Meat Quality Traits Using Gelatin–Green Tea Extract Hybrid Electrospun Nanofiber Active Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
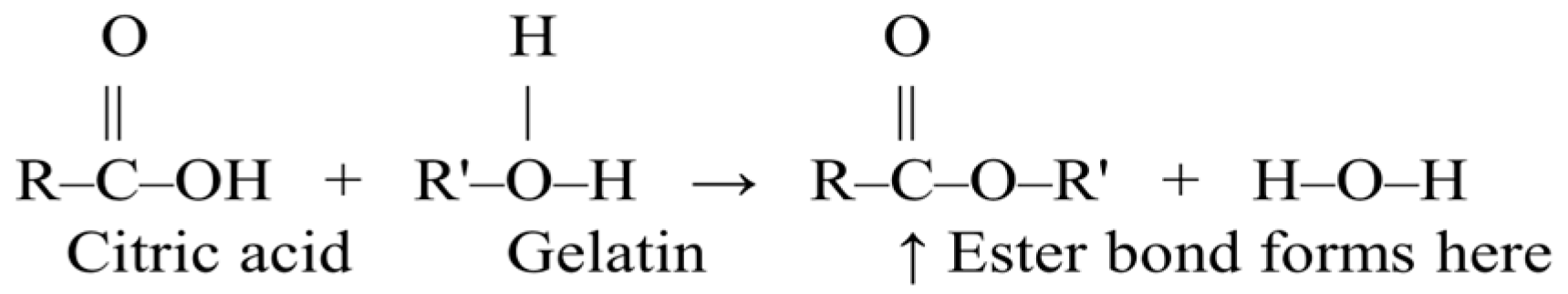
2.2. Hybrid Nanofiber Fabrication
2.2.1. Outer Layer
2.2.2. Inner Layer
2.2.3. Taylor Cone, Bead, and Nanofiber Fabrication
2.2.4. Stabilization and Crosslinking of Prepared Nanofibers
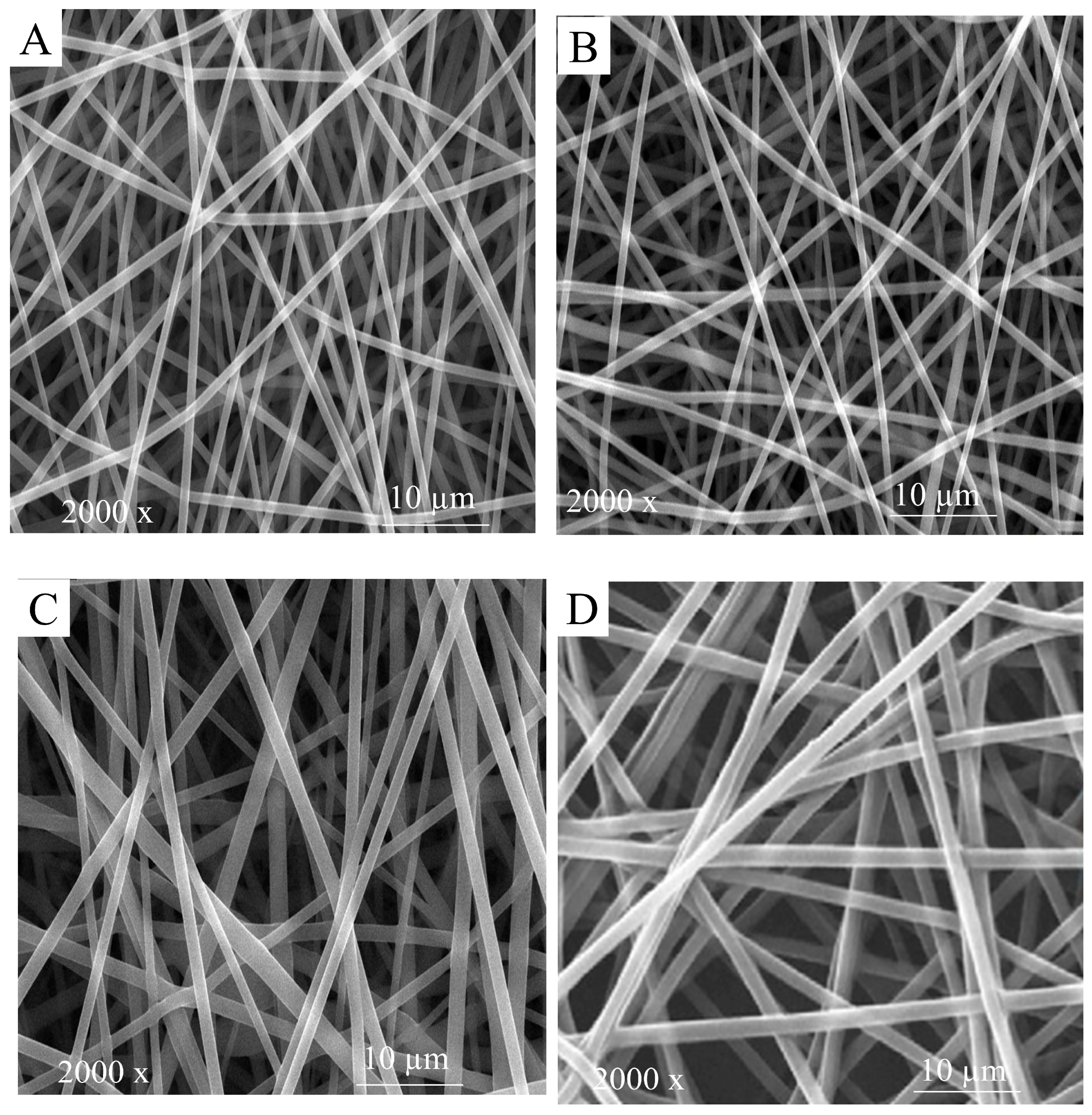
2.2.5. Scanning Electron Microscopy Images
2.2.6. In Vitro Degradation
2.2.7. Swelling Parameters
2.2.8. Mechanical Properties
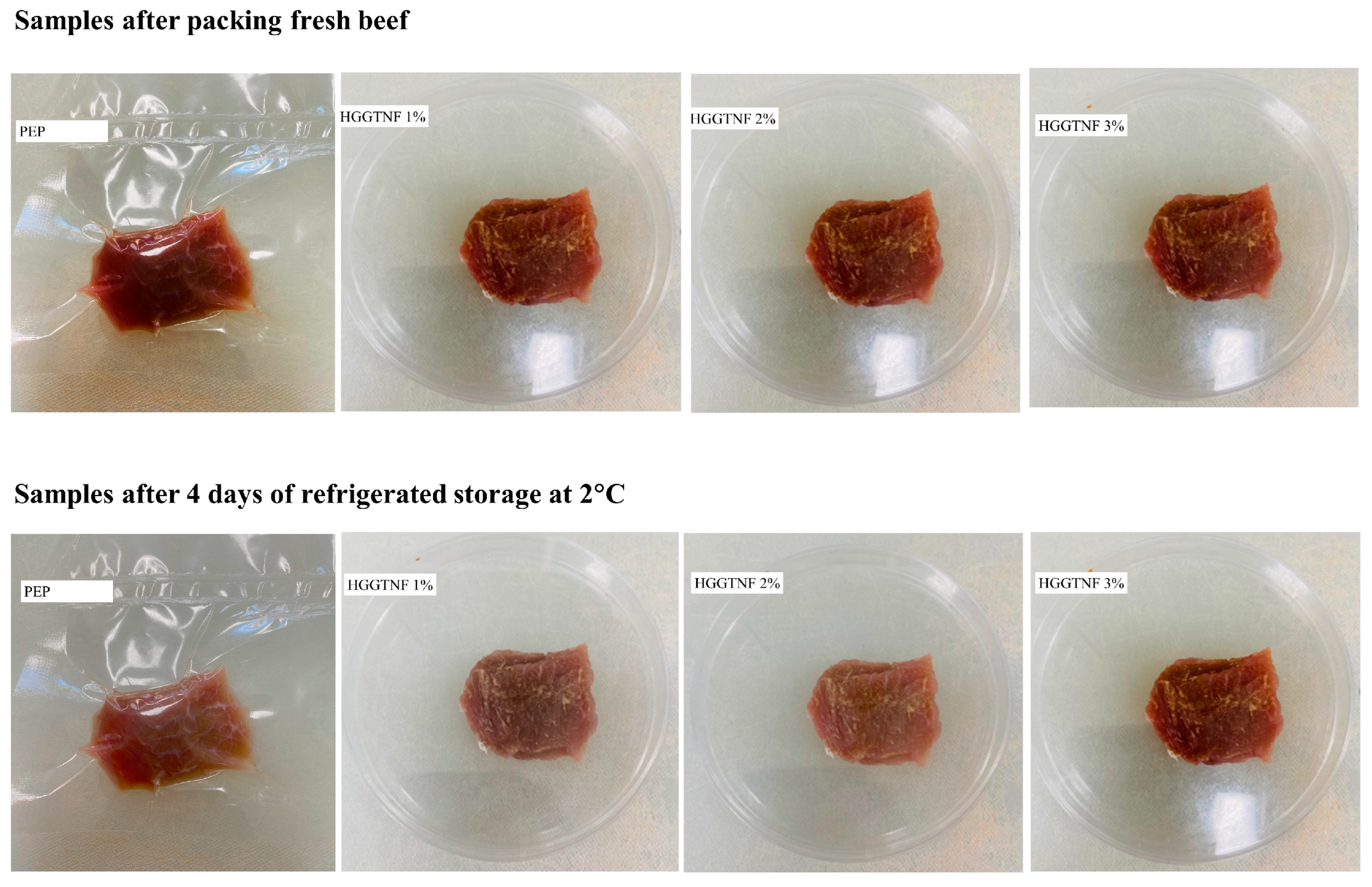
2.2.9. Application of Hybrid Nanofibers on Meat
2.2.10. Nutrition Profile
2.2.11. Fatty Acid Analysis
2.3. Color Analysis
2.4. Physicochemical Analysis
2.5. Oxidative Degradation Analysis
2.6. Investigation of Textural Parameters
2.7. Muscle Degradation Analysis
2.7.1. Activity of Cathepsin B and L
2.7.2. Total Collagen Content
2.7.3. Myofibrillar Fragmentation Index Assay
2.8. Histology of Meat Samples
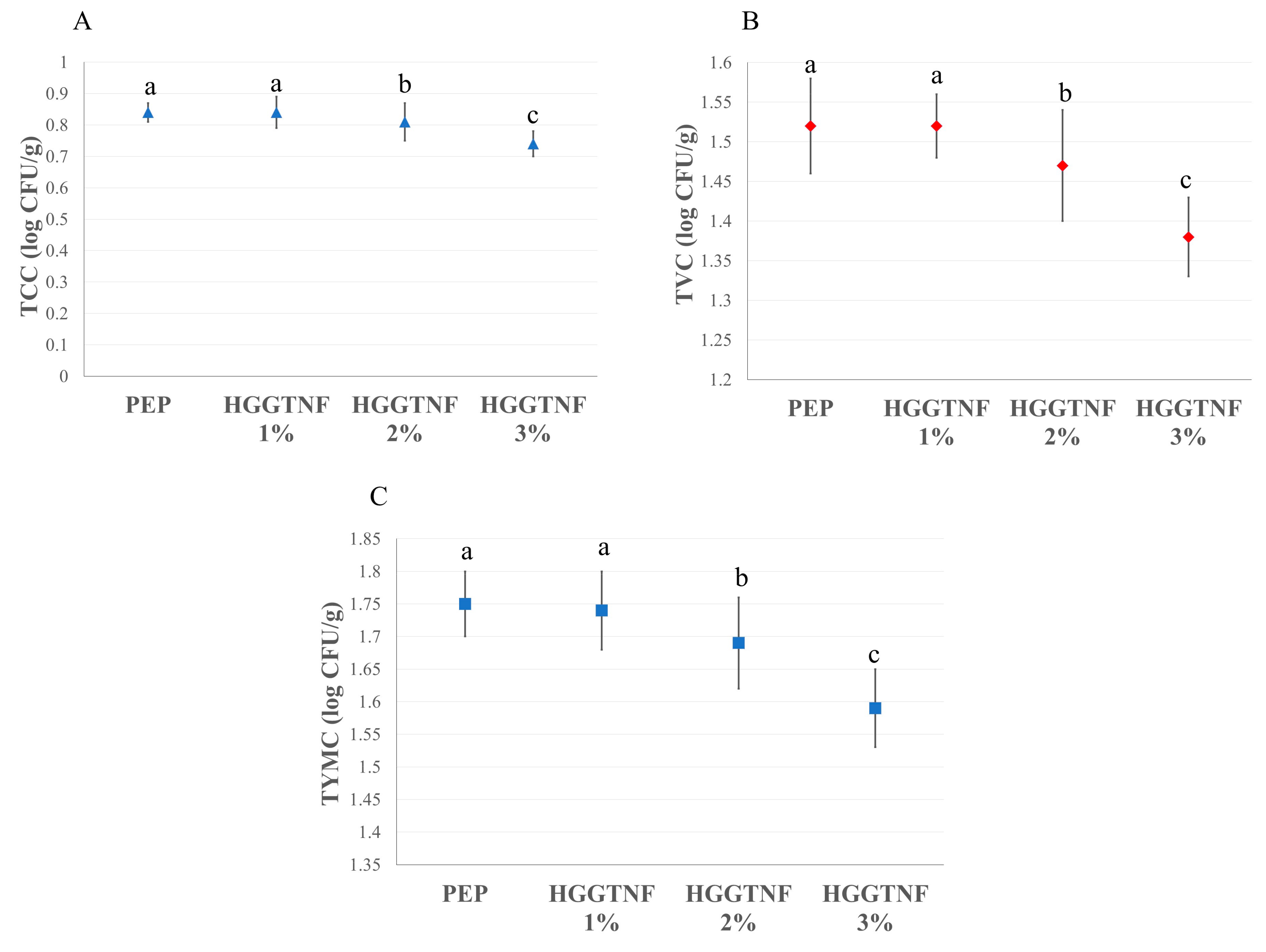
2.9. Microbiological Analysis
2.10. Taste Characteristics Assay by Taste Sensing System
2.11. Statistical Analysis
3. Results
3.1. Characterization of Taylor Cone, Bead, and Nanofiber Fabrication
3.2. Morphological Assessment
3.2.1. HGGTNF Physical Structure
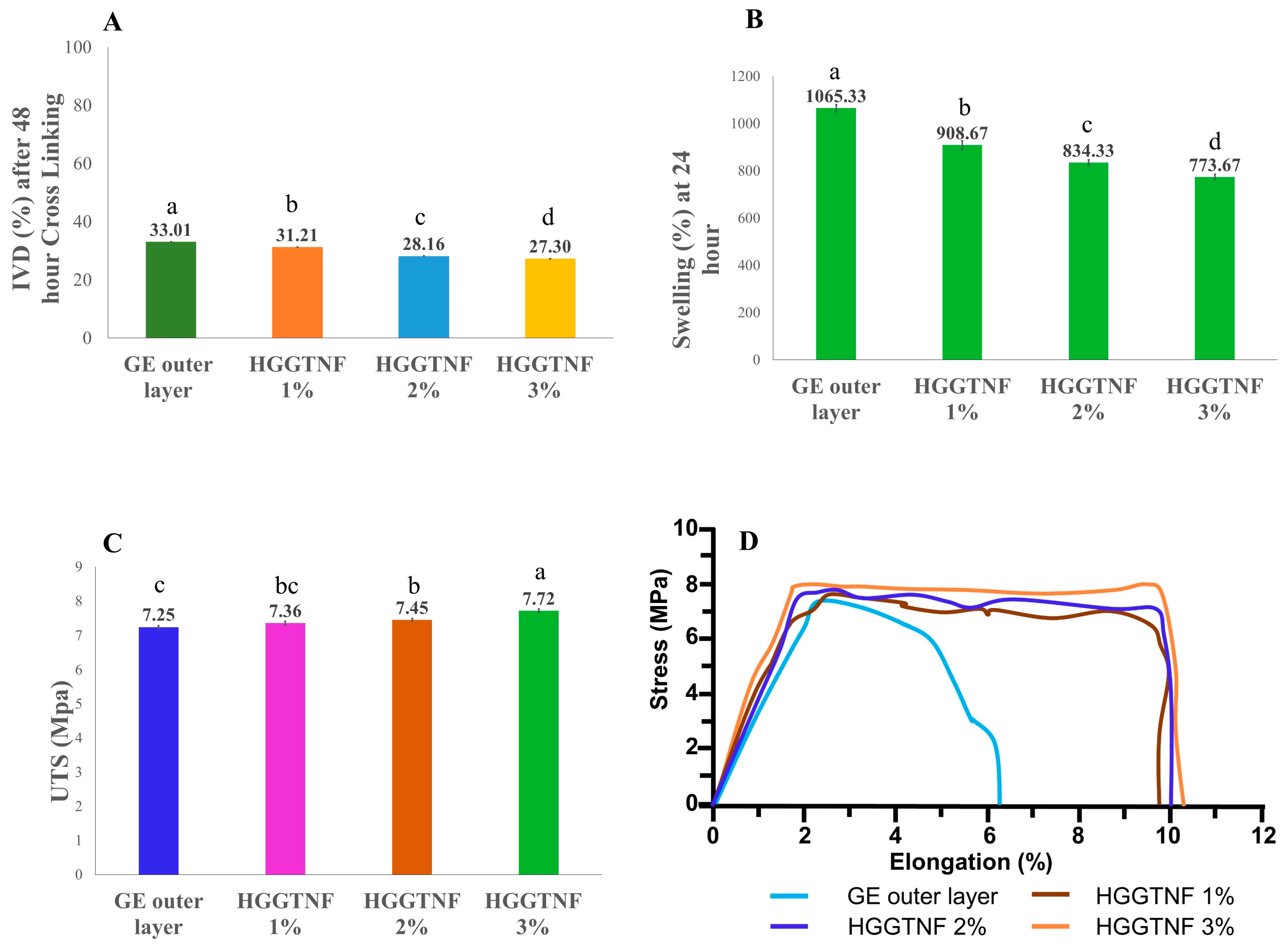
3.2.2. In Vitro Degradation
3.2.3. Swelling Parameters
3.2.4. Tensile Strength
3.3. Nutritional Profile
3.4. Fatty Acid Profile
3.5. Meat Color Parameters
3.6. Physicochemical Qualities
3.7. Oxidative Degradation Parameters
3.8. Meat Textural Parameters
3.9. Muscle Degradation Analysis
3.10. Histology of Meat Samples
3.11. Antimicrobial Effectiveness of Hybrid Nanofiber Mats
3.12. Taste Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ramachandraiah, K.; Hong, G.-P. Polymer based nanomaterials for strategic applications in animal food value chains. Food Rev. Int. 2022, 38, 1577–1606. [Google Scholar] [CrossRef]
- Savic, Z. Advances in the manufacture of sausage casings. In Advances in Meat, Poultry and Seafood Packaging; Elsevier: Amsterdam, The Netherlands, 2012; pp. 377–405. [Google Scholar]
- Batista, R.A.; Espitia, P.J.P.; Quintans, J.d.S.S.; Freitas, M.M.; Cerqueira, M.Â.; Teixeira, J.A.; Cardoso, J.C. Hydrogel as an alternative structure for food packaging systems. Carbohydr. Polym. 2019, 205, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Sothornvit, R. Nanostructured materials for food packaging systems: New functional properties. Curr. Opin. Food Sci. 2019, 25, 82–87. [Google Scholar] [CrossRef]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef] [PubMed]
- Goksen, G.; Demir, D.; Echegaray, N.; Bangar, S.P.; da Cruz, A.G.; Shao, P.; Lin, Y.; Lorenzo, J.M. New insights of active and smart natural-based electrospun mats for food safety in meat and meat products. Food Biosci. 2024, 104159. [Google Scholar] [CrossRef]
- Bölgen, N.; Demir, D.; Aşık, M.; Sakım, B.; Vaseashta, A. Introduction and fundamentals of electrospinning. In Electrospun Nanofibers: Principles, Technology and Novel Applications; Springer: Cham, Switzerland, 2022; pp. 3–34. [Google Scholar]
- Alam, A.N.; Kim, C.-J.; Kim, S.-H.; Kumari, S.; Lee, E.-Y.; Hwang, Y.-H.; Joo, S.-T. Scaffolding fundamentals and recent advances in sustainable scaffolding techniques for cultured meat development. Food Res. Int. 2024, 189, 114549. [Google Scholar] [CrossRef]
- Pateiro, M.; Gómez, B.; Munekata, P.E.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Lorenzo, J.M. Nanoencapsulation of promising bioactive compounds to improve their absorption, stability, functionality and the appearance of the final food products. Molecules 2021, 26, 1547. [Google Scholar] [CrossRef]
- HPS, A.K.; Saurabh, C.K.; Fazita, M.N.; Syakir, M.I.; Davoudpour, Y.; Rafatullah, M.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar]
- Niu, X.; Liu, Y.; Song, Y.; Han, J.; Pan, H. Rosin modified cellulose nanofiber as a reinforcing and co-antimicrobial agents in polylactic acid/chitosan composite film for food packaging. Carbohydr. Polym. 2018, 183, 102–109. [Google Scholar] [CrossRef]
- Arora, N.; Dua, S.; Purohit, S.V.; Dash, B.; Yadav, M.D.; Jena, B.K.; Senthilkumar, T. Polymer Composites: Synthesis, Application, and Basic Theoretical Aspects. In Polymer Composites: From Computational to Experimental Aspects; Springer: Singapore, 2024; pp. 1–41. [Google Scholar]
