Ultrasound-Assisted Polysaccharide Extraction from Grape Skin and Assessment of In Vitro Hypoglycemic Activity of Polysaccharides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. The Extraction and Fractionation of Polysaccharides from Grape Skin
2.2.1. Single-Factor Experiments for GSP Extraction
2.2.2. Box–Behnken Design and Statistical Evaluation
2.2.3. Purification of GSP
2.3. The Properties of the GSP Fraction
2.3.1. Molecular Weight (MW) Distribution
2.3.2. Chemical Composition of GSPs
2.3.3. Monosaccharide Profiling of GSPs
2.3.4. FT-IR
2.3.5. UV Spectroscopy
2.3.6. Scanning Electron Microscopy
2.4. The Anti-α-Amylase Activity
2.5. The Inhibitory Effect on α-Glucosidase Activity
2.6. Statistical Analysis
3. Results and Discussion
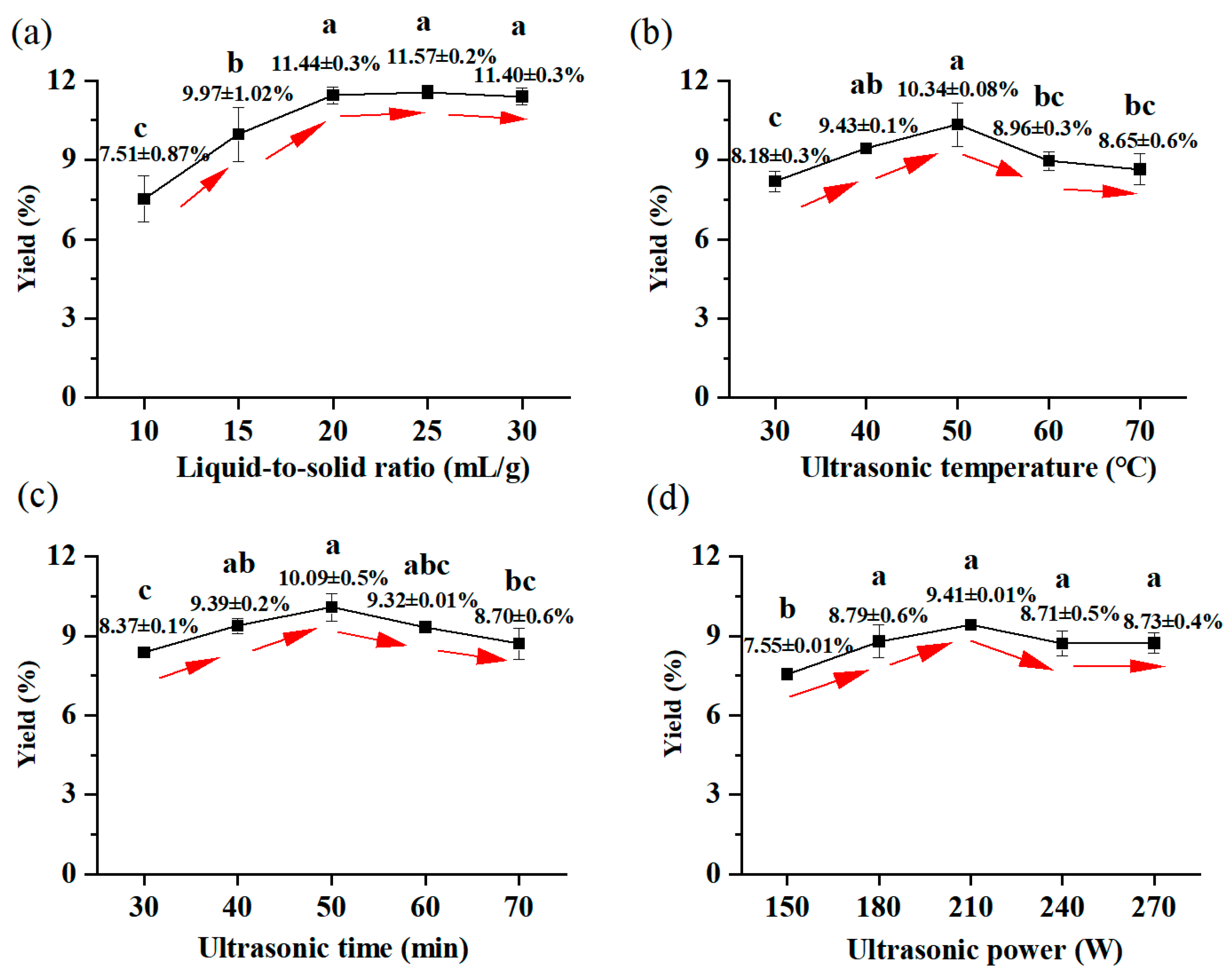
3.1. Analysis of Results of the Single-Factor Test
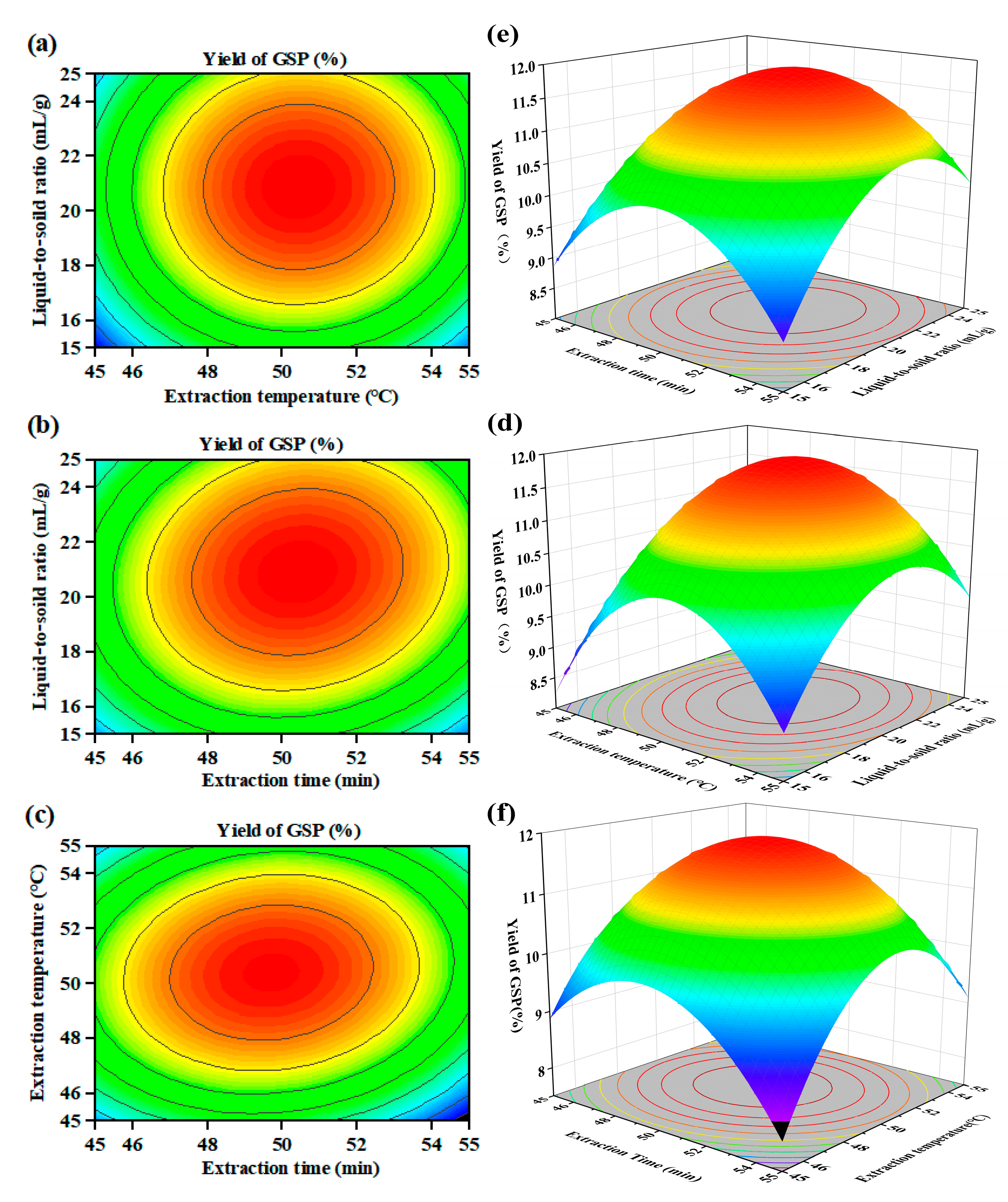
3.2. Optimization of Extraction Conditions Using RSM
+ 0.0027BC − 0.056255A2 − 0.068755B2 − 0.048965C2
3.3. Chemical Composition of GSP
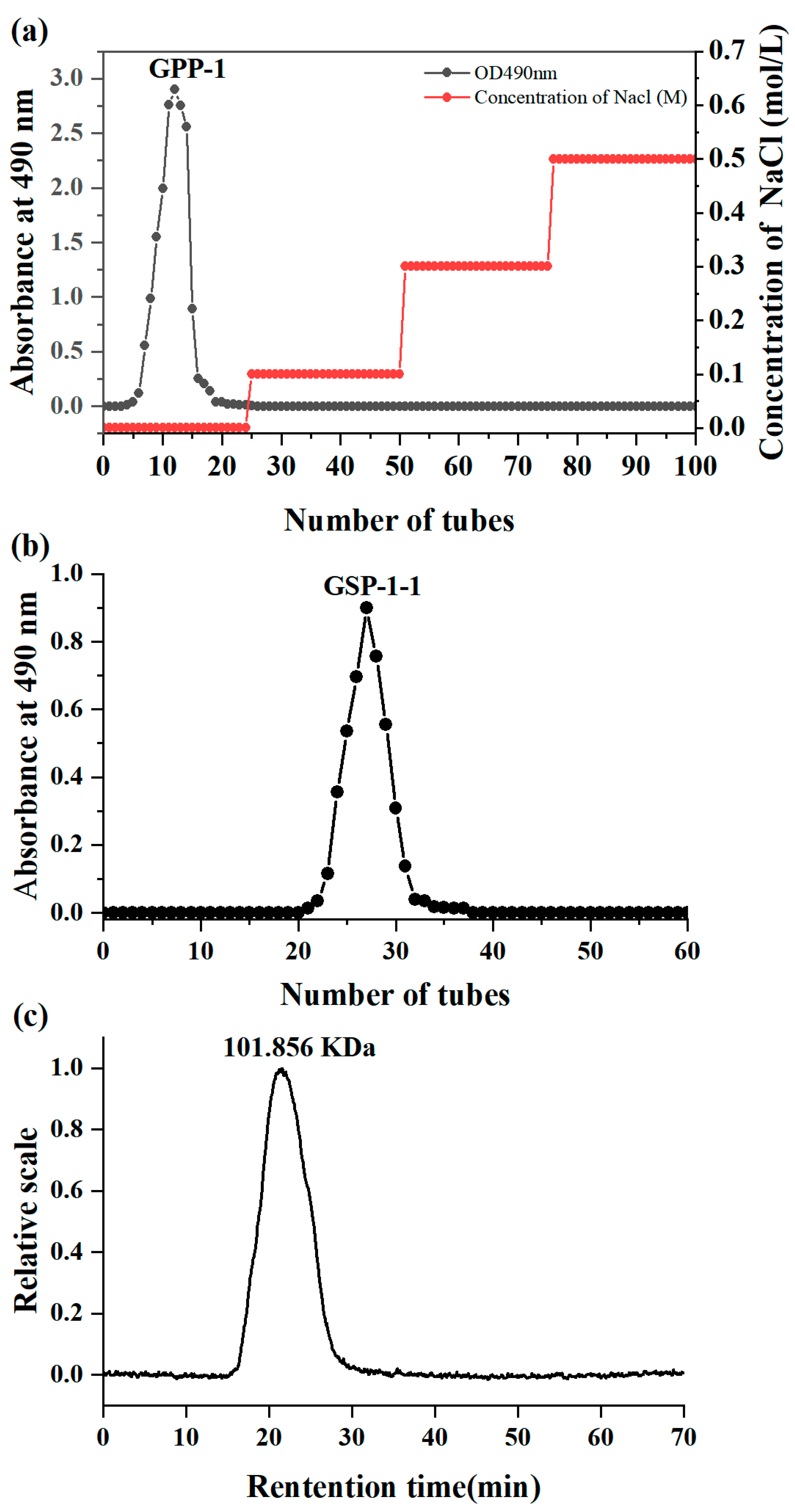
3.4. Elution Curve and Molecular Weight of GSPs
3.5. FT-IR Analysis and UV Spectroscopy
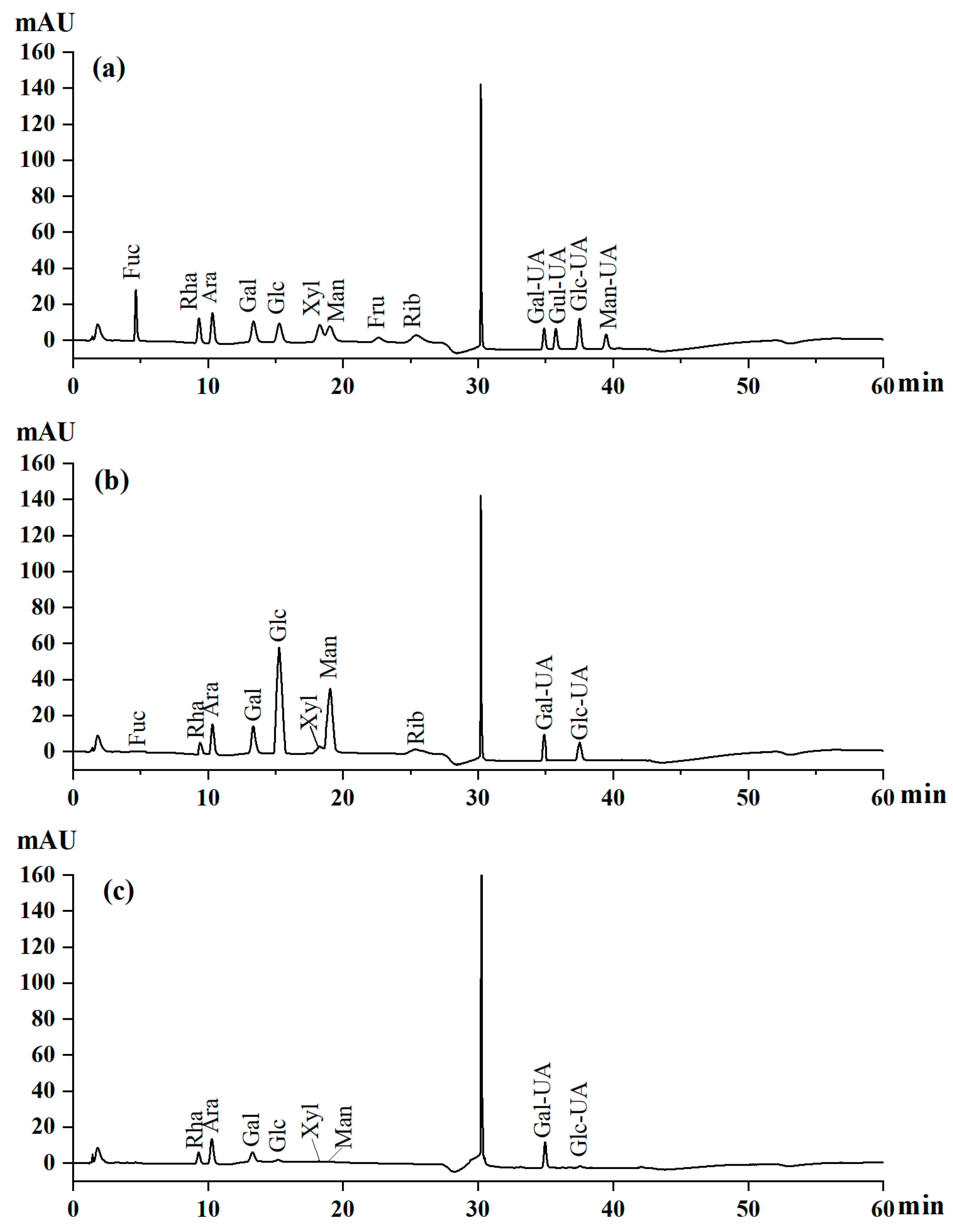
3.6. HPLC Analysis of Monosaccharide Composition
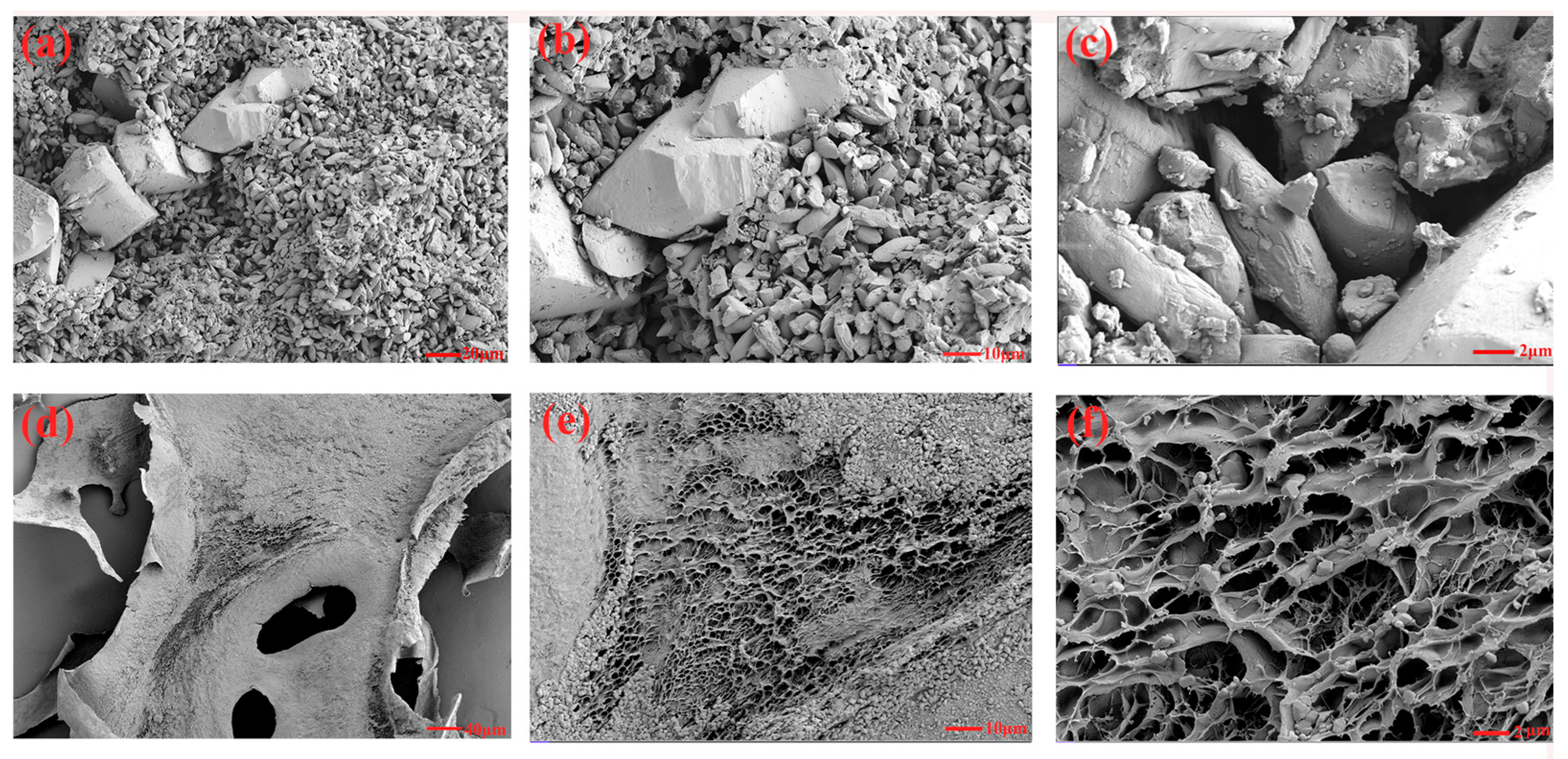
3.7. SEM Analysis
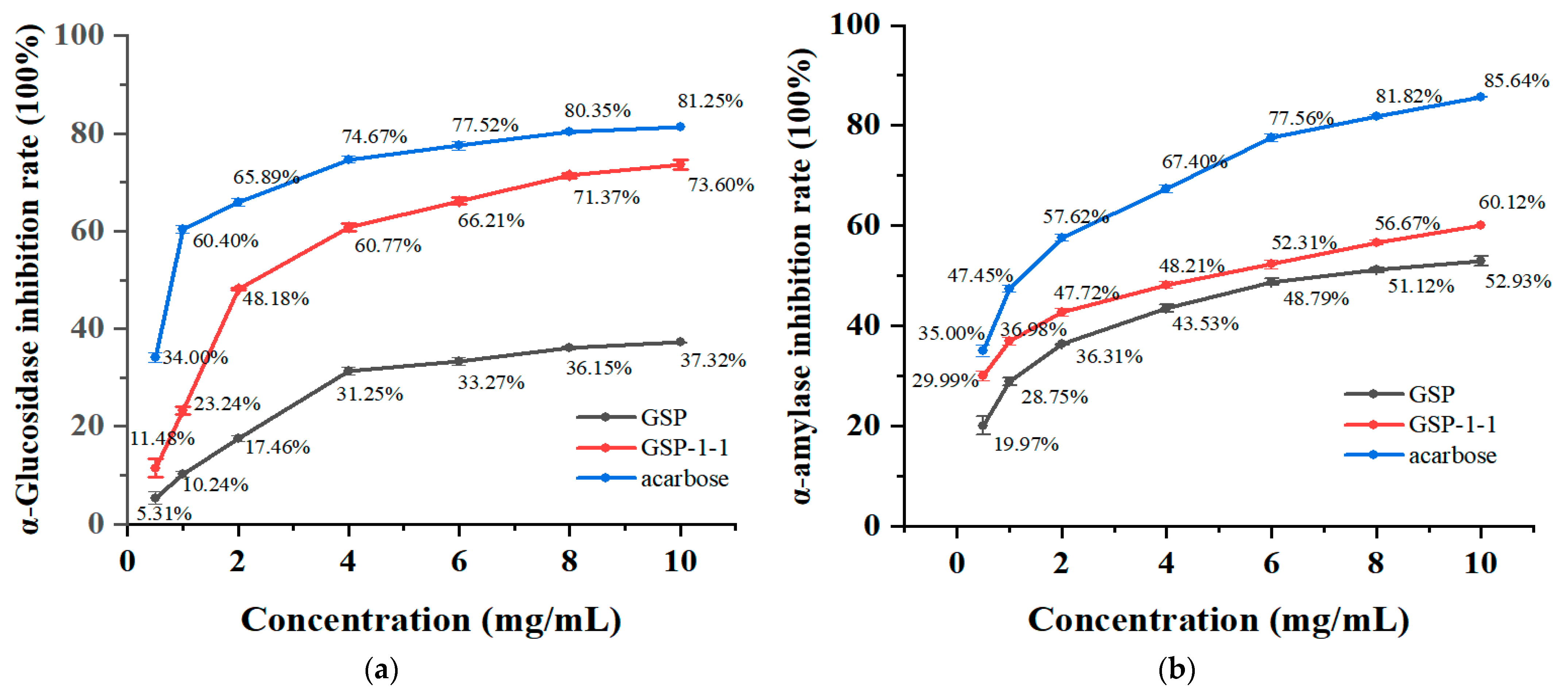
3.8. Inhibition of α-Amylase and α-Glucosidase by GSPs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ranjna, S.; Ayon, T.; Shikhangi, S.; Taru, N.; Vivek, K.G.; Edgard, G.; Bharathiraja, B.; Bharathiraja, D. Green processing and biotechnological potential of grape pomace: Current trends and opportunities for sustainable biorefinery. Bioresour. Technol. 2020, 314, 123771. [Google Scholar]
- Mariana, S.; Mircea, O. The potential of grape pomace varieties as a dietary source of pectic substances. Foods 2021, 10, 867. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Miao, W.; Li, J.; Liu, Y.; Mu, Q.; Li, Q. Physicochemical characteristics and biological activities of grape polysaccharides collected from different Cultivars. Food Res. Int. 2023, 163, 112161. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of grape pomace: An approach that is increasingly reaching its maturity—A review. Int. J. Food Sci. Tech. 2019, 4, 14118. [Google Scholar] [CrossRef]
