Ecological, Apicultural, and Therapeutic Value of Vachellia tortilis and Ziziphus spina-christi Honeys in the United Arab Emirates: A Model for Sustainable Use in Arid Ecosystems
Abstract
1. Introduction
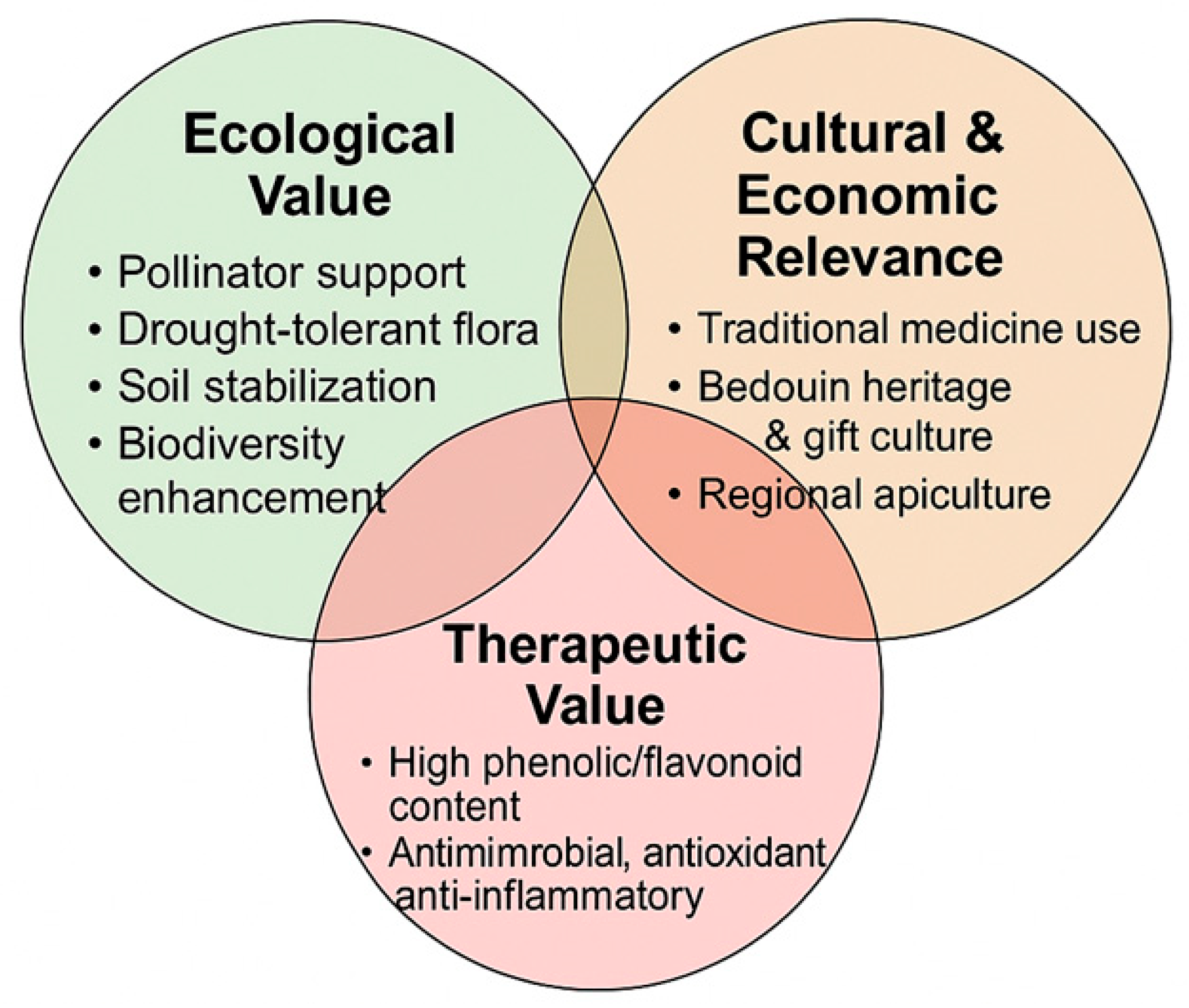
2. Ecological, Economic, and Cultural Significance of Vachellia tortilis and Ziziphus spina-christi
3. Apicultural and Medicinal Role of Vachellia tortilis in Arid Ecosystems
4. Ethnobotanical and Apicultural Importance of Ziziphus spina-christi
5. Integrating Ecology, Culture, and Sustainability
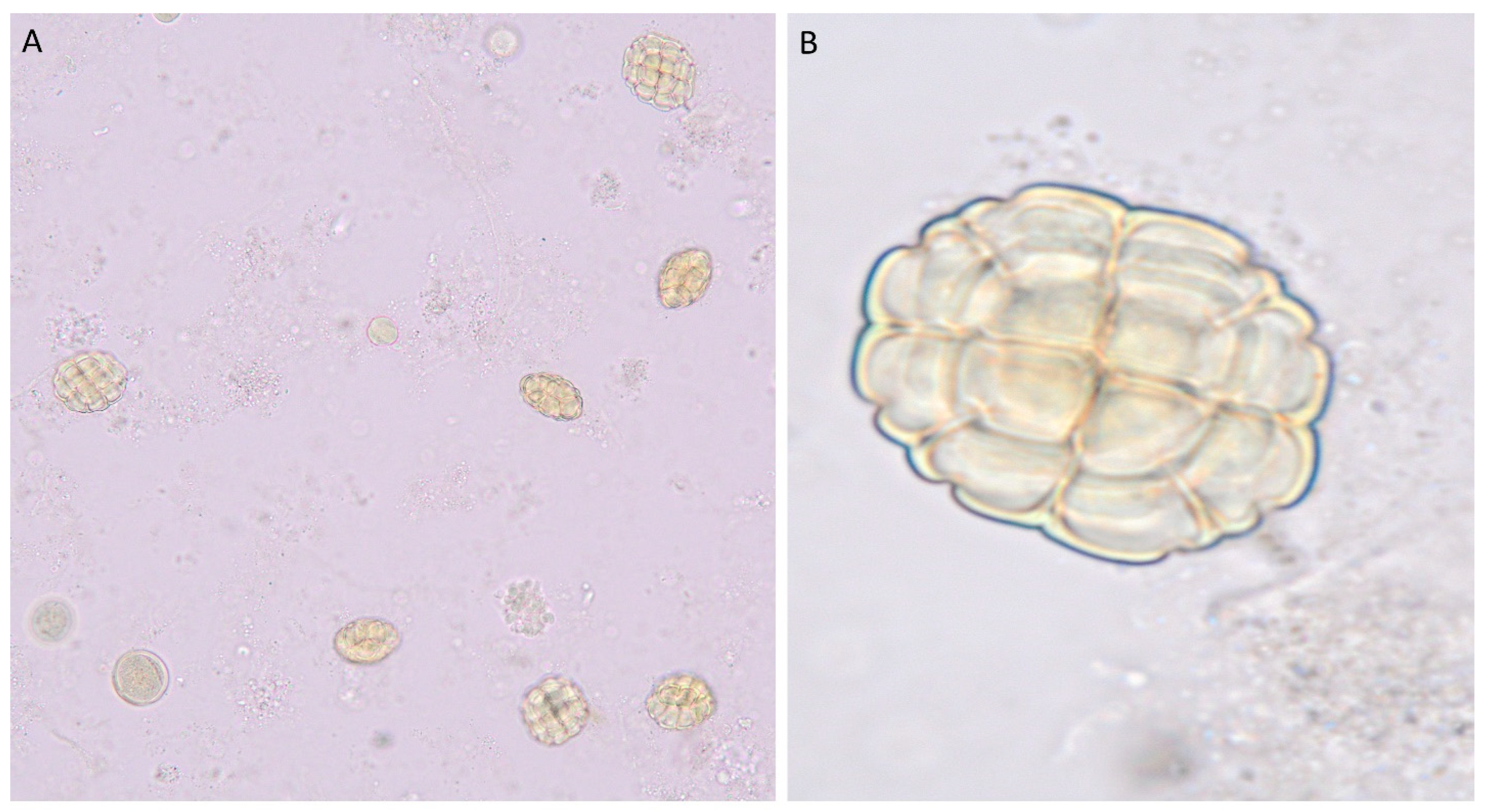
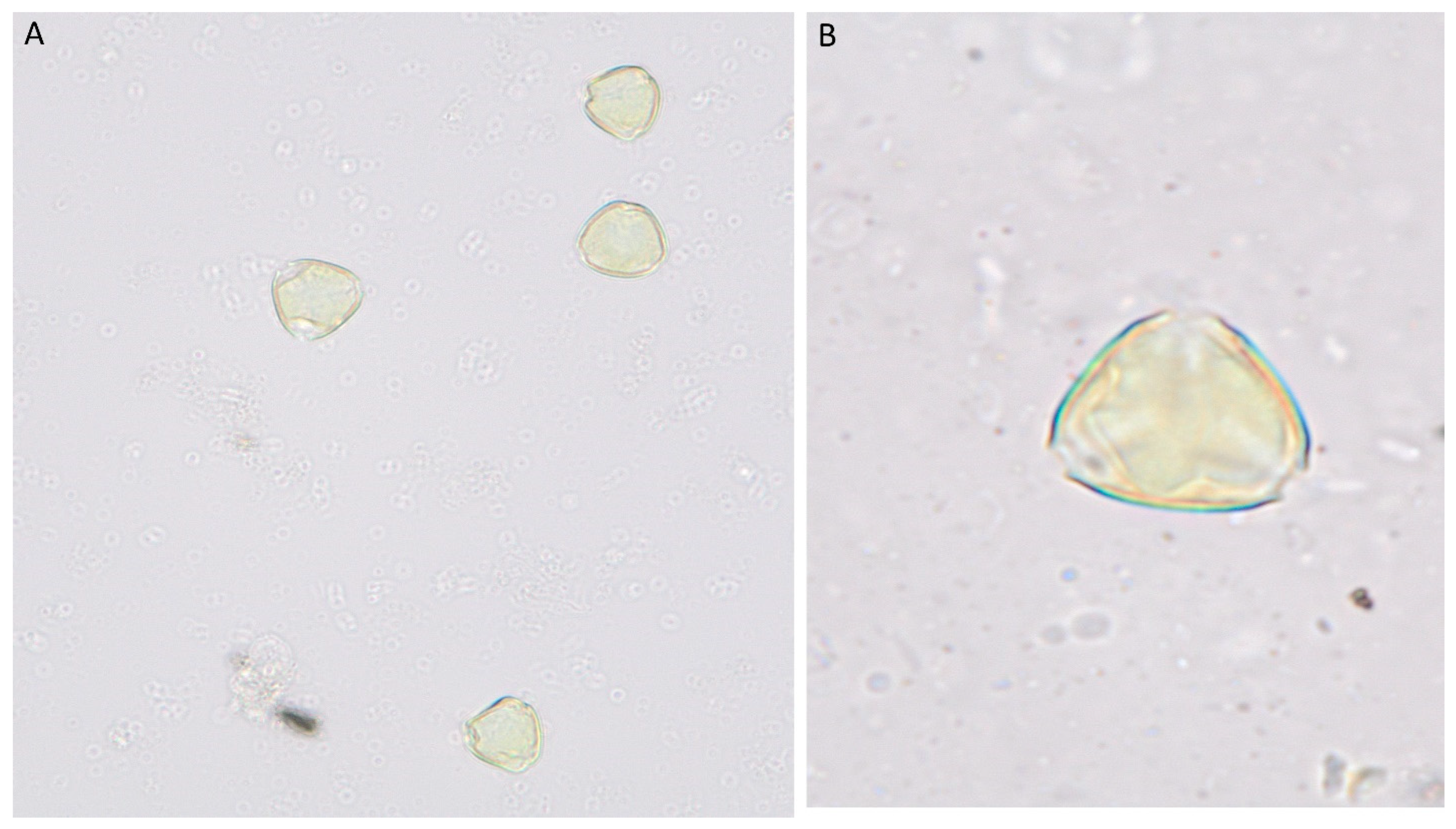
6. Pollen Grain Characterization of Vachellia tortilis and Ziziphus spina-christi
7. Phytochemical Constituents of Vachellia tortilis and Ziziphus spina-christi: Relevance to Honey Bioactivity
8. Therapeutic Compounds and Pharmacological Potentials of V. tortilis and Z. spina-christi Honeys
9. Characteristics of Produced Honey from Vachellia tortilis and Ziziphus spina-christi
10. Pharmacological Potentials of the Vachellia tortilis and Ziziphus spina-christi
11. Mechanisms and Evidence of Antimicrobial Activity in Emirati Honeys
12. Supplementary Therapeutic Insights from Propolis: Implications for Future Honeybee Product Research
13. Eco-Friendly ZS-Ag Nanoparticles for Health and Agriculture Application
14. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salomea, J.A. Comparison Analysis of Honeydew Honey Production and Quality in Fujairah, U.A.E and Other Regions of the World: A Review. Int. J. Innov. Sci. Res. Technol. 2022, 7, 55–63. [Google Scholar]
- Alaerjani, W.M.A.; Abu-Melha, S.A.; Khan, K.A.; Ghramh, H.A.; Alalmie, A.Y.A.; Alshareef, R.M.H.; Al-Shehri, B.M.; Mohammed, M.E.A. Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values. Open Chem. 2021, 19, 1162–1173. [Google Scholar] [CrossRef]
- FAO; WHO. Codex Alimentarius—International Food Standards; Standard for honey CXS 12-1981. Adopted in 1981, amended in 2019; FAO: Rome, Italy; WHO: Genewa, Switzerland, 2019. [Google Scholar]
- Owayss, A.A.; Elbanna, K.; Iqbal, J.; Abulreesh, H.H.; Organji, S.R.; Raweh, H.S.A.; Alqarni, A.S. In Vitro antimicrobial activities of Saudi honeys originating from Ziziphus spina-christi L. and Acacia gerrardii Benth. trees. Food Sci. Nutr. 2020, 8, 390–401. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Koulis, G.A.; Danezis, G.P.; Martakos, I.; Dasenaki, M.; Georgiou, C.A.; Thomaidis, N.S. Honey authenticity: Analytical techniques, state of the art and challenges. RSC Adv. 2021, 11, 11273–11294. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.; Adgaba, N.; Getachew, A.; Tadesse, Y. New approach for determination of an optimum honeybee colony’s carrying capacity based on productivity and nectar secretion potential of bee forage species. Saudi J. Biol. Sci. 2016, 23, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Vincent, B.; Bourillon, J.; Gotty, K.; Boukcim, H.; Selosse, M.