Storage Stability Enhancement of Lactic Acid Beverage Using Anti-MDA Lactiplantibacillus plantarum NJAU-01: The Antioxidant’s Role
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Preparation
2.2. Preparation and Content Determination of Malondialdehyde (MDA)
2.3. Kinetic Growth
2.4. Preparation of Cell-Free Extracts of NJAU-01
2.5. Determination of Oxidation and Antioxidant Indices of Cell-Free Extracts
2.5.1. Schiff Base
2.5.2. Determination of Total Sulfhydryl and Disulfide Bond Content
2.5.3. 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) Free Radical Scavenging Activity
2.5.4. 2,2′-Azino-Bis(3-ethylbenzothiazoline-6-sulfonic acid) Free Radical (ABTS) Scavenging Rate
2.5.5. Fe2+ Chelation Capacity
2.5.6. Fe3+ Reducing Capacity
2.5.7. Hydroxyl Radical Scavenging Rate (·OH)
2.5.8. Superoxide Anion Free Radical (O2−) Scavenging Activity
2.5.9. Anti-Peroxidation of Lipid (LP)
2.5.10. The Systematic Assessment of Antioxidant Activity
2.6. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.7. Determination of Quality Indicators for Lactic Acid Beverages
2.7.1. Sensory Evaluation
2.7.2. Response Surface Methodology (RSM)
2.7.3. The Count of Viable Bacteria
2.7.4. Titratable Acidity
- 6—The concentration of the sodium hydroxide standard solution (mol/L);
- V1—The volume of the sodium hydroxide standard solution consumed during titration (mL);
- V0—The volume of the sodium hydroxide standard solution consumed during blank titration (mL);
- 100—100 g of the sample;
- 10—The mass of the sample taken (g);
- 0.1—The molar concentration of sodium hydroxide defined by the acidity theory (mol/L).
2.7.5. Determination of Viscosity
2.7.6. Centrifugal Sedimentation Rate
2.7.7. Determination of Flavor Substances (GC-MS)
2.7.8. Odor Activity Value (OAV)
2.8. Statistical Analysis
3. Results and Discussions
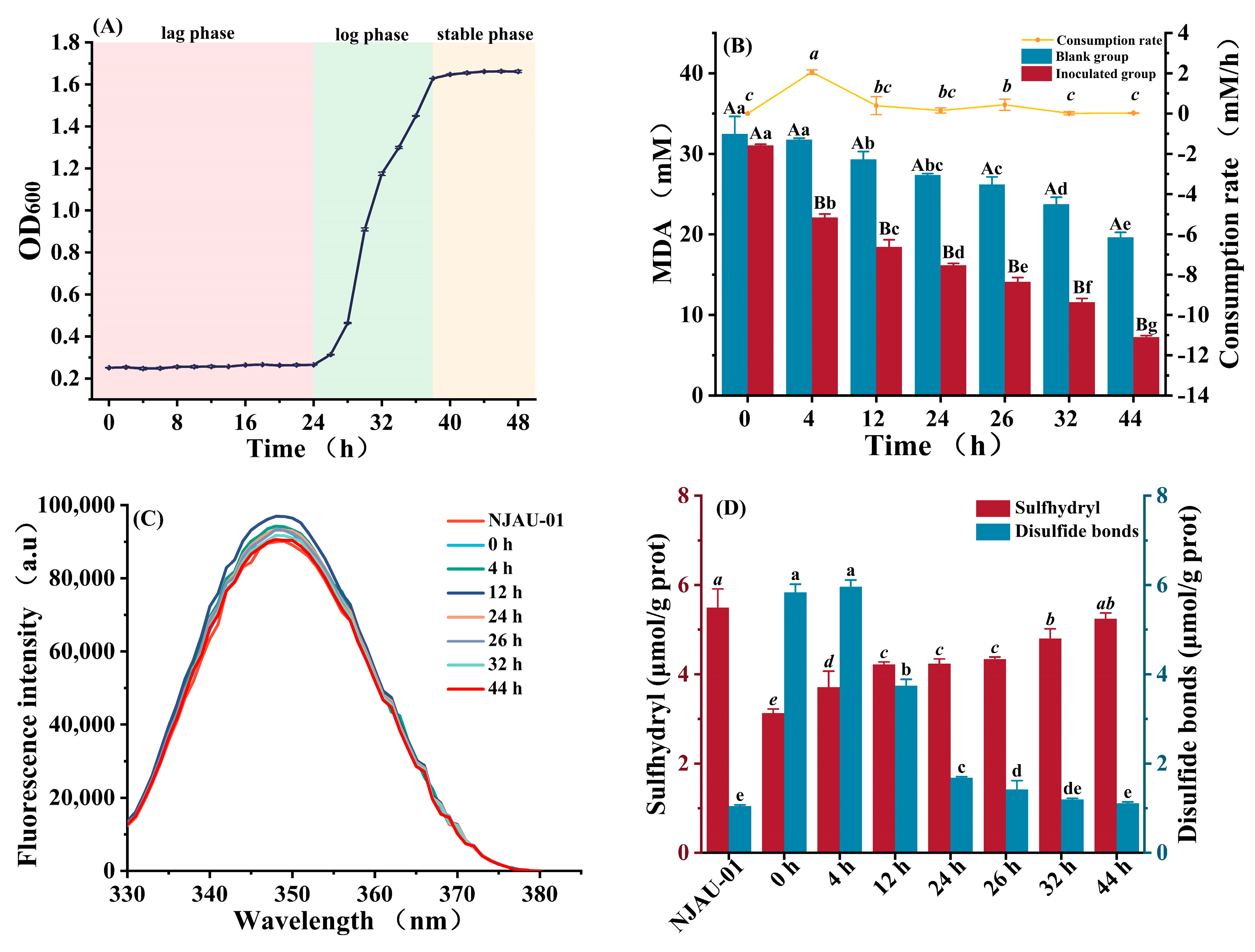
3.1. Oxidation Indices of NJAU-01 Under 40 mM MDA Stress
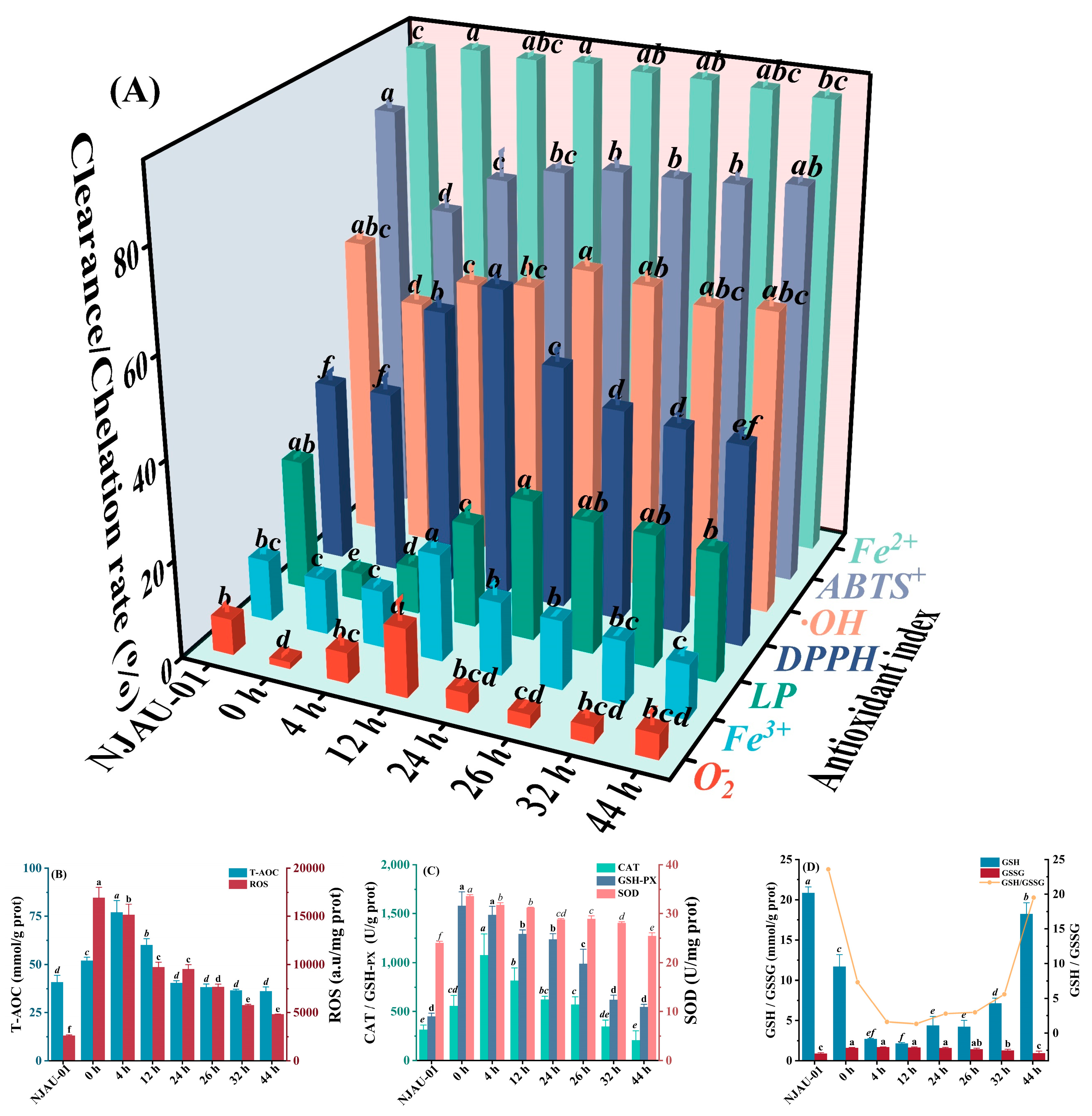
3.2. Changes in Antioxidant Activity and Antioxidant Enzyme Activity In Vitro
3.3. SDS-PAGE
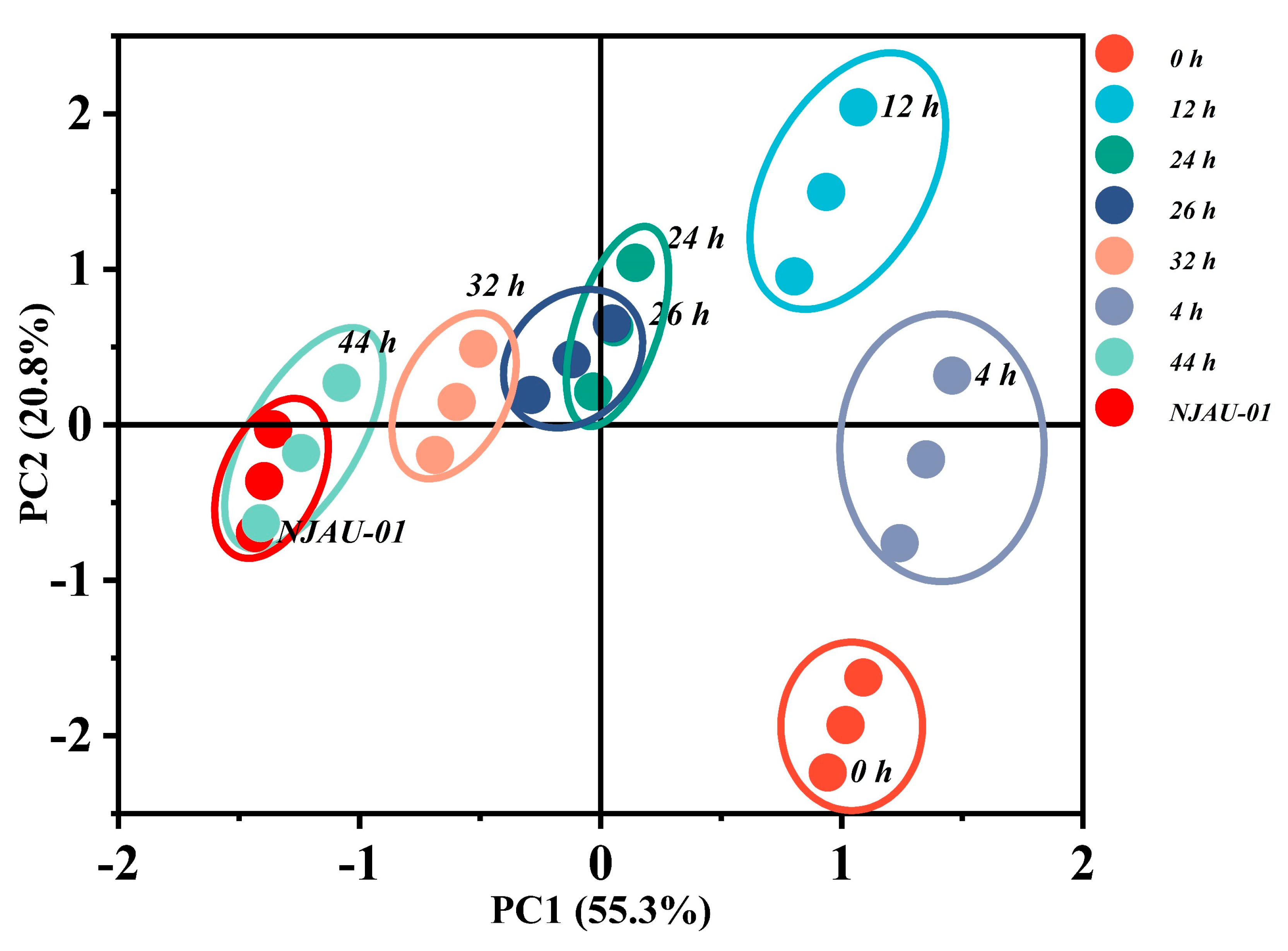
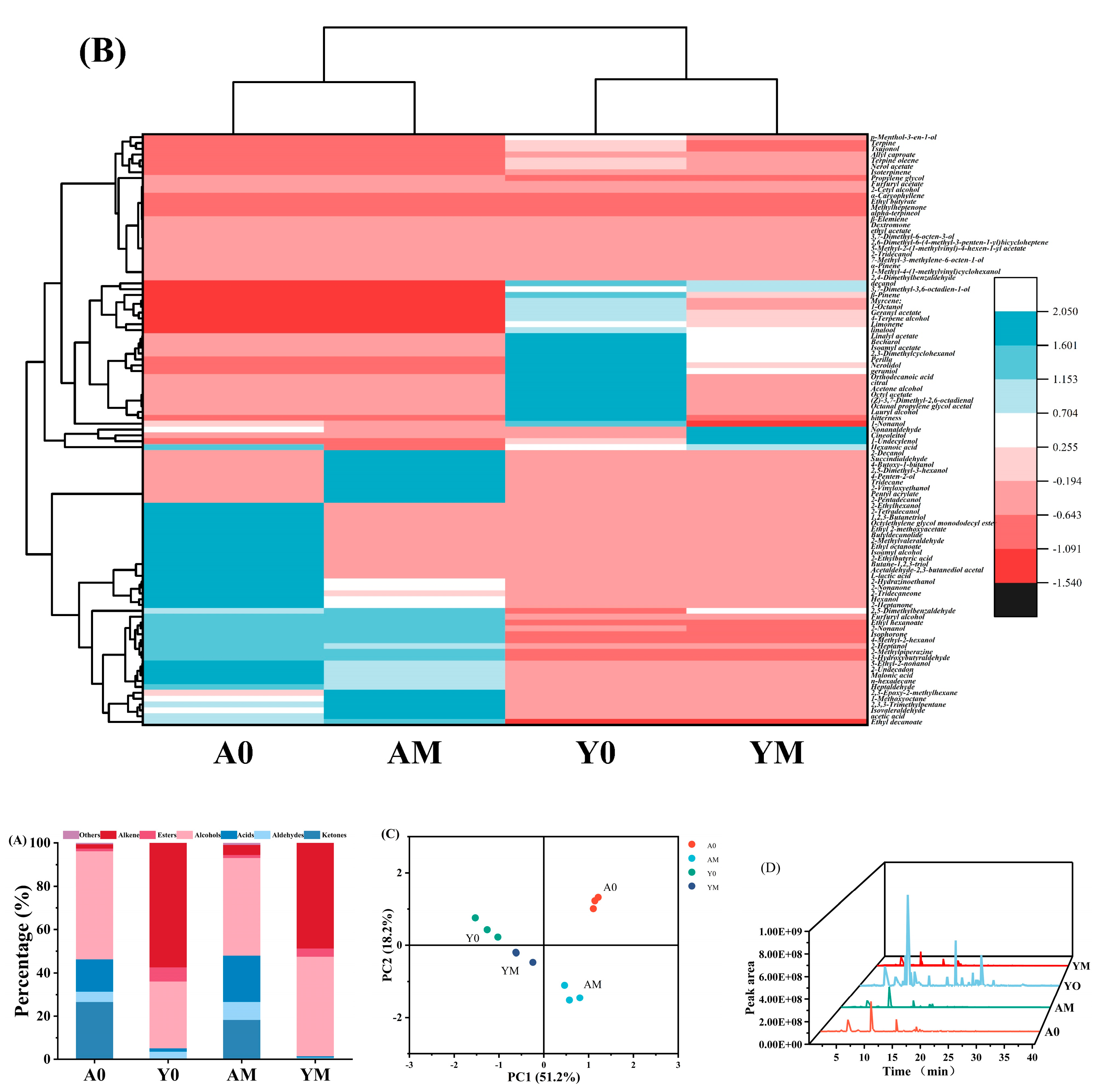
3.4. Principal Component Analysis (PCA)
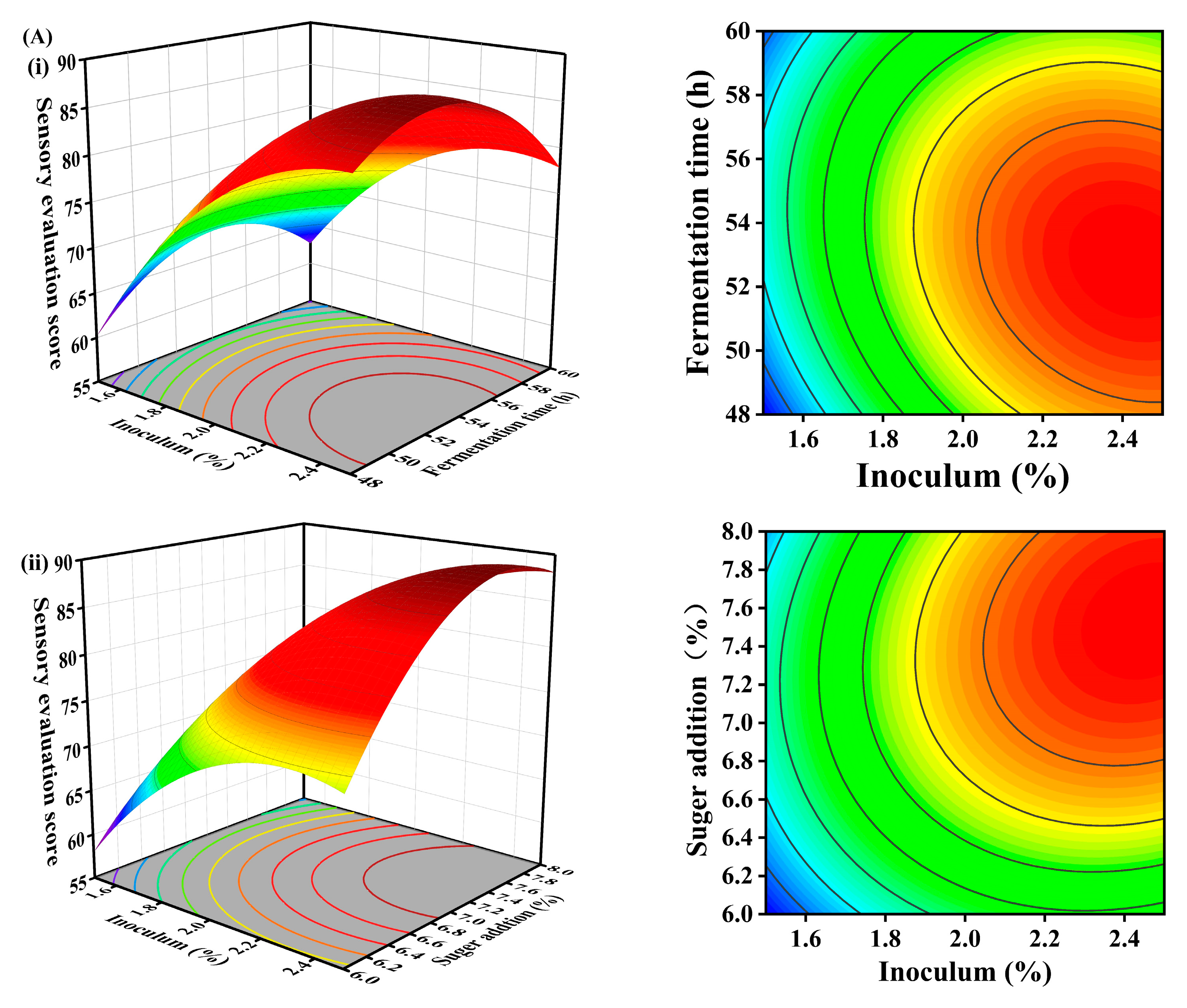
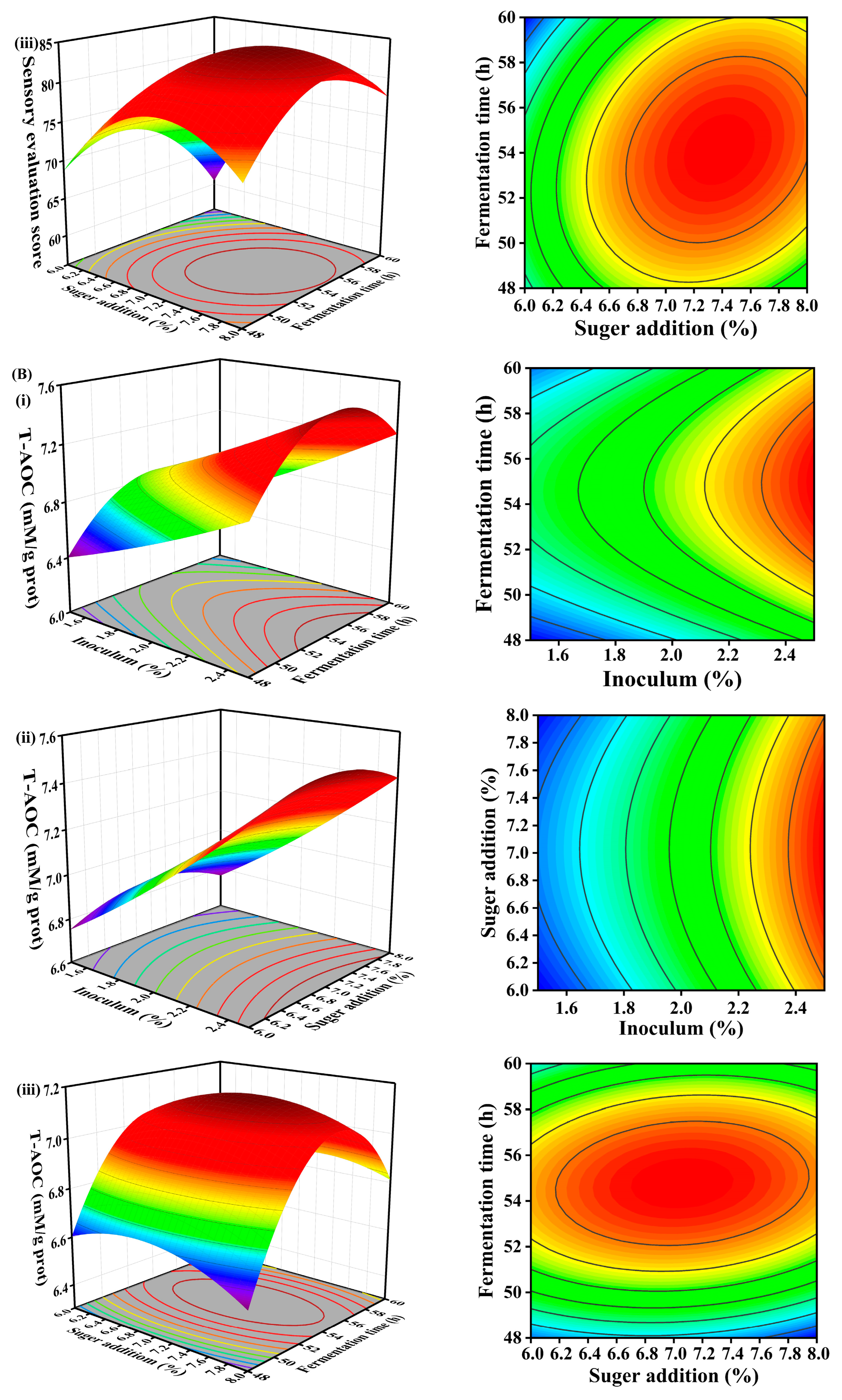
3.5. Response Surface
3.5.1. Effect of Three-Factor Interaction on Sensory Evaluation
3.5.2. Effect of Three-Factor Interaction on T-AOC
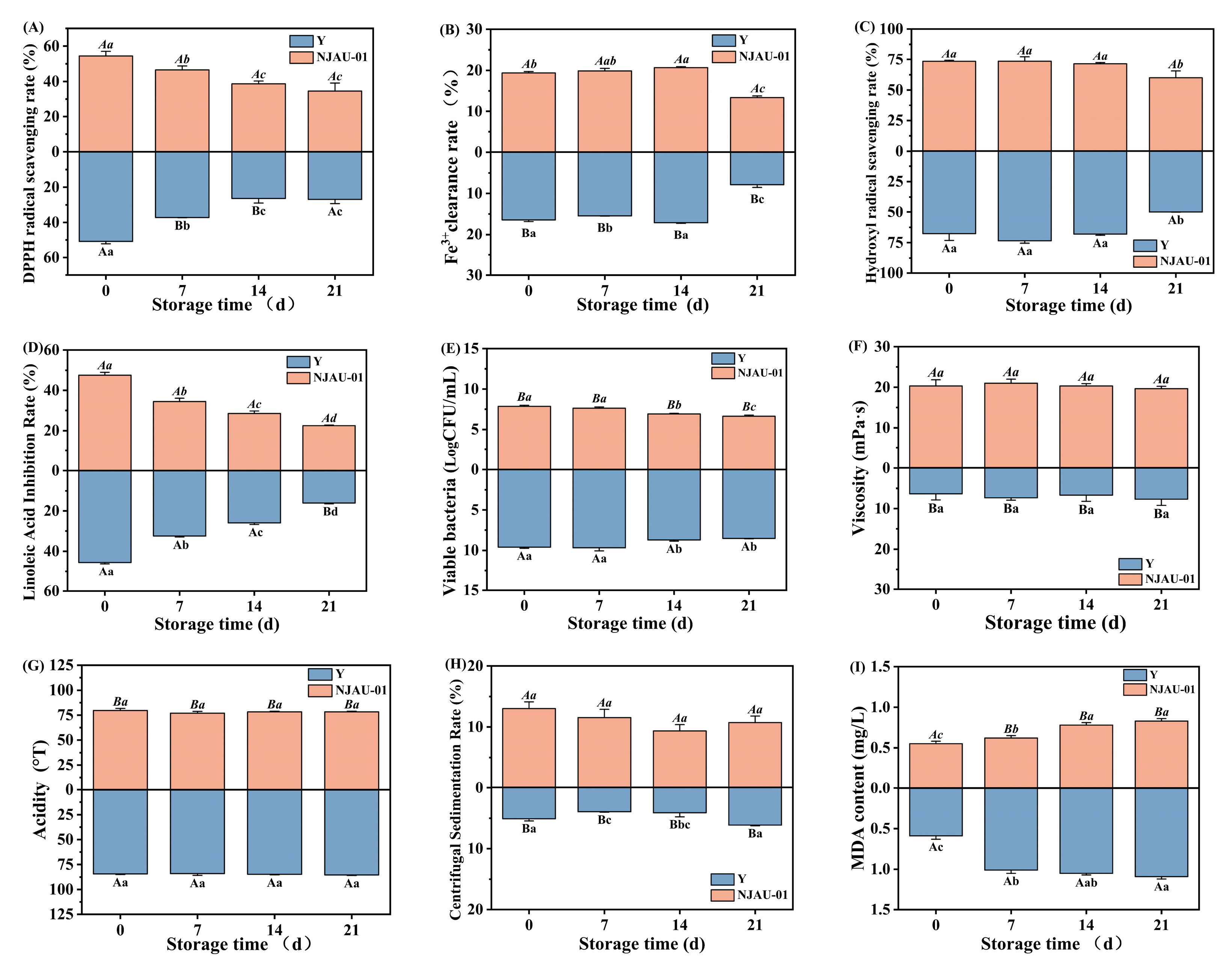
3.6. The Quality and Functions of Lactic Acid Beverages Throughout the Storage Period
3.7. GC-MS
3.7.1. Volatile Compound Profiles
3.7.2. Variations in Volatile Flavor Substance Contents in Beverages During Storage
3.7.3. Variations in Odor Activity Values (OAVs) of Beverages During Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anwer, M.; Wei, M.Q. Harnessing the power of probiotic strains in functional foods: Nutritive, therapeutic, and next-generation challenges. Food Sci. Biotechnol. 2024, 33, 2081–2095. [Google Scholar] [CrossRef] [PubMed]
- Zommiti, M.; Feuilloley, M.G.J.; Connil, N. Update of Probiotics in Human World: A Nonstop Source of Benefactions till the End of Time. Microorganisms 2020, 8, 1907. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Wang, B.K.; Zhan, X.A.; Wang, Y.B.; Li, W.F. Effects of glucose oxidase and its combination with B. amyloliquefaciens SC06 on intestinal microbiota, immune response and antioxidative capacity in broilers. Animal 2022, 16, 100473. [Google Scholar] [CrossRef]
- Amanatidou, A.; Smid, E.J.; Bennik, M.H.J.; Gorris, L.G.M. Antioxidative properties of upon exposure to elevated oxygen concentrations. FEMS Microbiol. Lett. 2001, 203, 87–94. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, J.; Hu, X.L.; Le, G.W.; Shi, Y.H. Lactobacilli prevent hydroxy radical production and inhibit and growth in system mimicking colon fermentation. Lett. Appl. Microbiol. 2010, 50, 264–269. [Google Scholar] [CrossRef]