- Yilmaz, M.T.; Hassanein, W.S.; Alkabaa, A.S.; Ceylan, Z. Electrospun eugenol-loaded gelatin nanofibers as bioactive packaging materials to preserve quality characteristics of beef. Food Packag. Shelf Life 2022, 34, 100968. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, J.; Huang, S.; Lan, X.; Wang, W.; Tang, Y. Functionalized Gelatin Electrospun Nanofibrous Membranes in Food Packaging: Modification Strategies for Fulfilling Evolving Functional Requirements. Polymers 2025, 17, 1066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ahmed, A.; Xu, L. Electrospun nanofibers for functional food packaging application. Materials 2023, 16, 5937. [Google Scholar] [CrossRef] [PubMed]
- Essa, R.Y.; Elsebaie, E.M. New fat replacement agent comprised of gelatin and soluble dietary fibers derived from date seed powder in beef burger preparation. LWT 2022, 156, 113051. [Google Scholar] [CrossRef]
- Zahmatkeshan, M.; Adel, M.; Bahrami, S.; Esmaeili, F.; Rezayat, S.M.; Saeedi, Y.; Mehravi, B.; Jameie, S.B.; Ashtari, K. Polymer-based nanofibers: Preparation, fabrication, and applications. In Handbook of Nanofibers; Springer: Cham, Switzerland, 2019; pp. 215–261. [Google Scholar]
- Fang, Z.; Zhao, Y.; Warner, R.D.; Johnson, S.K. Active and intelligent packaging in meat industry. Trends Food Sci. Technol. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef]
- Samanta, S. Potential bioactive components and health promotional benefits of tea (Camellia sinensis). J. Am. Nutr. Assoc. 2022, 41, 65–93. [Google Scholar] [CrossRef]
- Pusporini, P.; Edikresnha, D.; Sriyanti, I.; Suciati, T.; Munir, M.M.; Khairurrijal, K. Electrospun polyvinylpyrrolidone (PVP)/green tea extract composite nanofiber mats and their antioxidant activities. Mater. Res. Express 2018, 5, 054001. [Google Scholar] [CrossRef]
- Pateiro, M.; Lorenzo, J.M.; Amado, I.R.; Franco, D. Effect of addition of green tea, chestnut and grape extract on the shelf-life of pig liver pâté. Food Chem. 2014, 147, 386–394. [Google Scholar] [CrossRef]
- Jongberg, S.; Skov, S.H.; Tørngren, M.A.; Skibsted, L.H.; Lund, M.N. Effect of white grape extract and modified atmosphere packaging on lipid and protein oxidation in chill stored beef patties. Food Chem. 2011, 128, 276–283. [Google Scholar] [CrossRef]
- Alirezalu, K.; Hesari, J.; Eskandari, M.H.; Valizadeh, H.; Sirousazar, M. Effect of green tea, stinging nettle and olive leaves extracts on the quality and shelf life stability of frankfurter type sausage. J. Food Process. Preserv. 2017, 41, e13100. [Google Scholar] [CrossRef]
- Alav, A.; Kutlu, N.; Kına, E.; Meral, R. A novel green tea extract-loaded nanofiber coating for kiwi fruit: Improved microbial stability and nutritional quality. Food Biosci. 2024, 62, 105043. [Google Scholar] [CrossRef]
- Temel-Soylu, T.l.M.; Keçeciler-Emir, C.; Rababah, T.; Özel, C.; Yücel, S.; Basaran-Elalmis, Y.; Altan, D.; Kirgiz, O.m.; Seçinti, İ.E.; Kaya, U. Green electrospun poly (vinyl alcohol)/gelatin-based nanofibrous membrane by incorporating 45S5 bioglass nanoparticles and urea for wound dressing applications: Characterization and in vitro and in vivo evaluations. ACS Omega 2024, 9, 21187–21203. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wu, J.; Li, C.; Chen, X.; Cui, H. Fabrication of a dual-response intelligent antibacterial nanofiber and its application in beef preservation. LWT 2022, 154, 112606. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Hossain, M.J.; Lee, E.-Y.; Kim, S.-H.; Hwang, Y.-H.; Joo, S.-T. Characterization of imitated hybrid cultured meat patty to compare with beef meat. Food Sci. Anim. Resour. 2024. [Google Scholar] [CrossRef]
- Ulbricht, T.; Southgate, D. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- King, D.A.; Hunt, M.C.; Barbut, S.; Claus, J.R.; Cornforth, D.P.; Joseph, P.; Kim, Y.H.B.; Lindahl, G.; Mancini, R.A.; Nair, M.N. American Meat Science Association guidelines for meat color measurement. Meat Muscle Biol. 2023, 6, 12473. [Google Scholar] [CrossRef]
- Joo, S.-T. Determination of water-holding capacity of porcine musculature based on released water method using optimal load. Korean J. Food Sci. Anim. Resour. 2018, 38, 823. [Google Scholar] [CrossRef]