- Ferri, M.; Bin, S.; Vallini, V. Recovery of polyphenols from red grape pomace and assessment of their antioxidant and anti-cholesterol activities. N. Biotechnol. 2016, 33, 338–344. [Google Scholar] [CrossRef]
- Kobra, A.; Rezaei, M.; Hamidreza, T.; Heidari, P.; Khadivi, A. Morphological and pomological variability of a grape (Vitis vinifera L.) germplasm collection. Sci. Hortic. 2020, 266, 109285. [Google Scholar]
- Dong, X.; Zhu, C.; Huang, G.; Xiao, J. Fractionation and structural characterization of polysaccharides derived from red grape pomace. Process Biochem. 2021, 109, 37–45. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Wang, L.; Song, G.; Jiang, W.; Mu, L.; Li, J. Ficus carica polysaccharide extraction via ultrasound-assisted technique: Structure characterization, antioxidant, hypoglycemic and immunomodulatory activities. Ultrason. Sonochem. 2023, 101, 106680. [Google Scholar] [CrossRef]
- Hou, C.; Chen, L.; Yang, L.; Ji, X. An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 2020, 2, 315. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Q.; Zhang, H.; Wang, J.; Fu, Q.; Qiao, H.; Wang, Q. Insight into antibacterial mechanism of polysaccharides: A review. LWT 2021, 150, 11929. [Google Scholar] [CrossRef]
- Feng, Y.; Qiu, Y.; Ma, H. Characterization, antioxidant, antineoplastic and immune activities of selenium modified sagittaria sagittifolia L. polysaccharides. Food Res. Int. 2022, 153, 110913. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Yang, R.; Li, W. Structural characterization of an active polysaccharide of longan and evaluation of immunological activity. Carbohydr. Polym. 2019, 213, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Niu, X.; Yang, Y. Characterization, antioxidant and hypoglycemic activities of degraded polysaccharides from blackcurrant (Ribes nigrum L.) fruits. Food Chem. 2018, 243, 26–35. [Google Scholar] [CrossRef]
- Da, H.R.; Jwa, Y.C.; Nooruddin, B.S.; Jin, C.K.; Bokyun, L.; Muhammad, H.; Taek, S.L.; Hyoung, S.K.; Soo, H.P.; Chu, W.N.; et al. Optimization of antioxidant, anti-diabetic, and anti-inflammatory activities and ganoderic acid content of differentially dried ganoderma lucidum using response surface methodology—ScienceDirect. Food Chem. 2021, 335, 127645. [Google Scholar]