- Kim, K.J.; Kyung, S.; Jin, H.; Im, M.; Kim, J.W.; Kim, H.S.; Jang, S.E. Lactic Acid Bacteria Isolated from Human Breast Milk Improve Colitis Induced by 2,4,6-Trinitrobenzene Sulfonic Acid by Inhibiting NF-κB Signaling in Mice. J. Microbiol. Biotechnol. 2023, 33, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Arif, S.; Khan, R.S.; Nazir, W.; Khalid, N.; Maqsood, S. Opportunities and challenges for functional and medicinal beverages: Current and future trends. Trends Food Sci. Technol. 2019, 88, 513–526. [Google Scholar] [CrossRef]
- Shudong, P.; Guo, C.Q.; Wu, S.J.; Cui, H.C.; Suo, H.Y.; Duan, Z. Bioactivity and metabolomics changes of plant-based drink fermented by Bacillus coagulans VHProbi C08. LWT-Food Sci. Technol. 2022, 156, 113030. [Google Scholar] [CrossRef]
- Kadyan, S.; Rashmi, H.M.; Pradhan, D.; Kumari, A.; Chaudhari, A.; Deshwal, G.K. Effect of lactic acid bacteria and yeast fermentation on antimicrobial, antioxidative and metabolomic profile of naturally carbonated probiotic whey drink. LWT-Food Sci. Technol. 2021, 142, 111059. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Liu, P.P.; Fu, H.; Wang, D.D.; Zhao, D.; Zhang, J.C.; Wang, C.T.; Li, M. Effects of fermentation supernatant on skin aging caused by oxidative stress. J. Funct. Foods 2022, 96, 105222. [Google Scholar] [CrossRef]
- Medeiros, M.H.G. DNA Damage by Endogenous and Exogenous Aldehydes. J. Braz. Chem. Soc. 2019, 30, 2000–2009. [Google Scholar] [CrossRef]
- Tavakoli, M.; Najafi, M.B.H.; Mohebbi, M. Effect of the milk fat content and starter culture selection on proteolysis and antioxidant activity of probiotic yogurt. Heliyon 2019, 5, e01204. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.F.; Ge, P.W.; Jiang, D.L.; Du, N.; Chen, J.H.; Yuan, L.M.; Yu, H.; Xu, X.; Wu, M.G.; Zhang, W.G.; et al. A novel and simple cell-based electrochemical biosensor for evaluating the antioxidant capacity of strains isolated from Chinese dry-cured ham. Biosens. Bioelectron. 2018, 99, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.F.; Yang, B.; Liu, R.; Jiang, D.L.; Yu, H.; Wu, M.G.; Zhang, W.G. Antioxidant activity of Lactobacillus plantarum NJAU-01 in an animal model of aging. BMC Microbiol. 2021, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, R.; Li, S.Y.; Yu, H.; Ge, Q.F. Metabolism of hydrogen peroxide by Lactobacillus plantarum NJAU-01: A proteomics study. Food Microbiol. 2023, 112, 104246. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.X.; Jia, C.Y.; Yang, B.; Wu, Y.H.; Chen, L.; Liu, R.; Wu, M.G.; Yu, H.; Ge, Q.F. The ameliorative mechanism of NJAU-01 against D-galactose induced oxidative stress: A hepatic proteomics and gut microbiota analysis. Food Funct. 2024, 15, 6174–6188. [Google Scholar] [CrossRef]
- Ge, Q.F.; Chen, S.; Liu, R.; Chen, L.; Yang, B.; Yu, H.; Wu, M.G.; Zhang, W.G.; Zhou, G.H. Effects of NJAU-01 on the protein oxidation of fermented sausage. Food Chem. 2019, 295, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Kullisaar, T.; Zilmer, M.; Mikelsaar, M.; Vihalemm, T.; Annuk, H.; Kairane, C.; Kilk, A. Two antioxidative lactobacilli strains as promising probiotics. Int. J. Food Microbiol. 2002, 72, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, S.; Ito, M.; Sato, T.; Yokoi, W.; Yamamoto, Y.; Harada, K.; Ikemura, H.; Miyazaki, K. Intracellular GSH of Streptococcus thermophilus shows anti-oxidative activity against low-density lipoprotein oxidation in vitro and in a hyperlipidaemic hamster model. Benef. Microbes 2018, 9, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, P.G.; Villegas, J.M.; de Giori, G.S.; Saavedra, L.; Hebert, E.M. Enhancement of gamma-aminobutyric acid (GABA) production by Lactobacillus brevis CRL 2013 based on carbohydrate fermentation. Int. J. Food Microbiol. 2020, 333, 108792. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Y.; Gao, L.E.; Huang, W.K.; Ma, Y.; Muhammad, I.; Hanif, A.; Ding, Z.T.; Guo, X.S. Antioxidant properties of lactic acid bacteria isolated from traditional fermented yak milk and their probiotic effects on the oxidative senescence of Caenorhabditis elegans. Food Funct. 2022, 13, 3690–3703. [Google Scholar] [CrossRef] [PubMed]