- De Palo, P.; Tateo, A.; Maggiolino, A.; Centoducati, P. Effect of nutritive level on carcass traits and meat quality of IHDH foals. Anim. Sci. J. 2014, 85, 780–786. [Google Scholar] [CrossRef]
- Sadakuzzaman, M.; Rahman, M.M.; Hashem, M.A.; Ali, M.S.; Alam, A.N. Synergistic effects of gamma irradiation and butylated hydroxyanisole on the sensory, physicochemical, and microbiological quality of beef. Appl. Food Res. 2024, 4, 100547. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.-H.; Joo, S.-T. Interventions of two-stage thermal sous-vide cooking on the toughness of beef semitendinosus. Meat Sci. 2019, 157, 107882. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Luan, A.; Li, X.; Wang, F.; Huang, Y.; Li, A.; Liu, Y. Protein degradation and aggregation in silver carp (Hypophthalmichthys molitrix) muscle during hot air drying. LWT 2022, 163, 113540. [Google Scholar] [CrossRef]
- ISO 3496:1994; Meat and Meat Products. Determination of Hydroxyproline Content. International Organization Standard: Geneva, Switzerland, 1994.
- Xiong, X.; Zhang, H.; Zhuang, S.; Wang, K.; Ding, N.; Hong, H.; Chen, L.; Tan, Y.; Luo, Y. From texture to structure: The key role of caspase-3 on grass carp myofibrillar protein during postmortem storage. Food Biosci. 2024, 61, 104858. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.-H.; Joo, S.-T. Low-temperature and long-time heating regimes on non-volatile compound and taste traits of beef assessed by the electronic tongue system. Food Chem. 2020, 320, 126656. [Google Scholar] [CrossRef]
- Mahmud, M.M.; Zaman, S.; Perveen, A.; Jahan, R.A.; Islam, M.F.; Arafat, M.T. Controlled release of curcumin from electrospun fiber mats with antibacterial activity. J. Drug Deliv. Sci. Technol. 2020, 55, 101386. [Google Scholar] [CrossRef]
- Ceylan, Z.; Kutlu, N.; Meral, R.; Ekin, M.M.; Kose, Y.E. Protective effect of grape seed oil-loaded nanofibers: Limitation of microbial growth and lipid oxidation in kashar cheese and fish meat samples. Food Biosci. 2021, 42, 101076. [Google Scholar] [CrossRef]
- Zhang, Y.; Venugopal, J.; Huang, Z.-M.; Lim, C.T.; Ramakrishna, S. Crosslinking of the electrospun gelatin nanofibers. Polymer 2006, 47, 2911–2917. [Google Scholar] [CrossRef]
- Amirsadeghi, A.; Khorram, M.; Hashemi, S.S. Preparation of multilayer electrospun nanofibrous scaffolds containing soluble eggshell membrane as potential dermal substitute. J. Biomed. Mater. Res. Part A 2021, 109, 1812–1827. [Google Scholar] [CrossRef]
- Toledo, L.; Racine, L.; Pérez, V.; Henríquez, J.P.; Auzely-Velty, R.; Urbano, B.F. Physical nanocomposite hydrogels filled with low concentrations of TiO2 nanoparticles: Swelling, networks parameters and cell retention studies. Mater. Sci. Eng. C 2018, 92, 769–778. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, X.; Li, B.; Huang, G.; Xu, F.; Zhang, X. Solvent-free fabrication of carbon nanotube/silk fibroin electrospun matrices for enhancing cardiomyocyte functionalities. ACS Biomater. Sci. Eng. 2020, 6, 1630–1640. [Google Scholar] [CrossRef]
- Yim, D.-G.; Jo, C.; Mahabbat, A.; Park, J.-Y.; Lee, S.-Y.; Nam, K.-C. Combined effect of aging and irradiation on physicochemical quality of pork shoulder. Food Sci. Anim. Resour. 2019, 39, 510. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.-M.; Lee, E.-Y.; Alam, A.N.; Samad, A.; Hossain, M.J.; Hwang, Y.-H.; Seo, J.-K.; Kim, C.-B.; Choi, J.-H.; Joo, S.-T. The application of high-intensity ultrasound on wet-dry combined aged pork loin induces physicochemical and oxidative alterations. Food Sci. Anim. Resour. 2024, 44, 899. [Google Scholar] [CrossRef] [PubMed]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Ma, J.; Sun, D.-W. Enhancing physical and chemical quality attributes of frozen meat and meat products: Mechanisms, techniques and applications. Trends Food Sci. Technol. 2022, 124, 63–85. [Google Scholar] [CrossRef]
- Sameen, D.E.; Ahmed, S.; Lu, R.; Li, R.; Dai, J.; Qin, W.; Zhang, Q.; Li, S.; Liu, Y. Electrospun nanofibers food packaging: Trends and applications in food systems. Crit. Rev. Food Sci. Nutr. 2022, 62, 6238–6251. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun functional materials toward food packaging applications: A review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef]
