Analysis of Antioxidant Activity and Volatile Components in Rapeseed Flower-Enriched Persimmon Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Experimental Methods
2.2.1. Brewing of Rapeseed Flower–Persimmon Fermented Wine
Brewing Process
Key Operational Points
2.2.2. Qualitative Analysis of Monosaccharide Composition of Rapeseed Flower Polysaccharide
2.2.3. Molecular Docking
2.2.4. Molecular Dynamics Simulation
Simulated Conditions
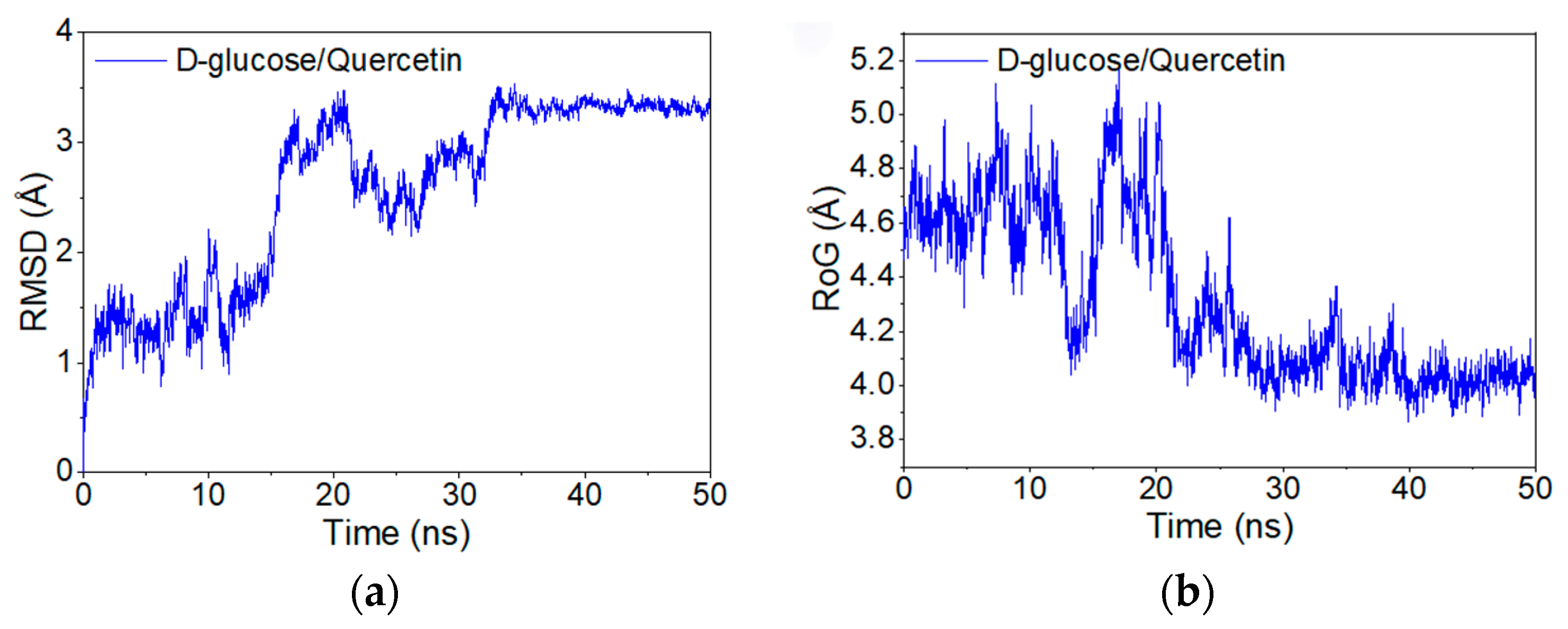
RMSF Analysis
2.2.5. Determination of Total Antioxidant Capacity (TAC) in Samples Using the FRAP Method
2.2.6. Determination of Hydroxyl Radical Scavenging Activity in Samples
2.2.7. Determination of DPPH Radical Scavenging Activity in Samples
2.2.8. Determination of Vitamin C Content in Samples
2.2.9. Determination of Flavoring Substances in Persimmon Wine
- Microextraction conditions:
- 2.
- Gas chromatography conditions:
- 3.
- Mass spectrometry conditions:
- 4.
- Qualitative and quantitative analysis of aroma components
- 5.
