Assessing the Impact of Microwaves and Other Disruptive Pretreatments on Lactiplantibacillus plantarum Growth and the Antioxidant Properties of Broccoli Stalks
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Microbial Strain
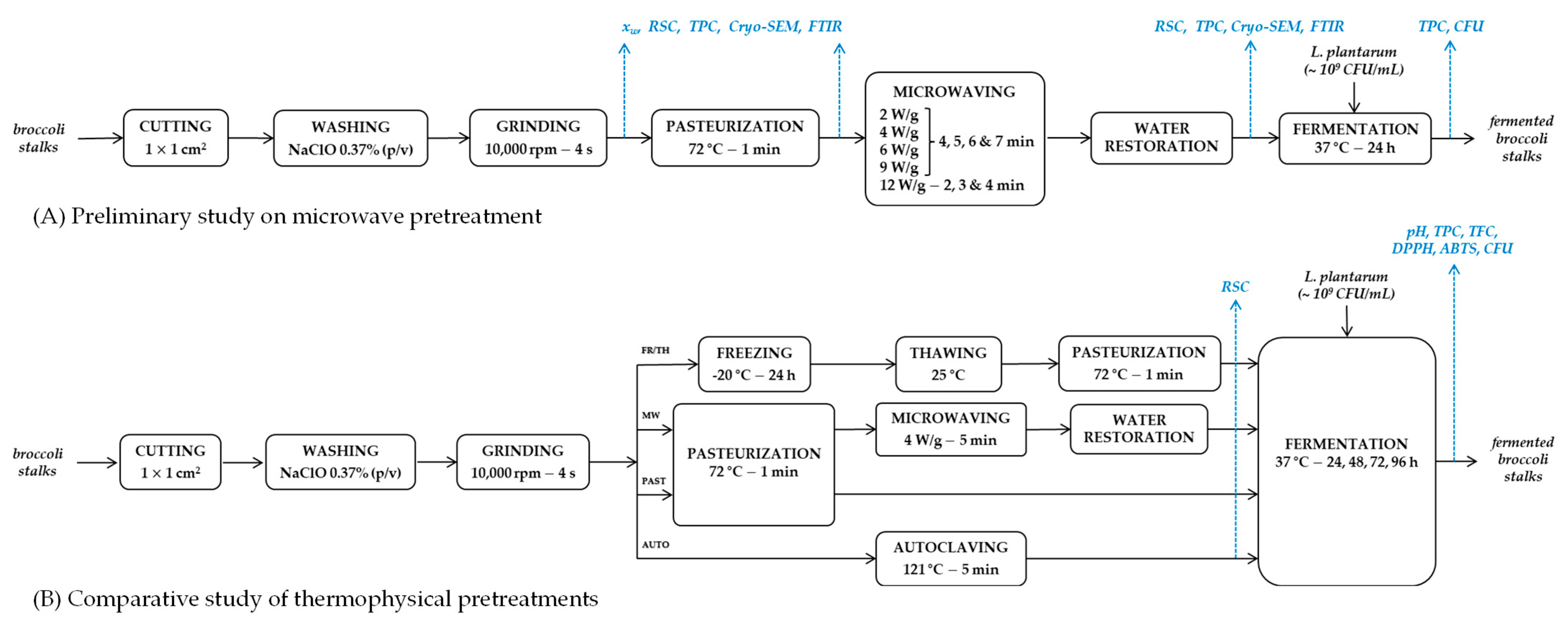
2.2. Thermophysical Pretreatments
2.3. Inoculation and Fermentation
2.4. Analytical Determinations
2.4.1. Moisture Content and pH
2.4.2. Reducing Sugar Content
2.4.3. Antioxidant Properties
2.4.4. Cryo-Field Emission Scanning Electron Microscopy (Cryo-FESEM)
2.4.5. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4.6. Microbial Counts
2.5. Statistical Analysis
3. Results and Discussion
3.1. Preliminary Study on Microwave Pretreatment
3.1.1. Impact on RSC, TPC, and Microbial Growth
3.1.2. Impact on Microstructure and FTIR-Spectra
3.2. Comparative Study of Thermophysical Pretreatments
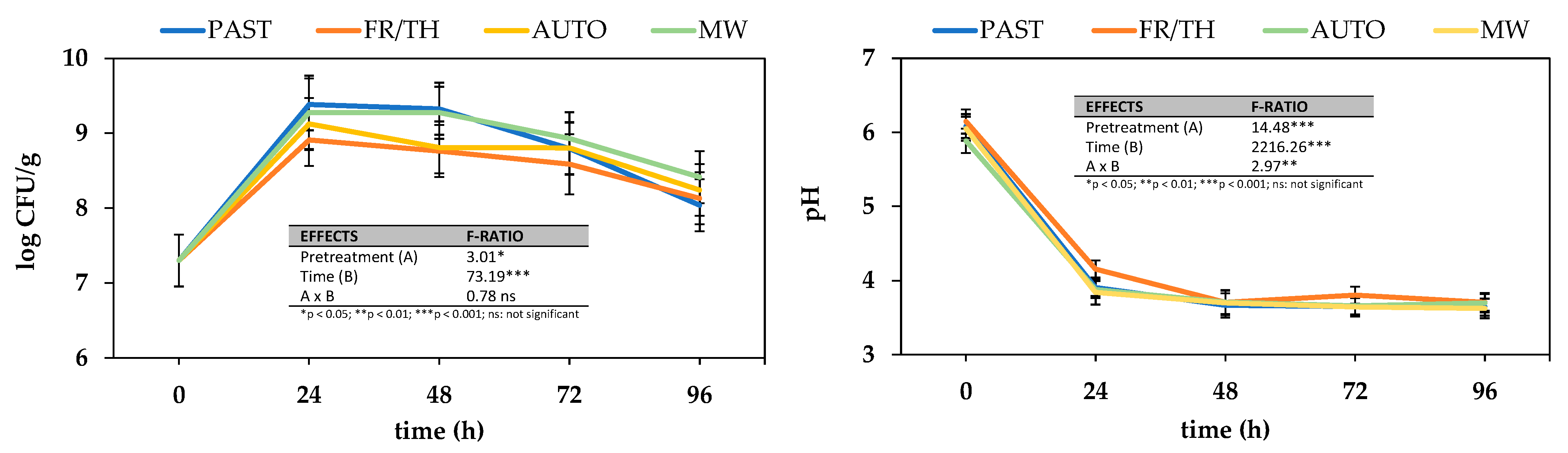
3.2.1. Impact on Microbial Growth
3.2.2. Impact on Antioxidant Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EUROSTAT. Food Waste and Food Waste Prevention–Estimates. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Food_waste_and_food_waste_prevention_-_estimates (accessed on 27 September 2024).
- FAO. The State of Food and Agriculture 2019: Moving Forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019. [Google Scholar] [CrossRef]
- Li, H.; Xia, Y.; Liu, H.Y.; Guo, H.; He, X.Q.; Liu, Y.; Wu, D.T.; Mai, Y.H.; Li, H.B.; Zou, L.; et al. Nutritional Values, Beneficial Effects, and Food Applications of Broccoli (Brassica oleracea var. italica Plenck). Trends Food Sci. Technol. 2022, 119, 288–308. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Ser, S.L.; Cumming, J.R.; Ku, K.-M. Comparative Phytonutrient Analysis of Broccoli By-Products: The Potentials for Broccoli By-Product Utilization. Molecules 2018, 23, 900. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.W.; Moon, J.K.; Shibamoto, T. Analysis and Antioxidant Activity of Extracts from Broccoli (Brassica oleracea L.) Sprouts. J. Agric. Food Chem. 2015, 63, 1169–1174. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Impact of Disruption and Drying Conditions on Physicochemical, Functional and Antioxidant Properties of Powdered Ingredients Obtained from Brassica Vegetable By-Products. Foods 2022, 11, 3663. [Google Scholar] [CrossRef]
- Nyangena, I.O.; Owino, W.O.; Imathiu, S.; Ambuko, J. Effect of Pretreatments Prior to Drying on Antioxidant Properties of Dried Mango Slices. Sci. Afr. 2019, 6, e00148. [Google Scholar] [CrossRef]
