Evaluation of Freezing-Induced Changes in Aroma Profiles of Pomegranate Juice by Quantitative Descriptive Sensory Analysis, Gas Chromatography–Mass Spectrometry/Olfactometry, Odor Activity Values, Orthogonal Partial Least Squares–Discriminant Analysis, and Odorant Addition Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Pomegranate Juice (PJ) Samples
2.3. Quantitative Descriptive Sensory Analysis (QDA)
2.4. Extraction of Volatiles by HS-SPME
2.5. Identification of Volatiles Using GC-MS
2.6. GC-O Analysis
2.7. Qualitative and Quantitative Analysis of Volatiles
2.8. Odor Activity Value (OAV)
2.9. Odorant Addition Experiment
2.10. Statistical Analysis
3. Results and Discussion
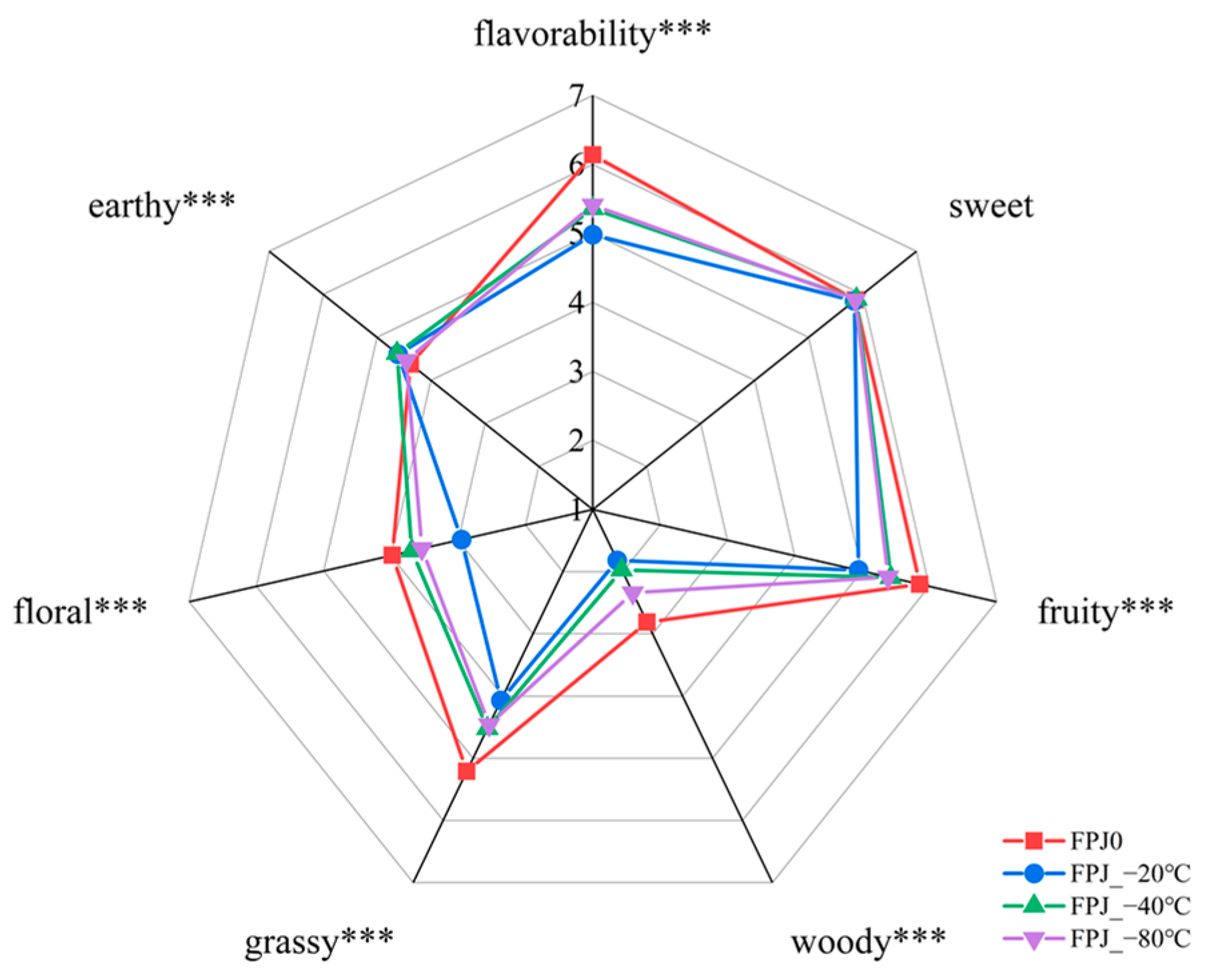
3.1. Aroma Profiles of Fresh and Frozen PJ
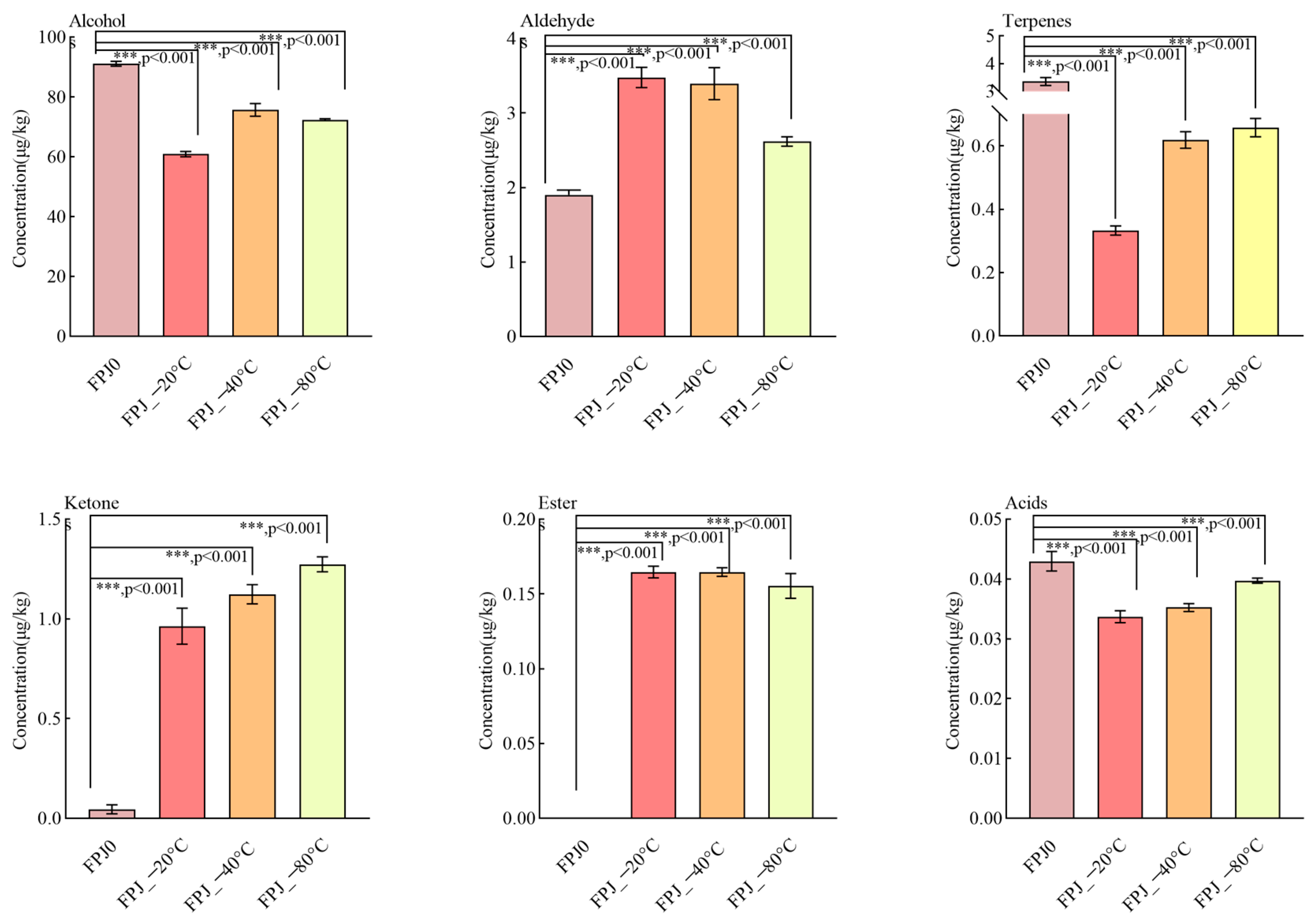
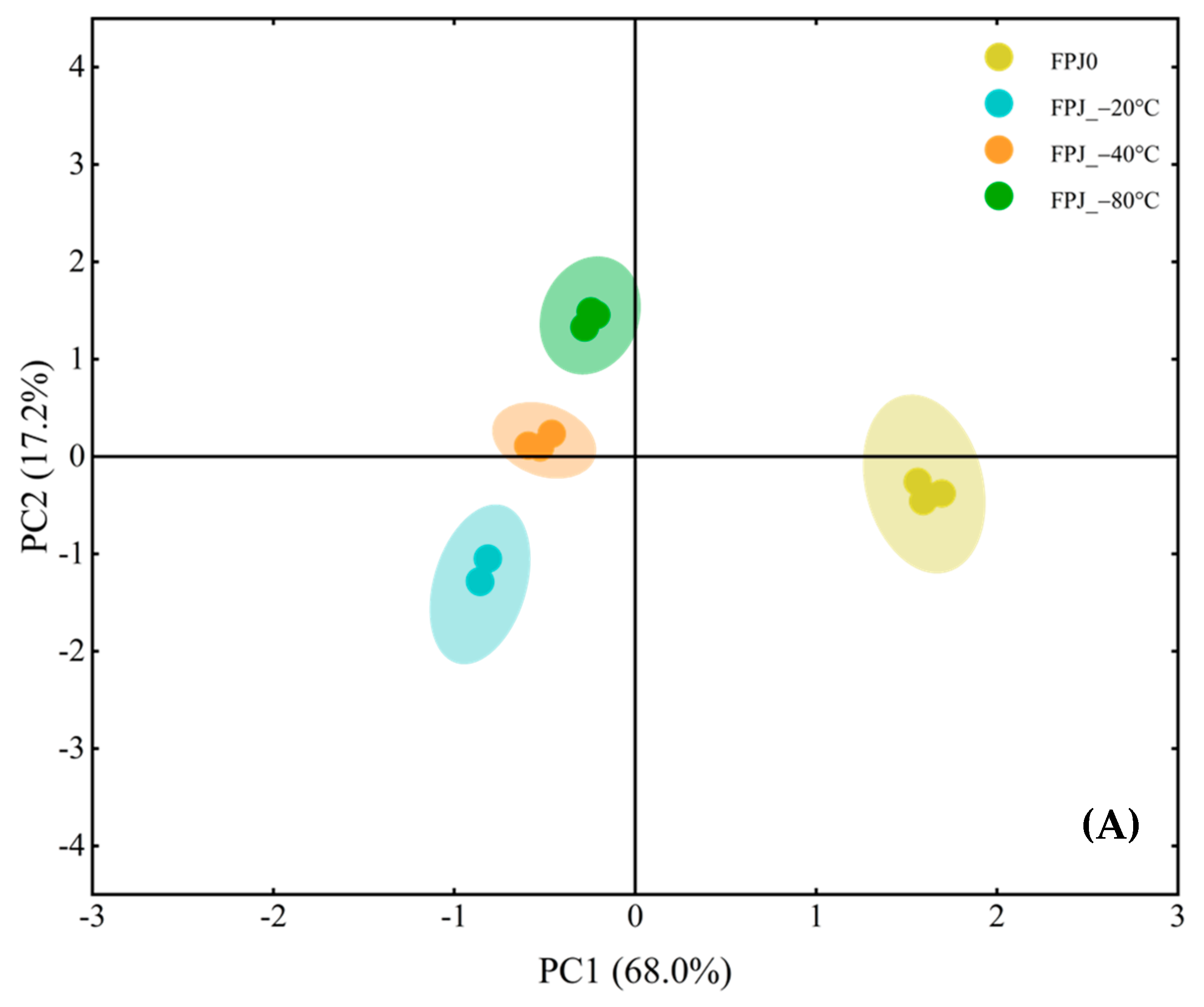
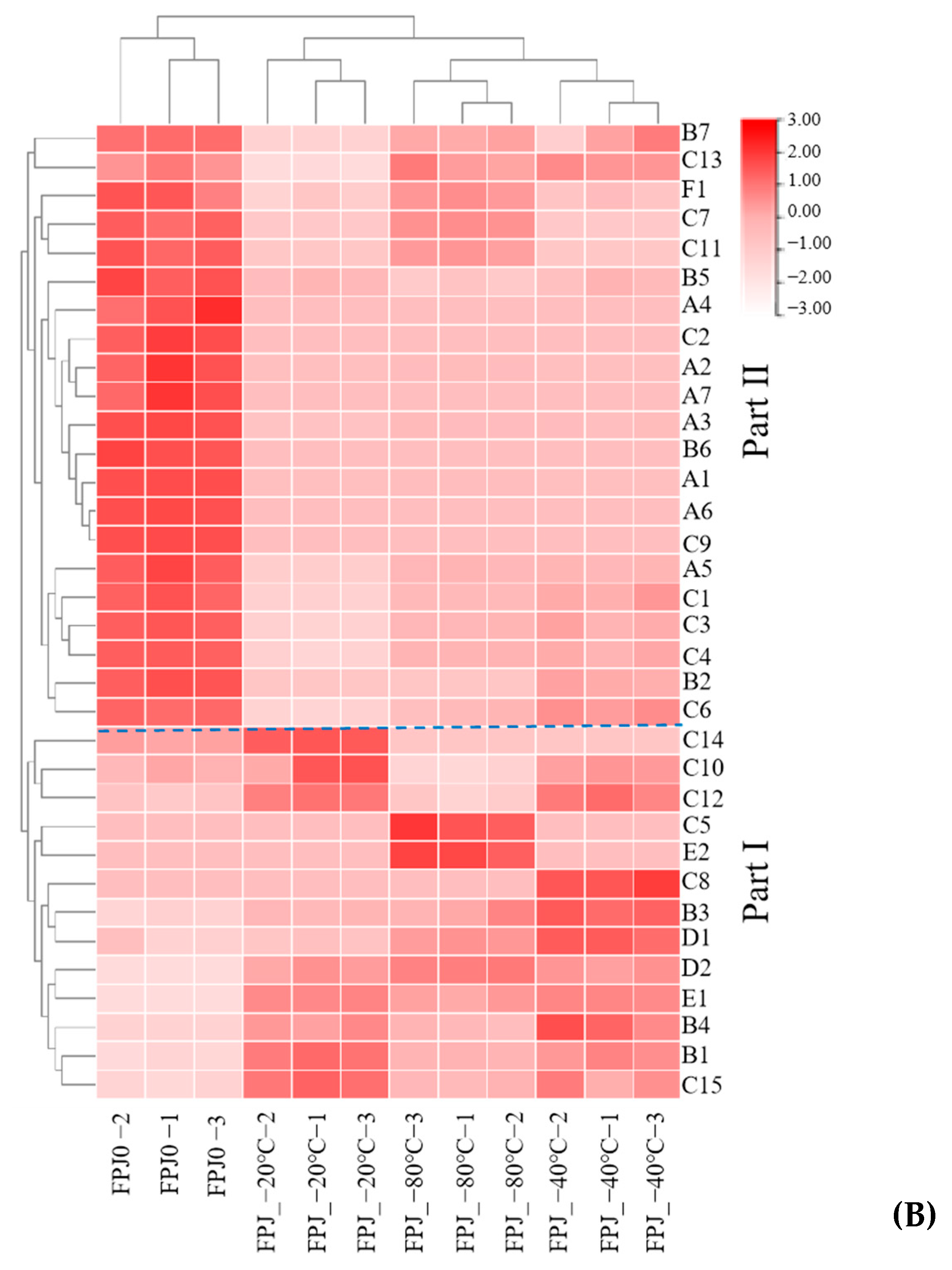
3.2. Comparative Analysis of Volatiles Between Fresh and Frozen PJs
3.3. Analysis of Aroma Active Compounds in Fresh and Frozen PJs Through GC-O/AI and OAV
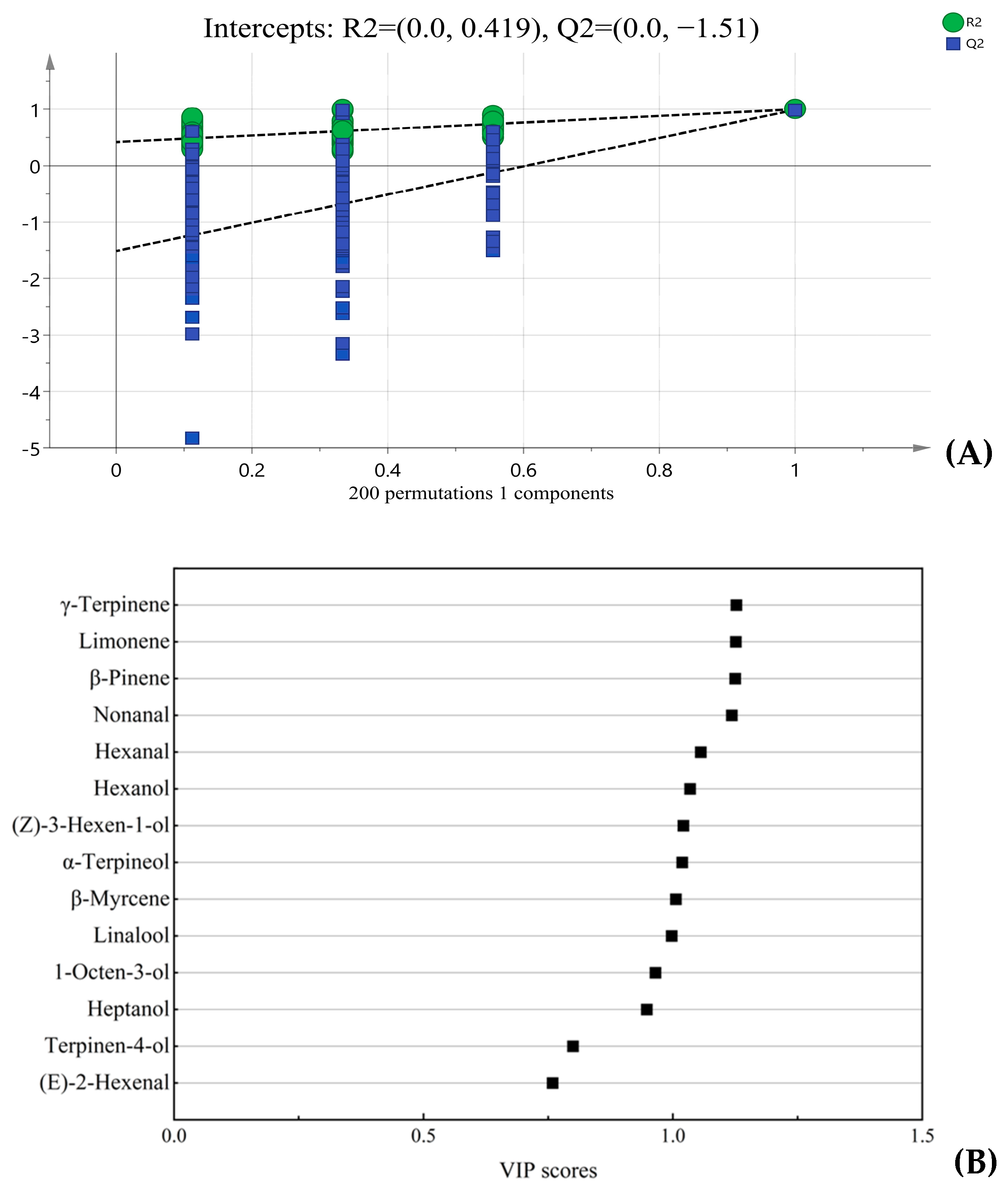
3.4. Key Differential Aroma Active Compounds Between FPJ0 and Frozen Samples
3.5. Odorant Addition Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCA | Hierarchical clustering analysis |
| GC-MS | Gas chromatography–mass spectrometry |
| HS-SPME | Headspace solid-phase microextraction |
| NIST | National Institute of Standards and Technology |
| OPLS-DA | Orthogonal partial least squares–discriminant analysis |
| PCA | Principal component analysis |
| OAV | Odor activity value |
| VIP | Variables important in the projection |
References
- Wan, R.; Song, J.; Lv, Z.; Qi, X.; Han, X.; Guo, Q.; Wang, S.; Shi, J.; Jian, Z.; Hu, Q.; et al. Genome-wide identification and comprehensive analysis of the AP2/ERF gene family in pomegranate fruit development and postharvest preservation. Genes 2022, 13, 895. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, H.; Wang, S.; Liu, X.; Hu, Q.; Jian, Z.-H.; Wan, R.; Song, J.; Shi, J. Comprehensive evaluation of 20 pomegranate (Punica granatum L.) cultivars in China. J. Integr. Agric. 2022, 21, 434–445. [Google Scholar]
- Al-Maiman, S.A.; Ahmad, D. Changes in physical and chemical properties during pomegranate (Punica granatum L.) fruit maturation. Food Chem. 2002, 76, 437–441. [Google Scholar] [CrossRef]