- Yang, Y.; Wu, H. Immunomodulatory function and anti-tumor mechanism of natural polysaccharides: A review. Front. Immunol. 2023, 14, 114741. [Google Scholar] [CrossRef]
- Miao, W.; Huang, R.; Li, Q. Physicochemical properties and in vivo hepatoprotective effect of polysaccharides from grape pomace. Antioxidants 2023, 12, 394. [Google Scholar] [CrossRef]
- Jie, G.; Lian, Z.L.; Bao, G.S.; Mou, M.Z. A comparison study on polysaccharides extracted from Laminaria japonica using different methods: Structural characterization and bile acid-binding capacity. Food Funct. 2017, 9, 3043–3052. [Google Scholar]
- Hong, M.S.; Hai, Z.Z.; Ran, L.; Meng, Y.D.; Hong, X.W.; Yu, J.L. Comparison of different extraction methods of polysaccharides from cup plant (Silphium perfoliatum L.). PLoS ONE 2017, 12, 183001. [Google Scholar]
- Sheng, Y.Z.; Atikur, R.; Jun, H.L.; Chao, Y.W.; Jian, L.C.; Robert, J.L.; Xing, Q.Y.; Shi, G.C. Extraction Methods Affect the Structure of Goji (Lycium barbarum) Polysaccharides. Molecules 2020, 25, 936. [Google Scholar] [CrossRef]
- Kun, C.Q.; Zun, J.L.; Ying, X.L.; Zhong, Y.L.; Wei, Z. Study on extraction methods of polysaccharides from a processed product of Aconitum carmichaeli Debx. RSC Adv. 2021, 11, 21259–21268. [Google Scholar]
- Long, X.; Yan, Q.; Cai, L.; Li, G.; Luo, X. Box-Behnken design-based optimization for deproteinization of crude polysaccharides in Lycium barbarum berry residue using the Sevag method. Heliyon 2020, 6, e03888. [Google Scholar] [CrossRef]
- Ji, X.; Yan, Y.; Hou, C.; Shi, M.; Liu, Y. Structural characterization of a galacturonic acid-rich polysaccharide from Ziziphus jujuba cv. Muzao. Int. J. Biol. Macromol 2019, 147, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; You, Q.; Zeng, H. Polysaccharide fractions from Fortunella margarita affect proliferation of Bifidobacterium adolescentis ATCC 15703 and undergo structural changes following fermentation. Int. J. Biol. Macromol. 2019, 123, 1070–1078. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Liu, C.; Du, P.; Guo, Y.; Xie, Y.; Qian, H. Extraction, characterization of aloe polysaccharides and the in-depth analysis of its prebiotic effects on mice gut microbiota. Carbohydr. Polym. 2021, 261, 117874. [Google Scholar] [CrossRef]