- Budaraju, S.; Mallikarjunan, K.; Annor, G.; Schoenfuss, T.; Raun, R. Effect of Pre-treatments on the Antioxidant Potential of Phenolic Extracts from Barley Malt Rootlets. Food Chem. 2018, 266, 31–37. [Google Scholar] [CrossRef]
- Conesa, C.; Seguí, L.; Laguarda-Miró, N.; Fito, P. Microwaves as a Pretreatment for Enhancing Enzymatic Hydrolysis of Pineapple Industrial Waste for Bioethanol Production. Food Bioprod. Process. 2016, 100 Pt A, 203–213. [Google Scholar] [CrossRef]
- Paciulli, M.; Ganino, T.; Carini, E.; Pellegrini, N.; Pugliese, A.; Chiavaro, E. Effect of Different Cooking Methods on Structure and Quality of Industrially Frozen Carrots. J. Food Sci. Technol. 2016, 53, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Cömert, E.D.; Gökmen, V. Antioxidants Bound to an Insoluble Food Matrix: Their Analysis, Regeneration Behavior, and Physiological Importance. Compr. Rev. Food Sci. Food Saf. 2017, 16, 382–399. [Google Scholar] [CrossRef]
- González, M.E.; Barret, D.M. Thermal, High Pressure, and Electric Field Processing Effects on Plant Cell Membrane Integrity and Relevance to Fruit and Vegetable Quality. J. Food Sci. 2010, 75, R121–R130. [Google Scholar] [CrossRef]
- Lizárraga-Chaidez, M.; Abadía-García, L.; Mendoza-Sánchez, M.J.; Huerta-Manzanilla, E.L.; Mendoza-Sánchez, M. Optimization of the Green Extraction Process of Antioxidants Derived from Grape Pomace. Sustain. Chem. Pharm. 2024, 37, 101396. [Google Scholar] [CrossRef]
- López-Hernández, A.A.; Ortega-Villarreal, A.S.; Vázquez-Rodríguez, J.A.; López-Cabanillas Lomelí, M.; González-Martínez, B.E. Application of Different Cooking Methods to Improve Nutritional Quality of Broccoli (Brassica oleracea var. italica) Regarding its Compounds Content with Antioxidant Activity. Int. J. Gastron. Food Sci. 2022, 28, 100510. [Google Scholar] [CrossRef]
- Czarnowska-Kujawska, M.; Draszanowska, A.; Starowicz, M. Effect of Different Cooking Methods on the Folate Content, Organoleptic and Functional Properties of Broccoli and Spinach. LWT 2022, 167, 113825. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Kamal, S.M.M.; Biak, D.R.A. Microwave-Assisted Pretreatment of Lignocellulosic Biomass: A review. J. Eng. Sci. Technol. 2015, 10, 97–109. [Google Scholar]
- Barrera, C.; Fito, P.; Castro-Giráldez, M.; Betoret, N.; Seguí, L. Microwaves Application to Food and Food Waste Processing. In Novel Technologies in Food Science; Chhikara, N., Panghal, A., Chaudhary, G., Eds.; Scrivener Publishing LLC.: Beverly, MA, USA, 2023; Chapter 6; pp. 235–269. [Google Scholar] [CrossRef]
