Integrated Chemical and Biological Evaluation of Linden Honeydew Honey from Bosnia and Herzegovina: Composition and Cellular Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Sample
2.2. Characterisation and Authentication of Linden Honeydew Honey Origin
2.2.1. Physicochemical Analysis
Water Content
Electrical Conductivity
Determination of Free Acidity
2.2.2. Colour Analysis
2.2.3. Pollen Analysis
2.2.4. Analysis of Volatile Compounds
2.3. Biological Activity
2.3.1. Antimicrobial Activity
Agar Well Diffusion Assay
Broth Dilution Method
2.3.2. Phenolic Content and Antioxidant Activity
Determination of Total Phenolic Content (TPC)
Determination of Total Flavones/Flavonols Content (TFC)
Determination of Total Flavanones/Dihydroflavonols Content
Determination of DPPH Radical Scavenging Activity
2.3.3. Assessment of Cytotoxicity (LC50)
2.3.4. Wound Healing Assay
3. Results and Discussion
3.1. Physicochemical Properties
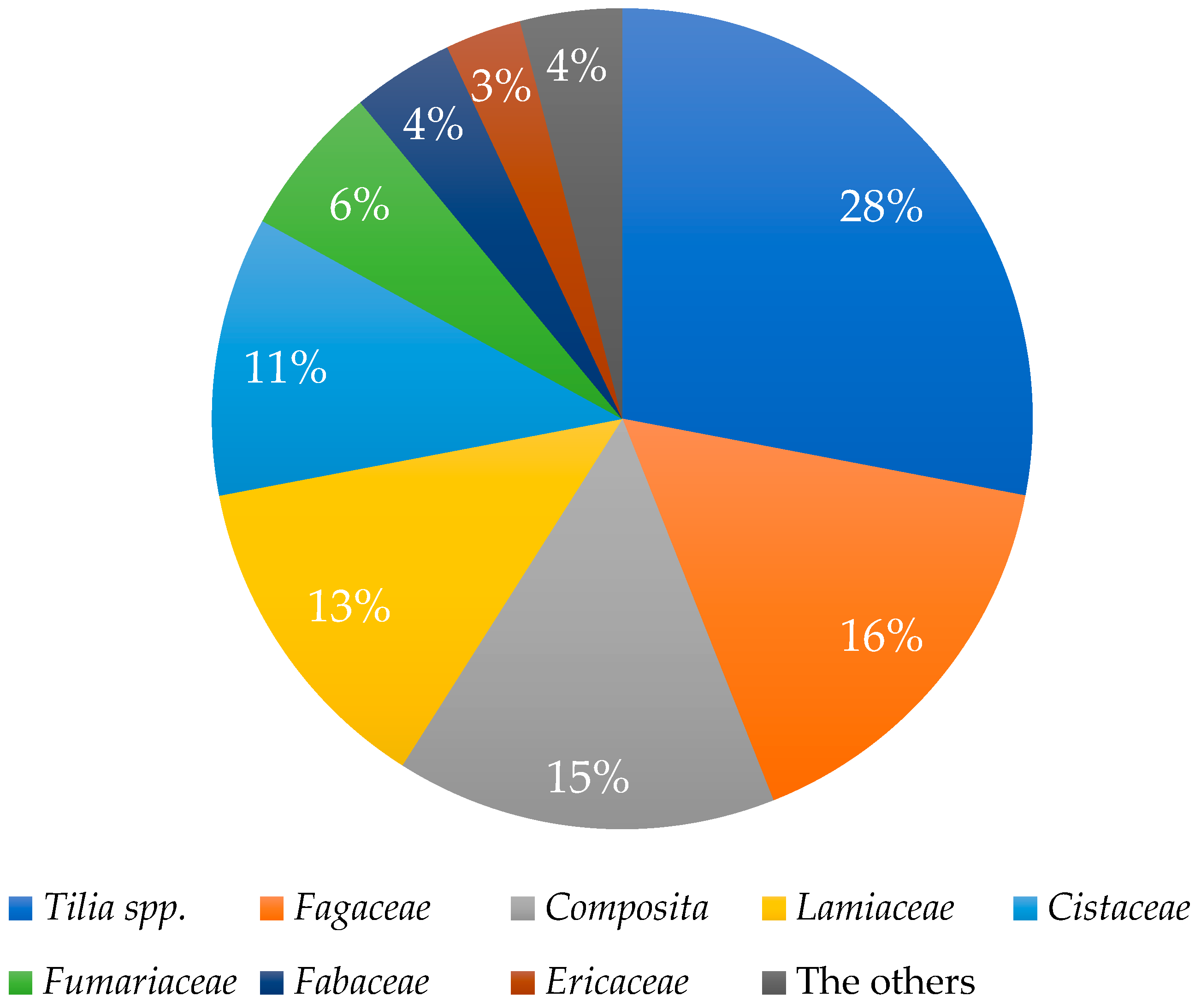
3.2. Pollen Analysis
3.3. Chemical Characterisation of Phenolic and Volatile Constituents
3.4. Biological Activity
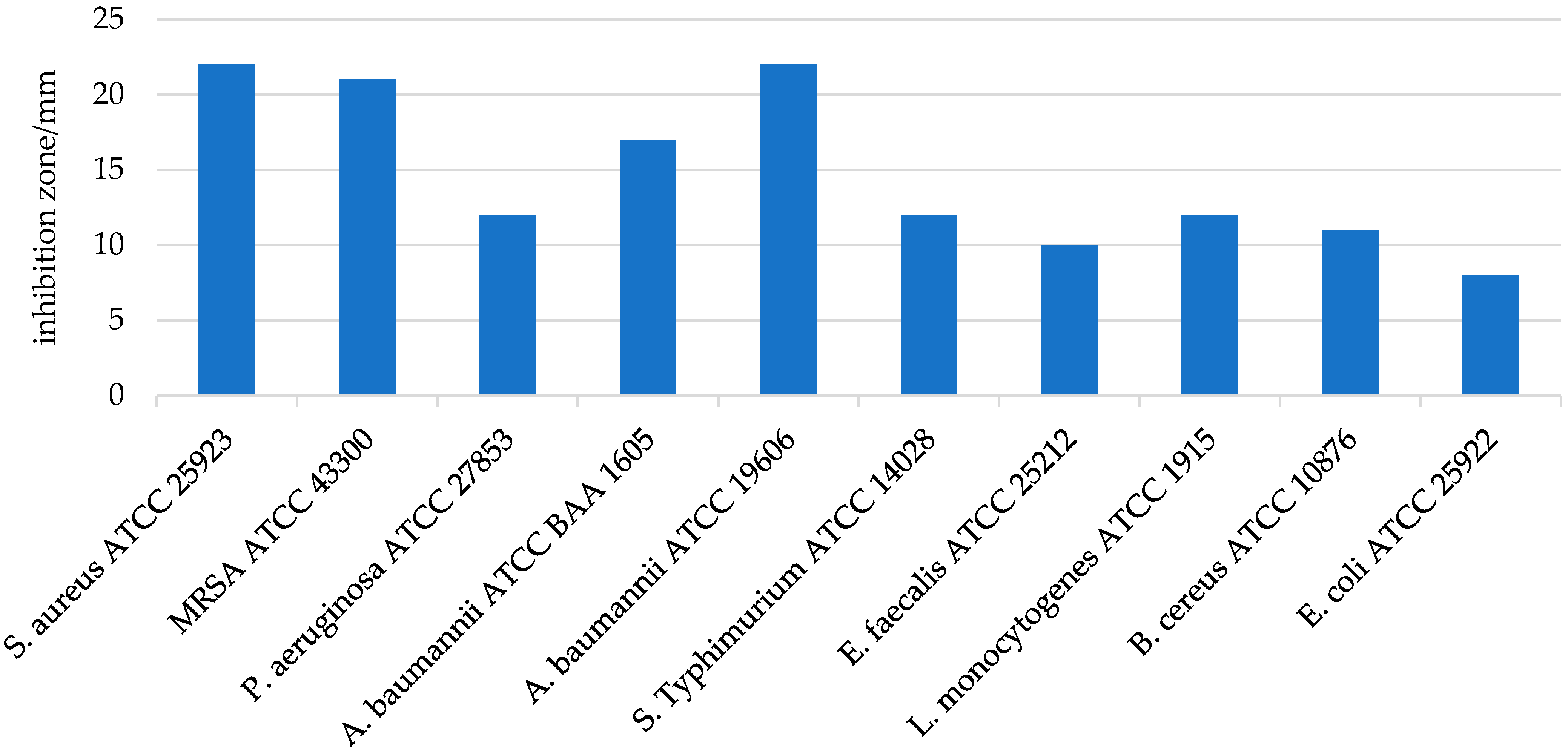
3.4.1. Antimicrobial Activity
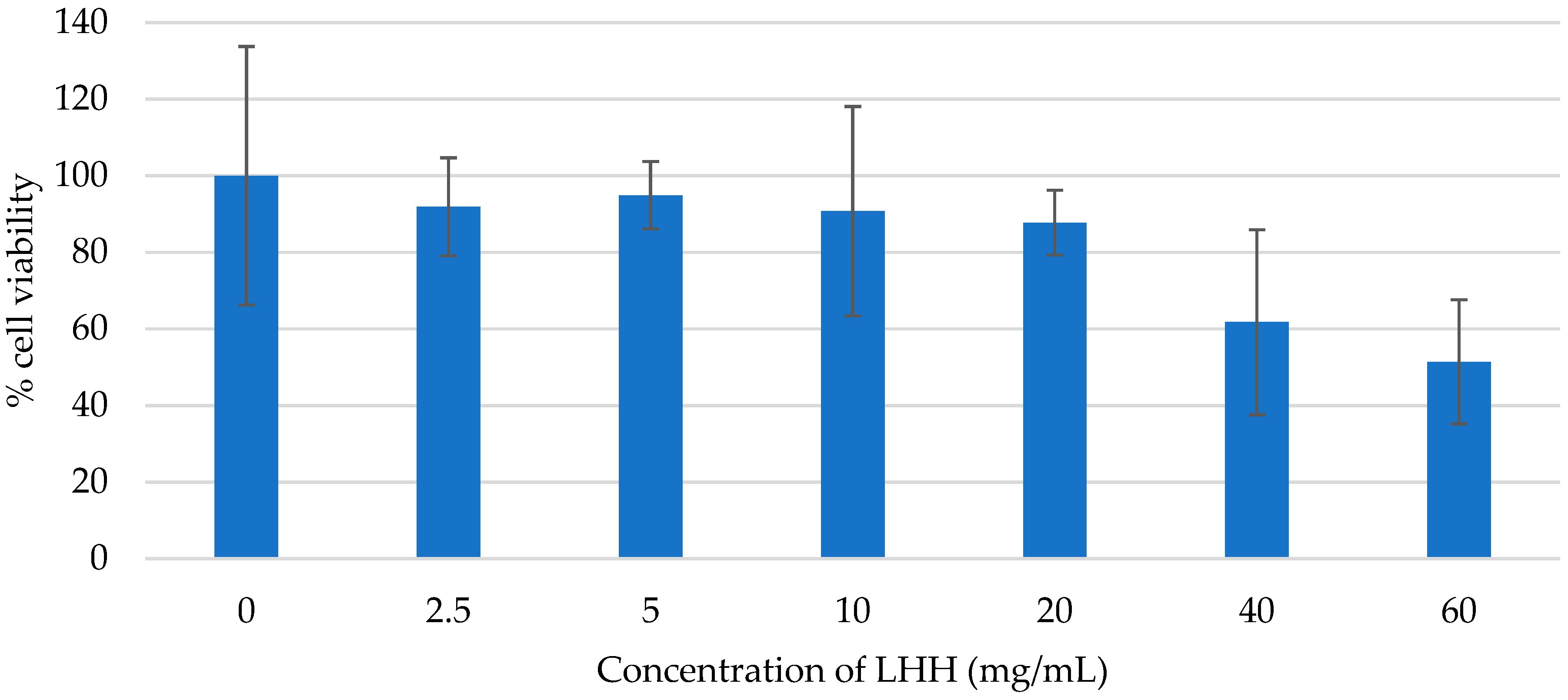
3.4.2. Assessment of Cytotoxicity (LC50)
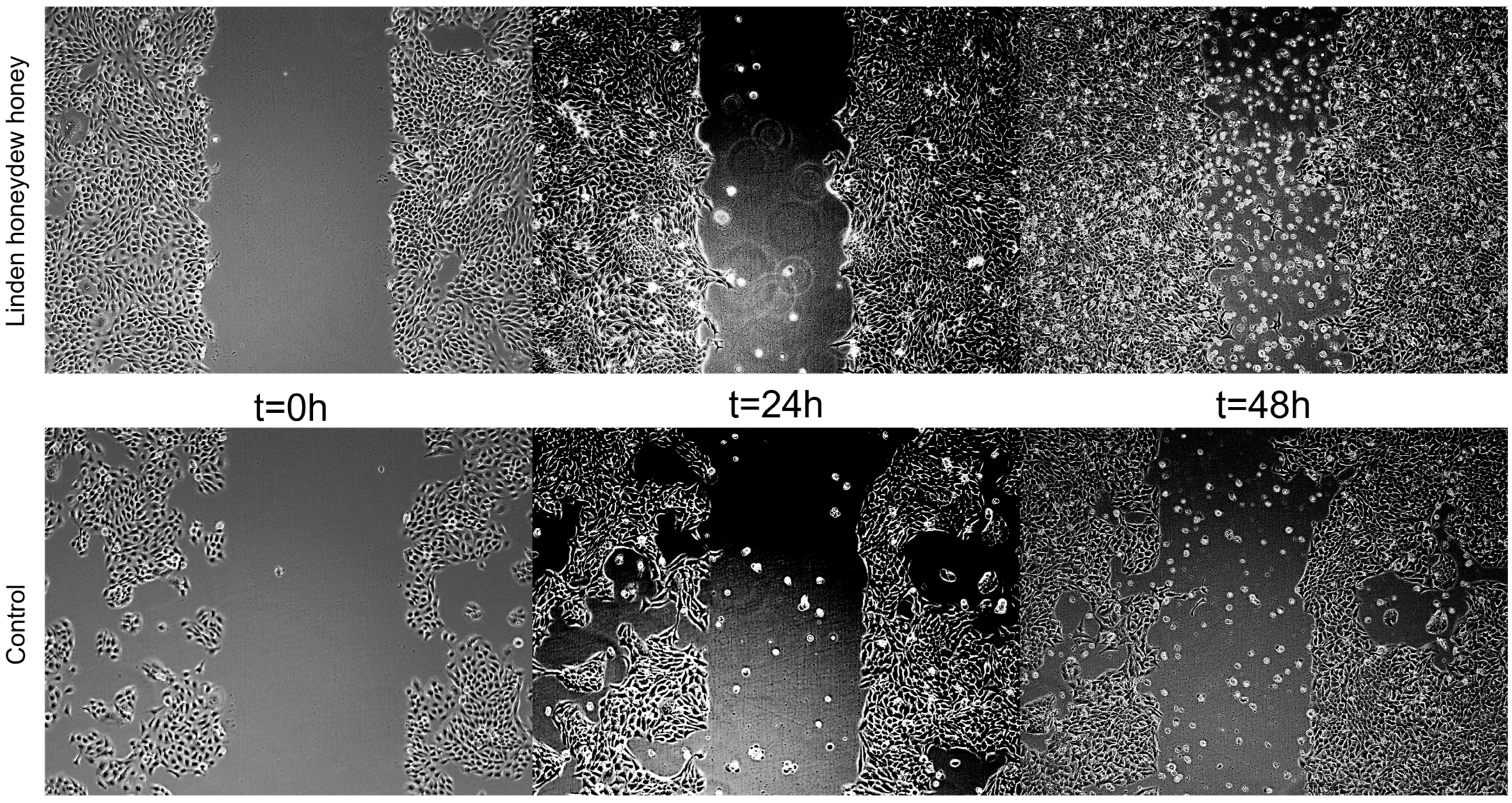
3.4.3. Wound Healing Assay
3.5. Strengths and Limitations of the Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luca, L.; Pauliuc, D.; Oroian, M. Honey Microbiota, Methods for Determining the Microbiological Composition and the Antimicrobial Effect of Honey–A Review. Food Chem. X 2024, 23, 101524. [Google Scholar] [CrossRef] [PubMed]
- Seraglio, S.K.T.; Silva, B.; Bergamo, G.; Brugnerotto, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. An Overview of Physicochemical Characteristics and Health-Promoting Properties of Honeydew Honey. Food Res. Int. 2019, 119, 44–66. [Google Scholar] [CrossRef]
- Azevedo, M.S.; Valentim-Neto, P.A.; Seraglio, S.K.T.; Da Luz, C.F.P.; Arisi, A.C.M.; Costa, A.C.O. Proteome Comparison for Discrimination between Honeydew and Floral Honeys from Botanical Species Mimosa Scabrella Bentham by Principal Component Analysis. J. Sci. Food Agric. 2017, 97, 4515–4519. [Google Scholar] [CrossRef]
- Dżugan, M.; Ciszkowicz, E.; Tomczyk, M.; Miłek, M.; Lecka-Szlachta, K. Coniferous Honeydew Honey: Antibacterial Activity and Anti-Migration Properties against Breast Cancer Cell Line (MCF-7). Appl. Sci. 2024, 14, 710. [Google Scholar] [CrossRef]
- Council Directive 2001/110/EC of 20 December 2001 Relating to Honey. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2002:010:0047:0052:EN:PDF (accessed on 22 January 2025).