-A.; Cambou, A.; Damasio, C.; Voisin, M.; Boivin, S.; Figura, T.; et al. Ecological aspects and relationships of the emblematic Vachellia spp. exposed to anthropic pressures and parasitism in natural hyper-arid ecosystems: Ethnobotanical elements, morphology, and biological nitrogen fixation. Planta 2024, 259, 132. [Google Scholar] [CrossRef]
- Yokota, H.; Abe, M.; Shoji, T.; Kiriiwa, Y.; Ozawa, K.; Yoshizaki, S.; Oishi, A. Effect of irrigation water salinity on the growth of trees for revegetation in the United Arab Emirates. In Plant Nutrition for Sustainable Food Production and Environment; Ando, T., Fujita, K., Mae, T., Matsumoto, H., Mori, S., Sekiya, J., Eds.; Developments in Plant and Soil Sciences 1997; Springer: Dordrecht, The Netherlands, 1997; Volume 78. [Google Scholar] [CrossRef]
- Fterich, A.; Mahdhi, M.; Mars, M. The effects of Acacia tortilis subsp. raddiana, soil texture and soil depth on soil microbial and biochemical characteristics in arid zones of Tunisia. Land. Degrad. Dev. 2014, 25, 143–152. [Google Scholar]
- Alyammahi, M.M. Physicochemical Characteristics of Mono Floral Emirati Honey. Master’s Thesis, United Arab Emirates University, Al Ain, United Arab Emirates, 2018. [Google Scholar]
- Osaili, T.M.; Odeh, W.A.M.B.; Al Sallagi, M.S.; Al Ali, A.A.S.A.; Obaid, R.S.; Garimella, V.; Bakhit, F.S.; Hasan, H.; Holley, R.; El Darra, N. Quality of Honey Imported into the United Arab Emirates. Foods 2023, 12, 729. [Google Scholar] [CrossRef] [PubMed]
- Escuredo, O.; Seijo, M.C. Authenticity of Honey: Characterization, Bioactivities and Sensorial Properties. Foods 2022, 11, 1301. [Google Scholar] [CrossRef]
- Taha, E.-K.A.; Al-Kahtani, S.; Taha, R. Comparison of Pollen Spectra and Amount of Mineral Content in Honey Produced by Apis florea Fand Apis mellifera L. J. Kans. Entomol. Soc. 2018, 91, 51–57. [Google Scholar] [CrossRef]
- Noumi, Z.; Abdallah, L.; Touzard, B.; Chaieb, M. Acacia tortilis (Forssk.) subsp. raddiana (Savi) Brenan as a foundation species: A test from the arid zone of Tunisia. Rangel. J. 2012, 34, 17–25. [Google Scholar] [CrossRef]
- Saied, A.S.; Gebauer, J.; Hammer, K.; Buerkert, A. Ziziphus spina-christi (L.) Willd.: A multipurpose fruit tree. Genet. Resour. Crop Evol. 2008, 55, 929–937. [Google Scholar] [CrossRef]
- Alqarni, A.S.; Hannan, M.; Owayss, A.; Engel, M.S. The indigenous honey bees of Saudi Arabia (Hymenoptera, Apidae, Apis mellifera jemenitica Ruttner): Their natural history and role in beekeeping. ZooKeys 2011, 134, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Asgarpanah, J.; Haghighat, E. Phytochemistry and pharmacologic properties of Ziziphus spina christi (L.) Willd. Afr. J. Pharm. Pharmacol. 2012, 6, 2332–2339. [Google Scholar] [CrossRef]
