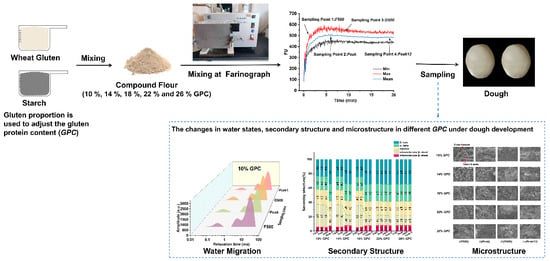
Dynamic Study on Water State and Water Migration during Gluten–Starch Model Dough Development under Different Gluten Protein Contents
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Gluten–Starch Model Dough
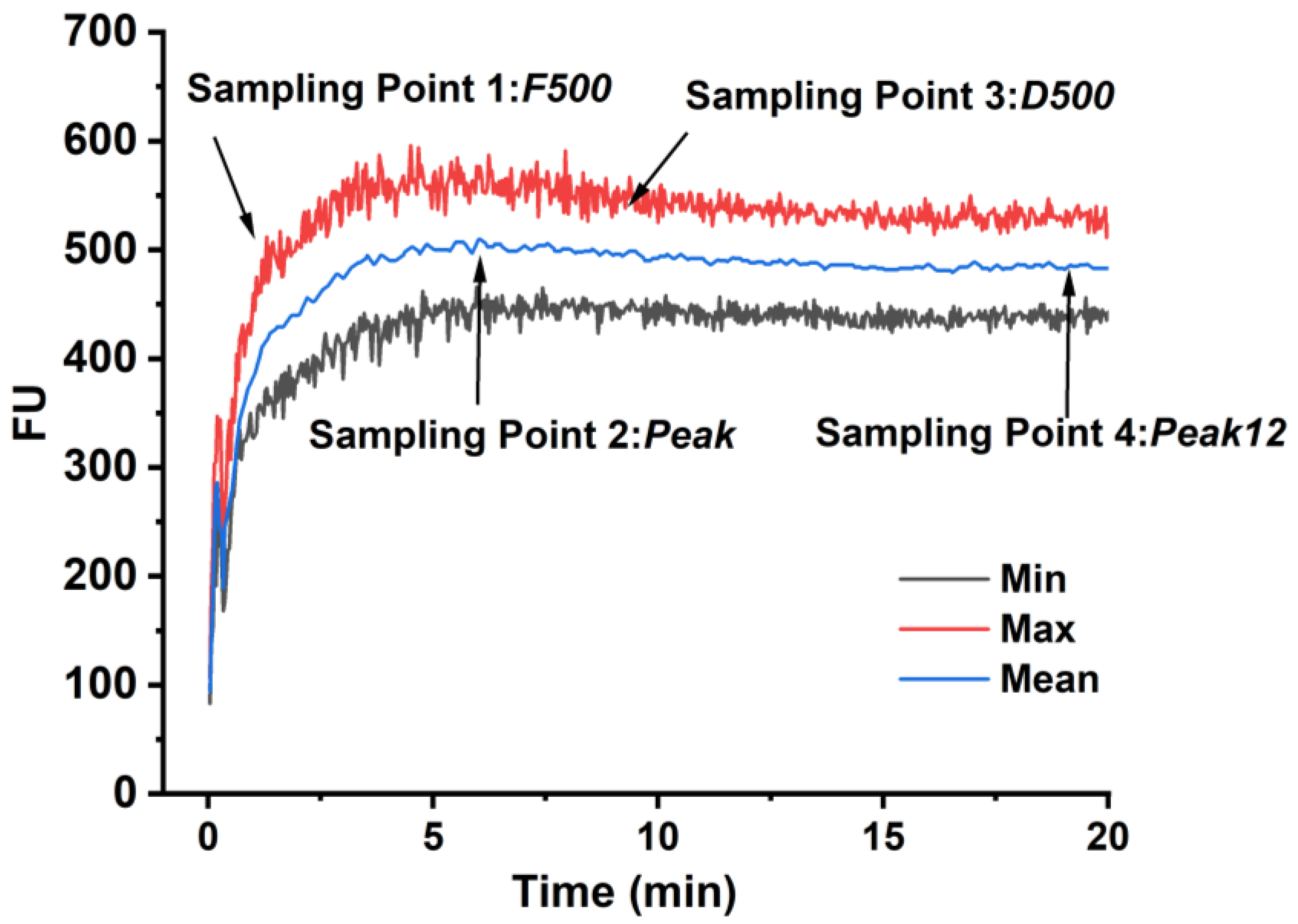
2.2. Farinograph Test and the Sampling Point
2.3. Low-Field Nuclear Magnetic Resonance (LF-NMR)
2.4. Secondary Structure of Dough
2.5. Scanning Electron Microscopy of the Dough Microstructure
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effects of Gluten Content on the Farinograph Parameters
3.2. The Present State of Water (T2) during Dough Development
3.2.1. The Relaxation Time of Strongly Bound Water (T21) during Dough Development
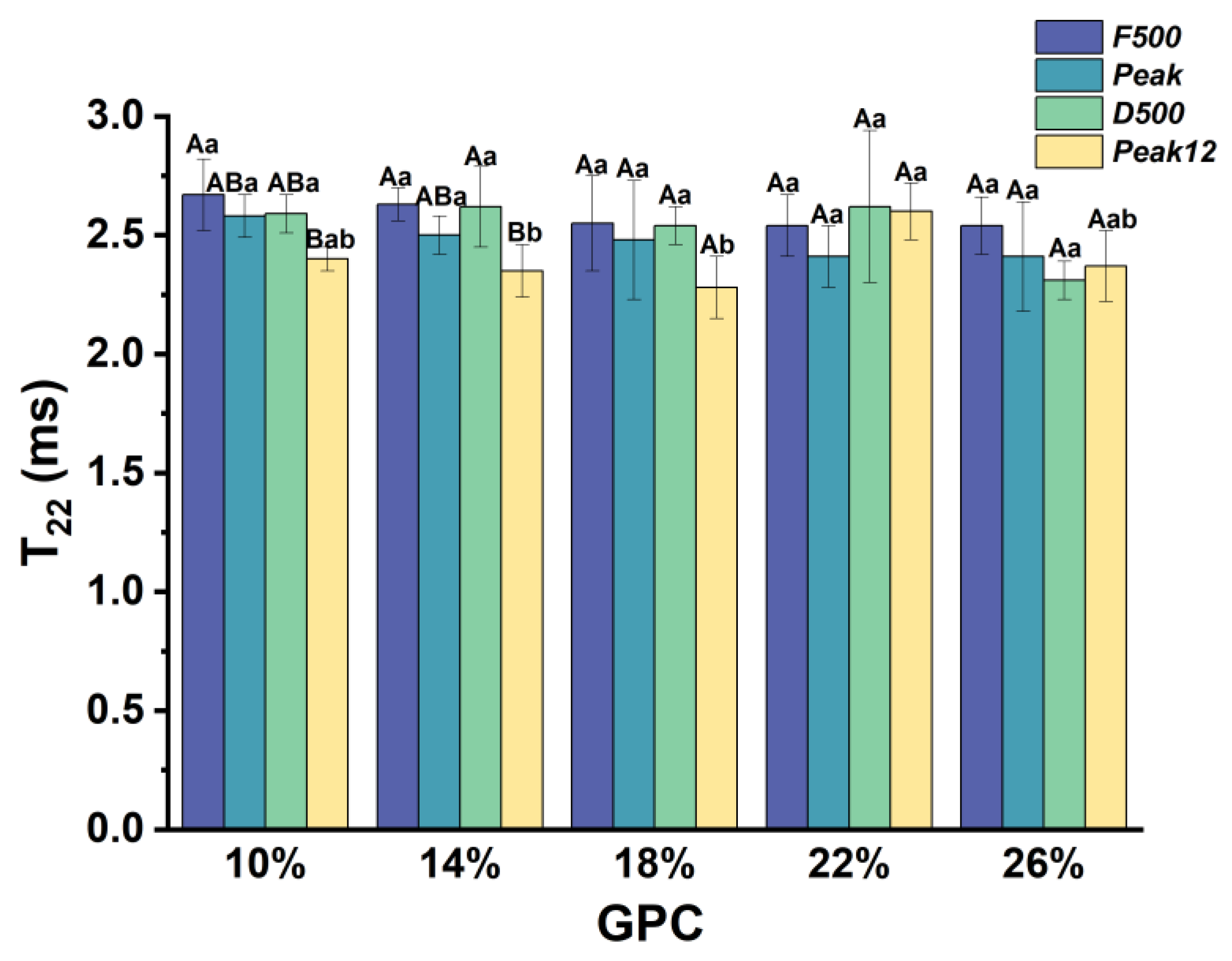
3.2.2. The Relaxation Time of Weakly Bound Water (T22) during Dough Development
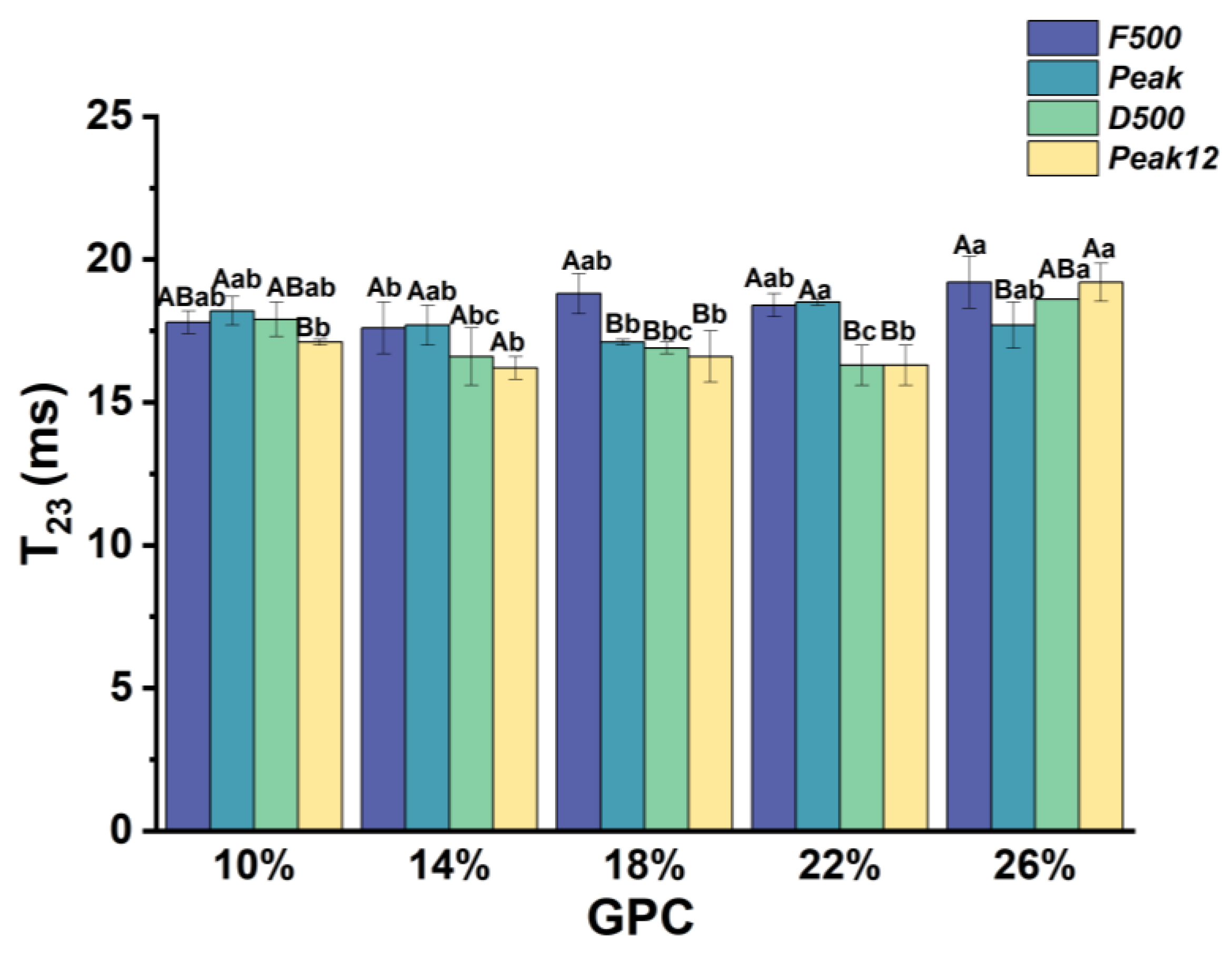
3.2.3. The Relaxation Time of Free Water (T23) during Dough Development
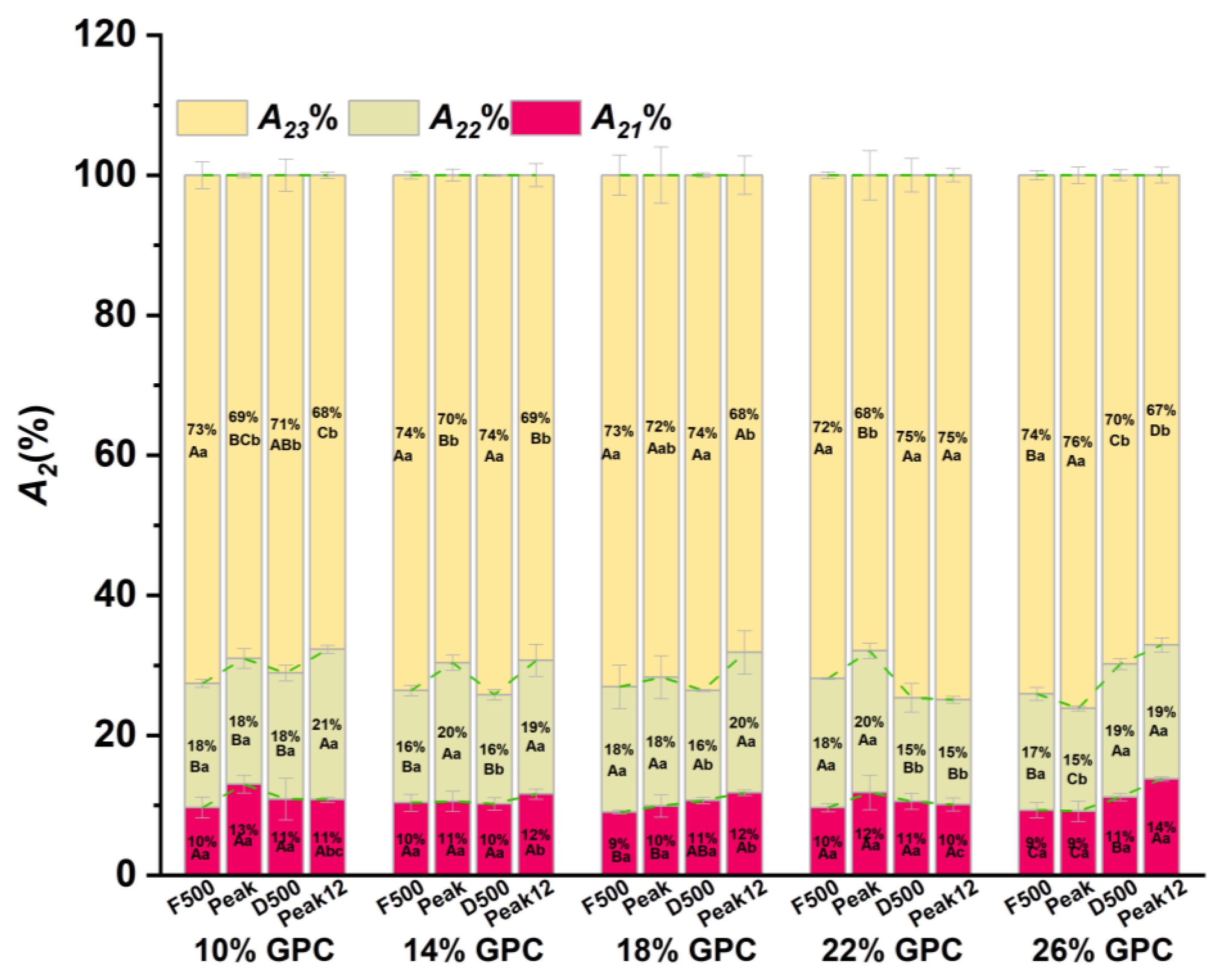
3.3. The Water Ratio (A2%) Changes during Dough Development
3.3.1. The Water Ratio of Strongly Bound Water during A21% Dough Development
3.3.2. The Water Ratio of Weakly Bound Water (A22%) during Dough Development
3.3.3. The Water Ratio of Free Water (A23%) during Dough Development