- Salvador, L.; Suganuma, T.; Kitahara, K.; Tanoue, H.; Ichiki, M. Monosaccharide composition of sweetpotato fiber and cell wall polysaccharides from sweetpotato, cassava, and potato analyzed by the high-performance anion exchange chromatography with pulsed amperometric detection method. Food Chem. 2000, 48, 3448–3454. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Huang, R.; Wen, P.; Song, Y.; Wang, H. Structural characterization and immunological activity of pectin polysaccharide from kiwano (Cucumis metuliferus) peels. Carbohydr. Polym. 2021, 254, 117371. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; Su, J.; Liang, G.; Tan, S.; Bi, Y.; Kong, F.; Wang, Z. Extraction of pithecellobium clypearia benth polysaccharides by dual-frequency ultrasound-assisted extraction: Structural characterization, antioxidant, hypoglycemic and anti-hyperlipidemic activities. Ultrason. Sonochem. 2024, 107, 106918. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.; Tran, H.; Xuan, T.; Ahmad, A.; Dat, T.; Khanh, T.; Teschke, R. Momilactones A and B are α-Amylase and α-Glucosidase inhibitors. Molecules 2019, 24, 482. [Google Scholar] [CrossRef]
- Samaram, S.; Mirhosseini, H.; Serjouie, A. Optimisation of ultrasound-assisted extraction of oil from papaya seed by response surface methodology: Oil recovery, radical scavenging antioxidant activity, and oxidation stability. Food Chem. 2015, 172, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Srivastav, S.; Sharanagat, V. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhou, C.; Zeng, Y.; Zhang, H.; Hossen, M.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Structures, physicochemical and bioactive properties of polysaccharides extracted from Panax notoginseng using ultrasonic/microwave-assisted extraction. LWT 2022, 154, 112446. [Google Scholar] [CrossRef]
- Olawuyi, I.; Kim, S.; Hahn, D.; Lee, W. Influences of combined enzyme-ultrasonic extraction on the physicochemical characteristics and properties of okra polysaccharides. Food Hydrocolloid. 2020, 100, 105293. [Google Scholar] [CrossRef]
- Zhu, C.; Zhai, X.; Li, B. Response surface optimization of ultrasound-assisted polysaccharides extraction from pomegranate peel. Food Chem. 2015, 177, 139–146. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Hu, X. Extraction optimization, characterization and the antioxidant activities in vitro and in vivo of polysaccharide from Pleurotus ferulae. Int. J. Biol. Macromol. 2020, 160, 380–389. [Google Scholar] [CrossRef]