- Saleena, L.A.K.; Chandran, D.; Geetha, R.; Radha, R.; Sathian, C.T.; Kumar, M.; Sureshkumar, R.; Marthandan, V.; Radha. Optimization and Identification of Lactic Acid Bacteria with Higher Mannitol Production Potential. Indian J. Anim. Res. 2023, 57, 1644–1651. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, C.M.; Hua, Y.F. Structural modification of soy protein by the lipid peroxidation product malondialdehyde. J. Sci. Food Agric. 2009, 89, 1416–1423. [Google Scholar] [CrossRef]
- Tang, W.; Xing, Z.Q.; Li, C.; Wang, J.J.; Wang, Y.P. Molecular mechanisms and antioxidant effects of MA2. Food Chem. 2017, 221, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, S.Y.; Yang, B.; Chen, L.; Ge, Q.F.; Xiong, G.Y.; Yu, H.; Wu, M.A.; Zhang, W.A. Investigation of the antioxidant capacity of cell-free extracts from NJAU-01 obtained by different cell disruption methods. LWT-Food Sci. Technol. 2021, 152, 112393. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhao, Y.J.; Zhang, L.; Zhang, X.; Huang, L.; Li, D.; Niu, C.H.; Yang, Z.N.; Wang, Q. Antioxidant activity of strains isolated from traditional Chinese fermented foods. Food Chem. 2012, 135, 1914–1919. [Google Scholar] [CrossRef] [PubMed]
- Padilla, P.; Andrade, M.J.; Peña, F.J.; Rodríguez, A.; Estévez, M. Molecular mechanisms of the disturbance caused by malondialdehyde on probiotic PL503. Microb. Biotechnol. 2022, 15, 668–682. [Google Scholar] [CrossRef]
- Lee, M.; Song, J.H.; Choi, E.J.; Yun, Y.R.; Lee, K.W.; Chang, J.Y. UPLC-QTOF-MS/MS and GC-MS Characterization of Phytochemicals in Vegetable Juice Fermented Using Lactic Acid Bacteria from Kimchi and Their Antioxidant Potential. Antioxidants 2021, 10, 1761. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, M.M.; Xiao, C.Q.; Zhao, Q.Z.; Su, G.W. Practical problems when using ABTS assay to assess the radical-scavenging activity of peptides: Importance of controlling reaction pH and time. Food Chem. 2016, 192, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.B.; Zhang, H.; Cheng, L.L.; Wang, L.; Qian, H.F.; Qi, X.G. In vitro and in vivo antioxidant activity of polyphenols extracted from black highland barley. Food Chem. 2016, 194, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Quatravaux, S.; Remize, F.; Bryckaert, E.; Colavizza, D.; Guzzo, J. Examination of lactate metabolism side effects in relation to the modulation of aeration parameters. J. Appl. Microbiol. 2006, 101, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.J.; Xiong, H.G.; Huang, X.; Guyonnet, V.; Ma, M.H.; Chen, X.Q.; Zheng, Y.T.; Wang, L.M.; Hu, G. Identification and molecular mechanisms of novel antioxidant peptides from two sources of eggshell membrane hydrolysates showing cytoprotection against oxidative stress: A combined in silico and in vitro study. Food Res. Int. 2022, 157, 111266. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.A.; Barril, C.; Bedgood, D.R.; Prenzler, P.D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Kim, J.S.; Kang, Y.M.; Lim, J.H.; Kim, Y.M.; Lee, M.S.; Jeong, M.H.; Ahn, C.B.; Je, J.Y. Antioxidant activity and γ-aminobutyric acid (GABA) content in sea tangle fermented by BJ20 isolated from traditional fermented foods. Food Chem. 2010, 122, 271–276. [Google Scholar] [CrossRef]