- Bergamo, G.; Seraglio, S.K.T.; Gonzaga, L.V.; Fett, R.; De Mello Castanho Amboni, R.D.; Dias, C.O.; Costa, A.C.O. Differentiation of Honeydew Honeys and Blossom Honeys: A New Model Based on Colour Parameters. J. Food Sci. Technol. 2019, 56, 2771–2777. [Google Scholar] [CrossRef]
- Brugnerotto, P.; Fuente-Ballesteros, A.; Martín-Gómez, B.; María Ares, A.; Valdemiro Gonzaga, L.; Fett, R.; Carolina Oliveira Costa, A.; Bernal, J. Free Amino Acid Profile in Mimosa Scabrella Honeydew Honey from Brazil and Chemometric Analysis for Geographical Discrimination. Food Res. Int. 2024, 177, 113856. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Muzolf-Panek, M.; Tomaszewska-Gras, J.; Konieczny, P. Predicting the Botanical Origin of Honeys with Chemometric Analysis According to Their Antioxidant and Physicochemical Properties. Pol. J. Food Nutr. Sci. 2019, 69, 191–201. [Google Scholar] [CrossRef]
- Pudelka, L.; Sleha, R.; Janovska, S.; Radochova, V.; Bostik, P. Czech Honeydew Honeys—A Potential Source of Local Medical Honey with Strong Antimicrobial Activity. Pharmaceuticals 2024, 17, 840. [Google Scholar] [CrossRef]
- Martinotti, S.; Calabrese, G.; Ranzato, E. Honeydew Honey: Biological Effects on Skin Cells. Mol. Cell. Biochem. 2017, 435, 185–192. [Google Scholar] [CrossRef]
- Kuś, P.M.; Jerković, I.; Marijanović, Z.; Tuberoso, C.I.G. Screening of Polish Fir Honeydew Honey Using GC/MS, HPLC-DAD, and Physical-Chemical Parameters: Benzene Derivatives and Terpenes as Chemical Markers. Chem. Biodivers. 2017, 14, e1700179. [Google Scholar] [CrossRef]
- Recklies, K.; Peukert, C.; Kölling-Speer, I.; Speer, K. Differentiation of Honeydew Honeys from Blossom Honeys and According to Their Botanical Origin by Electrical Conductivity and Phenolic and Sugar Spectra. J. Agric. Food Chem. 2021, 69, 1329–1347. [Google Scholar] [CrossRef] [PubMed]
- Puścion-Jakubik, A.; Karpińska, E.; Moskwa, J.; Socha, K. Content of Phenolic Acids as a Marker of Polish Honey Varieties and Relationship with Selected Honey-Quality-Influencing Variables. Antioxidants 2022, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Kędzierska-Matysek, M.; Stryjecka, M.; Teter, A.; Skałecki, P.; Domaradzki, P.; Florek, M. Relationships between the Content of Phenolic Compounds and the Antioxidant Activity of Polish Honey Varieties as a Tool for Botanical Discrimination. Molecules 2021, 26, 1810. [Google Scholar] [CrossRef]
- Fernández-Estellé, M.; Hernández-González, V.; Saurina, J.; Núñez, O.; Sentellas, S. Characterization and Classification of Spanish Honeydew and Blossom Honeys Based on Their Antioxidant Capacity. Antioxidants 2023, 12, 495. [Google Scholar] [CrossRef]
- Diez, M.J.; Andres, C.; Terrab, A. Physicochemical Parameters and Pollen Analysis of Moroccan Honeydew Honeys. Int. J. Food Sci. Technol. 2004, 39, 167–176. [Google Scholar] [CrossRef]
- Barbarić, A.; Saftić Martinović, L.; Milinčić, D.D.; Pešić, M.B.; Marijanović, Z.; Jakac, M.; Brčić Karačonji, I.; Brekalo, H.; Petrović, D.; Pavlešić, T.; et al. Characterization and Differentiation of Beech and Chestnut Honeydew Honeys: A Comparative Study. Food Chem. 2025, 477, 143446. [Google Scholar] [CrossRef]
- Pătruică, S.; Alexa, E.; Obiștioiu, D.; Cocan, I.; Radulov, I.; Berbecea, A.; Lazăr, R.N.; Simiz, E.; Vicar, N.M.; Hulea, A.; et al. Chemical Composition, Antioxidant and Antimicrobial Activity of Some Types of Honey from Banat Region, Romania. Molecules 2022, 27, 4179. [Google Scholar] [CrossRef]
- Naef, R.; Jaquier, A.; Velluz, A.; Bachofen, B. From the Linden Flower to Linden Honey–Volatile Constituents of Linden Nectar, the Extract of Bee-Stomach and Ripe Honey. Chem. Biodivers. 2004, 1, 1870–1879. [Google Scholar] [CrossRef] [PubMed]
- Frérot, E.; Velluz, A.; Decorzant, E.; Naef, R. From Linden Flower to Linden Honey. Part 2: Glycosidic Precursors of Cyclohexa-1,3-Diene-1-Carboxylic Acids. Chem. Biodivers. 2006, 3, 94–100. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, L.; Kong, L.; Dong, J.; Zhou, Z.; Zhang, H. Characteristic Components and Authenticity Evaluation of Rape, Acacia, and Linden Honey. J. Agric. Food Chem. 2020, 68, 9776–9788. [Google Scholar] [CrossRef]
- Zou, S.; Tao, H.; Chang, Y.-N. Characterization of Antioxidant Activity and Analysis of Phenolic Acids and Flavonoids in Linden Honey. Food Sci. Technol. 2022, 42, e76621. [Google Scholar] [CrossRef]
- Oraščanin, M.; Bektašević, M.; Šertović, E.; Sarić, Z.; Alibabić, V. Color, Total Phenols and Antioxidant Activity of Honey from Northwestern Bosnia and Herzegovina. Technol. Acta 2023, 16, 15–21. [Google Scholar] [CrossRef]