- Evaluation and Analysis of Fragrance Composition
3. Results
3.1. Qualitative Analysis of Monosaccharide Composition in Rapeseed Flower Polysaccharides
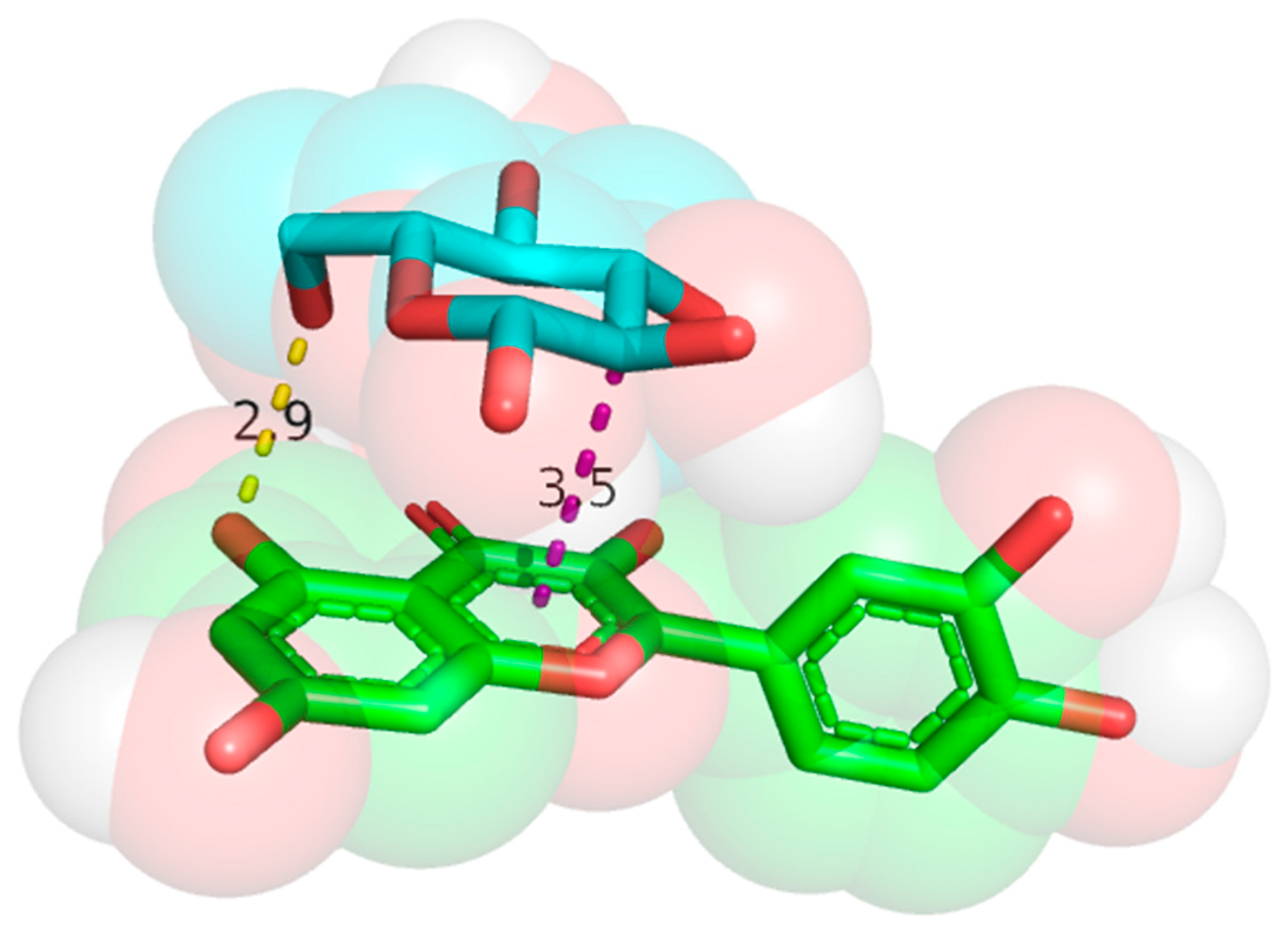
3.2. Molecular Docking Results
3.3. Molecular Simulation Dynamics Analysis