3.4. The Changes in Protein Secondary Structure during Dough Development
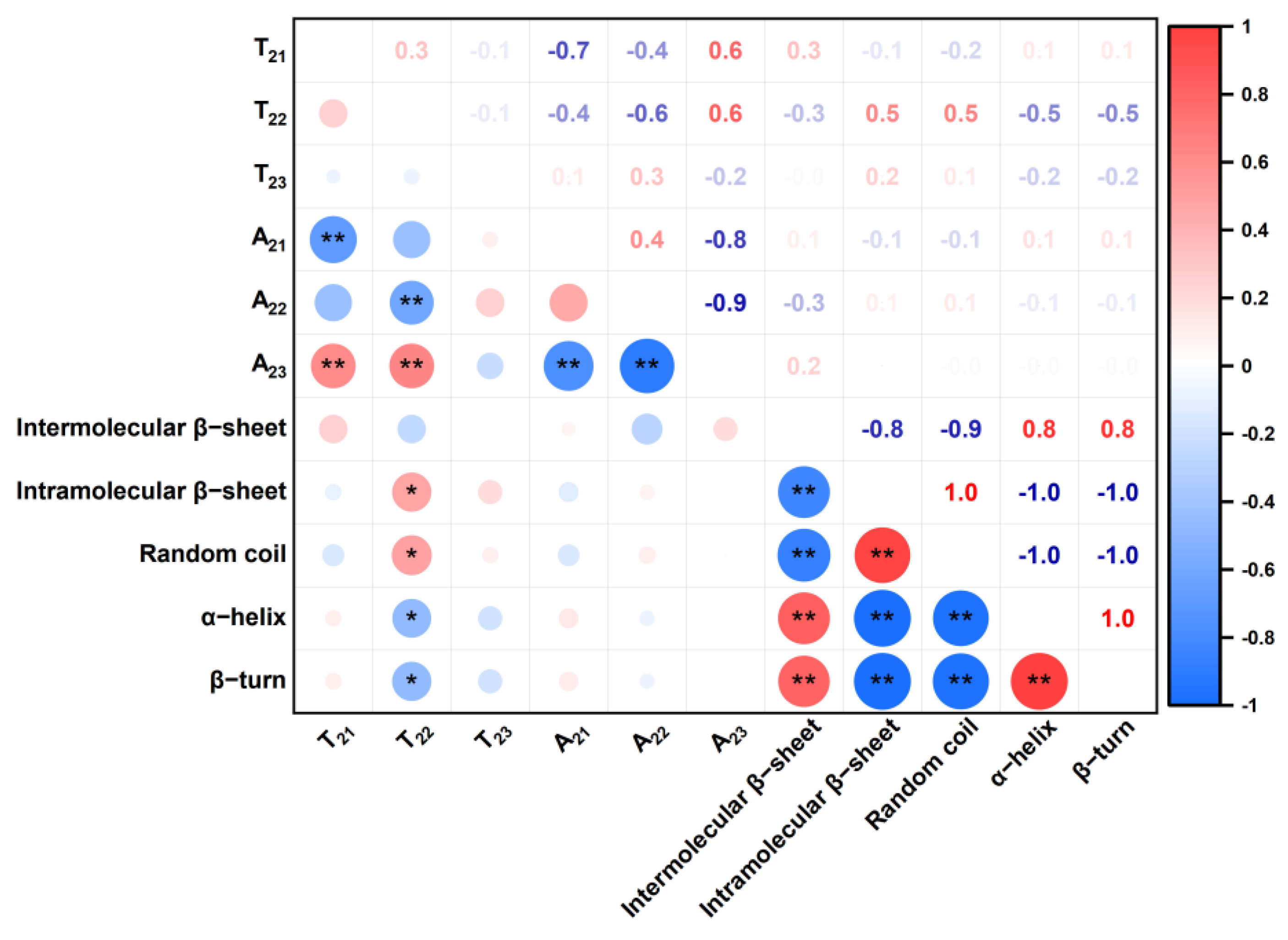
3.5. Correlation Analysis among Water States and the Protein Secondary Structure
3.6. Network Structure Changes during Dough Development
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dufour, M.; Chaunier, L.; Lourdin, D.; Réguerre, A.L.; Hugon, F.; Dugué, A.; Kansou, K.; Saulnier, S.; Della Valle, G. Unravelling the relationships between wheat dough extensional properties, gluten network and water distribution. Food Hydrocoll. 2024, 146, 109214. [Google Scholar] [CrossRef]
- Wang, Y.; Tacer-Caba, Z.; Immonen, M.; Kemell, M.; Varis, J.J.; Jian, C.; Maina, N.H. Understanding the influence of in situ produced dextran on wheat dough baking performance: Maturograph, biaxial extension, and dynamic mechanical thermal analysis. Food Hydrocoll. 2022, 131, 107844. [Google Scholar] [CrossRef]
- Dhal, S.; Anis, A.; Shaikh, H.M.; Alhamidi, A.; Pal, K. Effect of Mixing Time on Properties of Whole Wheat Flour-Based Cookie Doughs and Cookies. Foods 2023, 12, 941. [Google Scholar] [CrossRef]
- Yang, J.; Chen, L.; Guo, B.; Zhang, B.; Zhang, Y.; Li, M. Elucidation of rheological properties of frozen non-fermented dough with different thawing treatments: The view from protein structure and water mobility. J. Cereal Sci. 2022, 108, 103572. [Google Scholar] [CrossRef]