- Alsataf, S.; Başyiğit, B.; Karaaslan, M. Multivariate analyses of the antioxidant, antidiabetic, antimicrobial activity of pomegranate tissues with respect to pomegranate juice. Waste Biomass Valorization 2021, 12, 5909–5921. [Google Scholar] [CrossRef]
- Koppel, K.; Anderson, E.L.; Chambers IV, E. Influence of processing on pomegranate (Punica granatum L.) juice flavor and aroma. J. Sci. Food Agric. 2015, 95, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.; Auid-Orcid; Tian, Y.; Yang, X.; Sun, L.; Guo, Y. Freezing point temperature is in favor of not-from-concentrate apple juice storage. Food Sci. Nutr. 2019, 7, 2242–2251. [Google Scholar] [CrossRef]
- Teng, X.; Liu, Y.; Chen, L.; Xue, C.; Li, Z. Effects of liquid nitrogen freezing at different temperatures on the quality and flavor of Pacific oyster (Crassostrea gigas). Food Chem. 2023, 422, 136162. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Bi, Y.; Du, R.; Yuan, H.; Hou, Y.; Luo, R. The impact of freezing methods on the quality, moisture distribution, microstructure, and flavor profile of hand-grabbed mutton during long-term frozen storage. Food Res. Int. 2023, 173, 113346. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Y.; Ni, Y.; Zhu, L.; Fang, S. Sterilization of black carrot juice by thermosonication: Compound changes revealed by NMR and GC–MS metabolomics methods. J. Food Process Eng. 2023, 46, e14430. [Google Scholar] [CrossRef]
- Tan, F.; Wang, P.; Zhan, P.; Tian, H. Characterization of key aroma compounds in flat peach juice based on gas chromatography-mass spectrometry-olfactometry (GC-MS-O), odor activity value (OAV), aroma recombination, and omission experiments. Food Chem. 2022, 366, 130604. [Google Scholar] [CrossRef]
- Xiao, Z.; Wu, Q.; Niu, Y.; Wu, M.; Zhu, J.; Zhou, X.; Chen, X.; Wang, H.; Li, J.; Kong, J. Characterization of the key aroma compounds in five varieties of mandarins by gas chromatography–olfactometry, odor activity values, aroma recombination, and omission analysis. J. Agric. Food Chem. 2017, 65, 8392–8401. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, C.; Wang, Q.; Zhang, J.; Zhang, L. Identification and confirmation of key compounds causing cooked off-flavor in heat-treated tomato juice. J. Food Sci. 2022, 87, 2515–2526. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, C.; Song, H.L. Comparison of SPME Versus SAFE Processes for the Analysis of Flavor Compounds in Watermelon Juice. Food Anal. Methods 2018, 11, 1677–1689. [Google Scholar] [CrossRef]