- Landeka, V.; Cvrtila, Ž.; Kozačinski, L.; Drmać, M.; Sesar, A.; Aljičević, M. Microbiological and Physicochemical Quality of Honey in Bosnia and Herzegovina. Vet. Stanica 2022, 53, 561–571. [Google Scholar] [CrossRef]
- Correlation between Antioxidant and Physicochemical Parameters of Honey Samples from Bosnia and Herzegovina and Turkey. Glas. Hem. Tehnol. Bosne Hercegovine 2022, 59, 17–26. [CrossRef]
- Brčina, T.; Seferović, S.; Husejnagić, D.; Cvrk, R.; Bojanović, L. Health Safety and Sensory Properties of Honey from Two Different Areas in Tuzla Canton. Int. J. Res. Appl. Sci. Biotechnol. 2021, 8, 20–24. [Google Scholar] [CrossRef]
- Ferreira, T.; Nascimento-Gonçalves, E.; Macedo, S.; Borges, I.; Gama, A.; Gil Da Costa, R.M.; Neuparth, M.J.; Lanzarin, G.; Venâncio, C.; Félix, L.; et al. Toxicological and Anti-Tumor Effects of a Linden Extract (Tilia platyphyllos Scop.) in a HPV16-Transgenic Mouse Model. Food Funct. 2021, 12, 4005–4014. [Google Scholar] [CrossRef]
- Zhou, Y.-J.; Ren, Q.; Shen, Y.-B. Comprehensive Review of Tilia L.: Phytochemical Profiles, Edible Value, Therapeutic Potentials, and Ecological Significance. Food Med. Homol. 2025, 2, 9420035. [Google Scholar] [CrossRef]
- CXS 12-1981; Codex Alimentarius Commission Codex Standard for Honey. Food and Agriculture Organization: Rome, Italy, 2020.
- Official Gazette of Bosnia and Herzegovina, Food Safety Agency of Bosnia and Herzegovina Rules on Methods for Control of Honey and Other Bee Products 2009. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC148621/ (accessed on 22 January 2025).
- Bogdanov, S.; Vit, P.; Kilchenmann, V. Sugar Profiles and Conductivity of Stingless Bee Honeys from Venezuela. Apidologie 1996, 27, 445–450. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). Agricultural Marketing Service United States Standards for Grades of Extracted Honey 1985. Available online: https://www.ams.usda.gov/sites/default/files/media/Extracted_Honey_Standard%5B1%5D.pdf (accessed on 24 January 2025).
- Von Der Ohe, W.; Persano Oddo, L.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized Methods of Melissopalynology. Apidologie 2004, 35, S18–S25. [Google Scholar] [CrossRef]
- Gašić, U.; Kečkeš, S.; Dabić, D.; Trifković, J.; Milojković-Opsenica, D.; Natić, M.; Tešić, Ž. Phenolic Profile and Antioxidant Activity of Serbian Polyfloral Honeys. Food Chem. 2014, 145, 599–607. [Google Scholar] [CrossRef]
- Jerković, I.; Marijanović, Z.; Ljubičić, I.; Gugić, M. Contribution of the Bees and Combs to Honey Volatiles: Blank-Trial Probe for Chemical Profiling of Honey Biodiversity. Chem. Biodivers. 2010, 7, 1217–1230. [Google Scholar] [CrossRef]
- Kahlmeter, G.; Brown, D.F.J.; Goldstein, F.W.; MacGowan, A.P.; Mouton, J.W.; Odenholt, I.; Rodloff, A.; Soussy, C.-J.; Steinbakk, M.; Soriano, F.; et al. European Committee on Antimicrobial Susceptibility Testing (EUCAST) Technical Notes on Antimicrobial Susceptibility Testing. Clin. Microbiol. Infect. 2006, 12, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, A.G.; Al Guthami, F.M.; Al Gethami, A.F.M.; Allah, F.M.A.; Saleh, A.A.; Fouad, E.A. Potential Antibacterial Activity of Some Saudi Arabia Honey. Vet. World 2017, 10, 233–237. [Google Scholar] [CrossRef]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and Quantification of Methylglyoxal as the Dominant Antibacterial Constituent of Manuka (Leptospermum scoparium) Honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Juric, A.; Gasic, U.; Brcic-Karaconji, I.; Jurica, K.; Milojkovic-Opsenica, D. The Phenolic Profile of Strawberry Tree (Arbutus unedo L.) Honey. J. Serbian Chem. Soc. 2020, 85, 1011–1019. [Google Scholar] [CrossRef]
- Jurič, A.; Brčić Karačonji, I.; Žunec, S.; Katić, A.; Gašić, U.; Milojković Opsenica, D.; Kopjar, N. Protective Role of Strawberry Tree (Arbutus unedo L.) Honey against Cyto/Genotoxic Effects Induced by Ultraviolet B Radiation in Vitro. J. Apic. Res. 2024, 63, 513–522. [Google Scholar] [CrossRef]
- Brosin, A.; Wolf, V.; Mattheus, A.; Heise, H. Use of XTT-Assay to Assess the Cytotoxicity of Different Surfactants and Metal Salts in Human Keratinocytes (HaCaT). A Feasible Method for in Vitro Testing of Skin Irritants. Acta Derm. Venereol. 1997, 77, 26–28. [Google Scholar] [CrossRef]
- Marko, I.; Dražana, P.; Kristina, B. Honey Handbook 2014. Available online: https://faz.ba/wp-content/uploads/2023/03/Prirucnik-o-Medu_2014.pdf (accessed on 22 January 2025).