- Lan, X.; Luo, T.; Zhong, Z.; Huang, D.; Liang, C.; Liu, Y.; Wang, H.; Tang, Y. Green cross-linking of gelatin/tea polyphenol/ε-poly (L-lysine) electrospun nanofibrous membrane for edible and bioactive food packaging. Food Packag. Shelf Life 2022, 34, 100970. [Google Scholar] [CrossRef]
- Shao, P.; Niu, B.; Chen, H.; Sun, P. Fabrication and characterization of tea polyphenols loaded pullulan-CMC electrospun nanofiber for fruit preservation. Int. J. Biol. Macromol. 2018, 107, 1908–1914. [Google Scholar] [CrossRef]
- Gao, Y.; Hsu, Y.-I.; Uyama, H. Lignocellulosic Nanofibers Composite Films Reinforced with Green-Synthesized Copper Oxide Nanoparticles for Active Packaging. Polym. Degrad. Stab. 2025, 239, 111400. [Google Scholar] [CrossRef]
- Baysal, G.; Doğan, F. Investigation and preparation of biodegradable starch-based nanofilms for potential use of curcumin and garlic in food packaging applications. J. Biomater. Sci. Polym. Ed. 2020, 31, 1127–1143. [Google Scholar] [CrossRef]
- Durmuş, M.; Ozogul, Y.; Köşker, A.R.; Ucar, Y.; Boğa, E.K.; Ceylan, Z.; Ozogul, F. The function of nanoemulsion on preservation of rainbow trout fillet. J. Food Sci. Technol. 2020, 57, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Sykuła, A.; Janiak-Włodarczyk, I.; Kapusta, I.T. Formulation and Evaluation of the Antioxidant Activity of an Emulsion Containing a Commercial Green Tea Extract. Molecules 2025, 30, 197. [Google Scholar] [CrossRef] [PubMed]
- Abid, H.; Abid, Z.; Abid, S. Atherogenic indices in clinical practice and biomedical research: A short review. Baghdad J. Biochem. Appl. Biol. Sci. 2021, 2, 60–70. [Google Scholar] [CrossRef]
- Tsuzuki, W. Effects of antioxidants on heat-induced trans fatty acid formation in triolein and trilinolein. Food Chem. 2011, 129, 104–109. [Google Scholar] [CrossRef]
- Rehim, M.A.; Zahran, H.A.; Al-Moghazy, M. Synthesis of active packaging films from Lepidium sativum gum/polyvinyl alcohol composites and their application in preserving cheddar cheese. Sci. Rep. 2023, 13, 1647. [Google Scholar] [CrossRef]
- Salueña, B.H.; Gamasa, C.S.; Rubial, J.M.D.; Odriozola, C.A. CIELAB color paths during meat shelf life. Meat Sci. 2019, 157, 107889. [Google Scholar] [CrossRef]
- Dissanayake, K.; Samadiy, M.; Rifky, A.; Nurmukhamedov, K. Effect of myoglobin content on the beef meat color during storage: New technology for accurate evaluation of meat color. Sci. Innov. 2024, 3, 24–31. [Google Scholar] [CrossRef]
- Schilling, M.; Pham, A.; Williams, J.; Xiong, Y.; Dhowlaghar, N.; Tolentino, A.; Kin, S. Changes in the physiochemical, microbial, and sensory characteristics of fresh pork sausage containing rosemary and green tea extracts during retail display. Meat Sci. 2018, 143, 199–209. [Google Scholar] [CrossRef]
- Mendes, C.G.; Martins, J.T.; Lüdtke, F.L.; Geraldo, A.; Pereira, A.; Vicente, A.A.; Vieira, J.M. Chitosan coating functionalized with flaxseed oil and green tea extract as a bio-based solution for beef preservation. Foods 2023, 12, 1447. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sineiro, J.; Amado, I.R.; Franco, D. Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci. 2014, 96, 526–534. [Google Scholar] [CrossRef]
- Borzi, F.; Torrieri, E.; Wrona, M.; Nerín, C. Polyamide modified with green tea extract for fresh minced meat active packaging applications. Food Chem. 2019, 300, 125242. [Google Scholar] [CrossRef] [PubMed]
- Passos, R.S.F.T.; Barreto, B.G.; Leite, J.S.A.F.; Trevisan, A.B.; de Souza, C.O.; da Silva, M.C.A.; Cavalheiro, C.P. Green tea extract as natural preservative in chicken patties: Effects on physicochemical, microbiological, and sensory properties. J. Food Process. Preserv. 2022, 46, e16224. [Google Scholar] [CrossRef]
- Kırmızıkaya, G.; Karakaya, M.; Babaoğlu, A.S. Black, green, and white tea infusions and powder forms improve oxidative stability of minced beef throughout refrigerated storage. J. Food Process. Preserv. 2021, 45, e15359. [Google Scholar] [CrossRef]