- Sun, R.; Xing, R.; Zhang, J.; Wei, L.; Ge, Y.; Deng, T.; Zhang, W.; Chen, Y. Authentication and quality evaluation of not from concentrate and from concentrate orange juice by HS-SPME-GC-MS coupled with chemometrics. LWT 2022, 162, 113504. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Y.; Chen, J.; Xu, E.; Chen, S.; Ye, X.; Cheng, H.; Pan, H. Characteristic aroma improvement mechanisms of heat-sterilized bayberry juice regulated by exogenous polyphenols. Food Chem. 2023, 427, 136644. [Google Scholar] [CrossRef]
- Dong, T.; Tian, Z.; Wang, S.; Sun, J.; Chen, H.; Wang, S.; Sun, B. Identification of key off-flavor compounds during storage of fried pepper (Zanthoxylum bungeanum Maxim.) oils by sensory-directed flavor analysis and partial least squares regression (PLSR). J. Food Compos. Anal. 2024, 131, 14. [Google Scholar] [CrossRef]
- Tian, H.; Lu, C.; Zhan, P.; Wang, P.; Zhao, Y.; Tian, P. Effects of sterilization methods on pomegranate juice evaluated by descriptive sensory analysis and gas chromatography-mass spectrometry through partial least-squares regression. Flavour Fragr. J. 2020, 35, 674–685. [Google Scholar] [CrossRef]
- Xu, S.; Zhan, P.; Tian, H.; Wang, P. The presence of kiwifruit columella affects the aroma profiles of fresh and thermally treated kiwifruit juice. LWT 2022, 165, 113756. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Y.; Zhan, P.; Wang, P.; Tian, H. Characterization of the key aroma compounds in four varieties of pomegranate juice by gas chromatography-mass spectrometry, gas chromatography-olfactometry, odor activity value, aroma recombination, and omission tests. Food Sci. Hum. Wellness 2023, 12, 151–160. [Google Scholar] [CrossRef]
- Hao, Y.; Li, J.; Zhao, Z.; Xu, W.; Wang, L.; Lin, X.; Hu, X.; Li, C. Flavor characteristics of Shanlan rice wines fermented for different time based on HS-SPME-GC-MS-O, HS-GC-IMS, and electronic sensory analyses. Food Chem. 2024, 432, 137150. [Google Scholar] [CrossRef]
- Li, J.-X.; Schieberle, P.; Steinhaus, M. Insights into the Key Compounds of Durian (Durio zibethinus L. ‘Monthong’) Pulp Odor by Odorant Quantitation and Aroma Simulation Experiments. J. Agric. Food Chem. 2017, 65, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Gemert, L.J.V. Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners BV: Zeist, The Netherlands, 2003. [Google Scholar]
- Li, X.; Zhang, W.; Zeng, X.; Xi, Y.; Li, Y.; Hui, B.; Li, J. Characterization of the Major Odor-Active Off-Flavor Compounds in Normal and Lipoxygenase-Lacking Soy Protein Isolates by Sensory-Directed Flavor Analysis. J. Agric. Food Chem. 2023, 71, 8129–8139. [Google Scholar] [CrossRef]