- Wang, X.; Appels, R.; Zhang, X.; Bekes, F.; Diepeveen, D.; Ma, W.; Hu, X.; Islam, S. Solubility variation of wheat dough proteins: A practical way to track protein behaviors in dough processing. Food Chem. 2020, 312, 126038. [Google Scholar] [CrossRef] [PubMed]
- Ortolan, F.; Corrêa, G.P.; da Cunha, R.L.; Steel, C.J. Rheological properties of vital wheat glutens with water or sodium chloride. LWT 2017, 79, 647–654. [Google Scholar] [CrossRef]
- Ortolan, F.; Urbano, K.; Netto, F.M.; Steel, C.J. Chemical and structural characteristics of proteins of non-vital and vital wheat glutens. Food Hydrocoll. 2022, 125, 107383. [Google Scholar] [CrossRef]
- Jia, R.; Zhang, M.; Yang, T.; Ma, M.; Sun, Q.; Li, M. Evolution of the morphological, structural, and molecular properties of gluten protein in dough with different hydration levels during mixing. Food Chem. X 2022, 15, 100448. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Q.; Li, X.; Obadi, M.; Jiang, S.; Li, S.; Bin, X. Effects of dough resting time on the development of gluten network in different sheeting directions and the textural properties of noodle dough. LWT 2021, 141, 110920. [Google Scholar] [CrossRef]