- Chakraborty, P.; Kumar, R.; Chakrabortty, S.; Saha, S.; Chattaraj, S.; Roy, S.; Banerjee, A.; Tripathy, S.K.; Ghosh, A.K.; Jeon, B.H. Technological Advancements in the Pretreatment of Lignocellulosic Biomass for Effective Valorization: A Review of Challenges and Prospects. J. Ind. Eng. Chem. 2024, 137, 29–60. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.A. Microwave-Assisted Conversion of Biomass and Waste Materials to Biofuels. Renew. Sustain. Energy Rev. 2018, 82 Pt 1, 1149–1177. [Google Scholar] [CrossRef]
- Gao, Y.; Remón, J.; Matharu, A.S. Microwave-Assisted Hydrothermal Treatments for Biomass Valorisation: A Critical Review. Green Chem. 2021, 23, 3502–3525. [Google Scholar] [CrossRef]
- Xu, J.K.; Sun, Y.C.; Sun, R.C. Structural and Hydrolysis Characteristics of Cypress Pretreated by Ionic Liquids in a Microwave Irradiation Environment. Bioenerg. Res. 2014, 7, 1305–1316. [Google Scholar] [CrossRef]
- Yousefi, B.; Eslami, M.; Ghasemian, A.; Kokhaei, P.; Farrokhi, A.S.; Darabi, N. Probiotics Importance and Their Immunomodulatory Properties. J. Cell. Physiol. 2019, 234, 8008–8018. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Szkaradkiewicz, A.K. Characteristic of Bacteriocines and Their Application. Pol. J. Microbiol. 2013, 62, 223–235. [Google Scholar] [CrossRef]
- Iga-Buitrón, D.; Torres-Maravilla, E.; Bermúdez-Humaran, L.G.; Ascacio-Valdes, J.A.; Rodríguez-Herrera, R.; Aguilar, C.N.; Flores-Gallegos, A.C. Lactic Fermentation of Broccoli (Brassica oleracea var. italica) to Enhance the Antioxidant and Antiproliferative Activities. Fermentation 2023, 9, 122. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, X.; Fu, H.; Wang, X.; Guo, X.; Wang, M. Lactiplantibacillus plantarum: A Comprehensive Review of its Antifungal and Anti-Mycotoxic Effects. Trends Food Sci. Technol. 2023, 136, 224–238. [Google Scholar] [CrossRef]
- Corsetti, A.; Valomorri, S. Lactic Acid Bacteria|Lactobacillus spp.: Lactobacillus plantarum. In Encyclopedia of Dairy Sciences, 2nd ed.; John, W.F., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 111–118. [Google Scholar] [CrossRef]
- Fidanza, M.; Panigrahi, P.; Kollmann, T.R. Lactiplantibacillus plantarum–Nomad and Ideal Probiotic. Front. Microbiol. 2021, 12, 712236. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ). Update of the List of Qualified Presumption of Safety (QPS) Recommended Microbiological Agents Intentionally Added to Food or Feed as Notified to EFSA 17: Suitability of Taxonomic Units Notified to EFSA until September 2022. EFSA J. 2023, 21, e07746. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, N.; Battista, N.; Prete, R.; Corsetti, A. Health-Promoting Role of Lactiplantibacillus plantarum Isolated from Fermented Foods. Microorganisms 2021, 9, 349. [Google Scholar] [CrossRef]