- Pupan, N.; Dhamvithee, P.; Jangchud, A.; Boonbumrung, S. Influences of different freezing and thawing methods on the physico-chemical, flavor, and sensory properties of durian puree (cv. Monthong). J. Food Process. Preserv. 2018, 42, e13669. [Google Scholar] [CrossRef]
- Yi, Z.; Feng, T.; Zhuang, H.; Ye, R.; Li, M.; Liu, T. Comparison of different extraction methods in the analysis of volatile compounds in pomegranate juice. Food Anal. Methods 2016, 9, 2364–2373. [Google Scholar] [CrossRef]
- Vincenti, S.; Mariani, M.; Alberti, J.-C.; Jacopini, S.; Brunini-Bronzini de Caraffa, V.; Berti, L.; Maury, J. Biocatalytic synthesis of natural green leaf volatiles using the lipoxygenase metabolic pathway. Catalysts 2019, 9, 873. [Google Scholar] [CrossRef]
- Yu, H.; Xie, T.; He, L.; Xie, J.; Chen, C.; Tian, H. Characterization of aroma compounds in bayberry juice by sensory evaluation and gas chromatography–mass spectrometry. J. Food Meas. Charact. 2020, 14, 505–513. [Google Scholar] [CrossRef]
- Alves Filho, E.G.; Rodrigues, T.H.S.; Fernandes, F.A.N.; De Brito, E.S.; Cullen, P.J.; Frias, J.M.; Bourke, P.; Cavalcante, R.S.; Almeida, F.D.L.; Rodrigues, S. An untargeted chemometric evaluation of plasma and ozone processing effect on volatile compounds in orange juice. Innov. Food Sci. Emerg. Technol. 2017, 53, 63–69. [Google Scholar] [CrossRef]
- Pérez-López, A.J.; Saura, D.; Lorente, J.; Carbonell-Barrachina, Á.A. Limonene, linalool, α-terpineol, and terpinen-4-ol as quality control parameters in mandarin juice processing. Eur. Food Res. Technol. 2006, 222, 281–285. [Google Scholar] [CrossRef]
- Li, X.; Ren, J.-N.; Fan, G.; Pan, S.-Y. Changes of aroma compounds and qualities of freshly-squeezed orange juice during storage. J. Food Sci. Technol. 2018, 55, 4530–4543. [Google Scholar] [CrossRef]
- Fang, H.; Chen, J.; Tian, Y.; Liu, Y.; Li, H.; Cheng, H. Chemometrics characterization of volatile changes in processed bayberry juice versus intact fruit during storage by headspace solid-phase micro-extraction combined with GC–MS. J. Food Process. Preserv. 2020, 44, e14444. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Guan, X.; Qin, W.; Zhang, X.; Ding, Y.; Yang, W.; Zhou, J.; Yu, X. Characterization of key aroma compounds in melon spirits using the sensomics concept. LWT 2022, 161, 113341. [Google Scholar] [CrossRef]