- Leng, X.; Li, J.; Miao, W.; Liu, Y.; Haider, M.; Song, M.; Fang, J.; Li, Q. Comparison of physicochemical characteristics, antioxidant and immunomodulatory activities of polysaccharides from wine grapes. Int. J. Biol. Macromol. 2023, 239, 124164. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Yu, J.; Liu, A. Structural characterization of a low molecular weight polysaccharide from Grifola frondosa and its antitumor activity in H22 tumor-bearing mice. J. Funct. Foods. 2019, 61, 103472. [Google Scholar] [CrossRef]
- Oladzad, S.; Fallah, N.; Mahboubi, A.; Afsham, N.; Taherzadeh, M. Date fruit processing waste and approaches to its valorization: A Review. Bioresour. Technol. 2021, 340, 125625. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, Y.; Tian, Y.; Zhang, M.; Cheng, K.; Zhang, X.; Zhou, M.; Hui, M.; Zhang, Y. Extraction, characterization, and antioxidant activity of eucommia ulmoides polysaccharides. Molecules 2024, 29, 4793. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, Q.; Wang, Z.; Borjigin, G.; Sun, J.; Zhao, Y.; Li, Q.; Shi, X.; Shah, S.F.A.; Wang, X.; et al. Ultrasound-Assisted polysaccharide extraction from Fritillaria ussuriensis maxim and its structural characterization, antioxidant and immunological activity. Ultrason. Sonochem. 2024, 103, 106800. [Google Scholar] [CrossRef]
- Guo, L.; Kong, N.; Ma, H. Multimode ultrasonic extraction of polysaccharides from maca (Lepidium meyenii): Optimization, purification, and in vitro immunoregulatory activity. Ultrason. Sonochem. 2022, 88, 106062. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.J.; Chun, Y.H.; Yi, Z.Y.; Miao, M.S.; Yan, Q.L. Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba Mill.) fruit. Int. J. Biol. Macromol. 2020, 149, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.J.; Jian, H.G.; Teng, Z.C.; Ting, T.Z.; Yan, Q.L.; Yi, Z.Y. Review on mechanisms and structure-activity relationship of hypoglycemic effects of polysaccharides from natural resources. Food Sci. Hum. Well. 2023, 12, 1969–1980. [Google Scholar]
- Jun, C.C.; Lin, L.; Xin, Z.; Peng, Y.S.; Bing, L.; Xia, Z. Preliminary characterization and antioxidant and hypoglycemic activities in vivo of polysaccharides from Huidouba. Food Functdoi. 2018, 9, 6337–6348. [Google Scholar]