- Singh, I.; Singh, S. Honey Moisture Reduction and Its Quality. J. Food Sci. Technol. 2018, 55, 3861–3871. [Google Scholar] [CrossRef]
- Živkov Baloš, M.; Popov, N.; Jakšić, S.; Mihaljev, Ž.; Pelić, M.; Ratajac, R.; Ljubojević Pelić, D. Sunflower Honey—Evaluation of Quality and Stability during Storage. Foods 2023, 12, 2585. [Google Scholar] [CrossRef]
- Rulebook on Methods for the Control of Honey and Other Bee Products 2019. Available online: https://fsa.gov.ba/wp-content/uploads/2024/03/hr-Pravilnik_o_metodama_za_kontrolu_meda_i_drugih_pcelinjih_proizvoda_R01_84-19.pdf (accessed on 22 January 2025).
- Santos, E.I.; Meerhoff, E.; García Da Rosa, E.; Ferreira, J.; Raucher, M.; Quintana, W.; Martínez, A.; González, C.; Mancebo, Y. Color y Conductividad Eléctrica de Las Mieles Producidas Por Apis Mellifera En Uruguay. INNOTEC 2018. [Google Scholar] [CrossRef]
- Živkov Baloš, M.; Popov, N.; Vidaković, S.; Ljubojević Pelić, D.; Pelić, M.; Mihaljev, Ž.; Jakšić, S. Electrical Conductivity and Acidity of Honey. Arch. Vet. Med. 2018, 11, 91–101. [Google Scholar] [CrossRef]
- Majewska, E.; Drużyńska, B.; Derewiaka, D.; Ciecierska, M.; Pakosz, P. Comparison of Antioxidant Properties and Color of Selected Polish Honeys and Manuka Honey. Foods 2024, 13, 2666. [Google Scholar] [CrossRef]
- Patouna, A.; Vardakas, P.; Skaperda, Z.; Spandidos, D.; Kouretas, D. Evaluation of the Antioxidant Potency of Greek Honey from the Taygetos and Pindos Mountains Using a Combination of Cellular and Molecular Methods. Mol. Med. Rep. 2023, 27, 54. [Google Scholar] [CrossRef]
- Jaśkiewicz, K.; Szczęsna, T.; Jachuła, J. How Phenolic Compounds Profile and Antioxidant Activity Depend on Botanical Origin of Honey—A Case of Polish Varietal Honeys. Molecules 2025, 30, 360. [Google Scholar] [CrossRef] [PubMed]
- Cavia, M.M.; Fernández-Muiño, M.A.; Alonso-Torre, S.R.; Huidobro, J.F.; Sancho, M.T. Evolution of Acidity of Honeys from Continental Climates: Influence of Induced Granulation. Food Chem. 2007, 100, 1728–1733. [Google Scholar] [CrossRef]
- Tomczyk, M.; Bocian, A.; Sidor, E.; Miłek, M.; Zaguła, G.; Dżugan, M. The Use of HPTLC and SDS-PAGE Methods for Coniferous Honeydew Honey Fingerprinting Compiled with Mineral Content and Antioxidant Activity. Molecules 2022, 27, 720. [Google Scholar] [CrossRef] [PubMed]
- Kuś, P.M.; Szweda, P.; Jerković, I.; Tuberoso, C.I.G. Activity of Polish Unifloral Honeys against Pathogenic Bacteria and Its Correlation with Colour, Phenolic Content, Antioxidant Capacity and Other Parameters. Lett. Appl. Microbiol. 2016, 62, 269–276. [Google Scholar] [CrossRef]
- Atanassova, J.; Lazarova, M.; Yurukova, L. Significant Parameters of Bulgarian Honeydew Honey. J. Cent. Eur. Agric. 2016, 17, 640–651. [Google Scholar] [CrossRef][Green Version]
- Persano Oddo, L.; Bogdanov, S. Determination of Honey Botanical Origin: Problemsand Issues. Apidologie 2004, 35, S2–S3. [Google Scholar] [CrossRef]
- Pospiech, M.; Javůrková, Z.; Hrabec, P.; Čížková, H.; Titěra, D.; Štarha, P.; Ljasovská, S.; Kružík, V.; Podskalská, T.; Bednář, J.; et al. Physico-Chemical and Melissopalynological Characterization of Czech Honey. Appl. Sci. 2021, 11, 4989. [Google Scholar] [CrossRef]
- Gençay Çelemli, Ö.; ÖzeniRler, Ç.; Ecem Bayram, N.; Zare, G.; Sorkun, K. Kars Balının Coğrafi İşaretlemesi İçin Melissopalinolojik Analiz. Kafkas Univ. Vet. Fak. Derg. 2017, 24, 53–59. [Google Scholar] [CrossRef]
- Rodopoulou, M.; Tananaki, C.; Dimou, M.; Liolios, V.; Kanelis, D.; Goras, G.; Thrasyvoulou, A. The Determination of the Botanical Origin in Honeys with Over-represented Pollen: Combination of Melissopalynological, Sensory and Physicochemical Analysis. J. Sci. Food Agric. 2018, 98, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Toker, G.; Baser, K.H.C.; Kürkçüoglu, M.; Özek, T. The Composition of Essential Oils from Tilia L. Species Growing in Turkey. J. Essent. Oil Res. 1999, 11, 369–374. [Google Scholar] [CrossRef]