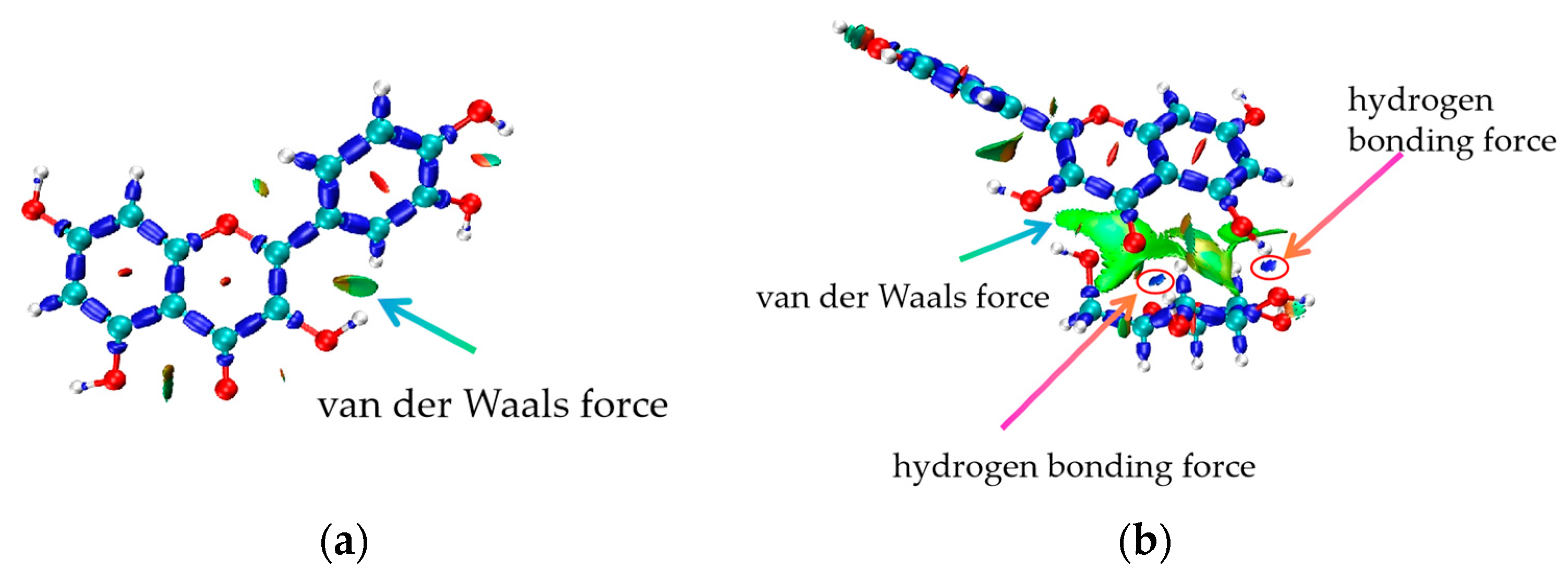
3.4. Analysis of IRI Hydrogen Bonding Force
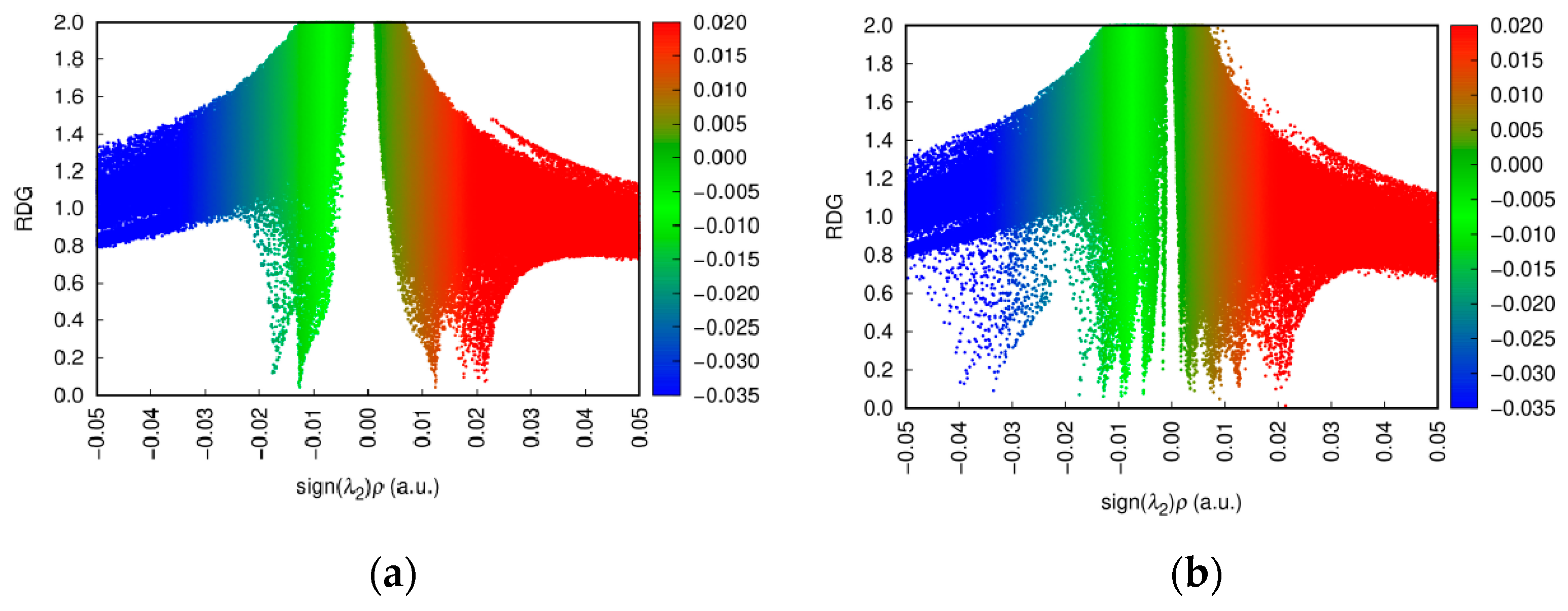
3.5. Analysis of RDG Hydrogen Bonding Force
- The distribution in the region of negative sign(λ2)ρ becomes denser, with a slightly extended range.