- Yen, O.C.; Lin, S.T.; Yang, P. Changes in Volatile Flavor Components of Guava Puree during Processing and Frozen Storage. J. Food Sci. 1992, 57, 679–681. [Google Scholar] [CrossRef]
- Beghè, D.; Cirlini, M.; Beneventi, E.; Miroslav, Č.; Tatjana, P.; Ganino, T.; Petruccelli, R.; Dall’Asta, C. Volatile profile of Italian and Montenegrine pomegranate juices for geographical origin classification. Eur. Food Res. Technol. 2021, 247, 211–220. [Google Scholar] [CrossRef]
- Andreu-Sevilla, A.J.; Mena, P.; Martí, N.; García Viguera, C.; Carbonell-Barrachina, Á.A. Volatile composition and descriptive sensory analysis of pomegranate juice and wine. Food Res. Int. 2013, 54, 246–254. [Google Scholar] [CrossRef]
- Mayuoni-kirshinbaum, L.; Tietel, Z.; Porat, R.; Ulrich, D. Identification of aroma-active compounds in ‘wonderful’pomegranate fruit using solvent-assisted flavour evaporation and headspace solid-phase micro-extraction methods. Eur. Food Res. Technol. 2012, 235, 277–283. [Google Scholar] [CrossRef]
- Tripathi, J.; Chatterjee, S.; Gamre, S.; Chattopadhyay, S.; Variyar, P.S.; Sharma, A. Analysis of free and bound aroma compounds of pomegranate (Punica granatum L.). LWT-Food Sci. Technol. 2014, 59, 461–466. [Google Scholar] [CrossRef]
- Ma, D.; Zhao, H.; Liu, Z.; Liu, M.; Qi, P.; Di, S.; Zhang, S.; Wang, X. Recent advances on mulberry volatile flavor: A review. J. Food Compos. Anal. 2023, 124, 105665. [Google Scholar] [CrossRef]
- Kaewtathip, T.; Charoenrein, S. Changes in volatile aroma compounds of pineapple (Ananas comosus) during freezing and thawing. Int. J. Food Sci. Technol. 2012, 47, 985–990. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Ren, J.-N.; Dong, M.; Tai, Y.-N.; Yang, S.-Z.; Shao, J.-H.; Pan, S.-Y.; Fan, G. Changes in the Physicochemical Characteristics, Free and Bound Aroma Compounds in the Raspberry Juice during Storage. J. Food Process. Preserv. 2015, 39, 2834–2843. [Google Scholar] [CrossRef]
- Ouyang, J.; Jiang, R.; Chen, H.; Liu, Q.; Yi, X.; Wen, S.; Huang, F.; Zhang, X.; Li, J.; Wen, H.; et al. Characterization of key odorants in ‘Baimaocha’ black teas from different regions. Food Chem. X 2024, 22, 101303. [Google Scholar] [CrossRef]