- Da Silva, A.R.P.; Costa, M.D.S.; Araújo, N.J.S.; De Freitas, T.S.; Dos Santos, A.T.L.; Gonçalves, S.A.; Da Silva, V.B.; Andrade-Pinheiro, J.C.; Tahim, C.M.; Lucetti, E.C.P.; et al. Antibacterial Activity and Antibiotic-Modifying Action of Carvacrol against Multidrug-Resistant Bacteria. Adv. Sample Prep. 2023, 7, 100072. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.; Barbieri, R.; Silva, A.; Nabavi, S.; Tsetegho Sokeng, A.; Izadi, M.; Jafari, N.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and Antifungal Activities of Thymol: A Brief Review of the Literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Tananaki, C.; Rodopoulou, M.-A.; Dimou, M.; Kanelis, D.; Liolios, V. The Total Phenolic Content and Antioxidant Activity of Nine Monofloral Honey Types. Appl. Sci. 2024, 14, 4329. [Google Scholar] [CrossRef]
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic Compounds in Honey and Their Relationship with Antioxidant Activity, Botanical Origin, and Color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Živković, J.; Sunarić, S.; Stanković, N.; Mihajilov-Krstev, T.; Spasić, A. Total Phenolic and Flavonoid Contents, Antioxidant and Antibacterial Activities of Selected Honeys Against Human Pathogenic Bacteria. Acta Pol. Pharm. Drug Res. 2019, 76, 671–681. [Google Scholar] [CrossRef]
- Kačániová, M.; Borotová, P.; Galovičová, L.; Kunová, S.; Štefániková, J.; Kowalczewski, P.Ł.; Šedík, P. Antimicrobial and Antioxidant Activity of Different Honey Samples from Beekeepers and Commercial Producers. Antibiotics 2022, 11, 1163. [Google Scholar] [CrossRef]
- Farkas, Á.; Balázs, V.L.; Kõszegi, T.; Csepregi, R.; Kerekes, E.; Horváth, G.; Szabó, P.; Gaál, K.; Kocsis, M. Antibacterial and Biofilm Degradation Effects of Hungarian Honeys Linked with Botanical Origin, Antioxidant Capacity and Mineral Content. Front. Nutr. 2022, 9, 953470. [Google Scholar] [CrossRef] [PubMed]
- Gruznov, D.V.; Gruznova, O.A.; Sokhlikov, A.B.; Lobanov, A.V.; Chesnokova, I.P. Changes in the Chemical Composition and Antimicrobial Activity of Linden, Buckwheat and Sunflower Honey Stored at Low Temperatures. Curr. Res. Nutr. Food Sci. J. 2024, 12, 824–840. [Google Scholar] [CrossRef]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-Mediated Production of Hydrogen Peroxide Is Crucial for High Antibacterial Activity of Honeydew Honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef] [PubMed]
- Hulea, A.; Obiștioiu, D.; Cocan, I.; Alexa, E.; Negrea, M.; Neacșu, A.-G.; Hulea, C.; Pascu, C.; Costinar, L.; Iancu, I.; et al. Diversity of Monofloral Honey Based on the Antimicrobial and Antioxidant Potential. Antibiotics 2022, 11, 595. [Google Scholar] [CrossRef]
- Mendoza-Meza, D.L.; España-Puccini, P. Cytotoxic and Genotoxic Activity of Phenolic Fractions from Ulomoides Dermestoides Fairmaire, 1893 (Coleoptera, Tenebrionidae), in Hacat Cells. TIP 2016, 19, 83–91. [Google Scholar] [CrossRef]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT Keratinocytes Response on Antimicrobial Atelocollagen Substrates: Extent of Cytotoxicity, Cell Viability and Proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef]
- Torres Di Bello, D.; Narváez, D.M.; Groot De Restrepo, H.; Vives, M.J. Cytotoxic Evaluation in HaCaT Cells of the Pa.7 Bacteriophage from Cutibacterium (Propionibacterium) acnes, Free and Encapsulated Within Liposomes. PHAGE 2023, 4, 26–34. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A.F. Cutaneous Wound Healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Blažević, F.; Milekić, T.; Romić, M.D.; Juretić, M.; Pepić, I.; Filipović-Grčić, J.; Lovrić, J.; Hafner, A. Nanoparticle-Mediated Interplay of Chitosan and Melatonin for Improved Wound Epithelialisation. Carbohydr. Polym. 2016, 146, 445–454. [Google Scholar] [CrossRef]
- Fouché, M.; Willers, C.; Hamman, S.; Malherbe, C.; Steenekamp, J. Wound Healing Effects of Aloe Muth-Muth: In Vitro Investigations Using Immortalized Human Keratinocytes (HaCaT). Biology 2020, 9, 350. [Google Scholar] [CrossRef]
- Suguna, L.; Chandrakasan, G.; Ramamoorthy, U.; Joseph, K.T. Influence of Honey on Biochemical and Biophysical Parameters of Wounds in Rats. J. Clin. Biochem. Nutr. 1993, 14, 91–99. [Google Scholar] [CrossRef]