- More blue regions emerge in the low-RDG areas, representing specific strong interaction zones within the attractive region. This indicates a significant increase in strong non-covalent attractive interactions, predominantly hydrogen bonding.
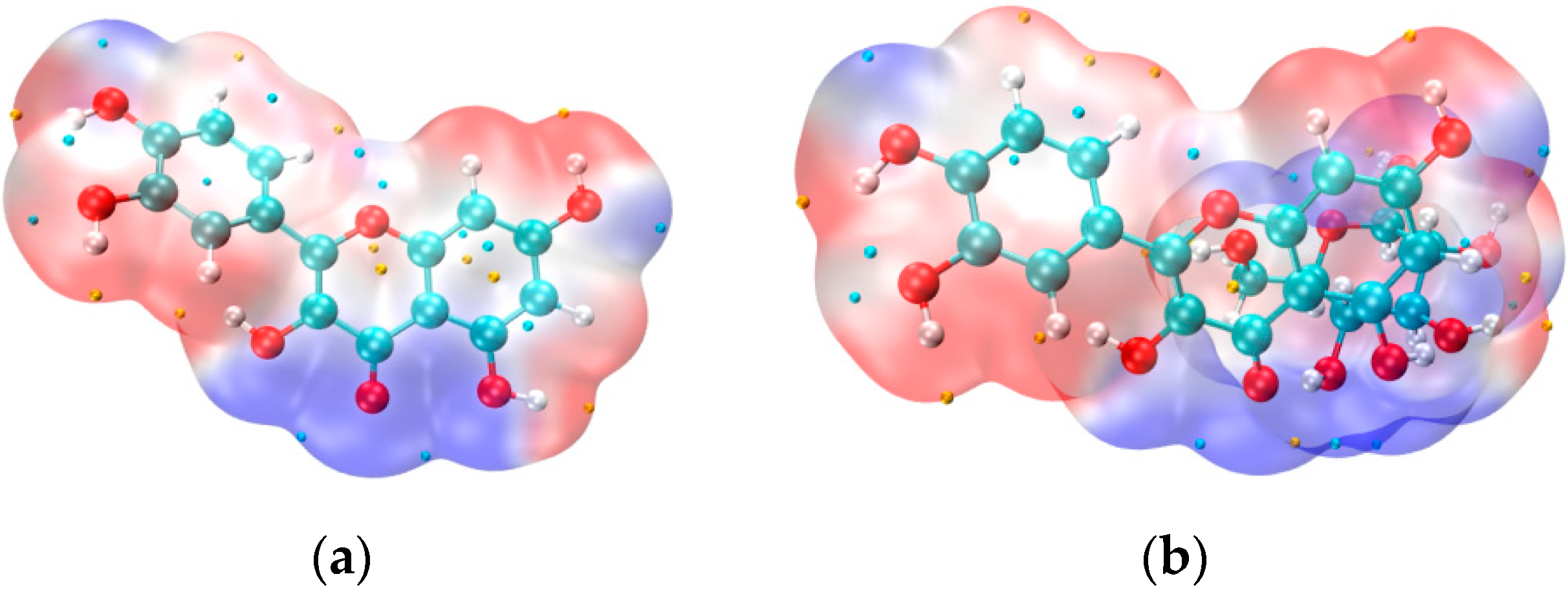
3.6. Electron Density Distribution (VMD Imaging)
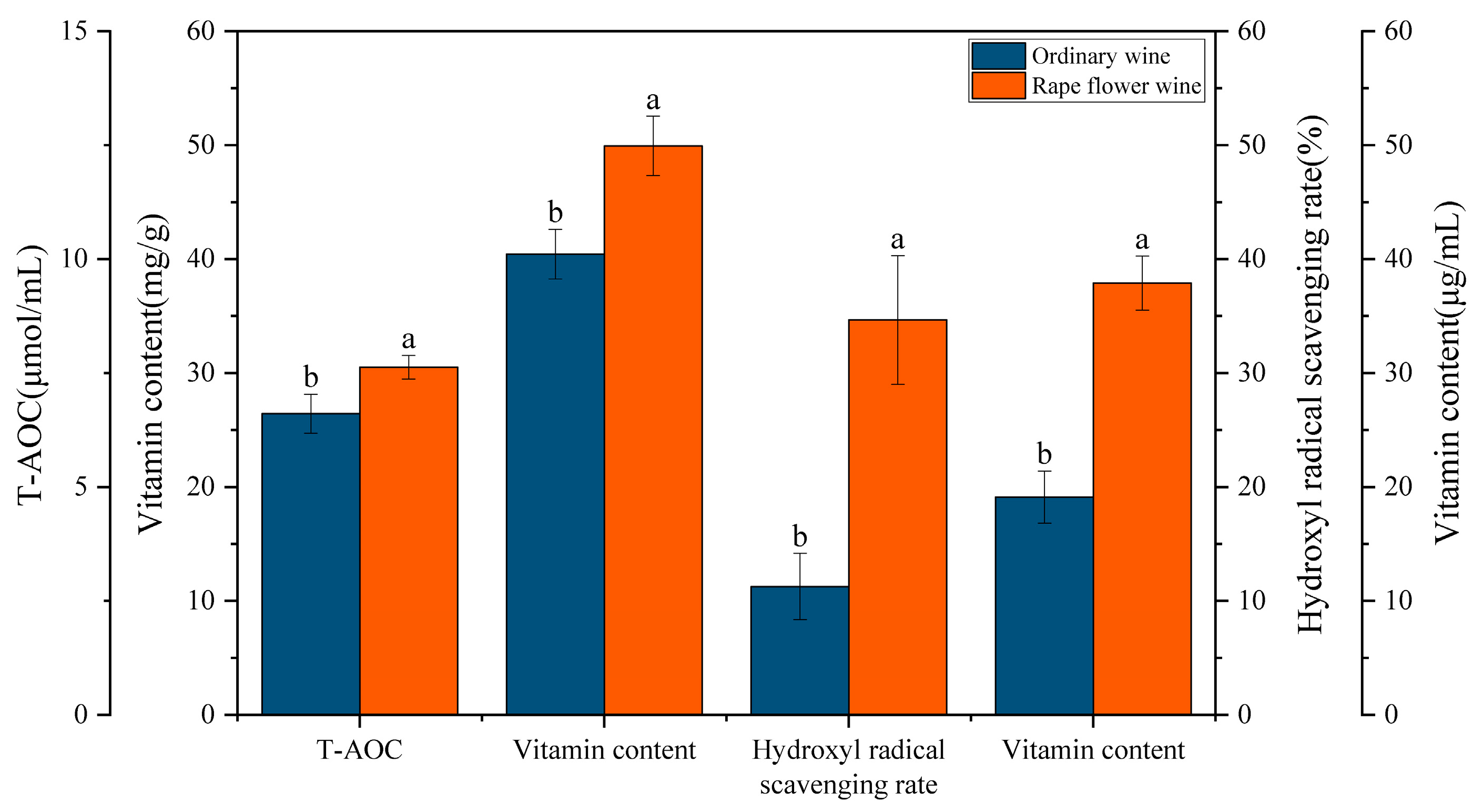
3.7. In Vitro Antioxidant Assay
3.8. Composition of Flavoring Substances in Persimmon Wine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verocai, M.; González-Barrios, P.; Mazzilli, S.R. A Comparative Study of Yield Components and Their Trade-off in Oilseed Crops (Brassica napus L. and Brassica carinata A. Braun). Eur. J. Agron. 2024, 161, 127377. [Google Scholar] [CrossRef]
- Abraham, A.; Kattoor, A.J.; Saldeen, T.; Mehta, J.L. Vitamin E and Its Anticancer Effects. Crit. Rev. Food Sci. Nutr. 2019, 59, 2831–2838. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Jiang, L.; Mason, A.S.; Xiao, M.; Zhu, L.; Li, L.; Zhou, Q.; Shen, C.; Huang, C. Research Progress and Strategies for Multifunctional Rapeseed: A Case Study of China. J. Integr. Agric. 2016, 15, 1673–1684. [Google Scholar] [CrossRef]
- Han, Y.Y.; Zhang, Q.H.; Chen, W.S.; Li, Z.L.; Xie, D.; Zhang, S.L.; Lu, H.; Wang, L.W.; Xu, Z.H.; Zhang, L.Z. Fermented Rape Pollen Powder Can Alleviate Benign Prostatic Hyperplasia in Rats by Reducing Hormone Content and Changing Gut Microbiota. Benef. Microbes 2023, 14, 503–524. [Google Scholar] [CrossRef]
- Tileuberdi, N.; Turgumbayeva, A.; Yeskaliyeva, B.; Sarsenova, L.; Issayeva, R. Extraction, Isolation of Bioactive Compounds and Therapeutic Potential of Rapeseed (Brassica napus L.). Molecules 2022, 27, 8824. [Google Scholar] [CrossRef]
- Butt, M.S.; Sultan, M.T.; Aziz, M.; Naz, A.; Ahmed, W.; Kumar, N.; Imran, M. Persimmon (Diospyros Kaki) Fruit: Hidden Phytochemicals and Health Claims. EXCLI J. 2015, 14, 542–561. [Google Scholar] [CrossRef]
- Hosseininejad, S.; Moraga, G.; Hernando, I. Valorizing Astringent ‘Rojo Brillante’ Persimmon Through the Development of Persimmon-Based Bars. Foods 2024, 13, 3748. [Google Scholar] [CrossRef]
- Hyun, J.-E.; Kim, J.-Y.; Kim, E.-M.; Kim, J.-C.; Lee, S.-Y. Changes in Microbiological and Physicochemical Quality of Dried Persimmons (Diospyros kaki Thunb.) Stored at Various Temperatures. J. Food Qual. 2019, 2019, 6256409. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Lv, J.; Ma, Y.; Guan, X. Changes in the Physicochemical Components, Polyphenol Profile, and Flavor of Persimmon Wine during Spontaneous and Inoculated Fermentation. Food Sci. Nutr. 2020, 8, 2728–2738. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Podio, N.S.; Wang, X.-Y.; Jiang, S.; Xu, S.; Qiu, X.; Zeng, Z.; Gong, W.; Wang, S.; et al. Untargeted Metabolomics Profiling of Purple Rice Phenolics and Their Antioxidant Activities. LWT 2024, 214, 117127. [Google Scholar] [CrossRef]