- Hussein, A.S. Ziziphus spina-christi: Analysis of Bioactivities and Chemical Composition. In Wild Fruits: Composition, Nutritional Value and Products; Mariod, A.A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 175–197. [Google Scholar]
- Khan, S.U.; Zafar, M.; Ahmad, M.; Anjum, F.; Sultana, S.; Kilic, O.; Ozdemir, F.A.; Nazir, A.; Yaseen, G.; Aabidin, S.Z.U.; et al. Pollen micromorphological analysis of tribe Acacieae (Mimosaceae) with LM and SEM techniques. Microsc. Res. Tech. 2019, 82, 1610–1620. [Google Scholar] [CrossRef]
- Sajwani, A.; Farooq, S.A.; Bryant, V.M. Studies of bee foraging plants and analysis of pollen pellets from hives in Oman. Palynology 2014, 38, 207–223. [Google Scholar] [CrossRef]
- Muhaisen, H.M.H. Extraction, Isolation and Characterization of Valuable Worked on Acacia Tortilis. Egypt. J. Chem. 2021, 64, 6731–6747. [Google Scholar] [CrossRef]
- Taha, D.; El Hajjaji, S.; Mourabit, Y.; Bouyahya, A.; Lee, L.H.; El Menyiy, N.; Tarik, A.; Benali, T.; El Moudden, H.; Gallo, M.; et al. Traditional Knowledge, Phytochemistry, and Biological Properties of Vachellia tortilis. Plants 2022, 11, 3348. [Google Scholar] [CrossRef]
- Gabr, S.; Nikles, S.; Pferschy Wenzig, E.M.; Ardjomand-Woelkart, K.; Hathout, R.M.; El-Ahmady, S.; Motaal, A.A.; Singab, A.; Bauer, R. Characterization and optimization of phenolics extracts from Acacia species in relevance to their anti-inflammatory activity. Biochem. Syst. Ecol. 2018, 78, 21–30. [Google Scholar] [CrossRef]
- Lakhera, A.K.; Kumar, V. Monosaccharide composition of acidic gum exudates from Indian Acacia tortilis ssp. raddiana (Savi) Brenan. Int. J. Biol. Macromol. 2017, 94, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Khale, S.M.J. Studying the Heavy Metals Composition and the Impact of Different Common Solvents on the Extraction Efficiency of Phytochemical Secondary Metabolites from the Leaves of Ziziphus spina-christi Grown in Jordan. Pak. J. Nutr. 2018, 17, 392–398. [Google Scholar] [CrossRef]
- Ads, E.N.; Rajendrasozhan, S.; Hassan, S.; Sharawy, S.; Humaidi, J. Phytochemical-screening-of-different-organic-crude-extracts-from-the-stem-bark-of-ziziphus-spinachristi-l. Biomed. Res. 2018, 29, 1645–1652. [Google Scholar]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxidative Med. Cell. Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef]
- Alajmi, M.F.; Alam, P.; Alqasoumi, S.I.; Ali Siddiqui, N.; Basudan, O.A.; Hussain, A.; Husain, F.M.; Khan, A.A. Comparative anticancer and antimicrobial activity of aerial parts of Acacia salicina, Acacia laeta, Acacia hamulosa and Acacia tortilis grown in Saudi Arabia. Saudi Pharm. J. 2017, 25, 1248–1252. [Google Scholar] [CrossRef]