- Scepankova, H.; Combarros-Fuertes, P.; Fresno, J.M.; Tornadijo, M.E.; Dias, M.S.; Pinto, C.A.; Saraiva, J.A.; Estevinho, L.M. Role of Honey in Advanced Wound Care. Molecules 2021, 26, 4784. [Google Scholar] [CrossRef] [PubMed]
- Lusby, P. Honey: A Potent Agent for Wound Healing? J. WOCN 2002, 29, 295–300. [Google Scholar] [CrossRef]

| Physicochemical Parameter | LHH | Reference Values |
|---|---|---|
| Water (%) | 15.10 | ≤20% [30] |
| Electrical conductivity (ms/cm) | 1.21 | ≥0.8 mS/cm [30] |
| Free acidity (meq/kg) | 35 | ≤50 [30] |
| pH | 4.93 | 4.5–6.5 [42] |
| Compound | LHH | |
|---|---|---|
| RI 1 | Area (%) | |
| Ethanol | <900 | 1.00 |
| Aceton | <900 | 2.21 |
| Dimethyl sulphide | <900 | 1.43 |
| Formic acid * | <900 | 5.24 |
| 2-Methylfuran * | <900 | 1.97 |
| Dimethyl disulfide | <900 | 0.67 |
| Methylbenzene (Toluene) | <900 | 0.66 |
| 2-furan carboxaldehyde (furfural) * | <900 | 7.80 |
| 2-Furanmethanol | <900 | 0.59 |
| 2-acetylfuran | 914 | 0.42 |
| Benzaldehyde | 965 | 1.39 |
| Hexanoic acid | 974 | 0.18 |
| Octanal | 1006 | 0.31 |
| δ-3-carene | 1016 | 4.01 |
| p-cimen | 1028 | 0.58 |
| Benzyl alcohol | 1037 | 0.57 |
| 2-Phenylacetaldehyde | 1048 | 1.30 |
| Trans-Linalool oxid | 1076 | 0.33 |
| α-terpinolene | 1090 | 17.40 |
| Linalool | 1101 | 0.93 |
| Hotrienol | 1106 | 2.99 |
| 2-Phenylethanol | 1116 | 1.61 |
| Phenylacetonitrile | 1140 | 0.18 |
| 2,3-Dihydro-2,5-dihydoxy-6-methyl-4-H-pyran-4-one * | 1144 | 0.28 |
| Methyl salicylate | 1195 | 0.11 |
| Decanal | 1207 | 2.09 |
| 5-Hydroxymethylfurfural | 1230 | 0.74 |
| 4-(1-methylethyl)-benzaldehyde | 1243 | 0.32 |
| Nonanoic acid | 1273 | 0.59 |
| Carvacrol | 1300 | 1.34 |
| Thymol | 1303 | 1.09 |
| Analysis | LHH |
|---|---|
| TPC (mg GAE 1/kg) | 816.38 ± 24.84 |
| TFC-Al (mg QE 2/kg) | 245.21 ± 10.57 |
| TFC-DNP (g NAR 3/kg) | 18.21 ± 3.02 |
| DPPH (mmol TE 4/kg) | 1.11 ± 0.02 |
| Honey Sample | Antibiotic Control | |||||
|---|---|---|---|---|---|---|
| Bacteria | LHH | Vancomycin | Meropenem | |||
| MIC | MBC | MIC | MBC | MIC | MBC | |
| S. aureus ATCC 25923 | 0.0125 | 0.0125 | 10−6 | 10−6 | ND * | ND |
| MRSA ATCC 43300 | 0.0125 | 0.0125 | 5 × 10−7 | 10−6 | ND | ND |
| P. aeruginosa ATCC 27853 | 0.1 | 0.1 | ND | ND | 6.4 × 10−8 | 6.4 × 10−8 |
| E. coli ATCC 25922 | 0.05 | 0.1 | ND | ND | 6 × 10−9 | 6 × 10−9 |
| A. baumannii 19606 | 0.05 | 0.05 | ND | ND | >3.2 × 10–5 | >3.2 × 10–5 |
| S. Typhimurium ATCC 14028 | 0.1 | 0.1 | 2.0 × 10−6 | 2.0 × 10−6 | ND | ND |
| E. faecalis ATCC 25212 | 0.1 | 0.2 | 1.0 × 10−6 | 1.0 × 10−6 | ND | ND |
| L. monocytogenes ATCC 1915 | 0.1 | 0.1 | 1.0 × 10−6 | 1.0 × 10−6 | ND | ND |
| B. cereus ATCC 10876 | 0.1 | 0.1 | 2.0 × 10−6 | 2.0 × 10−6 | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbarić, A.; Saftić Martinović, L.; Marijanović, Z.; Juretić, L.; Jurič, A.; Petrović, D.; Šoljić, V.; Gobin, I. Integrated Chemical and Biological Evaluation of Linden Honeydew Honey from Bosnia and Herzegovina: Composition and Cellular Effects. Foods 2025, 14, 1668. https://doi.org/10.3390/foods14101668
Barbarić A, Saftić Martinović L, Marijanović Z, Juretić L, Jurič A, Petrović D, Šoljić V, Gobin I. Integrated Chemical and Biological Evaluation of Linden Honeydew Honey from Bosnia and Herzegovina: Composition and Cellular Effects. Foods. 2025; 14(10):1668. https://doi.org/10.3390/foods14101668
Chicago/Turabian StyleBarbarić, Ana, Lara Saftić Martinović, Zvonimir Marijanović, Lea Juretić, Andreja Jurič, Danijela Petrović, Violeta Šoljić, and Ivana Gobin. 2025. "Integrated Chemical and Biological Evaluation of Linden Honeydew Honey from Bosnia and Herzegovina: Composition and Cellular Effects" Foods 14, no. 10: 1668. https://doi.org/10.3390/foods14101668
APA StyleBarbarić, A., Saftić Martinović, L., Marijanović, Z., Juretić, L., Jurič, A., Petrović, D., Šoljić, V., & Gobin, I. (2025). Integrated Chemical and Biological Evaluation of Linden Honeydew Honey from Bosnia and Herzegovina: Composition and Cellular Effects. Foods, 14(10), 1668. https://doi.org/10.3390/foods14101668