- Montaño-Sánchez, E.; Torres-Martínez, B.d.M.; Vargas-Sánchez, R.D.; Huerta-Leidenz, N.; Sánchez-Escalante, A.; Beriain, M.J.; Torrescano-Urrutia, G.R. Effects of chitosan coating with green tea aqueous extract on lipid oxidation and microbial growth in pork chops during chilled storage. Foods 2020, 9, 766. [Google Scholar] [CrossRef]
- Yoon, J.; Bae, S.M.; Gwak, S.H.; Jeong, J.Y. Use of green tea extract and rosemary extract in naturally cured pork sausages with white kimchi powder. Food Sci. Anim. Resour. 2021, 41, 840. [Google Scholar] [CrossRef]
- Fang, Z.; Lin, D.; Warner, R.D.; Ha, M. Effect of gallic acid/chitosan coating on fresh pork quality in modified atmosphere packaging. Food Chem. 2018, 260, 90–96. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, G.; Wang, J.; Li, C.; Cui, H.; Lin, L. Application of glycyrrhiza polysaccharide nanofibers loaded with tea tree essential oil/gliadin nanoparticles in meat preservation. Food Biosci. 2021, 43, 101270. [Google Scholar] [CrossRef]
- Bertram, H.C.; Kristensen, M.; Østdal, H.; Baron, C.P.; Young, J.F.; Andersen, H.J. Does oxidation affect the water functionality of myofibrillar proteins? J. Agric. Food Chem. 2007, 55, 2342–2348. [Google Scholar] [CrossRef]
- Kalem, I.K.; Bhat, Z.; Kumar, S.; Desai, A. Terminalia arjuna: A novel natural preservative for improved lipid oxidative stability and storage quality of muscle foods. Food Sci. Hum. Wellness 2017, 6, 167–175. [Google Scholar] [CrossRef]
- Lin, L.; Mei, C.; Shi, C.; Li, C.; Abdel-Samie, M.A.; Cui, H. Preparation and characterization of gelatin active packaging film loaded with eugenol nanoparticles and its application in chicken preservation. Food Biosci. 2023, 53, 102778. [Google Scholar] [CrossRef]
- Hamann, D.; Puton, B.M.S.; Comin, T.; Colet, R.; Valduga, E.; Zeni, J.; Steffens, J.; Junges, A.; Backes, G.T.; Cansian, R.L. Active edible films based on green tea extract and gelatin for coating of fresh sausage. Meat Sci. 2022, 194, 108966. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Peng, C.; Zeng, D.; Yang, Z.; Wang, X.; Zhang, S.; Bai, Y.; Kuang, L.; Guo, L.; Qin, Y. Electrospinning is a potential way for preparing edible plant protein-based nanofibers with porous surface using safflower seed meal. Food Hydrocoll. 2024, 146, 109201. [Google Scholar] [CrossRef]
- Warner, R.D.; Wheeler, T.L.; Ha, M.; Li, X.; Bekhit, A.E.-D.; Morton, J.; Vaskoska, R.; Dunshea, F.R.; Liu, R.; Purslow, P. Meat tenderness: Advances in biology, biochemistry, molecular mechanisms and new technologies. Meat Sci. 2022, 185, 108657. [Google Scholar] [CrossRef]
- Bellucci, E.R.B.; Bis-Souza, C.V.; Domínguez, R.; Bermúdez, R.; Barretto, A.C.d.S. Addition of natural extracts with antioxidant function to preserve the quality of meat products. Biomolecules 2022, 12, 1506. [Google Scholar] [CrossRef]
- Han, J.H.; Keum, D.H.; Kothuri, V.; Kim, Y.-J.; Kwon, H.C.; Jung, H.S.; Han, S.G. Enhancing emulsion, texture, rheological and sensory properties of plant-based meat analogs with green tea extracts. Food Chem. X 2024, 24, 101807. [Google Scholar] [CrossRef]
- Calder, P.C. Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Calder, P.C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef]
- Xing, W.; Liu, W.; Li, H.; Xing, S. Preservative effect of tea polyphenols and hydrogen peroxide on quality of chilled meat. Emir. J. Food Agric. 2024, 36, 1–5. [Google Scholar] [CrossRef]
- Chongsrimsirisakhol, O.; Pirak, T. Total polyphenol content and antioxidant properties of cold brew coffee extracts as affected by ultrasound treatment and their application in low fat pork sausage. Int. J. Food Prop. 2022, 25, 813–826. [Google Scholar] [CrossRef]
- Nikoo, M.; Regenstein, J.M.; Ahmadi Gavlighi, H. Antioxidant and antimicrobial activities of (-)-epigallocatechin-3-gallate (EGCG) and its potential to preserve the quality and safety of foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 732–753. [Google Scholar] [CrossRef] [PubMed]
- U Nisa, I.; Ashwar, B.A.; Shah, A.; Gani, A.; Gani, A.; Masoodi, F.A. Development of potato starch based active packaging films loaded with antioxidants and its effect on shelf life of beef. J. Food Sci. Technol. 2015, 52, 7245–7253. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Liu, Z.; Fan, L.; Dong, T.; Jin, Y.; Saldaña, M.D.; Sun, W. Sustained-release antibacterial pads based on nonwovens polyethylene terephthalate modified by β-cyclodextrin embedded with cinnamaldehyde for cold fresh pork preservation. Food Packag. Shelf Life 2020, 26, 100554. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Noipha, S. Active film from chitosan incorporating green tea extract for shelf life extension of pork sausages. Food Hydrocoll. 2012, 27, 102–108. [Google Scholar] [CrossRef]