- Song, H.; Liu, J. GC-O-MS technique and its applications in food flavor analysis. Food Res. Int. 2018, 114, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Sohail, A.; Al-Dalali, S.; Wang, J.; Xie, J.; Shakoor, A.; Asimi, S.; Shah, H.; Patil, P. Aroma compounds identified in cooked meat: A review. Food Res. Int. 2022, 157, 111385. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.A.A.; Kılıç-Büyükkurt, Ö.; Aboul Fotouh, M.M.; Selli, S. Aroma active compounds of honey: Analysis with GC-MS, GC-O, and molecular sensory techniques. J. Food Compos. Anal. 2024, 134, 106545. [Google Scholar] [CrossRef]



| Aroma Active Compounds | Linear Equations | R2 | OTs (µg/kg) a | Concentration (μg/kg) | OAVs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FPJ0 | FPJ_−20 °C | FPJ_−40 °C | FPJ_−80 °C | FPJ0 | FPJ_−20 °C | FPJ_−40 °C | FPJ_−80 °C | ||||
| Hexanal | y = 0.0667x − 0.1147 | 0.9963 | 6.9 | 19.215 | 48.196 | 42.792 | 34.348 | 2.785 | 6.985 | 6.202 | 4.978 |
| (E)-2-Hexenal | y = 0.0869x − 0.0279 | 0.9999 | 17 | 1.121 | 0.000 | 0.815 | 0.000 | 0.066 | 0.000 | 0.048 | 0.000 |
| Nonanal | y = 0.0144x + 0.0123 | 0.9998 | 1 | 0.000 | 5.041 | 5.559 | 4.493 | 0.000 | 5.041 | 5.559 | 4.493 |
| Linalool | y = 0.0899x − 0.1 | 0.9984 | 0.22 | 6.029 | 1.917 | 4.308 | 3.542 | 27.403 | 8.714 | 19.583 | 16.101 |
| Hexanol | y = 0.0524x − 0.0539 | 0.9967 | 5.6 | 1294.896 | 888.859 | 1089.149 | 1044.915 | 231.231 | 158.725 | 194.491 | 186.592 |
| (Z)-3-Hexen-1-ol | y = 0.1119x − 0.1106 | 0.9988 | 3.9 | 183.150 | 101.996 | 143.198 | 137.825 | 46.962 | 26.153 | 36.717 | 35.340 |
| 1-Octen-3-ol | y = 0.0993x − 0.1079 | 0.997 | 0.005 | 1.878 | 2.013 | 2.250 | 2.068 | 375.550 | 402.629 | 450.044 | 413.520 |
| Heptanol | y = 0.0816x − 0.0391 | 0.9996 | 5.4 | 2.211 | 0.000 | 0.000 | 1.100 | 0.409 | 0.000 | 0.000 | 0.204 |
| Terpinen-4-ol | y = 0.1047x − 0.0764 | 0.9985 | 340 | 1.677 | 2.566 | 2.558 | 1.540 | 0.005 | 0.008 | 0.008 | 0.005 |
| α-Terpineol | y = 0.1355x − 0.1672 | 0.9967 | 280 | 11.714 | 16.262 | 15.098 | 13.792 | 0.23 | 0.32 | 0.34 | 0.25 |
| β-Pinene | y = 0.0965x − 0.0598 | 0.9993 | 140 | 10.684 | 0.000 | 0.000 | 1.285 | 0.076 | 0.000 | 0.000 | 0.009 |
| β-Myrcene | y = 0.0761x − 0.1092 | 0.9972 | 1.2 | 4.962 | 0.000 | 0.000 | 2.494 | 4.135 | 0.000 | 0.000 | 2.078 |
| Limonene | y = 0.0103x − 0.0598 | 0.9996 | 10 | 1.844 | 1.109 | 1.181 | 1.192 | 0.184 | 0.111 | 0.118 | 0.119 |
| γ-Terpinene | y = 0.1022x − 0.0758 | 0.9999 | 65 | 2.256 | 0.000 | 0.000 | 0.000 | 0.035 | 0.000 | 0.000 | 0.000 |
| No. | Compounds | Identification | RIa | RIb | FPJ0 | Frozen-Treated Samples | ||
|---|---|---|---|---|---|---|---|---|
| FPJ_−20 °C | FPJ_−40 °C | FPJ_−80 °C | ||||||
| A1 | β-Pinene | RI MS St | 1096 | 1110 | 0.93 ± 0.01 a | ND | ND | 0.03 ± 0.00 b |