- Ads, E.N.; Hassan, S.I.; Sharawy, S.M.; Humaidi, J.R. Phytochemical, antimicrobial and cytotoxic evaluation of Ziziphus spinachristi (L.) stem bark. Biomed. Res. 2017, 28, 6646. [Google Scholar]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef]
- Martinez-Armenta, C.; Camacho-Rea, M.C.; Martínez-Nava, G.A.; Espinosa-Velázquez, R.; Pineda, C.; Gomez-Quiroz, L.E.; López-Reyes, A. Therapeutic Potential of Bioactive Compounds in Honey for Treating Osteoarthritis. Front. Pharmacol. 2021, 12, 642836. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Al Guthami, F.M.; Al Gethami, A. Physiochemical analysis of some Saudi Arabia honey. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 1441–1448. [Google Scholar] [CrossRef]
- Al-Khalifa, A.S.; Al-Arify, I.A. Physicochemical characteristics and pollen spectrum of some Saudi honeys. Food Chem. 1999, 67, 21–25. [Google Scholar] [CrossRef]
- El-Metwally, A. Factors Affecting the Physical and Chemical Characteristics of Egyptian Beehoney. Ph.D. Thesis, Cairo University, Giza, Egypt, 2015; pp. 306–320. [Google Scholar]
- El Sohaimy, S.A.; Masry, S.H.D.; Shehata, M.G. Physicochemical characteristics of honey from different origins. Ann. Agric. Sci. 2015, 60, 279–287. [Google Scholar] [CrossRef]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, W.D.M.; Azmat, A. Pharmacological evidence of neuro-pharmacological activity of Acacia tortilis leaves in mice. Metab. Brain Dis. 2016, 31, 881–885. [Google Scholar] [CrossRef]
- Alharbi, W.; Azmat, A. Hypoglycemic and hypocholesterolemic effects of Acacia tortilis (Fabaceae) growing in Makkah. Pak. J. Pharmacol. 2011, 28, 1–8. [Google Scholar]
- Karar, M.; Quiet, L.; Rezk, A.; Jaiswal, R.; Rehders, M.; Ullrich, M.; Brix, K.; Kuhnert, N. Phenolic profile and in vitro assessment of cytotoxicity and antibacterial activity of Ziziphus spina-christi leaf extracts. Med. Chem. 2016, 6, 143–156. [Google Scholar]
- Márcio, C.; Isabel, C.F.R.F. The Role of Phenolic Compounds in the Fight against Cancer—A Review. Anti-Cancer Agents Med. Chem. 2013, 13, 1236–1258. [Google Scholar]
- Ziani, B.E.C.; Carocho, M.; Abreu, R.M.V.; Bachari, K.; Alves, M.J.; Calhelha, R.C.; Talhi, O.; Barros, L.; Ferreira, I.C.F.R. Phenolic profiling, biological activities and in silico studies of Acacia tortilis (Forssk.) Hayne ssp. raddiana extracts. Food Biosci. 2020, 36, 100616. [Google Scholar] [CrossRef]
- Ekhtelat, M.; Ravaji, K.; Parvari, M. Effect of Iranian Ziziphus honey on growth of some foodborne pathogens. J. Nat. Sci. Biol. Med. 2016, 7, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Kilty, S.J.; Duval, M.; Chan, F.T.; Ferris, W.; Slinger, R. Methylglyoxal: (active agent of manuka honey) in vitro activity against bacterial biofilms. Int. Forum Allergy Rhinol. 2011, 1, 348–350. [Google Scholar] [CrossRef]
- Majtan, J. Methylglyoxal-a potential risk factor of manuka honey in healing of diabetic ulcers. Evid. Based Complement. Altern. Med. 2011, 2011, 295494. [Google Scholar] [CrossRef] [PubMed]