- Negrean, O.-R.; Farcas, A.C.; Pop, O.L.; Socaci, S.A. Blackthorn—A Valuable Source of Phenolic Antioxidants with Potential Health Benefits. Molecules 2023, 28, 3456. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Moccia, F.; Tedesco, I.; Adabbo, E.; Panzella, L.; Russo, G.L.; Napolitano, A. Stillage Waste from Strawberry Spirit Production as a Source of Bioactive Compounds with Antioxidant and Antiproliferative Potential. Antioxidants 2023, 12, 421. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qin, C.; He, X.; Chen, B.; Tang, J.; Liu, G.; Li, L.; Yang, Y.; Ye, D.; Li, J.; et al. Development of Green Banana Fruit Wines: Chemical Compositions and In Vitro Antioxidative Activities. Antioxidants 2022, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Liu, Y.; Wu, Y.; Ren, F.; Liu, H. Exploring the Application of Molecular Simulation Technology in Food Sector: Focusing on Food Component Interactions and Food Quality. Food Biosci. 2024, 62, 105480. [Google Scholar] [CrossRef]
- Chen, G.; Huang, K.; Miao, M.; Feng, B.; Campanella, O.H. Molecular Dynamics Simulation for Mechanism Elucidation of Food Processing and Safety: State of the Art. Comp. Rev. Food Sci. Food Safe 2019, 18, 243–263. [Google Scholar] [CrossRef]
- Cala, O.; Pinaud, N.; Simon, C.; Fouquet, E.; Laguerre, M.; Dufourc, E.J.; Pianet, I. NMR and Molecular Modeling of Wine Tannins Binding to Saliva Proteins: Revisiting Astringency from Molecular and Colloidal Prospects. FASEB J. 2010, 24, 4281–4290. [Google Scholar] [CrossRef]
- Ramos-Pineda, A.M.; García-Estévez, I.; Brás, N.F.; Martín Del Valle, E.M.; Dueñas, M.; Escribano Bailón, M.T. Molecular Approach to the Synergistic Effect on Astringency Elicited by Mixtures of Flavanols. J. Agric. Food Chem. 2017, 65, 6425–6433. [Google Scholar] [CrossRef]
- Ahmad, I.; Huang, P.-J.; Malak, N.; Khan, A.; Asad, F.; Chen, C.-C. Antioxidant Potential of Alkaloids and Polyphenols of Viola Canescens Wall Using in Vitro and in Silico Approaches. Front. Chem. 2024, 12, 1379463. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, X.; Lan, Q.; Wan, F.; Yang, Z.; Nie, X.; Cai, Z.; Hu, B.; Tang, J.; Zhu, C.; et al. Comparison of Aroma and Taste Profiles of Kiwi Wine Fermented with/without Peel by Combining Intelligent Sensory, Gas Chromatography-Mass Spectrometry, and Proton Nuclear Magnetic Resonance. Foods 2024, 13, 1729. [Google Scholar] [CrossRef]
