes of Volatile Compounds in Fresh and Dried Tricholoma Matsutake Singer by HS-GC-IMS and HS-SPME-GC-MS. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2018, 1099, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Roncero-Ramos, I.; Delgado-Andrade, C. The Beneficial Role of Edible Mushrooms in Human Health. Curr. Opin. Food Sci. 2017, 14, 122–128. [Google Scholar] [CrossRef]
- Li, M.; Du, H.; Lin, S. Flavor Changes of Tricholoma Matsutake Singer under Different Processing Conditions by Using HS-GC-IMS. Foods 2021, 10, 531. [Google Scholar] [CrossRef]
- Hoshi, H.; Yagi, Y.; Iijima, H.; Matsunaga, K.; Ishihara, Y.; Yasuhara, T. Isolation and Characterization of a Novel Immunomodulatory α-Glucan−Protein Complex from the Mycelium of Tricholoma Matsutakein Basidiomycetes. J. Agric. Food Chem. 2005, 53, 8948–8956. [Google Scholar] [CrossRef]
- Ding, X.; Hou, Y. Identification of Genetic Characterization and Volatile Compounds of Tricholoma Matsutake from Different Geographical Origins. Biochem. Syst. Ecol. 2012, 44, 233–239. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Li, W.; Li, X.; Huang, W.; Yang, H.; Zheng, L. Chemical Compositions and Volatile Compounds of Tricholoma Matsutake from Different Geographical Areas at Different Stages of Maturity. Food Sci. Biotechnol. 2016, 25, 71–77. [Google Scholar] [CrossRef]
- Ouzouni, P.K.; Koller, W.; Badeka, A.V.; Riganakos, K.A. Volatile Compounds from the Fruiting Bodies of Three Hygrophorus Mushroom Species from Northern Greece. Int. J. Food Sci. Tech. 2009, 44, 854–859. [Google Scholar] [CrossRef]
- Tranchida, P.Q.; Franchina, F.A.; Dugo, P.; Mondello, L. Comprehensive Two-dimensional Gas Chromatography-mass Spectrometry: Recent Evolution and Current Trends. Mass. Spec. Rev. 2014, 35, 524–534. [Google Scholar] [CrossRef]
- Fang, X.; Chen, Y.; Gao, J.; Run, Z.; Chen, H.; Shi, R.; Li, Y.; Zhang, H.; Liu, Y. Application of GC–TOF/MS and GC×GC–TOF/MS to Discriminate Coffee Products in Three States (Bean, Powder, and Brew). Foods 2023, 12, 3123. [Google Scholar] [CrossRef]
- Yu, Y.; Nie, Y.; Chen, S.; Xu, Y. Characterization of the Dynamic Retronasal Aroma Perception and Oral Aroma Release of Baijiu by Progressive Profiling and an Intra-Oral SPME Combined with GC × GC-TOFMS Method. Food Chem. 2022, 405, 134854. [Google Scholar] [CrossRef]
- Schütt, J.; Schieberle, P. Quantitation of Nine Lactones in Dairy Cream by Stable Isotope Dilution Assays Based on Novel Syntheses of Carbon-13-Labeled γ-Lactones and Deuterium-Labeled δ-Lactones in Combination with Comprehensive Two-Dimensional Gas Chromatography with Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 2017, 65, 10534–10541. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Y.P.; Blank, I.; Li, F.; Li, C.; Liu, Y. GC × GC-ToF-MS and GC-IMS Based Volatile Profile Characterization of the Chinese Dry-Cured Hams from Different Regions. Food Res. Int. 2021, 142, 110222. [Google Scholar] [CrossRef]
- Garg, N.; Sethupathy, A.; Tuwani, R.; Nk, R.; Dokania, S.; Iyer, A.; Gupta, A.; Agrawal, S.; Singh, N.; Shukla, S.; et al. FlavorDB: A Database of Flavor Molecules. Nucl. Acids Res. 2017, 46, D1210–D1216. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for Large-Scale Metabolic Profiling of Serum and Plasma Using Gas Chromatography and Liquid Chromatography Coupled to Mass Spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef] [PubMed]