- Shahina, S.J.; Krishnan, P. Comparison of the antimicrobial activity of Manuka honey and native honey against methicillin resistant staphylococci from asymptomatic healthcare workers. Int. J. Phytomed. 2013, 5, 262. [Google Scholar]
- Al-Brahim, J.S.; Mohammed, A.E. Antioxidant, cytotoxic and antibacterial potential of biosynthesized nanoparticles using bee honey from two different floral sources in Saudi Arabia. Saudi J. Biol. Sci. 2020, 27, 363–373. [Google Scholar] [CrossRef]
- Gebrehiwot, H.; Ensermu, U.; Dekebo, A.; Endale, M.; Nefo Duke, T. In Vitro Antibacterial and Antioxidant Activities, Pharmacokinetics, and In Silico Molecular Docking Study of Phytochemicals from the Roots of Ziziphus spina-christi. Biochem. Res Int. 2024, 2024, 7551813. [Google Scholar] [CrossRef]
- Mahdi, S.A.A. Antibacterial Activity of Ziziphus Spina-Christi Leaf Extract and Its Effect on Gene Expression of Some Virulence Factors of Some Pathogenic Bacteria. Microbiol. Res. J. Int. 2025, 35, 106–113. [Google Scholar] [CrossRef]
- Dash, N.; Panigrahi, D.; Al-Zarouni, M. Antimicrobial effect of honey from the Arabian Gulf region against bacterial isolates from pus and wound swabs. Adv. Microbiol. 2016, 6, 745–752. [Google Scholar] [CrossRef]
- Al-Waili, N.S.; Akmal, M.; Al-Waili, F.S.; Saloom, K.Y.; Ali, A. The antimicrobial potential of honey from United Arab Emirates on some microbial isolates. Med. Sci. Monit. 2005, 11, Br433-8. [Google Scholar]
- Sung, S.-H.; Choi, G.-H.; Lee, N.-W.; Shin, B.-C. External Use of Propolis for Oral, Skin, and Genital Diseases: A Systematic Review and Meta-Analysis. Evid. Based Complement. Altern. Med. 2017, 2017, 8025752. [Google Scholar] [CrossRef] [PubMed]
- Gardana, C.; Scaglianti, M.; Pietta, P.; Simonetti, P. Analysis of the polyphenolic fraction of propolis from different sources by liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2007, 45, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Yosri, N.; Abd El-Wahed, A.A.; Ghonaim, R.; Khattab, O.M.; Sabry, A.; Ibrahim, M.A.A.; Moustafa, M.F.; Guo, Z.; Zou, X.; Algethami, A.F.M.; et al. Anti-Viral and Immunomodulatory Properties of Propolis: Chemical Diversity, Pharmacological Properties, Preclinical and Clinical Applications, and In Silico Potential against SARS-CoV-2. Foods 2021, 10, 1776. [Google Scholar] [CrossRef]
- Zainullin, R.; Kunakova, R.; Gareev, V.; Galyautdinov, I.; Sadretdinova, Z.; Muslimov, Z.; Odinokov, V. Flavanones and flavones from Bashkir propolis. Chem. Nat. Compd. 2018, 54, 975–977. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016, 4, 481–511. [Google Scholar] [CrossRef]