- Ojo, O.O.; Ogunleke, T.; Ajeoge, J.; Olorunsogo, O.O. Experimental and Molecular Docking Studies of Quercetin and Vitamin E with Diabetes-Associated Mitochondrial-ATPase as Anti-Apoptotic Therapeutic Strategies. J. Diabetes Metab. Disord. 2022, 21, 1717–1729. [Google Scholar] [CrossRef]
- Ali, M.; Hassan, M.; Ansari, S.A.; Alkahtani, H.M.; Al-Rasheed, L.S.; Ansari, S.A. Quercetin and Kaempferol as Multi-Targeting Antidiabetic Agents against Mouse Model of Chemically Induced Type 2 Diabetes. Pharmaceuticals 2024, 17, 757. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, T.; Xie, J.; Cheng, M.; Sun, L.; Zhang, S.; Xin, J.; Zhang, N. A Review on Application of Molecular Simulation Technology in Food Molecules Interaction. Curr. Res. Food Sci. 2022, 5, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Zu, G.; Sun, K.; Li, L.; Zu, X.; Han, T.; Huang, H. Mechanism of Quercetin Therapeutic Targets for Alzheimer Disease and Type 2 Diabetes Mellitus. Sci. Rep. 2021, 11, 22959. [Google Scholar] [CrossRef] [PubMed]
- Madhubalaji, C.K.; Mudaliar, S.N.; Chauhan, V.S.; Sarada, R. Evaluation of Drying Methods on Nutritional Constituents and Antioxidant Activities of Chlorella Vulgaris Cultivated in an Outdoor Open Raceway Pond. J. Appl. Phycol. 2021, 33, 1419–1434. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Liu, Y.; Zhong, J.; Wang, J.; Sun, L.; Yu, L.; Wang, Y.; Li, Q.; Jin, W.; et al. Remodeling Liver Microenvironment by L-Arginine Loaded Hollow Polydopamine Nanoparticles for Liver Cirrhosis Treatment. Biomaterials 2023, 295, 122028. [Google Scholar] [CrossRef]
- Cui, D.; Yin, Y.; Sun, H.; Wang, X.; Zhuang, J.; Wang, L.; Ma, R.; Jiao, Z. Regulation of Cellular Redox Homeostasis in Arabidopsis Thaliana Seedling by Atmospheric Pressure Cold Plasma-Generated Reactive Oxygen/Nitrogen Species. Ecotoxicol. Environ. Saf. 2022, 240, 113703. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Yang, Y. Impact of Pediococcus Pentosaceus YF01 on the Exercise Capacity of Mice through the Regulation of Oxidative Stress and Alteration of Gut Microbiota. Front. Microbiol. 2024, 15, 1421209. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, S.; Zheng, B.; Wu, P.; He, X.; Zhao, Y.; Zhong, Z.; Zhang, X.; Guan, J.; Wang, H.; et al. Lubricating and Dual-Responsive Injectable Hydrogels Formulated From ZIF-8 Facilitate Osteoarthritis Treatment by Remodeling the Microenvironment. Small 2025, 21, 2407885. [Google Scholar] [CrossRef]