- Serna-Barrera, M.A.; Bas-Bellver, C.; Seguí, L.; Betoret, N.; Barrera, C. Exploring Fermentation with Lactic Acid Bacteria as a Pretreatment for Enhancing Antioxidant Potential in Broccoli Stem Powders. AIMS Microbiol. 2024, 10, 255–272. [Google Scholar] [CrossRef]
- Clifford, P.A. Report on Moisture in Dried Fruit. J. AOAC Int. 1934, 17, 215–228. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L.; Harasym, J. IV-Range Carrot Waste Flour Enhances Nutritional and Functional Properties of Rice-Based Gluten-Free Muffins. Foods 2024, 13, 1312. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Luximon-Ramma, A.; Bahorun, T.; Soobrattee, M.A.; Aruoma, O.I. Antioxidant Activities of Phenolic, Proanthocyanidin, and Flavonoid Components in Extracts of Cassia fistula. J. Agric. Food Chem. 2002, 50, 5042–5047. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Agustika, D.K.; Mercuriani, I.; Purnomo, C.W.; Hartono, S.; Triyana, K.; Iliescu, D.D.; Leeson, M.K. Fourier Transform Infrared Spectrum Pre-Processing Technique Selection for Detecting PYLCV-Infected Chilli Plants. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 278, 121339. [Google Scholar] [CrossRef]
- Rosa, E.; David, M.; Gomes, M.H. Glucose, Fructose and Sucrose Content in Broccoli, White Cabbage and Portuguese Cabbage Grown in Early and Late Seasons. J. Sci. Food Agric. 2001, 81, 1145–1149. [Google Scholar] [CrossRef]
- Núñez-Gómez, V.; González-Barrio, R.; Baenas, N.; Moreno, D.A.; Periago, M.J. Dietary-Fibre-Rich Fractions Isolated from Broccoli Stalks as a Potential Functional Ingredient with Phenolic Compounds and Glucosinolates. Int. J. Mol. Sci. 2022, 23, 13309. [Google Scholar] [CrossRef]
- Zhan, L.; Pang, L.; Ma, Y.; Zhang, C. Thermal Processing Affecting Phytochemical Contents and Total Antioxidant Capacity in Broccoli (Brassica oleracea L.). J. Sci. Food Agric. 2017, 42, e13548. [Google Scholar] [CrossRef]
- Sew, C.C.; Ghazali, H.M.; Martín-Belloso, O.; Noranizan, M.A. Effects of Combining Ultraviolet and Mild Heat Treatments on Enzymatic Activities and Total Phenolic Contents in Pineapple Juice. Innov. Food Sci. Emerg. Technol. 2014, 26, 511–516. [Google Scholar] [CrossRef]
- Cardoso, H.B.; Frommhagen, M.; Wierenga, P.A.; Gruppen, H.; Schols, H.A. Maillard Induced Saccharide Degradation and its Effects on Protein Glycation and Aggregation. Food Chem. Adv. 2023, 2, 100165. [Google Scholar] [CrossRef]