- Chaijan, S.; Panpipat, W.; Panya, A.; Cheong, L.-Z.; Chaijan, M. Preservation of chilled Asian sea bass (Lates calcarifer) steak by whey protein isolate coating containing polyphenol extract from ginger, lemongrass, or green tea. Food Control 2020, 118, 107400. [Google Scholar] [CrossRef]
- Karim, M.; Fathi, M.; Soleimanian-Zad, S. Nanoencapsulation of cinnamic aldehyde using zein nanofibers by novel needle-less electrospinning: Production, characterization and their application to reduce nitrite in sausages. J. Food Eng. 2021, 288, 110140. [Google Scholar] [CrossRef]
- Schulte, M.D.; Johnson, L.G.; Zuber, E.A.; Patterson, B.M.; Outhouse, A.C.; Fedler, C.A.; Steadham, E.M.; King, D.A.; Prusa, K.J.; Huff-Lonergan, E. Influence of postmortem aging and post-aging freezing on pork loin quality attributes. Meat Muscle Biol. 2019, 3. [Google Scholar] [CrossRef]
- Valenzuela, C.; Garcia-Galicia, I.A.; Paniwnyk, L.; Alarcon-Rojo, A.D. Physicochemical characteristics and shelf life of beef treated with high-intensity ultrasound. J. Food Process. Preserv. 2021, 45, e15350. [Google Scholar] [CrossRef]
- Parés, D.; Pèlach, M.À.; Toldrà, M.; Saguer, E.; Tarrés, Q.; Carretero, C. Nanofibrillated cellulose as functional ingredient in emulsion-type meat products. Food Bioprocess Technol. 2018, 11, 1393–1401. [Google Scholar] [CrossRef]
- Toldrá, F.; Etherington, D.J. Examination of cathepsins B, D, H and L activities in dry-cured hams. Meat Sci. 1988, 23, 1–7. [Google Scholar] [CrossRef]
- Kaur, L.; Hui, S.X.; Morton, J.D.; Kaur, R.; Chian, F.M.; Boland, M. Endogenous proteolytic systems and meat tenderness: Influence of post-mortem storage and processing. Food Sci. Anim. Resour. 2021, 41, 589. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Luan, A.; Yi, S.; Wu, J.; Wang, F.; Liu, Y.; Li, X. Moderate protein degradation and lipid oxidation induced by cold plasma and its effect on the quality of dried fish products. J. Food Compos. Anal. 2023, 123, 105636. [Google Scholar] [CrossRef]
- Zhou, C.-Y.; Cao, J.-X.; Zhuang, X.-B.; Bai, Y.; Li, C.-B.; Xu, X.-L.; Zhou, G.-H. Evaluation of the secondary structure and digestibility of myofibrillar proteins in cooked ham. CyTA-J. Food 2019, 17, 78–86. [Google Scholar] [CrossRef]
- Wang, R.; Guo, F.; Zhao, J.; Feng, C. Myofibril degradation and structural changes in myofibrillar proteins of porcine longissimus muscles during frozen storage. Food Chem. 2024, 435, 137671. [Google Scholar] [CrossRef]
- Keller, M.R.; Dörr, T. Bacterial metabolism and susceptibility to cell wall-active antibiotics. Adv. Microb. Physiol. 2023, 83, 181–219. [Google Scholar] [CrossRef]
- Sejuk, S. Extraction of pomegranate peel and green tea leaves and their effects on the microbial, physicochemical, microstructural and sensorial properties of chilled-stored chicken meat. Malays. J. Anal. Sci. 2021, 25, 569–583. [Google Scholar]
- Sun, Y.; Zhang, M.; Bhandari, B.; Bai, B. Nanoemulsion-based edible coatings loaded with fennel essential oil/cinnamaldehyde: Characterization, antimicrobial property and advantages in pork meat patties application. Food Control 2021, 127, 108151. [Google Scholar] [CrossRef]
- Jiang, S.; Guo, T.; Liu, J.; Liu, T.; Gong, W. Biodegradable antimicrobial films prepared in a continuous way by melt extrusion using plant extracts as effective components. Food Chem. 2025, 464, 141643. [Google Scholar] [CrossRef]
- Duan, M.; Sun, J.; Huang, Y.; Jiang, H.; Hu, Y.; Pang, J.; Wu, C. Electrospun gelatin/chitosan nanofibers containing curcumin for multifunctional food packaging. Food Sci. Hum. Wellness 2023, 12, 614–621. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Wang, S.; Qin, W.; Zhang, Q. Electrospun antimicrobial polylactic acid/tea polyphenol nanofibers for food-packaging applications. Polymers 2018, 10, 561. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.; Bautista, D. Antibacterial activity of green tea polyphenols against Escherichia coli K 12. Nahrung 2000, 44, 60–62. [Google Scholar] [CrossRef]