- Amaraweera, S.; Gunathilake, C.; Gunawardene, O.; Dassanayake, R.; Fernando, N.; Wanninayaka, D.; Rajapaksha, S.; Manamperi, A.; Gangoda, M.; Manchanda, A. Preparation and characterization of dual-modified cassava starch-based biodegradable foams for sustainable packaging applications. ACS Omega 2022, 7, 19579–19590. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, J.; Wang, S.; Li, X.; Miao, Y.; Zhou, J.; Liu, Z. Hydrophobic Ti3C2Tx/TEMPO oxidized cellulose nanofibers com.posite aerogel for efficient oil-water separation. Polymers 2025, 17, 273. [Google Scholar] [CrossRef] [PubMed]
- Paiheerding, M.; Rehebati, N.; Chun, F.L.; Haibaier, H.; Aytursun, A.; Abulimiti, Y. Extraction, purification, and characterization of polysaccharides from Alhagi pseudoalhagi with antioxidant and hypoglycemic activities. Process Biochem. 2022, 121, 339–348. [Google Scholar]
- Ren, B.; Chen, C.; Li, C. Optimization of microwave-assisted extraction of sargassum thunbergii polysaccharides and its antioxidant and hypoglycemic activities. Carbohydr. Polym. 2017, 173, 192–201. [Google Scholar] [CrossRef]
- Nie, C.; Zhu, P.; Wang, M.; Ma, S.; Wei, Z. Optimization of water-soluble polysaccharides from stem lettuce by response surface methodology and study on its characterization and bioactivities. Int. J. Biol. Macromol. 2017, 105, 912–923. [Google Scholar] [CrossRef]
- Fu, X.; Yang, H.; Ma, C.; Li, X.; Wang, L. Characterization and inhibitory activities on α-amylase and α-glucosidase of the polysaccharide from blue honeysuckle berries. Int. J. Biol. Macromol. 2020, 163, 414–422. [Google Scholar] [CrossRef]
- Mei, M.Z.; Jing, W.B.; Xue, Y.B.; Yu, T.Y.; Li, B.W.; Yu, Y.; Ya, Q.X. Characterization of selenized polysaccharides from Ribes nigrum L. and its inhibitory effects on α-amylase and α-glucosidase. Carbohyd. Polym. 2021, 259, 117729. [Google Scholar]
- Do, Y.H.; Zhen, L.L.; Darren, Y.S.L.; Siah, Y.T.; Sivakumar, M.; Khang, W.T.; Zhen, H.B. Ultrasonic cavitation: An effective cleaner and greener intensification technology in the extraction and surface modification of nanocellulose. Ultrason. Sonochem. 2022, 90, 106176. [Google Scholar]