- Eggleston, G.; Vercellotti, J.R. Degradation of Sucrose, Glucose and Fructose in Concentrated Aqueous Solutions Under Constant pH Conditions at Elevated Temperature. J. Carbohydr. Chem. 2000, 19, 1305–1318. [Google Scholar] [CrossRef]
- Gulati, A.; Rawat, R.; Singh, B.; Ravindranath, S.D. Application of Microwave Energy in the Manufacture of Enhanced-Quality Green Tea. J. Agric. Food Chem. 2003, 51, 4764–4768. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, D.; Zhang, J.; Li, T.; Niu, H.; Xin, X.; Zhao, S.; He, C.; Zhang, C. Enhancing the Formation of Functional Glucosinolate Degradation Products in Fermented Broccoli Stalk By-Product with Lactic Acid Bacteria. Food Chem. 2025, 464 Pt I, 141689. [Google Scholar] [CrossRef]
- Zdziobek, P.; Jodłowski, G.S.; Strzelec, E.A. Biopreservation and Bioactivation Juice from Waste Broccoli with Lactiplantibacillus plantarum. Molecules 2023, 28, 4594. [Google Scholar] [CrossRef]
- Ye, J.H.; Huang, L.Y.; Terefe, N.S.; Augustin, M.A. Fermentation-Based Biotransformation of Glucosinolates, Phenolics and Sugars in Retorted Broccoli Puree by Lactic Acid Bacteria. Food Chem. 2019, 286, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, R.; Lo Vecchio, G.; Rando, R.; Leonardi, M.; Cicero, N.; Costa, R. A Sustainable Strategy for the Conversion of Industrial Citrus Fruit Waste into Bioethanol. Sustainability 2023, 15, 9647. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant Extracts Rich in Polyphenols: Antibacterial Agents and Natural Preservatives for Meat and Meat Products. Crit. Rev. Food Sci. Nutr 2020, 61, 149–178. [Google Scholar] [CrossRef] [PubMed]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 829. [Google Scholar] [CrossRef]
- Salas-Millán, J.A.; Aznar, A.; Conesa, E.; Conesa-Bueno, A.; Aguayo, E. Functional Food Obtained from Fermentation of Broccoli By-Products (Stalk): Metagenomics Profile and Glucosinolate and Phenolic Compounds Characterization by LC-ESI-QqQ-MS/MS. LWT 2022, 169, 113915. [Google Scholar] [CrossRef]
- Wightman, R. An Overview of Cryo-Scanning Electron Microscopy Techniques for Plant Imaging. Plants 2022, 11, 1113. [Google Scholar] [CrossRef]
- Seguí, L.; Fito, P.J.; Fito, P. A Study on the Rehydration Ability of Isolated Apple Cells After Osmotic Dehydration Treatments. J. Food Eng. 2013, 115, 145–153. [Google Scholar] [CrossRef]
- Assani, A.; Moundanga, S.; Beney, L.; Gervais, P. Vesicle Formation in the Membrane of Onion Cells (Allium cepa) During Rapid Osmotic Dehydration. Ann Bot. 2009, 104, 1389–1395. [Google Scholar] [CrossRef]