- Baudouin, F.; Nogueira, T.L.; van Der Mijnsbrugge, A.; Frederix, S.; Redl, A.; Morel, M.H. Mechanochemical activation of gluten network development during dough mixing. J. Food Eng. 2020, 283, 110035. [Google Scholar] [CrossRef]
- Abang Zaidel, D.N.; Chin, N.L.; Yusof, Y.A.; Abdul Rahman, R.; Karim, R. Statistical modelling of gluten production by varying mixing time, salt and water levels during dough mixing. Int. J. Food Eng. 2009, 5. [Google Scholar] [CrossRef]
- Bosmans, G.M.; Lagrain, B.; Deleu, L.J.; Fierens, E.; Hills, B.P.; Delcour, J.A. Assignments of proton populations in dough and bread using NMR relaxometry of starch, gluten, and flour model systems. J. Agr. Food Chem. 2012, 60, 5461–5470. [Google Scholar] [CrossRef]
- Ling, X.; Tang, N.; Zhao, B.; Zhang, Y.; Guo, B.; Wei, Y.M. Study on the water state, mobility and textural property of Chinese noodles during boiling. Int. J. Food Sci. Technol. 2020, 55, 1716–1724. [Google Scholar] [CrossRef]
- Kamal, T.; Cheng, S.; Khan, I.A.; Nawab, K.; Zhang, T.; Song, Y.; Wang, S.; Nadeem, M.; Raiz, M.; Khan MA, U.; et al. Potential uses of LF-NMR and MRI in the study of water dynamics and quality measurement of fruits and vegetables. J. Food Process. 2019, 43, e14202. [Google Scholar] [CrossRef]