- Rivera-Yañez, N.; Rivera-Yañez, C.R.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Reyes-Reali, J.; Mendoza-Ramos, M.I.; Méndez-Cruz, A.R.; Nieto-Yañez, O. Effects of Propolis on Infectious Diseases of Medical Relevance. Biology 2021, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A. Perspectives for Uses of Propolis in Therapy against Infectious Diseases. Molecules 2022, 27, 4594. [Google Scholar] [CrossRef]
- Schnitzler, P.; Neuner, A.; Nolkemper, S.; Zundel, C.; Nowack, H.; Sensch, K.H.; Reichling, J. Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother. Res. 2010, 24 (Suppl. 1), S20–S28. [Google Scholar] [CrossRef]
- Yagoub, A.E.; Alshammari, G.M.; Subash-Babu, P.; Mohammed, M.A.; Yahya, M.A.; Alhosain, A.I. Synthesis of Ziziphus spina-christi (Jujube) Root Methanol Extract Loaded Functionalized Silver Nanoparticle (ZS-Ag-NPs); Physiochemical Characterization and Effect of ZS-Ag-NPs on Adipocyte Maturation, Adipokine and Vascular Smooth Muscle Cell Interaction. Nanomaterials 2021, 11, 2563. [Google Scholar] [CrossRef]
- Zayed, M.F.; Eisa, W.H.; Abdel-Moneam, Y.K.; El-kousy, S.M.; Atia, A. Ziziphus spina-christi based bio-synthesis of Ag nanoparticles. J. Ind. Eng. Chem. 2015, 23, 50–56. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; Elshaer, M.A.; Abd-Elraheem, M.A.; Ali, O.M.O.M.; Haggag, M.I.; El-Sayyad, G.S.; Attia, M.S. Ziziphus spina-christi extract-stabilized novel silver nanoparticle synthesis for combating Fusarium oxysporum-causing pepper wilt disease: In Vitro and in vivo studies. Arch. Microbiol. 2023, 205, 69. [Google Scholar] [CrossRef]

| Quality Parameter | Vachellia tortilis | Ziziphus spina-christi | CODEX Standards |
|---|---|---|---|
| Moisture (%) | 14.7 ± 0.51 | 16.9 ± 0.49 | Less than 20% |
| Ph | 4.5 ± 0.2 | 4.7 ± 0.07 | 3.4–6.1 |
| Acidity (meq acid/100 g) | 39 ± 1.7 | 27.5 ± 3.5 | Not more than 50 meq acid/100 g |
| Fructose (%) | 51.4 ± 0.32 | 41.2 ± 2.1 | NA |
| Glucose (%) | 33.5 ± 0.47 | 35.7 ± 3.4 | NA |
| Fructose + Glucose (%) | 84.9 | 76.9 | Not less than 60 g/100 g |
| Sucrose (%) | 0.08 ± 0.02 | 1.01 ± 0.21 | Not more than 5 g/100 g |
| Hydroxymethylfurfural (HMF) (mg/kg) | 0.0 ± 0.0 | 1.3 ± 0.4 | Not more than 40 mg/kg |
| Diastase (Diastase Number—DN) | 12 ± 2.6 | 7.5 ± 0.7 | Not less than 8 DN (Schade) |
| Total Protein (µg/g) | 222.54 ± 23.0 | 560.56 ± 32.7 | NA |
| Honey Type | Key Phytochemicals | Measured Bioactive Content | Pharmacological Effects | References |
|---|---|---|---|---|
| V. tortilis (Samar, UAE) | Quercetin, Rutin, Catechin | 1624–2898 mg GAE/kg | Antioxidant, Antibacterial (S. aureus inhibition up to 23 mm) | [2,4,29] |
| Z. spina-christi (Sidr, UAE) | Quercetin, Catechin, Peptides | 972–1520 mg GAE/kg | Antimicrobial (E. coli, C. albicans), Wound healing | [2,4,6] |
| Manuka (New Zealand) | Methylglyoxal (MGO), DHA | ~563–785 mg GAE/kg | Antibacterial (non-peroxide-based), Wound healing | [31,37] |