| A2 | β-Myrcene | RI MS St | 1148 | 1146 | 0.20 ± 0.03 a | ND | ND | 0.01 ± 0.00 b |
| A3 | Limonene | RI MS St | 1183 | 1160 | 1.48 ± 0.03 a | 0.28 ± 0.01 c | 0.40 ± 0.02 b | 0.42 ± 0.02 b |
| A4 | γ-Terpinene | RI MS St | 1216 | 1205 | 0.11 ± 0.02 | ND | ND | ND |
| A5 | p-Cymene | RI MS St | 1244 | 1259 | 0.43 ± 0.03 a | ND | 0.14 ± 0.00 b | 0.14 ± 0.01 b |
| A6 | trans-α-Bergamotene | RI MS | 1575 | 1573 | 0.06 ± 0.00 | ND | ND | ND |
| A7 | β-copaene | MS | 1588 | 1603 | 0.08 ± 0.01 | ND | ND | ND |
| B1 | Hexanal | RI MS St | 1080 | 1102 | 1.07 ± 0.06 d | 3.03 ± 0.10 a | 2.67 ± 0.12 b | 2.10 ± 0.03 c |
| B2 | (E)-2-Hexenal | RI MS St | 1200 | 1201 | 0.06 ± 0.00 a | ND | 0.03 ± 0.00 b | ND |
| B3 | Octanal | RI MS St | 1286 | 1279 | 0.07 ± 0.01 d | 0.16 ± 0.01 c | 0.29 ± 0.01 a | 0.2 ± 0.03 b |
| B4 | Nonanal | RI MS St | 1390 | 1384 | 0.00 ± 0.00 d | 0.04 ± 0.00 b | 0.06 ± 0.01 a | 0.02 ± 0.00 c |
| B5 | Decanal | RI MS St | 1472 | 1433 | 0.5 ± 0.02 a | 0.25 ± 0.01 b | 0.25 ± 0.03 b | 0.18 ± 0.02 c |
| B6 | Benzaldehyde | RI MS St | 1502 | 1508 | 0.01 ± 0.00 | ND | ND | ND |
| B7 | Undecanal | RI MS St | 1622 | 1613 | 0.19 ± 0 a | ND | 0.10 ± 0.07 b | 0.11 ± 0.00 ab |
| C1 | Linalool | RI MS St | 1544 | 1550 | 0.38 ± 0.02 a | 0.04 ± 0.00 c | 0.22 ± 0.02 b | 0.16 ± 0.00 b |
| C2 | (Z)-2-Penten-1-ol | RI MS St | 1304 | 1315 | 0.05 ± 0.00 | ND | ND | ND |
| C3 | Hexanol | RI MS St | 1345 | 1351 | 67.76 ± 0.55 a | 46.49 ± 0.45 d | 56.98 ± 1.29 b | 54.67 ± 0.12 c |
| C4 | (Z)-3-Hexen-1-ol | RI MS St | 1378 | 1378 | 20.45 ± 0.11 a | 11.28 ± 0.34 c | 15.84 ± 0.42 b | 15.24 ± 0.13 b |
| C5 | 2,4-Hexadien-1-ol | RI MS | 1523 | 1500 | ND | ND | ND | 0.01 ± 0.00 |
| C6 | 1-Octen-3-ol | RI MS St | 1430 | 1447 | 0.06 ± 0.00 a | 0.01 ± 0.00 d | 0.05 ± 0.00 b | 0.03 ± 0.00 c |
| C7 | Heptanol | RI MS St | 1447 | 1451 | 0.11 ± 0.01 a | ND | ND | 0.07 ± 0.00 b |
| C8 | (Z)-2-Hepten-1-ol | MS | - | 1475 | ND | ND | 0.06 ± 0.01 | ND |
| C9 | (E)-2-Hepten-1-ol | RI MS | 1517 | 1517 | 0.02 ± 0.00 | ND | ND | ND |
| C10 | Octanol | RI MS St | 1550 | 1558 | 0.15 ± 0.01 a | 0.18 ± 0.02 a | 0.16 ± 0.00 a | 0.10 ± 0.00 b |
| C11 | Verbenol | RI MS | 1675 | 1680 | 0.09 ± 0.01 a | ND | ND | 0.05 ± 0.00 b |
| C12 | Terpinen-4-ol | RI MS St | 1586 | 1591 | 0.30 ± 0.00 b | 0.39 ± 0.00 a | 0.39 ± 0.01 a | 0.29 ± 0.01 b |
| C13 | (E)-2-Octen-1-ol | MS | - | 1606 | 0.18 ± 0.01 a | 0.06 ± 0.00 b | 0.18 ± 0.00 a | 0.17 ± 0.02 a |