- Zhao, L.L.; Zhang, M.; Wang, H.X.; Devahastin, S. Effect of carbon dots in combination with aqueous chitosan solution on shelf life and stability of soy milk. Int. J. Food Microbiol. 2020, 326, 108650. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Hwang, Y.R.; Kim, M.S.; Chung, M.S.; Kim, Y.S. Comparison of Volatile and Nonvolatile Compounds in Rice Fermented by Different Lactic Acid Bacteria. Molecules 2019, 24, 1183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mi, S.; Liu, R.B.; Sang, Y.X.; Wang, X.H. Evaluation of Volatile Compounds during the Fermentation Process of Yogurts by Streptococcus thermophilus Based on Odor Activity Value and Heat Map Analysis. Int. J. Anal. Chem. 2020, 2020, 3242854. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, H.R.; Hou, Y.Q.; Li, J.; Zou, T.T.; Zhang, D.J.; Wen, R.; Li, H.L.; Song, H.L. Characterization of Key Odor-Active Off-Flavor Compounds in Aged Pasteurized Yogurt by Sensory-Directed Flavor Analysis. J. Agric. Food Chem. 2022, 70, 14439–14447. [Google Scholar] [CrossRef] [PubMed]
- Dan, T.; Hu, H.M.; Li, T.; Dai, A.N.; He, B.B.; Wang, Y.A. Screening of mixed-species starter cultures for increasing flavour during fermentation of milk. Int. Dairy J. 2022, 135, 105473. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, H.W.; Liu, T.J.; Gong, P.M.; Wang, Y.W.; Wang, H.Z.; Tian, X.Y.; Liu, Q.Q.; Cui, Q.Y.; Xie, X.; et al. Aroma classification and flavor characterization of fermented milk by HS-GC-IMS and HS-SPME-GC-TOF/MS. Food Biosci. 2022, 49, 101832. [Google Scholar] [CrossRef]
- Fritz, K.S.; Petersen, D.R. An overview of the chemistry and biology of reactive aldehydes. Free. Radic. Biol. Med. 2013, 59, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.X.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed]
- Basu Thakur, P.; Long, A.R.; Nelson, B.J.; Kumar, R.; Rosenberg, A.F.; Gray, M.J. Complex Responses to Hydrogen Peroxide and Hypochlorous Acid by the Probiotic Bacterium Lactobacillus reuteri. mSystems 2019, 4, e00453-19. [Google Scholar] [CrossRef] [PubMed]
- Vandemoortele, A.; Heynderickx, P.M.; Leloup, L.; De Meulenaer, B. Kinetic modeling of malondialdehyde reactivity in oil to simulate actual malondialdehyde formation upon lipid oxidation. Food Res. Int. 2021, 140, 110063. [Google Scholar] [CrossRef] [PubMed]
- Vandemoortele, A.; Babat, P.; Yakubu, M.; De Meulenaer, B. Reactivity of Free Malondialdehyde during In Vitro Simulated Gastrointestinal Digestion. J. Agric. Food Chem. 2017, 65, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Chen, L.; Wu, H.; Xu, X.; Zhou, G.; Zhu, B.; Feng, X. (-)-Epigallocatechin-3-gallate-mediated formation of myofibrillar protein emulsion gels under malondialdehyde-induced oxidative stress. Food Chem. 2019, 285, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xiong, Z.; Lu, S.; Walayat, N.; Hu, C.; Xiong, H. Effects of oxidative modification on the functional, conformational and gelling properties of myofibrillar proteins from Culter alburnus. Int. J. Biol. Macromol. 2020, 162, 1442–1452. [Google Scholar] [CrossRef]
- Wu, X.J.; Li, F.; Wu, W. Effects of rice bran rancidity on the oxidation and structural characteristics of rice bran protein. LWT-Food Sci. Technol. 2020, 120, 108943. [Google Scholar] [CrossRef]
- Gatellier, P.; Santé-Lhoutellier, V.; Portanguen, S.; Kondjoyan, A. Use of meat fluorescence emission as a marker of oxidation promoted by cooking. Meat Sci. 2009, 83, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Sobral, M.M.C.; Casal, S.; Faria, M.A.; Cunha, S.C.; Ferreira, I.M.P.L.V.O. Influence of culinary practices on protein and lipid oxidation of chicken meat burgers during cooking and gastrointestinal digestion. Food Chem. Toxicol. 2020, 141, 111401. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Arcanjo, N.O.; Andrade, M.J.; Padilla, P.; Rodríguez, A.; Madruga, M.S.; Estévez, M. Resveratrol protects against HO- induced oxidative stress and stimulates antioxidant defenses through upregulation of the gene. Free Radic. Biol. Med. 2019, 135, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Li, Z.W.; Wang, K.D.; Kong, B.H.; Chen, Q. L-glycine and L-glutamic acid protect R1 against oxidative damage induced by hydrogen peroxide. Food Microbiol. 2022, 101, 103897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liao, C.M.; Yang, Y.W.; Wang, Y.Z.; Ding, K.; Huo, D.Q.; Hou, C.J. Response of lipid biosynthesis into intracellular reactive oxygen species level under stress conditions. Bioresour. Technol. 