- Yang, Y.; Guan, E.; Zhang, T.; Li, M.; Bian, K. Influence of water addition methods on water mobility characterization and rheological properties of wheat flour dough. J. Cereal Sci. 2019, 89, 102791. [Google Scholar] [CrossRef]
- Liu, H.; Xing, Y.; Liu, D.; Yang, Q.; Xiao, S.; Fu, Y.; Wang, X. Quality deterioration of frozen dough bread during terminal freezing and thawing: From the insight of moisture and starch properties. Food Biosci. 2023, 56, 103435. [Google Scholar] [CrossRef]
- Li, M.; Liu, C.; Zheng, X.; Hong, J.; Bian, L.; Li, L. Interaction between A-type/B-type starch granules and gluten in dough during mixing. Food Chem. 2021, 358, 129870. [Google Scholar] [CrossRef]
- Sissons, M.J.; Soh, H.N.; Turner, M.A. Role of gluten and its components in influencing durum wheat dough properties and spaghetti cooking quality. J. Sci. Food Agric. 2007, 87, 1874–1885. [Google Scholar] [CrossRef]
- Gras, P.W.; Carpenter, H.C.; Anderssen, R.S. Modelling the developmental rheology of wheat-flour dough using extension tests. J. Cereal Sci. 2020, 31, 1–13. [Google Scholar] [CrossRef]