- Liu, C.; Jia, Y.; He, L.; Li, H.; Song, J.; Ji, L.; Wang, C. Integrated Transcriptome and DNA Methylome Analysis Reveal the Biological Base of Increased Resistance to Gray Leaf Spot and Growth Inhibition in Interspecific Grafted Tomato Scions. BMC Plant Biol. 2024, 24, 130. [Google Scholar] [CrossRef]
- Sun, H.; Wang, S.; Wang, H.; Zeng, Q.; Wang, F.; Zheng, Y.; Haick, H.; Shi, G.; Zhang, M. Stick-and-Sensing Microneedle Patch for Personalized Nutrition Management. Sens. Actuators B Chem. 2024, 418, 136207. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Liang, G.; Xiang, S.; Han, G. Volatile Composition Changes in Lemon during Fruit Maturation by HS-SPME-GC-MS. J. Sci. Food Agric. 2022, 102, 3599–3606. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Yang, H.; Sun, L.; Xia, H.; Sun, W.; Wang, Z.; Zhang, J. Bacterial Communities Related to Aroma Formation during Spontaneous Fermentation of ‘Cabernet Sauvignon’ Wine in Ningxia, China. Foods 2022, 11, 2775. [Google Scholar] [CrossRef]



| Number | Peak Time/min | Molecular Weight (AMU) | Monosaccharide IUPAC Name | Monosaccharide Abbreviation | Relative Content% |
|---|---|---|---|---|---|
| 1 | 17.888 | 438.23 | (3R,4S,5S,6R)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol | D-glucopyranose | 5.26% |
| 2 | 18.14 | 554.24 | (3R,4S,5S,6R)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol | D-glucopyranose | 1.53% |
| 3 | 18.36 | 378.171 | (3R,4S,5R)-oxane-2,3,4,5-tetrol | D-xylopyranose | 34.00% |
| 4 | 19.04 | 438.211 | (3R,4S,5S)-oxane-2,3,4,5-tetrol | L-arabinopyranose | 1.02% |
| 5 | 19.188 | 452.22 | (3R,4S,5R,6R)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol | D-galactopyranose | 5.40% |
| 6 | 19.57 | 496.253 | (3R,4S,5R,6R)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol | D-galactopyranose | 5.45% |
| 7 | 20.275 | 466.206 | (3R,4S,5S,6R)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol | D-glucopyranose | 40.40% |
| 8 | 21.207 | 554.24 | (2S,3S,4S,5R)-3,4,5,6-tetrahydroxyoxane-2-carboxylic acid | D-glucopyranuronic acid | 2.05% |
| 9 | 21.504 | 540.26 | (3R,4S,5S,6R)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol | D-glucopyranose | 4.88% |
| Serial Number | Name of Substance | Amount Present (μg/L) | ROAV | Flavor Threshold (μg/L) | Fragrance Description | ||
|---|---|---|---|---|---|---|---|
| Pure Persimmon Wine | Rapeseed Flower– Persimmon Wine | Pure Persimmon Wine | Rapeseed Flower– Persimmon Wine | ||||
| 1 | butane-2,3-diol | 32.34 | 206.78 | 0.0000072 | 0.000046 | 4,500,000 | Molasses |
| 2 | hexan-1-ol | — | 107.8 | — | 0.0135 | 8000 | Fruity |
| 3 | phenethyl alcohol | 3089.94 | 1546.44 | 0.221 | 0.11 | 14,000 | Saussurea costus |
| 4 | heptan-1-ol | — | 24.598 | — | — | — | Grassy flavor |