2019, 287, 121414. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.F.; Li, R.N.; Dai, P.Y.; Li, Z.J.; Li, Y.S.; Li, C.M. Deoxynivalenol induced apoptosis and inflammation of IPEC-J2 cells by promoting ROS production. Environ. Pollut. 2019, 251, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.Q.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jové, M.; Mota-Martorell, N.; Pradas, I.; Martín-Gari, M.; Ayala, V.; Pamplona, R. The Advanced Lipoxidation End-Product Malondialdehyde-Lysine in Aging and Longevity. Antioxidants 2020, 9, 1132. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Qu, X.W.; Xia, Q.N.; Wang, H.X.; Chen, P.; Li, X.D.; Wang, L.N.; Yang, W.S. Effect of on the antioxidant activity of Cheddar cheese during ripening and under simulated gastrointestinal digestion. LWT-Food Sci. Technol. 2018, 95, 99–106. [Google Scholar] [CrossRef]
- Wang, A.R.; Hou, K.R.; Mu, G.Q.; Ma, C.L.; Tuo, Y.F. Antioxidative effect of soybean milk fermented by Y16 on 2, 2 -azobis (2-methylpropionamidine) dihydrochloride (ABAP)-damaged HepG2 cells. Food Biosci. 2021, 44, 101120. [Google Scholar] [CrossRef]
- Shori, A.B.; Aljohani, G.S.; Al-zahrani, A.J.; Al-sulbi, O.S.; Baba, A.S. Viability of probiotics and antioxidant activity of cashew milk-based yogurt fermented with selected strains of probiotic Lactobacillus spp. LWT-Food Sci. Technol. 2022, 153, 112482. [Google Scholar] [CrossRef]
- Fan, X.K.; Du, L.H.; Xu, J.; Shi, Z.H.; Zhang, T.; Jiang, X.X.; Zeng, X.Q.; Wu, Z.; Pan, D.D. Effect of single probiotics CGMCC1.5956 and CGMCC1.5954 and their combination on the quality of yogurt as fermented milk. LWT-Food Sci. Technol. 2022, 163, 113530. [Google Scholar] [CrossRef]
- Zahrani, A.J.A.; Shori, A.B. Viability of probiotics and antioxidant activity of soy and almond milk fermented with selected strains of probiotic. LWT-Food Sci. Technol. 2023, 176, 114531. [Google Scholar] [CrossRef]
- Shori, A.B.; Rashid, F.; Baba, A.S. Effect of the addition of phytomix-3+mangosteen on antioxidant activity, viability of lactic acid bacteria, type 2 diabetes key-enzymes, and sensory evaluation of yogurt. LWT-Food Sci. Technol. 2018, 94, 33–39. [Google Scholar] [CrossRef]
- Chi, X.L.; Shao, Y.W.; Pan, M.H.; Yang, Q.Y.; Yang, Y.; Zhang, X.M.; Ai, N.S.; Sun, B.G. Distinction of volatile flavor profiles in various skim milk products via HS-SPME-GC-MS and E-nose (Mar, 10.1007/s00217-021-03730-0, 2021). Eur. Food Res. Technol. 2021, 247, 1553–1556. [Google Scholar] [CrossRef]
- Jo, Y.; Benoist, D.M.; Barbano, D.M.; Drake, M.A. Flavor and flavor chemistry differences among milks processed by high-temperature, short-time pasteurization or ultra-pasteurization. J. Dairy Sci. 2018, 101, 3812–3828. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.T.H.; Cozzolino, D.; Zisu, B.; Chandrapala, J. Effects of high and low frequency ultrasound on the production of volatile compounds in milk and milk products—A review. J. Dairy Res. 2020, 87, 501–512. [Google Scholar] [CrossRef]
- Toelstede, S.; Hofmann, T. Sensomics mapping and identification of the key bitter metabolites in Gouda cheese. J. Agric. Food Chem. 2008, 56, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Z.J.; Xu, L.Y.; Wang, B.; Zhang, J.H.; Li, B.Z.; Cao, Y.P.; Tan, L. Key aroma compounds identified in Cheddar cheese with different ripening times by aroma extract dilution analysis, odor activity value, aroma recombination, and omission. J. Dairy Sci. 2021, 104, 1576–1590. [Google Scholar] [CrossRef] [PubMed]
- Iranmanesh, M.; Ezzatpanah, H.; Akbari-Adergani, B.; Karimi Torshizi, M.A. SPME/GC-MS characterization of volatile compounds of Iranian traditional dried Kashk. Int. J. Food Prop. 2018, 21, 1067–1079. [Google Scholar] [CrossRef]