- Parvez, M.A.K.; Saha, K.; Rahman, J.; Munmun, R.A.; Rahman, M.A.; Dey, S.K.; Rahman, M.S.; Islam, S.; Shariare, M.H. Antibacterial activities of green tea crude extracts and synergistic effects of epigallocatechingallate (EGCG) with gentamicin against MDR pathogens. Heliyon 2019, 5, e02126. [Google Scholar] [CrossRef] [PubMed]
- Vodnar, D.C. Inhibition of Listeria monocytogenes ATCC 19115 on ham steak by tea bioactive compounds incorporated into chitosan-coated plastic films. Chem. Cent. J. 2012, 6, 74. [Google Scholar] [CrossRef]
- Mickymaray, S. Efficacy and mechanism of traditional medicinal plants and bioactive compounds against clinically important pathogens. Antibiotics 2019, 8, 257. [Google Scholar] [CrossRef]
- Hossain, M.J.; Alam, A.N.; Hwang, Y.-H.; Samad, A.; Joo, S.-T. Metabolomic profiling of free amino acids and nucleotides to uncover the key mechanisms enhancing umami taste, complemented by an in-depth investigation of physicochemical and textural changes in Hanwoo beef during wet aging. Food Biosci. 2025, 68, 106631. [Google Scholar] [CrossRef]
- Alam, A.; Lee, E.-Y.; Hossain, M.J.; Kim, S.-H.; Kim, C.-J.; Hwang, Y.-H.; Joo, S.-T. Physicochemical and sensory characteristics of hybrid flexitarian pork loin steak combined with different plant ingredients. Food Sci. Anim. Resour. 2024, 45, 468–483. [Google Scholar] [CrossRef]
- Guo, X.; Jiang, S.; Wang, L.; Jin, K.; Ma, J.; Zhou, Q. Insight into the taste profiles of green tea made with fresh tea leaves plucked in summer. Br. Food J. 2025. [Google Scholar] [CrossRef]
- Zhang, D.; Ge, X.; Jiao, Y.; Liu, Y. The protective effects of black tea as nitrite replacer on the oxidation, physicochemical and sensory properties of steamed beef. LWT 2023, 188, 115375. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Lin, L. Plasma-treated poly (ethylene oxide) nanofibers containing tea tree oil/beta-cyclodextrin inclusion complex for antibacterial packaging. Carbohydr. Polym. 2018, 179, 360–369. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Li, C.; Liu, R.; Lin, L. Fabrication of chitosan nanofibers containing tea tree oil liposomes against Salmonella spp. in chicken. LWT 2018, 96, 671–678. [Google Scholar] [CrossRef]
- Xia, Y.; Tang, X.; Wu, X.; Li, Y.; Xu, C.; Chen, S.; Wu, P. Electrospun chitosan-based nanofibers loading tea tree oil for fresh salmon fillet shelf-life extension. J. Food Sci. 2023, 88, 3075–3089. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Alam, A.N.; Hossain, M.J.; Lee, E.-Y.; Hwang, Y.-H.; Joo, S.-T. Sensory Evaluation of Plant-Based Meat: Bridging the Gap with Animal Meat, Challenges and Future Prospects. Foods 2023, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kumari, S.; Kim, C.-J.; Lee, E.-Y.; Alam, A.N.; Chung, Y.-S.; Hwang, Y.-H.; Joo, S.-T. Effect of Adding Cultured Meat Tissue on Physicochemical and Taste Characteristics of Hybrid Cultured Meat Manufactured Using Wet-Spinning. Food Sci. Anim. Resour. 2024, 44, 1440. [Google Scholar] [CrossRef] [PubMed]


| Nutritional Parameters | PEP | HGGTNF 1% | HGGTNF 2% | HGGTNF 3% | p Value |
|---|---|---|---|---|---|
| DM | 35.68 ± 0.02 a | 34.04 ± 0.02 c | 34.49 ± 0.03 b | 34.67 ± 0.04 b | 0.0001 |
| Moisture | 64.32 ± 0.02 d | 65.92 ± 0.02 a | 65.51 ± 0.02 c | 65.63 ± 0.03 b | 0.0001 |
| CP | 21.11 ± 0.03 d | 22.17 ± 0.02 c | 22.41 ± 0.02 b | 22.50 ± 0.02 a | 0.0001 |
| CF | 10.98 ± 0.02 d | 11.11 ± 0.02 c | 11.20 ± 0.02 b | 11.34 ± 0.03 a | 0.0001 |
| Ash | 1.04 ± 0.02 b | 1.08 ± 0.02 ab | 1.15 ± 0.02 a | 1.15 ± 0.03 a | 0.0206 |
| Fatty acid profile | PEP | HGGTNF 1% | HGGTNF 2% | HGGTNF 3% | p Value |
| Lauric acid (C12:0) | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 | NS |
| Myristic acid (C14:0) | 2.36 ± 0.00 d | 2.39 ± 0.01 c | 2.41 ± 0.01 b | 2.52 ± 0.01 a | 0.0001 |
| Myristoleic acid (C14:1) | 0.74 ± 0.01 b | 0.75 ± 0.01 b | 0.75 ± 0.01 b | 0.78 ± 0.01 a | 0.0181 |
| Palmitic acid (C16:0) | 19.46 ± 0.01 d | 19.81 ± 0.00 c | 19.98 ± 0.01 b | 20.24 ± 0.00 a | 0.0001 |
| Palmitoleic acid (C16:1) | 4.45 ± 0.00 d | 4.61 ± 0.01 c | 4.65 ± 0.01 b | 4.81 ± 0.00 a | 0.0001 |