- Ritota, M.; Gianferri, R.; Bucci, R.; Brosio, E. Proton NMR relaxation study of swelling and gelatinisation process in rice starch–water samples. Food Chem. 2008, 110, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Uthayakumaran, S.; Newberry, M.; Keentok, M.; Stoddard, F.L.; Bekes, F. Basic rheology of bread dough with modified protein content and glutenin-to-gliadin ratios. Cereal Chem. 2020, 77, 744–749. [Google Scholar] [CrossRef]
- Schurer, F.; Kieffer, R.; Wieser, H.; Koehler, P. Effect of hydrostatic pressure and temperature on the chemical and functional properties of wheat gluten II. Studies on the influence of additives. J. Cereal Sci. 2007, 46, 39–48. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhang, Y.R.; Yang, Y.Y.; Shen, J.Q.; Zhang, Q.M.; Zhang, G.Z. Effect of wheat gluten addition on the texture, surface tackiness, protein structure, and sensory properties of frozen cooked noodles. LWT 2022, 161, 113348. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, X.; Xu, X.; Feng, X.; Wang, Z.; Meng, L.; Wu, D.; Tang, X. Effects of soaking conditions on the quality and in vitro starch digestibility of extruded whole buckwheat noodles. J. Cereal Sci. 2022, 108, 103584. [Google Scholar] [CrossRef]
- Dufour, M.; Foucat, L.; Hugon, F.; Dugué, A.; Chiron, H.; Della Valle, G.; Saulnier, L. Water mobility and microstructure of gluten network during dough mixing using TD NMR. Food Chem. 2023, 409, 135329. [Google Scholar] [CrossRef]