- Hiraide, M.; Miyazaki, Y.; Shibata, Y. The Smell and Odorous Components of Dried Shiitake Mushroom, Lentinula Edodes I: Relationship between Sensory Evaluations and Amounts of Odorous Components. J. Wood Sci. 2004, 50, 358–364. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Pei, Z.; Wei, P.; Xiang, D.; Cao, X.; Shen, X.; Li, C. Volatile Flavour Components and the Mechanisms Underlying Their Production in Golden Pompano (Trachinotus Blochii) Fillets Subjected to Different Drying Methods: A Comparative Study Using an Electronic Nose, an Electronic Tongue and SDE-GC-MS. Food Res. Int. 2019, 123, 217–225. [Google Scholar] [CrossRef]
- Bao, C.; Guan, C.; Xin, M.; Teng, X.; Liu, T.; Wang, D. Effect of Roasting on Volatile Flavor Compounds of Stropharia rugoso-annulata Analyzed by Headspace-Solid Phase Microextraction-Gas Chromatography-Mass Spectrometry Combined with Electronic Nose. Food Sci. 2022, 14, 226–233. [Google Scholar]
- Cho, I.H.; Kim, S.Y.; Choi, H.-K.; Kim, Y.-S. Characterization of Aroma-Active Compounds in Raw and Cooked Pine-Mushrooms (Tricholoma Matsutake Sing.). J. Agric. Food Chem. 2006, 54, 6332–6335. [Google Scholar] [CrossRef]
- Pueschel, V.A.; Schieberle, P. Changes in the Key Aroma Compounds of Matsutake Mushroom (Tricholoma Matsutake Sing.) from Canada during Pan-Frying Elucidated by Application of the Sensomics Approach. Eur. Food Res. Technol. 2020, 247, 51–65. [Google Scholar] [CrossRef]
- Wurzenberger, M.; Grosch, W. The Formation of 1-Octen-3-Ol from the 10-Hydroperoxide Isomer of Linoleic Acid by a Hydroperoxide Lyase in Mushrooms (Psalliota Bispora). Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1984, 794, 25–30. [Google Scholar] [CrossRef]
- Combet, E.; Eastwood, D.C.; Burton, K.S.; Henderson, J. Eight-Carbon Volatiles in Mushrooms and Fungi: Properties, Analysis, and Biosynthesis. Mycoscience 2006, 47, 317–326. [Google Scholar] [CrossRef]
- Dong, H.; Lu, H.; Jiang, N.; Li, Q.; Li, Y.; Shang, X.; Li, Z.; Wang, Z.; Song, C.; Zhou, F. GC-IMS analyses of volatile organic compounds in Lentinula edodes at different ages. Mycosystema 2023, 42, 1330–1344. [Google Scholar]
- Hou, H.; Liu, C.; Lu, X.; Fang, D.; Hu, Q.; Zhang, Y.; Zhao, L. Characterization of Flavor Frame in Shiitake Mushrooms (Lentinula Edodes) Detected by HS-GC-IMS Coupled with Electronic Tongue and Sensory Analysis: Influence of Drying Techniques. LWT 2021, 146, 111402. [Google Scholar] [CrossRef]
- Guo, Q.; Adelina, N.M.; Hu, J.; Zhang, L.; Zhao, Y. Comparative Analysis of Volatile Profiles in Four Pine-Mushrooms Using HS-SPME/GC-MS and E-Nose. Food Control 2022, 134, 108711. [Google Scholar] [CrossRef]
- Lai, P.; Li, L.; Wei, Y.; Sun, J.; Tang, B.; Yang, Y.; Chen, J.; Wu, L. GC-IMS-Based Volatile Characteristic Analysis of Hypsizygus Marmoreus Dried by Different Methods. Foods 2024, 13, 1322. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, Y.; Dong, Q.; Shu, X.; Zhang, S.; Xie, L.; Peng, W.; Wang, H. Analysis and evaluation of volatile compounds in different fruiting bodies of different strains of Morchella. Mycosystema 2025, 1–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, P.; Zhang, W.; Zhu, H.; Wang, C.; Xie, N.; Wang, Y.; Pang, X.; Marie-Laure, F.; Lü, J.; et al. Free Fatty Acid Hydrolyzed with Lipases and Their Effects on Enzyme-Modified Cheese Flavor. Food Sci. Anim. Prod. 2023, 1, 9240031. [Google Scholar] [CrossRef]
- Yang, H.; Li, W.; Zi, L.; Xu, N.; Guo, Z.; Chen, B.; Hua, Y.; Guo, L. Comprehensive Analysis of the Dynamic Changes of Volatile and Non-Volatile Metabolites in Boletus Edulis during Processing by HS-SPME-GC-MS and UPLC-MS/MS Analysis. Food Chem. X 2024, 22, 101487. [Google Scholar] [CrossRef]