- Xiao, Y.L.; Jing, S.Z.; Teng, Y.W.; Jia, P.S.; Tian, H.G.; Li, J.Z.; Guo, P.Y.; Xiu, F.X. Antidiabetic activity of Armillaria mellea polysaccharides: Joint ultrasonic and enzyme assisted extraction. Ultrason. Sonochem. 2023, 95, 106370. [Google Scholar]
- Raouf, A.; Mohammed, S.A.; Asgar, A.; Yang, T.; Sivakumar, M. A chemometric approach to evaluate the effects of probe-type ultrasonication on the enzyme inactivation and quality attributes of fresh amla juice. Ultrason. Sonochem. 2023, 92, 106268. [Google Scholar]
- Zi, C.W.; Xue, Y.Z.; Li, L.S.; Di, Z.; Xin, X.Z.; Ya, P.P.; Xiao, X.Y.; Xiao, N.L.; Qi, W.; Bao, S.W.; et al. Effect of ultrasonic degradation on the structural feature, physicochemical property and bioactivity of plant and microbial polysaccharides: A review. Int. J. Biol. Macromol. 2023, 236, 123924. [Google Scholar]
- Bei, W.; Ling, L.Y.; Shu, C.G.; Ling, W.; Meng, L.Y.; Liang, F.; Xiao, B.J. Structural elucidation, modification, and structure-activity relationship of polysaccharides in chinese herbs: A. Review. Front. Nutr. 2022, 9, 908175. [Google Scholar]
- Yang, Y.; Olusola, L.; Jie, S.; Li, M.W.; Ting, M.; Hong, X.W. Activity diversity structure-activity relationship of polysaccharides from lotus root varieties. Carbohydr. Polym. 2018, 190, 67–76. [Google Scholar]
- Yuan, W.; Bing, H.L.; Miao, M.C.; Bing, L.; Liang, L.J. Research progress on ginger polysaccharides: Extraction, purification and structure-bioactivity relationship. Food Funct. 2023, 14, 10651–10666. [Google Scholar]
- Jeremy, P.H.W.; Noémie, C.; Michaela, F.S.; Samuel, C.Z.; Tom, J.B.; Alexandre, P. Bacteroides thetaiotaomicron metabolic activity decreases with polysaccharide molecular weight. mBio 2024, 15, 3. [Google Scholar]

| Level | Factor | ||
|---|---|---|---|
| Z1: Ultrasonic Time (min) | Z2: Ultrasonic Temperature (°C) | Z3: Liquid-to-Solid Ratio (mL/g) | |
| −1 | 45 | 45 | 15 |
| 0 | 50 | 50 | 20 |
| 1 | 55 | 55 | 25 |
| Run | Factor | Yield Rate (%) | ||
|---|---|---|---|---|
| Z1 | Z2 | Z3 | ||
| 1 | −1 | 1 | 0 | 9.27 ± 0.01 |
| 2 | 0 | 0 | 0 | 11.69 ± 0.10 |
| 3 | 0 | 1 | −1 | 8.73 ± 0.03 |
| 4 | 0 | 0 | 0 | 11.98 ± 0.01 |
| 5 | 1 | −1 | 0 | 8.70 ± 0.03 |
| 6 | 0 | 0 | 0 | 11.82 ± 0.09 |
| 7 | 1 | 1 | 0 | 8.97 ± 0.02 |
| 8 | 0 | −1 | −1 | 8.50 ± 0.01 |
| 9 | 0 | 1 | 1 | 9.43 ± 0.02 |
| 10 | 1 | 0 | −1 | 8.63 ± 0.01 |
| 11 | 1 | 0 | 1 | 10.30 ± 0.01 |
| 12 | −1 | −1 | 0 | 7.93 ± 0.01 |