| C14 | Nonanol | RI MS St | 1640 | 1649 | 0.27 ± 0.01 | 0.55 ± 0.01 | ND | ND |
| C15 | α-Terpineol | RI MS St | 1669 | 1684 | 1.31 ± 0.02 d | 1.93 ± 0.03 a | 1.77 ± 0.09 b | 1.60 ± 0.03 c |
| D1 | 6-Methyl-5-Hepten-2-one | RI MS St | 1323 | 1327 | 0.04 ± 0.02 d | 0.06 ± 0.00 c | 0.18 ± 0.01 a | 0.13 ± 0.00 b |
| D2 | 2-Nonanone | RI MS St | 1387 | 1367 | ND | 0.90 ± 0.07 b | 0.94 ± 0.04 b | 1.15 ± 0.03 a |
| E1 | Hexyl acetate | RI MS St | 1267 | 1308 | ND | 0.16 ± 0.00 a | 0.16 ± 0.03 a | 0.13 ± 0.01 a |
| E2 | (Z)-2-Hexen-1-ol acetate | RI MS | - | 1302 | ND | ND | ND | 0.02 ± 0.00 |
| F1 | 3-Octenoic acid | MS | - | 1401 | 0.04 ± 0.00 a | 0.03 ± 0.00 c | 0.04 ± 0.00 c | 0.04 ± 0.00 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, P.; He, W.; Tian, H.; Geng, J.; Zhang, R.; Zhan, P. Evaluation of Freezing-Induced Changes in Aroma Profiles of Pomegranate Juice by Quantitative Descriptive Sensory Analysis, Gas Chromatography–Mass Spectrometry/Olfactometry, Odor Activity Values, Orthogonal Partial Least Squares–Discriminant Analysis, and Odorant Addition Experiment. Foods 2025, 14, 1811. https://doi.org/10.3390/foods14101811
Chen Y, Wang P, He W, Tian H, Geng J, Zhang R, Zhan P. Evaluation of Freezing-Induced Changes in Aroma Profiles of Pomegranate Juice by Quantitative Descriptive Sensory Analysis, Gas Chromatography–Mass Spectrometry/Olfactometry, Odor Activity Values, Orthogonal Partial Least Squares–Discriminant Analysis, and Odorant Addition Experiment. Foods. 2025; 14(10):1811. https://doi.org/10.3390/foods14101811
Chicago/Turabian StyleChen, Yixiu, Peng Wang, Wanying He, Honglei Tian, Jingzhang Geng, Runguang Zhang, and Ping Zhan. 2025. "Evaluation of Freezing-Induced Changes in Aroma Profiles of Pomegranate Juice by Quantitative Descriptive Sensory Analysis, Gas Chromatography–Mass Spectrometry/Olfactometry, Odor Activity Values, Orthogonal Partial Least Squares–Discriminant Analysis, and Odorant Addition Experiment" Foods 14, no. 10: 1811. https://doi.org/10.3390/foods14101811
APA StyleChen, Y., Wang, P., He, W., Tian, H., Geng, J., Zhang, R., & Zhan, P. (2025). Evaluation of Freezing-Induced Changes in Aroma Profiles of Pomegranate Juice by Quantitative Descriptive Sensory Analysis, Gas Chromatography–Mass Spectrometry/Olfactometry, Odor Activity Values, Orthogonal Partial Least Squares–Discriminant Analysis, and Odorant Addition Experiment. Foods, 14(10), 1811. https://doi.org/10.3390/foods14101811