- Liao, Y.; Ding, Y.; Wu, Y.; Du, Q.; Xia, J.; Jia, J.; Lin, H.; Benjakul, S.; Zhang, B.; Hu, Y. Analysis of Volatile Compounds and Flavor Fingerprint in Hairtail (Trichiurus Lepturus) during Air-Drying Using Headspace-Gas Chromatography-Ion Mobility Spectrometry (HS-GC-IMS). Front. Nutr. 2023, 9, 1088128. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Niu, L.; Yu, W.; Deng, L.; Ye, L.; Wu, X.; He, X.; Fan, Y. Analyses of volatile components of Armillaria spp. in the Changbai Mountain, Northeast China. Mycosystema 2023, 42, 823–834. [Google Scholar]
- Li, W.; Zi, L.; Xu, N.; Yang, H.; Dong, S.; Qin, F.; Guo, L. Identification of Characteristic Flavor Compounds of Boletus Edulis from Different Regions Based on by E-Nose, HS-GC-IMS and HS-SPME-GC-MS. Food Chem. X 2024, 23, 101601. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, H.; Huang, F.; Qiang, Y.; Han, D.; Jia, Q.; Zhang, C. Analysis of differences in nutritional quality and volatile flavor components of daylily from different regions. China Condiment 2023, 48, 25–32. [Google Scholar]
- Huang, L.; He, C.; Si, C.; Shi, H.; Duan, J. Nutritional, Bioactive, and Flavor Components of Giant Stropharia (Stropharia Rugoso-Annulata): A Review. JoF 2023, 9, 792. [Google Scholar] [CrossRef]
- Liu, Q.; Bau, T.; Jin, R.; Cui, X.; Zhang, Y.; Kong, W. Comparison of Different Drying Techniques for Shiitake Mushroom (Lentinus Edodes): Changes in Volatile Compounds, Taste Properties, and Texture Qualities. LWT 2022, 164, 113651. [Google Scholar] [CrossRef]
- Pantoja-Benavides, A.D.; Garces-Varon, G.; Restrepo-Díaz, H. Foliar Growth Regulator Sprays Induced Tolerance to Combined Heat Stress by Enhancing Physiological and Biochemical Responses in Rice. Front. Plant Sci. 2021, 12, 702892. [Google Scholar] [CrossRef]
- He, Y.; Liu, Z.; Qian, M.; Yu, X.; Xu, Y.; Chen, S. Unraveling the Chemosensory Characteristics of Strong-Aroma Type Baijiu from Different Regions Using Comprehensive Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry and Descriptive Sensory Analysis. Food Chem. 2020, 331, 127335. [Google Scholar] [CrossRef]




| Name | Collection Places | Geographic Position |
|---|---|---|
| CX | Nanhua, Chuxiong, Yunnan province, China | N 24°51′29″, E 100°48′32″ |
| DL | Jianchuan, Dali, Yunnan province, China | N 26°35′9″, E 99°40′9″ |
| DQ | Shangri-La, Diqing, Yunnan province, China | N 27°54′14″, E 99°38′14″ |
| JL | Yanji, Jilin province, China | N 42°51′1″, E 129°29′59″ |
| SC | Xiaojin, Aba, Sichuan province, China | N 30°57′49″, E 102°17′58″ |
| XZ | Linzhi, Xizang province, China | N 29°53′53″, E 93°26′42″ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Liu, S.; Fang, Y.; Li, J.; Ma, M.; Yang, Z.; Shang, L.; Guo, X.; Hua, R.; Sun, D. Volatile Flavor of Tricholoma matsutake from the Different Regions of China by Using GC×GC-TOF MS. Foods 2025, 14, 1824. https://doi.org/10.3390/foods14101824
Feng Y, Liu S, Fang Y, Li J, Ma M, Yang Z, Shang L, Guo X, Hua R, Sun D. Volatile Flavor of Tricholoma matsutake from the Different Regions of China by Using GC×GC-TOF MS. Foods. 2025; 14(10):1824. https://doi.org/10.3390/foods14101824
Chicago/Turabian StyleFeng, Yunli, Shaoxiong Liu, Yuan Fang, Jianying Li, Ming Ma, Zhenfu Yang, Lue Shang, Xiang Guo, Rong Hua, and Dafeng Sun. 2025. "Volatile Flavor of Tricholoma matsutake from the Different Regions of China by Using GC×GC-TOF MS" Foods 14, no. 10: 1824. https://doi.org/10.3390/foods14101824
APA StyleFeng, Y., Liu, S., Fang, Y., Li, J., Ma, M., Yang, Z., Shang, L., Guo, X., Hua, R., & Sun, D. (2025). Volatile Flavor of Tricholoma matsutake from the Different Regions of China by Using GC×GC-TOF MS. Foods, 14(10), 1824. https://doi.org/10.3390/foods14101824