| Parameter | Ethanolic Extract | Decoction Extract | Notes |
|---|---|---|---|
| Cell Lines Tested | Four tumor cell lines + PLP2 (normal) | Four tumor cell lines + PLP2 (normal) | Cytotoxicity tested on tumor and normal lines [40] |
| GI50 Values (HepG2 tumor cells) | 33 ± 1 μg/mL | 52.4 ± 0.5 μg/mL | Lower values indicate greater inhibition [40] |
| GI50 Range (tumor cells) | 33.3 to 53.0 μg/mL | 33.3 to 53.0 μg/mL | Consistent effects across tumor cell lines [41] |
| GI50 Values (PLP2 normal cells) | 253 ± 0.02 μg/mL | 259 ± 0.05 μg/mL | Demonstrates selectivity to cancer cells [40] |
| Interpretation of Potency | More effective against HepG2 | Less effective against HepG2 | Suggests tumor-specific targeting [42] |
| Effect on Tumor vs. Normal Cells | Higher response in tumor cells | Higher response in tumor cells | Tumor cells more reactive to phenolic compounds [40] |
| Mechanisms Affected | Cell cycle, apoptosis, and cell death | Cell cycle, apoptosis, and cell death | Likely apoptosis/cell cycle disruption mechanisms [42] |
| Plant/Extract | Dose/Model | Observed Effect | Pharmacological Category | Reference |
|---|---|---|---|---|
| Vachellia tortilis (aqueous leaf extract) | 400 and 800 mg/kg orally in mice (behavioral models) | Anxiolytic and antidepressant effects, increased time in the light–dark box test | Anxiolytic/Neurobehavioral | [38] |
| Vachellia tortilis (aqueous leaf extract) | 800 mg/kg orally for 7 days in rats (lipid profile) | Significant decrease in blood glucose, total cholesterol, LDL; increase in HDL | Hypolipidemic/Antidiabetic | [39] |
| Vachellia tortilis (ethanolic extract) | Topical or in vitro antimicrobial assays | Broad-spectrum antimicrobial action against bacteria and fungi; MIC: 0.4–0.8 mg/mL | Antimicrobial | [24] |
| Ziziphus spina-christi (aqueous extract) | Antifungal test against Aspergillus and Geotrichum spp. | Inhibition of fungal growth with enhanced cytotoxic response | Antifungal | [30] |
| Ziziphus spina-christi (alkaline ethyl acetate extract) | Cytotoxic test on colon/breast carcinoma cells | Dose-dependent inhibition of cancer cells; IC50: 196–400 μg/well | Anticancer/Cytotoxic | [30] |
| Ziziphus spina-christi (ethanolic extract) | HepG2 tumor cells and PLP2 normal cell assays | Selective inhibition of tumor cells over normal cells; GI50 ~33–53 μg/mL | Anticancer/Selective cytotoxicity | [40,41,42] |
| Honey Type/Floral Source | Target Microbes/Organisms | Key Findings | Reference |
|---|---|---|---|
| Vachellia tortilis (UAE) | S. aureus, E. coli, P. aeruginosa | Strong inhibition zones up to 23 mm; dose-dependent efficacy | [50] |
| Ziziphus spina-christi (UAE) | E. coli, C. albicans, S. aureus | Growth suppressed within 24 h at ≥80% concentration; dose- and time-dependent inhibition | [51] |