| 5 | tetradecan-1-ol | — | 33.32 | — | — | — | Molasses |
| 6 | 3-Methylacetic acid-butan-1-ol | — | 808.5 | — | 0.027 | 30,000 | Fruity |
| 7 | Ethyl acetate | — | 45.08 | — | 0.003 | 17,000 | Fruity |
| 8 | Ethyl hexanoate | 178.365 | 1117.3 | 2.347 | 14.701 | 76 | Fruity |
| 9 | Ethyl benzoate | — | 12.152 | — | 0.008 | 1434 | Cherry scent |
| 10 | Diethyl succinate | — | 80.36 | — | 0.0008 | 100,000 | Fruity |
| 11 | Ethyl octanoate | 746.79 | 1661.1 | 3.112 | 6.921 | 240 | Brandy flavor |
| 12 | Ethyl decanoate | 36.26 | 341.09 | 0.363 | 3.411 | 100 | Coconut scent |
| 13 | octanoic acid | 549.78 | 485.12 | 0.037 | 0.0323 | 15,000 | Fruity |
| 14 | 2,2,4,4,6,6-hexamethyl-1,3,5,2,4,6-trioxatrisilinane | 267.54 | 225.45 | — | — | — | Pungent odor |
| 15 | 2,2,4,4,6,6,8,8,10,10-decamethyl-1,3,5,7,9,2,4,6,8,10-pentaoxapentasilecane | 245.98 | 158.76 | — | — | — | Pungent odor |
| 16 | bis[[dimethyl(trimethylsilyloxy)silyl]oxy]-dimethylsilane | 255.78 | 115.64 | 2.56 | 1.16 | 100 | Pungent odor |
| 17 | Nonanal | 47.04 | 78.41 | — | — | — | Citrus zest |
| 18 | Decanal | 37.24 | 28.42 | 0.372 | 0.284 | 100 | Citrus zest |
| 19 | (NE)-N-[1-(2-methoxyphenyl)propan-2-ylidene]hydroxylamine | 545.86 | 37.142 | — | — | — | Earthy, flavor |
| Name of Substance | ROAV | |
|---|---|---|
| Pure Persimmon Wine | Rapeseed Flower–Persimmon Wine | |
| butane-2,3-diol | 0.0000072 | 0.000046 |
| hexan-1-ol | — | 0.0135 |
| phenethyl alcohol | 0.221 | 0.11 |
| 3-Methylacetic acid-butan-1-ol | — | 0.027 |
| Ethyl hexanoate | 2.347 | 14.701 |
| Ethyl octanoate | 3.112 | 6.921 |
| Ethyl decanoate | 0.363 | 3.411 |
| octanoic acid | 0.037 | 0.0323 |
| bis[[dimethyl(trimethylsilyloxy)silyl]oxy]-dimethylsilane | 2.560 | 1.160 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Sun, K.; Wang, Y.; Yu, F.; Liu, Z. Analysis of Antioxidant Activity and Volatile Components in Rapeseed Flower-Enriched Persimmon Wine. Foods 2025, 14, 1804. https://doi.org/10.3390/foods14101804
Li Z, Sun K, Wang Y, Yu F, Liu Z. Analysis of Antioxidant Activity and Volatile Components in Rapeseed Flower-Enriched Persimmon Wine. Foods. 2025; 14(10):1804. https://doi.org/10.3390/foods14101804
Chicago/Turabian StyleLi, Zhijie, Kaishuo Sun, Yanyan Wang, Fang Yu, and Zhiwen Liu. 2025. "Analysis of Antioxidant Activity and Volatile Components in Rapeseed Flower-Enriched Persimmon Wine" Foods 14, no. 10: 1804. https://doi.org/10.3390/foods14101804
APA StyleLi, Z., Sun, K., Wang, Y., Yu, F., & Liu, Z. (2025). Analysis of Antioxidant Activity and Volatile Components in Rapeseed Flower-Enriched Persimmon Wine. Foods, 14(10), 1804. https://doi.org/10.3390/foods14101804