- Liu, W.; Pu, X.; Sun, J.; Shi, X.; Cheng, W.; Wang, B. Effect of Lactobacillus plantarum on functional characteristics and flavor profile of fermented walnut milk. LWT 2022, 160, 113254. [Google Scholar] [CrossRef]
- Liu, A.; Liu, Q.Q.; Bu, Y.S.; Hao, H.N.; Liu, T.J.; Gong, P.M.; Zhang, L.W.; Chen, C.; Tian, H.X.; Yi, H.X. Aroma classification and characterization of subsp fermented milk. Food Chem. X 2022, 15, 100385. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Tong, L.J.; Chi, X.L.; Ai, N.S.; Cao, Y.G.; Sun, B.G. Comparison of Sensory and Electronic Tongue Analysis Combined with HS-SPME-GC-MS in the Evaluation of Skim Milk Processed with Different Preheating Treatments. Molecules 2019, 24, 1650. [Google Scholar] [CrossRef]
- Dursun, A.; Güler, Z.; Sekerli, Y.E. Characterization of volatile compounds and organic acids in ultra-high-temperature milk packaged in tetra brik cartons. Int. J. Food Prop. 2017, 20, 1511–1521. [Google Scholar] [CrossRef]
- De Santis, D.; Fidaleo, M. Effect of aging pit on volatile compounds and sensory attributes of traditional Italian Fossa cheese. LWT-Food Sci. Technol. 2022, 153, 112507. [Google Scholar] [CrossRef]
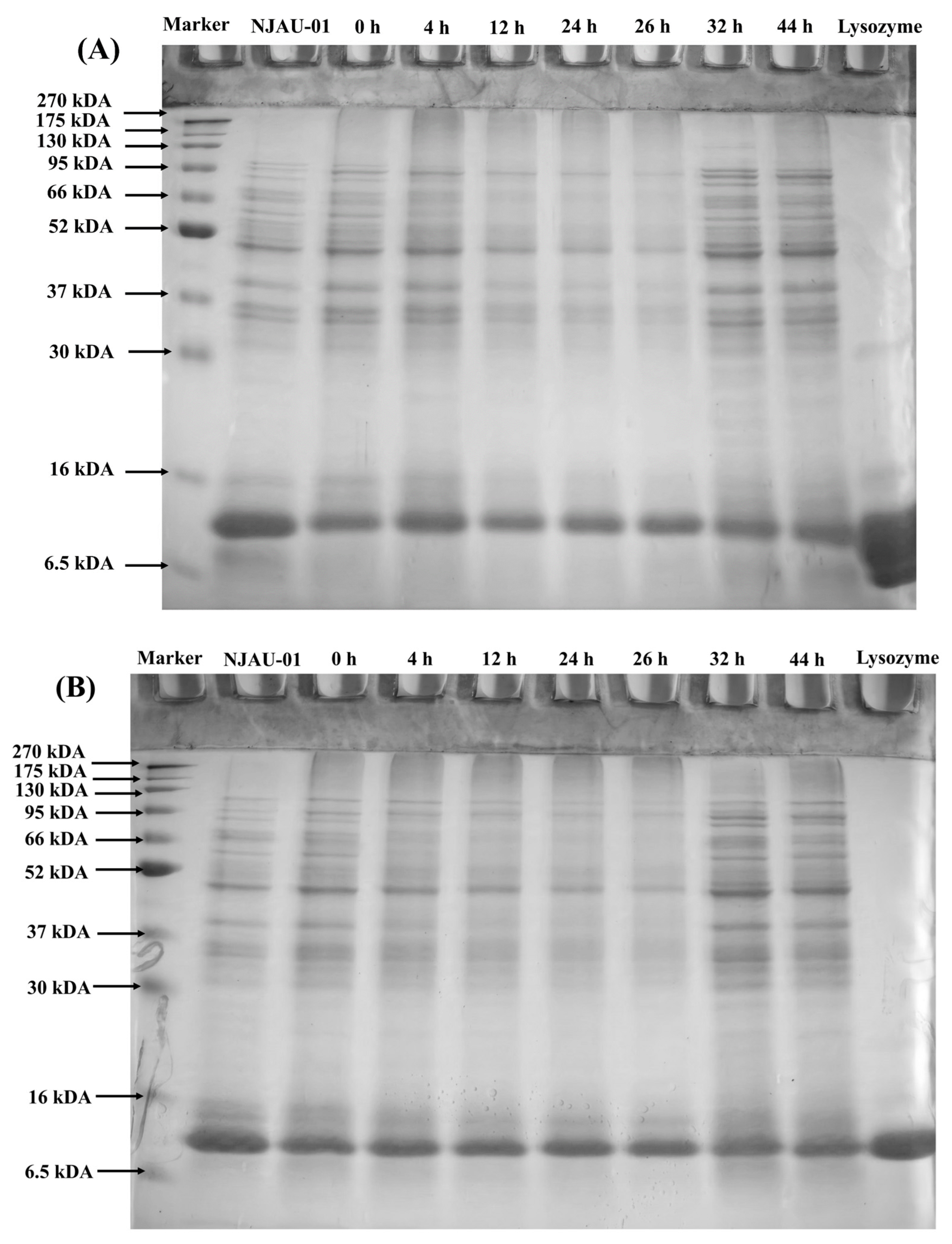
| Sample | +Mercaptoethanol | −Mercaptoethanol |
|---|---|---|
| NJAU-01 | 295,378 | 468,606 |
| 0 h | 386,947 | 507,988 |
| 4 h | 423,444 | 618,450 |
| 12 h | 417,184 | 565,550 |
| 24 h | 411,552 | 462,140 |
| 26 h | 394,399 | 466,192 |
| 32 h | 386,211 | 482,419 |
| 44 h | 339,259 | 481,844 |
| (A) | ||||||
| Number | (A) Inoculum | (B) Fermentation Time | (C) Sugar Addition | Sensory Score | T-AOC (mM/g prot) | |
| 1 | 1 | 0 | −1 | 71.56 | 7.40 | |
| 2 | −1 | 1 | 0 | 61.10 | 6.57 | |
| 3 | 1 | 1 | 0 | 78.34 | 7.16 | |
| 4 | 0 | 0 | 0 | 84.42 | 7.07 | |
| 5 | 0 | 0 | 0 | 82.48 | 7.16 | |
| 6 | 0 | 0 | 0 | 83.04 | 7.18 | |
| 7 | 0 | −1 | −1 | 68.06 | 6.63 | |
| 8 | 0 | 0 | 0 | 83.16 | 7.20 | |
| 9 | 1 | −1 | 0 | 85.28 | 6.96 | |
| 10 | −1 | 0 | −1 | 59.14 | 6.66 | |
| 11 | 0 | 0 | 0 | 85.52 | 7.16 | |
| 12 | 0 | 1 | 1 | 78.26 | 6.79 | |
| 13 | 0 | 1 | −1 | 60.86 | 6.78 | |
| 14 | −1 | 0 | 1 | 64.50 | 6.75 | |
| 15 | 1 | 0 | 1 | 86.92 | 7.50 | |
| 16 | 0 | −1 | 1 | 72.70 | 6.46 | |
| 17 | −1 | −1 | 0 | 59.64 | 6.48 | |
| (B) | ||||||
| Source | Sum of Squares | df | Mean Squares | F-Value | p-Value | Significance |
| Model | 1690.20 | 9 | 187.80 | 81.22 | <0.0001 | *** |
| A-Inoculum | 755.05 | 1 | 755.05 | 326.56 | <0.0001 | *** |
| B-Fermentation time | 6.34 | 1 | 6.34 | 2.74 | 0.1418 | |
| C-sugar addition | 228.55 | 1 | 228.55 | 98.85 | <0.0001 | *** |
| AB | 17.64 | 1 | 17.64 | 7.63 | 0.0280 | * |
| AC | 25.00 | 1 | 25.00 | 10.81 | 0.0133 | * |
| BC | 40.70 | 1 | 40.70 | 17.60 | 0.0041 | ** |
| A2 | 153.45 | 1 | 153.45 | 66.37 | <0.0001 | *** |
| B2 | 183.24 | 1 | 183.24 | 79.25 | <0.0001 | *** |
| C2 | 215.67 | 1 | 215.67 | 93.28 | <0.0001 | *** |
| Residual | 16.18 | 7 | 2.31 | |||
| Lack of Fit | 10.14 | 3 | 3.38 | 2.24 | 0.2262 | |
| Pure Error | 6.04 | 4 | 1.51 | |||
| Cor Total | 1706.38 | 16 | ||||
| Model | R2 = 0.9905 | R2Adj = 0.9783 | ||||
| (C) | ||||||
| Source | Sum of Squares | df | Mean Squares | F-Value | p-Value | Significance |
| Model | 1.60 | 9 | 0.18 | 23.52 | 0.0002 | *** |
| A-Inoculum | 0.82 | 1 | 0.82 | 108.12 | <0.0001 | *** |
| B-Fermentation time | 0.076 | 1 | 0.076 | 10.11 | 0.0155 | * |
| C-Sugar addition | 0.0003 | 1 | 0.0003 | 0.040 | 0.8475 | |
| AB | 0.0035 | 1 | 0.0035 | 0.46 | 0.5187 | |
| AC | 4.000 × 10−6 | 1 | 4.000 × 10−6 | 0.0005 | 0.9823 | |
| BC | 0.0078 | 1 | 0.0078 | 1.04 | 0.3421 | |
| A2 | 0.0029 | 1 | 0.0029 | 0.39 | 0.5529 | |
| B2 | 0.63 | 1 | 0.63 | 83.57 | <0.0001 | *** |
| C2 | 0.043 | 1 | 0.043 | 5.74 | 0.0478 | |
| Residual | 0.053 | 7 | 0.0075 | |||
| Lack of Fit | 0.042 | 3 | 0.014 | 5.32 | 0.0702 | |
| Pure Error | 0.011 | 4 | 0.0026 | |||
| Cor Total | 1.65 | 16 | ||||
| Model | R2 = 0.9680 | R2Adj = 0.9268 | ||||
| A0 μg/L | Y0 μg/L | AM μg/L | YM μg/L | ||
|---|---|---|---|---|---|