| 13 | 0 | 0 | 0 | 12.04 ± 0.10 |
| 14 | 0 | 0 | 0 | 11.68 ± 0.07 |
| 15 | −1 | 0 | 1 | 9.28 ± 0.05 |
| 16 | 0 | −1 | 1 | 8.93 ± 0.04 |
| 17 | −1 | 0 | −1 | 8.63 ± 0.01 |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 33.1700 | 9 | 3.6900 | 62.2900 | <0.0001 | ** |
| Z1 | 0.2742 | 1 | 0.2742 | 4.6300 | 0.0684 | - |
| Z2 | 0.6827 | 1 | 0.6827 | 11.5400 | 0.0100 | * |
| Z3 | 1.4800 | 1 | 1.4800 | 1.0500 | 0.0016 | * |
| Z1Z2 | 0.2846 | 1 | 0.2846 | 4.8100 | 0.0644 | - |
| Z1Z3 | 0.1700 | 1 | 0.1700 | 4.3400 | 0.0756 | - |
| Z2Z3 | 0.0182 | 1 | 0.0182 | 0.3080 | 0.5962 | - |
| Z12 | 8.3300 | 1 | 8.3300 | 140.7300 | <0.0001 | ** |
| Z22 | 12.4400 | 1 | 12.4400 | 210.2200 | <0.0001 | ** |
| Z32 | 6.3100 | 1 | 6.3100 | 106.6200 | <0.0001 | ** |
| Residual | 0.4142 | 7 | 0.0592 | |||
| Lack of Fit | 0.3062 | 3 | 0.1021 | 3.7800 | 0.1159 | - |
| Pure Error | 0.1081 | 4 | 0.0270 | |||
| Total | 33.5900 | 16 |
| Item | Carbohydrate (%) | Protein (%) | Uronic Acid (%) | Sulfuric Radical (%) |
|---|---|---|---|---|
| GSP | 37.68 ± 1.67% | 1.69 ± 0.23% | 4.26 ± 0.20% | 5.87 ± 0.11% |
| GSP-1-1 | 87.79 ± 0.51% | - | 9.31 ± 0.15% | 0.67 ± 0.05% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wang, N.; Xu, T.; Du, Q.; Yang, R.; Ai, M.; Han, X.; Wang, W. Ultrasound-Assisted Polysaccharide Extraction from Grape Skin and Assessment of In Vitro Hypoglycemic Activity of Polysaccharides. Foods 2025, 14, 1801. https://doi.org/10.3390/foods14101801
Li W, Wang N, Xu T, Du Q, Yang R, Ai M, Han X, Wang W. Ultrasound-Assisted Polysaccharide Extraction from Grape Skin and Assessment of In Vitro Hypoglycemic Activity of Polysaccharides. Foods. 2025; 14(10):1801. https://doi.org/10.3390/foods14101801
Chicago/Turabian StyleLi, Wei, Na Wang, Ting Xu, Qingping Du, Rui Yang, Mingxun Ai, Xinyao Han, and Wei Wang. 2025. "Ultrasound-Assisted Polysaccharide Extraction from Grape Skin and Assessment of In Vitro Hypoglycemic Activity of Polysaccharides" Foods 14, no. 10: 1801. https://doi.org/10.3390/foods14101801
APA StyleLi, W., Wang, N., Xu, T., Du, Q., Yang, R., Ai, M., Han, X., & Wang, W. (2025). Ultrasound-Assisted Polysaccharide Extraction from Grape Skin and Assessment of In Vitro Hypoglycemic Activity of Polysaccharides. Foods, 14(10), 1801. https://doi.org/10.3390/foods14101801