- Fox, A.R.; Scochera, F.; Laloux, T.; Filik, K.; Degand, H.; Morsomme, P.; Alleva, K.; Chaumont, F. Plasma Membrane Aquaporins Interact with the Endoplasmic Reticulum Resident VAP27 Proteins at ER-PM Contact Sites and Endocytic Structures. New Phytol. 2020, 228, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Harahap, A.F.P.; Husnil, Y.A.; Ramadhana, M.Y.A.; Sahlan, M.; Hermansyah, H.; Prasetya, B.; Gozan, M. Effect of Microwave Pretreatment on Some Properties of Bamboo (Gigantochloa apus) for Bioethanol Production. Int. J. Adv. Sci. Eng. Inf. Technol. 2022, 12, 365. [Google Scholar] [CrossRef]
- Alajaji, S.A.; El-Adawy, T.A. Nutritional Composition of Chickpea (Cicer arietinum L.) as Affected by Microwave Cooking and Other Traditional Cooking Methods. J. Food Compos. Anal. 2006, 19, 806–812. [Google Scholar] [CrossRef]
- Meriles, S.P.; Steffolani, M.E.; Penci, M.C.; Curet, S.; Boillereaux, L.; Ribotta, P.D. Effects of Low-Temperature Microwave Treatment of Wheat Germ. J. Sci. Food Agric. 2022, 102, 2538–2544. [Google Scholar] [CrossRef]
- Shokri, S.; Jegasothy, H.; Hliang, M.M.; Augustin, M.A.; Terefe, N.S. Thermosonication of Broccoli Florets Prior to Fermentation Increases Bioactive Components in Fermented Broccoli Puree. Fermentation 2022, 8, 236. [Google Scholar] [CrossRef]
- Atasoy, M.; Álvarez-Ordóñez, A.; Cenian, A.; Djukić-Vuković, A.; Lund, P.A.; Ozogul, F.; Trček, J.; Ziv, C.; De Biase, D. Exploitation of Microbial Activities at Low pH to Enhance Planetary Health. FEMS Microbiol. Rev. 2024, 48, fuad062. [Google Scholar] [CrossRef]
- González-Hidalgo, I.; Moreno, D.A.; García-Viguera, C.; Ros-García, J.M. Effect of Industrial Freezing on the Physical and Nutritional Quality Traits in Broccoli. Food Sci. Technol. Int. 2019, 25, 56–65. [Google Scholar] [CrossRef]
- Severini, C.; Giuliani, R.; De Filippis, A.; Derossi, A.; De Pilli, T. Influence of Different Blanching Methods on Colour, Ascorbic Acid and Phenolics Content of Broccoli. J. Food Sci. Technol. 2016, 53, 501–510. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Barrera, C.; Seguí, L. Impact of Thermophysical and Biological Pretreatments on Antioxidant Properties and Phenolic Profile of Broccoli Stem Products. Foods 2024, 13, 3585. [Google Scholar] [CrossRef]
- Hou, F.; Cai, Y.; Wang, J. Antioxidant Capacity Changes and Untargeted Metabolite Profile of Broccoli during Lactic Acid Bacteria Fermentation. Fermentation 2023, 9, 474. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of Antioxidant Activity. J. Funt. Foods 2015, 18 Pt B, 757–781. [Google Scholar] [CrossRef]