| Ketones | 2-heptanone | 60.40 ± 4.93 | / | 27.09 ± 1.70 | / |
| 2-tridecanone | 15.39 ± 2.04 | / | 4.47 ± 0.16 | / | |
| 2-undecanone | 23.81 ± 2.24 | / | 16.02 ± 2.99 | / | |
| Isophorone | 2.83 ± 0.45 | / | 3.13 ± 0.51 | / | |
| 2-nononone | 43.84 ± 0.51 | / | 14.86 ± 2.58 | / | |
| Aldehydes | 2, 5-dimethylbenzaldehyde | 1.22 ± 0.14 | / | 1.50 ± 0.34 | 1.02 ± 0.45 |
| Acetaldehyde-2, 3-butane diol acetal | 0.66 ± 0.12 | / | / | / | |
| 3-hydroxybutyraldehyde | 13.54 ± 2.62 | / | 12.58 ± 0.55 | / | |
| Heptanal | 5.71 ± 1.39 | / | 4.33 ± 0.20 | / | |
| Isovaleraldehyde | 4.29 ± 0.16 | / | 8.18 ± 1.24 | / | |
| Nonanal | 1.25 ± 0.17 | / | / | 2.84 ± 0.28 | |
| Butanedial | / | / | 2.78 ± 0.57 | / | |
| 2-methylvaleraldehyde | 0.60 ± 0.05 | / | / | / | |
| Citral | / | 6.95 ± 1.41 | / | / | |
| Perillaldehyde | / | 6.49 ± 0.72 | / | 2.74 ± 0.10 | |
| (Z)-3, 7-dimethyl-2, 6-octanedienal | / | 5.63 ± 1.19 | / | / | |
| Octyl propanediol acetal | / | 16.06 ± 1.64 | / | / | |
| Acids | Acetic acid | 41.13 ± 11.02 | / | 66.24 ± 10.08 | / |
| Malonic acid | 13.30 ± 2.43 | / | 8.54 ± 2.15 | / | |
| L-lactic acid | 18.98 ± 2.92 | / | 1.69 ± 0.04 | / | |
| 2-ethylbutyric acid | 2.42 ± 0.14 | / | / | / | |
| N-hexylic acid | 6.15 ± 0.29 | / | / | / | |
| Octanoic acid | / | 8.29 ± 1.59 | / | / | |
| Caproic acid | / | 4.19 ± 1.64 | / | 4.88 ± 0.10 | |
| N-decanoic acid | / | 2.37 ± 1.08 | / | / | |
| Alcohols | Linalool | / | 115.59 ± 11.20 | / | 97.59 ± 15.68 |
| decyl alcohol | / | 79.33 ± 13.71 | / | 67.63 ± 0.15 | |
| 4-terpenol | / | 25.82 ± 8.32 | / | 12.30 ± 2.34 | |
| Geraniol | / | 21.39 ± 3.40 | / | 9.64 ± 0.38 | |
| 1-octanol | / | 9.35 ± 3.24 | / | 3.54 ± 0.69 | |
| 2-nonyl alcohol | 37.60 ± 4.58 | 0.84 ± 0.16 | 37.00 ± 1.98 | / | |
| 2-hydrazyl ethanol | 126.42 ± 12.48 | / | 41.12 ± 1.55 | / | |
| 2-tetradecyl alcohol | 14.21 ± 2.71 | / | / | / | |
| 2-heptanol | 24.32 ± 4.91 | / | 20.37 ± 2.57 | / | |
| Furfuryl alcohol | 12.05 ± 1.31 | 4.40 ± 0.61 | 12.13 ± 1.72 | 3.96 ± 0.46 | |
| 1-nonyl alcohol | 7.30 ± 0.28 | 14.22 ± 3.01 | 4.73 ± 1.23 | / | |
| 4-methyl-2-hexanol | 8.26 ± 2.30 | / | 7.24 ± 0.66 | / | |
| Butane-1,2, 3-triol | 2.22 ± 0.39 | / | / | / | |
| Isoamylol | 3.33 ± 1.40 | / | / | / | |
| Hexyl alcohol | 2.88 ± 0.70 | / | 1.43 ± 0.22 | / | |
| Propylene glycol | 12.98 ± 0.78 | / | 11.30 ± 2.30 | / | |
| 2-ethyl hexanol | 15.74 ± 2.38 | / | / | / | |
| 5-ethyl-2-nonanol | 7.01 ± 1.33 | / | 4.75 ± 0.12 | / | |
| 1,2, 3-butanediol | 0.90 ± 0.04 | / | / | / | |
| 2-pentadecyl alcohol | / | / | 6.53 ± 0.29 | / | |
| 4-pentene-2-ol | / | / | 6.84 ± 2.07 | / | |
| 2-decanol | / | / | 3.38 ± 0.27 | / | |
| 2, 5-dimethyl-3-hexanol | / | / | 2.73 ± 0.11 | / | |
| 2-ethylxyethanol | / | / | 0.72 ± 0.15 | / | |
| 4-butoxy-1-butanol | / | / | 0.98 ± 0.27 | / | |
| Eudesmol | / | 2.71 ± 0.98 | / | 137.17 ± 7.29 | |
| Fenchol | / | 5.07 ± 1.15 | / | 2.11 ± 0.32 | |
| Thujyl alcohol | / | 2.45 ± 0.87 | / | / | |
| Nerol | / | 2.37 ± 0.45 | / | 0.89 ± 0.13 | |
| L-undecanol | / | 2.84 ± 0.67 | / | 7.99 ± 0.30 | |
| p-menthyl-3-ene-1-ol | / | 1.60 ± 0.65 | / | 0.51 ± 0.08 | |
| Laurinol | / | 2.12 ± 0.53 | / | / | |
| 3, 7-dimethyl-3, 6-octadiene-1-ol | / | 4.38 ± 1.28 | / | 5.33 ± 1.70 | |
| 2, 3-dimethylcyclohexanol | / | 1.02 ± 0.08 | / | 0.49 ± 0.19 | |
| Perilla alcohol | / | 0.75 ± 0.30 | / | / | |
| Acetone alcohol | / | 0.72 ± 0.09 | / | / | |
| Esters | Ethyl caprate | 2.76 ± 0.41 | / | 3.46 ± 0.78 | / |
| Butyl decyllactone | 0.73 ± 0.15 | / | / | / | |
| Octyl ethylene glycol mono-n-dodecyl ester | 0.42 ± 0.13 | / | / | / | |
| Pentyl acrylate | / | / | 0.77 ± 0.09 | / | |
| Ethyl orthoformate | 0.74 ± 0.26 | / | 0.75 ± 0.07 | / | |
| 2-Methoxyethyl acetate | 1.15 ± 0.63 | / | / | / | |
| Ethyl caprylate | 0.55 ± 0.08 | / | / | / | |
| Ethyl caprylate | / | 24.73 ± 4.41 | / | 12.01 ± 1.72 | |
| Geranyl acetate | / | 21.80 ± 4.25 | / | 10.75 ± 0.82 | |