| Stearic acid (C18:0) | 7.78 ± 0.01 d | 7.89 ± 0.01 c | 7.97 ± 0.01 b | 8.19 ± 0.01 a | 0.0001 |
| Oleic acid (C18:1n9c) | 46.17 ± 0.01 d | 47.94 ± 0.00 c | 48.11 ± 0.00 b | 48.64 ± 0.00 a | 0.0001 |
| Linoleic acid (C18:2n6c) | 2.79 ± 0.01 d | 2.89 ± 0.01 c | 2.95 ± 0.01 b | 3.07 ± 0.00 a | 0.0001 |
| α-Linolenic acid (C18:3n3) | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.00 | 0.16 ± 0.01 | NS |
| Arachidic acid (C20:0) | 0.16 ± 0.01 c | 0.16 ± 0.00 bc | 0.17 ± 0.00 b | 0.19 ± 0.01 a | 0.0138 |
| arachidonic acid (C20:4n6) | 0.30 ± 0.01 c | 0.31 ± 0.01 bc | 0.33 ± 0.01 ab | 0.34 ± 0.00 a | 0.0292 |
| eicosapentaenoic acid (C20:5n3) | 0.31 ± 0.02 | 0.32 ± 0.01 | 0.34 ± 0.03 | 0.35 ± 0.00 | NS |
| C22:6n3 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | NS |
| SFA | 29.8 ± 0.01 d | 30.29 ± 0.02 c | 30.57 ± 0.02 b | 31.18 ± 0.00 a | 0.0001 |
| MUFA | 51.35 ± 0.02 d | 53.29 ± 0.01 c | 53.50 ± 0.02 b | 54.22 ± 0.01 a | 0.0001 |
| PUFA | 3.57 ± 0.04 d | 3.69 ± 0.00 c | 3.79 ± 0.03 b | 3.93 ± 0.01 a | 0.0013 |
| AI | 0.40 ± 0.00 a | 0.39 ± 0.00 c | 0.39 ± 0.00 b | 0.39 ± 0.00 b | 0.0002 |
| Color Parameters | PEP | HGGTNF 1% | HGGTNF 2% | HGGTNF 3% | p-Value |
|---|---|---|---|---|---|
| L* | 36.53 ± 0.07 | 36.58 ± 0.06 | 36.76 ± 0.19 | 36.78 ± 0.15 | NS |
| a* | 16.55 ± 0.2 c | 17.10 ± 0.21 c | 18.14 ± 0.36 b | 19.41 ± 0.30 a | 0.0004 |
| b* | 8.88 ± 0.14 | 8.55 ± 0.16 | 8.53 ± 0.16 | 8.39 ± 0.14 | NS |
| C* | 18.78 ± 0.24 c | 19.12 ± 0.21 c | 20.05 ± 0.30 b | 21.14 ± 0.30 a | 0.001 |
| h (°) | 28.2 ± 0.08 a | 26.58 ± 0.51 b | 25.21 ± 0.74 b | 23.39 ± 0.34 c | 0.0006 |
| Physicochemical attributes | |||||
| pH | 5.66 ± 0.02 b | 5.71 ± 0.02 b | 5.7 ± 0.02 b | 5.78 ± 0.01 a | 0.0100 |
| DL (%) | 1.21 ± 0.01 a | 1.20 ± 0.01 a | 1.17 ± 0.01 b | 1.15 ± 0.00 b | 0.0005 |
| CL (%) | 20.26 ± 0.03 a | 19.91 ± 0.01 b | 19.05 ± 0.04 c | 18.13 ± 0.03 d | 0.0001 |
| ERV (mL) | 20.55 ± 0.07 b | 20.52 ± 0.03 a | 19.27 ± 0.02 b | 19.09 ± 0.04 c | 0.0001 |
| PL (%) | 91.52 ± 0.05 a | 91.39 ± 0.06 a | 90.28 ± 0.04 b | 90.09 d ± 0.06 c | 0.0001 |
| Oxidative degradation parameters | |||||
| FFA (%) | 0.14 ± 0.01 a | 0.14 ± 0.02 a | 0.13 ± 0.01 ab | 0.12 ± 01 b | 0.0226 |
| POV (meq/kg) | 1.16 ± 0.07 a | 1.16 ± 0.06 ab | 1.15 ± 0.05 ab | 1.14 ± 0.06 b | 0.0407 |
| TBARS (mg-MDA/kg) | 0.29 ± 0.01 a | 0.26 ± 0.01 b | 0.25 ± 0.01 b | 0.23 ± 0.01 c | 0.0006 |
| Textural parameters | |||||
| WBSF (kg/cm2) | 4.66 ± 0.09 | 4.79 ± 0.11 | 4.92 ± 0.09 | 4.96 ± 0.12 | NS |
| Hardness (N) | 37.74 ± 0.21 b | 37.84 ± 0.14 b | 38.53 ± 0.14 a | 38.97 ± 0.14 a | 0.0001 |
| Springiness (cm) | 0.92 ± 0.01 | 0.93 ± 0.02 | 0.90 ± 0.02 | 0.87 ± 0.03 | NS |
| Guminess (N) | 23.25 ± 0.26 c | 24.60 ± 0.82 bc | 27.04 ± 1.0 ab | 27.35 ± 1.05 a | 0.0085 |
| Chewiness (N/cm) | 21.47 ± 0.20 | 22.95 ± 0.73 | 24.47 ± 1.17 | 23.85 ± 1.55 | NS |
| Cohesiveness | 0.62 ± 0.01 b | 0.65 ± 0.02 ab | 0.70 ± 0.03 a | 0.70 ± 0.03 a | 0.035 |
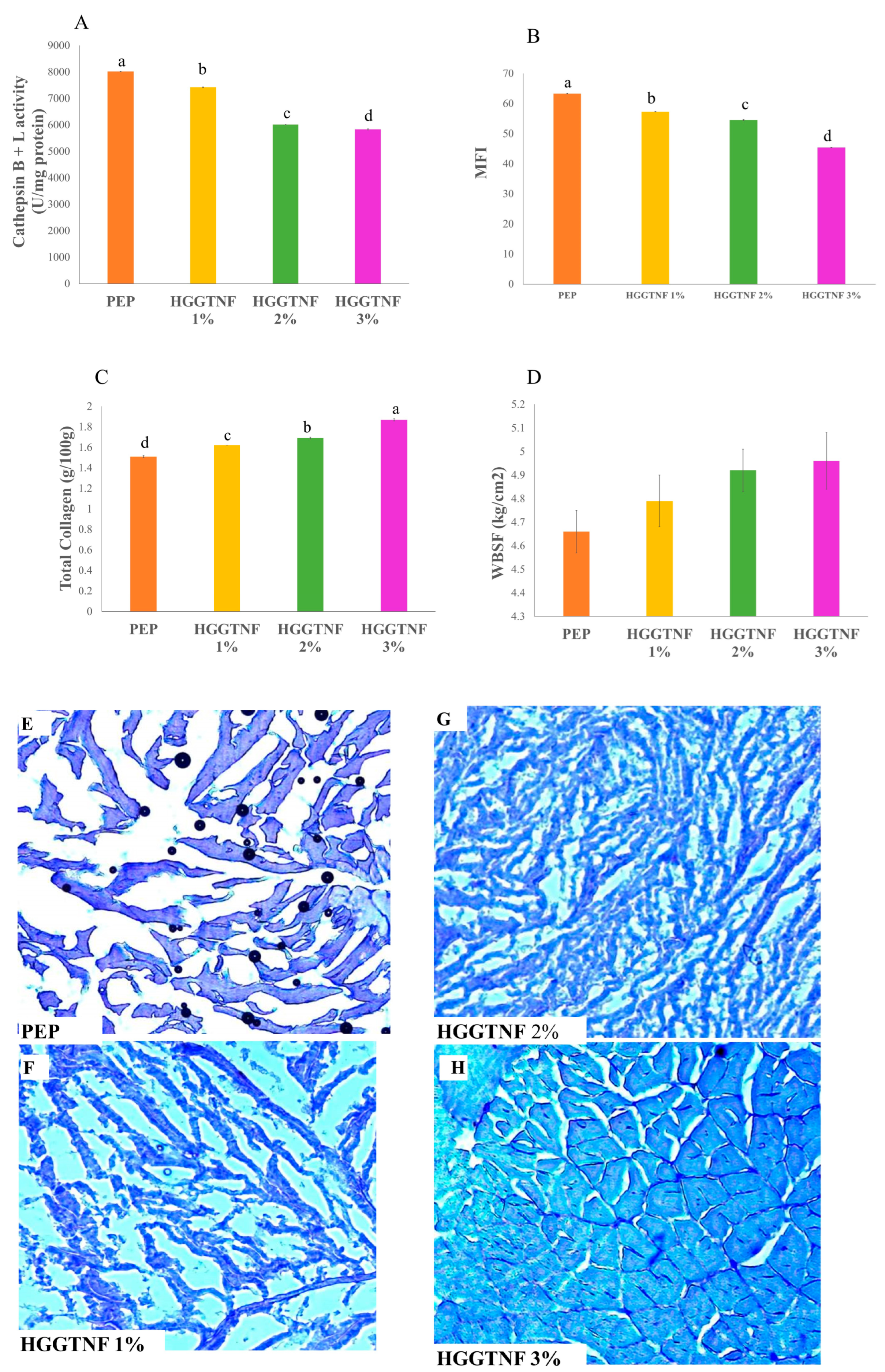
| Muscle degradation parameters | |||||
| Cathepsin B + L activity (U/mg protein) | 8009.80 ± 2.50 a | 7417.80 ± 16.06 b | 6009.80 ± 3.90 c | 5822.80 ± 20.16 d | 0.0001 |
| Total Collagen (g/100 g) | 1.51 ± 0.01 d | 1.62 ± 0.00 c | 1.69 ± 0.01 b | 1.87 ± 0.01 a | 0.0001 |
| MFI | 63.25 ± 0.07 a | 57.28 ± 0.07 b | 54.48 ± 0.17 c | 45.42 ± 0.06 d | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, A.M.M.N.; Hwang, Y.-H.; Samad, A.; Joo, S.-T. Meat Quality Traits Using Gelatin–Green Tea Extract Hybrid Electrospun Nanofiber Active Packaging. Foods 2025, 14, 1734. https://doi.org/10.3390/foods14101734
Alam AMMN, Hwang Y-H, Samad A, Joo S-T. Meat Quality Traits Using Gelatin–Green Tea Extract Hybrid Electrospun Nanofiber Active Packaging. Foods. 2025; 14(10):1734. https://doi.org/10.3390/foods14101734
Chicago/Turabian StyleAlam, A. M. M. Nurul, Young-Hwa Hwang, Abdul Samad, and Seon-Tea Joo. 2025. "Meat Quality Traits Using Gelatin–Green Tea Extract Hybrid Electrospun Nanofiber Active Packaging" Foods 14, no. 10: 1734. https://doi.org/10.3390/foods14101734
APA StyleAlam, A. M. M. N., Hwang, Y.-H., Samad, A., & Joo, S.-T. (2025). Meat Quality Traits Using Gelatin–Green Tea Extract Hybrid Electrospun Nanofiber Active Packaging. Foods, 14(10), 1734. https://doi.org/10.3390/foods14101734