| TREATMENT | RSC (mg GE/gdw) | TPC (mg GAE/gdw) | L. plantarum (Log CFU/g) | |
|---|---|---|---|---|
| RAW | 144 ± 2 ab | 1.97 ± 0.06 abc | NF | |
| PASTEURIZED | 195 ± 5 f | 2.59 ± 0.08 cde | NF | |
| 2 W/g | 4′ | 193.2 ± 1.3 ef | 2.55 ± 0.02 bcd | 9.46 ± 0.05 ab |
| 5′ | 180.7 ± 1.2 def | 2.32 ± 0.10 abcd | 9.29 ± 0.02 ab | |
| 6′ | 171.7 ± 1.1 bcdef | 2.56 ± 0.02 bcd | 9.23 ± 0.09 ab | |
| 7’ | 147.3 ± 1.4 abc | 2.1 ± 0.3 abc | 9.044 ± 0.003 a | |
| 4 W/g | 4′ | 167 ± 3 bcde | 1.795 ± 0.008 ab | 9.0 ± 0.2 a |
| 5′ | 147.7 ± 0.9 abc | 2.31 ± 0.13 abcd | 9.48 ± 0.13 ab | |
| 6′ | 146 ± 2 ab | 1.91 ± 0.04 abc | 9.46 ± 0.11 ab | |
| 7′ | 177 ± 2 cdef | 1.55 ± 0.04 a | 9.70 ± 0.08 bc | |
| 6 W/g | 4′ | 166 ± 7 bcde | 2.62 ± 0.11 bcd | 9.21 ± 0.10 ab |
| 5′ | 156 ± 5 abcd | 2.7 ± 0.3 bcd | 9.22 ± 0.12 ab | |
| 6′ | 147 ± 4 ab | 2.18 ± 0.11 abcd | 9.28 ± 0.05 ab | |
| 7′ | 156 ± 8 abcd | 3.02 ± 0.04 d | 9.23 ± 0.02 ab | |
| 9 W/g | 4′ | 171 ± 2 bcdef | 2.61 ± 0.13 bcd | 10.13 ± 0.14 c |
| 5′ | 180 ± 3 def | 2.6 ± 0.2 bcd | 10.16 ± 0.12 c | |
| 6′ | 133 ± 9 a | 2.7 ± 0.2 cd | 10.2 ± 0.3 c | |
| 7′ | - | 3.0 ± 0.2 d | NF | |
| 12 W/g | 2′ | - | 2.37 ± 0.11 bcd | NF |
| 3′ | - | 2.25 ± 0.02 bcd | NF | |
| 4′ | - | 3.40 ± 0.10 f | NF | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldassa, S.; Barrera, C.; Muñoz-Ibáñez, M.; Seguí, L. Assessing the Impact of Microwaves and Other Disruptive Pretreatments on Lactiplantibacillus plantarum Growth and the Antioxidant Properties of Broccoli Stalks. Foods 2025, 14, 1809. https://doi.org/10.3390/foods14101809
Baldassa S, Barrera C, Muñoz-Ibáñez M, Seguí L. Assessing the Impact of Microwaves and Other Disruptive Pretreatments on Lactiplantibacillus plantarum Growth and the Antioxidant Properties of Broccoli Stalks. Foods. 2025; 14(10):1809. https://doi.org/10.3390/foods14101809
Chicago/Turabian StyleBaldassa, Simone, Cristina Barrera, Marta Muñoz-Ibáñez, and Lucía Seguí. 2025. "Assessing the Impact of Microwaves and Other Disruptive Pretreatments on Lactiplantibacillus plantarum Growth and the Antioxidant Properties of Broccoli Stalks" Foods 14, no. 10: 1809. https://doi.org/10.3390/foods14101809
APA StyleBaldassa, S., Barrera, C., Muñoz-Ibáñez, M., & Seguí, L. (2025). Assessing the Impact of Microwaves and Other Disruptive Pretreatments on Lactiplantibacillus plantarum Growth and the Antioxidant Properties of Broccoli Stalks. Foods, 14(10), 1809. https://doi.org/10.3390/foods14101809