| Ziziphus spina-christi (Iran) | S. aureus, E. coli, L. monocytogenes, S. typhimurium | Log 3–7.5 CFU reduction over 120 h; potent antimicrobial activity | [43] |
| Z. spina-christi and Vachellia gerrardi (Saudi Arabia) | B. cereus, T. mentagrophytes, E. coli, S. aureus | Diluted honey exceeded antibiotic activity; strong antifungal effects | [4] |
| Manuka (Leptospermum spp., New Zealand) | Various Gram-positive/negative bacteria; biofilms | High non-peroxide activity due to MGO; effective against resistant strains and biofilms | [44,45] |
| Manuka vs. native honey (India) | MRSA, MRCoNS | Manuka showed stronger inhibition zones (≥30 mm) and lower MICs (6.3–12.5%) than native honey (50%) | [46] |
| Z. spina-christi (biosynthesized AgNPs) | MRSA, E. coli, P. Aeruginosa | AgNPs from honey showed strong antibacterial and anticancer activity | [47] |
| Z. spina-christi (roots) | P. aeruginosa and DPPH free radicals | Root compounds showed strong antioxidant activity and microbial inhibition | [48] |
| Z. spina-christi (leaf extract) | S. aureus, K. pneumoniae | Significant MIC/MBC; downregulated virulence genes agrA and fimH | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtar, F.A.; Elhawary, S.S.; Fakhry, A.M.; Almoalla, A.A.; Alyammahi, K.M.; Belaid, Y.; Abdelazim, K.; Zabady, A.H.; Yassin, H.A.; Elnahas, H.M.; et al. Ecological, Apicultural, and Therapeutic Value of Vachellia tortilis and Ziziphus spina-christi Honeys in the United Arab Emirates: A Model for Sustainable Use in Arid Ecosystems. Foods 2025, 14, 2859. https://doi.org/10.3390/foods14162859
Mokhtar FA, Elhawary SS, Fakhry AM, Almoalla AA, Alyammahi KM, Belaid Y, Abdelazim K, Zabady AH, Yassin HA, Elnahas HM, et al. Ecological, Apicultural, and Therapeutic Value of Vachellia tortilis and Ziziphus spina-christi Honeys in the United Arab Emirates: A Model for Sustainable Use in Arid Ecosystems. Foods. 2025; 14(16):2859. https://doi.org/10.3390/foods14162859
Chicago/Turabian StyleMokhtar, Fatma Alzahraa, Seham S. Elhawary, Amal M. Fakhry, Aseela Abdulla Almoalla, Khawla Mohammed Alyammahi, Youssouf Belaid, Karim Abdelazim, Ahmed Hamdy Zabady, Heba A. Yassin, Hanan M. Elnahas, and et al. 2025. "Ecological, Apicultural, and Therapeutic Value of Vachellia tortilis and Ziziphus spina-christi Honeys in the United Arab Emirates: A Model for Sustainable Use in Arid Ecosystems" Foods 14, no. 16: 2859. https://doi.org/10.3390/foods14162859
APA StyleMokhtar, F. A., Elhawary, S. S., Fakhry, A. M., Almoalla, A. A., Alyammahi, K. M., Belaid, Y., Abdelazim, K., Zabady, A. H., Yassin, H. A., Elnahas, H. M., & El-Keblawy, A. (2025). Ecological, Apicultural, and Therapeutic Value of Vachellia tortilis and Ziziphus spina-christi Honeys in the United Arab Emirates: A Model for Sustainable Use in Arid Ecosystems. Foods, 14(16), 2859. https://doi.org/10.3390/foods14162859