| Isoamyl acetate | / | 6.52 ± 2.53 | / | 2.88 ± 0.12 | |
| Octyl acetate | / | 4.28 ± 1.11 | / | / | |
| Allyl hexanoate | / | 3.51 ± 0.51 | / | 1.55 ± 0.28 | |
| Linalyl acetate | / | 1.96 ± 0.33 | / | 0.80 ± 0.03 | |
| Alkyl alkenes | L-methoxyoctane | 1.23 ± 0.16 | / | 2.81 ± 0.71 | / |
| 2,3, 3-trimethylpentane | 3.28 ± 0.22 | / | 5.79 ± 0.87 | / | |
| N-Hexadecane | 5.33 ± 0.35 | / | 3.13 ± 0.53 | / | |
| 2, 3-epoxy-2-methylhexane | 1.19 ± 0.29 | / | 3.76 ± 1.52 | / | |
| Tridecane | / | / | 0.78 ± 0.03 | / | |
| Limonene | / | 426.63 ± 35.03 | / | 328.72 ± 18.45 | |
| Terpinene | / | 59.04 ± 13.08 | / | 9.10 ± 1.56 | |
| Beta-pinene | / | 35.88 ± 4.99 | / | 14.74 ± 0.76 | |
| Myrcene | / | 15.69 ± 2.77 | / | 5.83 ± 0.47 | |
| Terpinolene | / | 10.50 ± 2.83 | / | 6.41 ± 1.25 | |
| Terpinolene | / | 2.57 ± 0.74 | / | 3.67 ± 0.40 | |
| Else | 2-methylpiperazine | 2.97 ± 0.42 | / | 2.69 ± 0.15 | / |
| Total | 551.10 ± 7.49 | 960.12 ± 68.48 | 356.65 ± 20.61 | 757.11 ± 91.96 |
| Substance | Flavor Characteristics | Threshold Value (mg/L) | A0 | Y0 | AM | YM |
|---|---|---|---|---|---|---|
| Heptan-2-one | Cream, fruit flavor | 140 | 0.43 | / | 0.19 | / |
| 2-undecone | Orange, grass flavor | 80 | 0.29 | / | 0.26 | / |
| 2-nononone | Grass, fruit flavor | 100 | 0.44 | / | 0.15 | / |
| Heptanal | Fresh fruity scent | 3000 | 0.02 | / | 0.01 | / |
| Isovaleral | Floral and fruity | 3.6 | 1.19 | / | 2.27 | / |
| Nonanal | Floral, citrus, honey aromas | 1 | 1.25 | / | / | 2.85 |
| Acetic acid | Acetic | 99 | 0.41 | / | 0.67 | / |
| N-hexylic acid | Acetic | 0.98 | 6.27 | / | / | / |
| Octanoic acid | Acetic | 3 | / | 2.76 | / | / |
| N-capric acid | / | 10 | / | 0.24 | / | / |
| Linalool | Floral and fruity | 6 | / | 19.27 | / | 16.27 |
| Geraniol | Rose flower fragrance | 26 | / | 0.82 | / | 0.37 |
| 2-nonyl alcohol | Rose and orange Fragrance | 25 | 1.5 | 0.034 | 1.48 | / |
| 2-heptanol | Soil, oil | 55 | 0.4 | / | 0.4 | / |
| Furfuralcohol | Coffee, nutty | 5000 | 0.01 | 0.01 | 0.02 | 0.01 |
| 1-nonyl alcohol | Rose and orange fragrance | 0.045 | 162.29 | 315.83 | 105.04 | / |
| Hexyl alcohol | Fruity | 0.0056 | 514.44 | / | 255.27 | / |
| Nerol | Rose flower fragrance | 0.01 | / | 237.16 | / | 88.82 |
| Butyl decyllactone | Peach flavor | 0.06 | 12.13 | / | / | / |
| Ethyl n-caproate | Tropical fruit fragrance | 1 | 0.74 | / | 0.75 | / |
| Linalool | Floral and fruity | 6 | / | 19.27 | / | 16.27 |
| Isoamyl acetate | Banana, pear flavor | 73 | / | 0.09 | / | 0.05 |
| Limonene | Lemon, mint flavor | 200 | / | 2.13 | / | 1.64 |
| Terpinene | Herbaceous | 108 | / | 0.55 | / | 0.09 |
| β-Pinene | / | 140 | / | 0.26 | / | 0.11 |
| Myrcene | Light balsam flavor | 100 | / | 0.16 | / | 0.06 |
| α-pinene | / | 6 | / | 0.1 | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhao, M.; Li, S.; Liu, S.; Gao, S.; Liu, R.; Wu, M.; Yu, H.; Ge, Q. Storage Stability Enhancement of Lactic Acid Beverage Using Anti-MDA Lactiplantibacillus plantarum NJAU-01: The Antioxidant’s Role. Foods 2025, 14, 52. https://doi.org/10.3390/foods14010052
Wu Y, Zhao M, Li S, Liu S, Gao S, Liu R, Wu M, Yu H, Ge Q. Storage Stability Enhancement of Lactic Acid Beverage Using Anti-MDA Lactiplantibacillus plantarum NJAU-01: The Antioxidant’s Role. Foods. 2025; 14(1):52. https://doi.org/10.3390/foods14010052
Chicago/Turabian StyleWu, Yuehao, Menghao Zhao, Suyun Li, Siyu Liu, Song Gao, Rui Liu, Mangang Wu, Hai Yu, and Qingfeng Ge. 2025. "Storage Stability Enhancement of Lactic Acid Beverage Using Anti-MDA Lactiplantibacillus plantarum NJAU-01: The Antioxidant’s Role" Foods 14, no. 1: 52. https://doi.org/10.3390/foods14010052
APA StyleWu, Y., Zhao, M., Li, S., Liu, S., Gao, S., Liu, R., Wu, M., Yu, H., & Ge, Q. (2025). Storage Stability Enhancement of Lactic Acid Beverage Using Anti-MDA Lactiplantibacillus plantarum NJAU-01: The Antioxidant’s Role. Foods, 14(1), 52. https://doi.org/10.3390/foods14010052