- Liu, H.; Wan, L.; Xiao, S.; Fu, Y.; Wang, X. Changes in the physicochemical and protein distribution properties of dough with the wheat oligopeptide incorporation. Food Biosci. 2023, 52, 102354. [Google Scholar] [CrossRef]
- Yang, Y.L.; Guan, E.Q.; Zhang, L.L.; Pang, J.Y.; Li, M.M.; Bian, K. Effects of vacuum degree, mixing speed, and water amount on the moisture distribution and rheological properties of wheat flour dough. J. Food Sci. 2021, 86, 2421–2433. [Google Scholar] [CrossRef] [PubMed]
- Šćepanović, P.; Goudoulas, T.B.; Germann, N. Numerical investigation of microstructural damage during kneading of wheat dough. Food Struct. 2018, 16, 8–16. [Google Scholar] [CrossRef]
- Liu, L.; Hu, X.; Zou, L. Wheat polysaccharides and gluten effect on water migration and structure in noodle doughs: An 1H LF-NMR study. J. Cereal Sci. 2023, 110, 103628. [Google Scholar] [CrossRef]
- Berton, B.; Scher, J.; Villieras, F.; Hardy, J. Measurement of hydration capacity of wheat flour: Influence of composition and physical characteristics. Powder Technol. 2020, 128, 326–331. [Google Scholar] [CrossRef]
- Guo, L.; Fang, F.; Zhang, Y.; Xu, D.; Jin, Z.; Xu, X. Glutathione affects rheology and water distribution of wheat dough by changing gluten conformation and protein depolymerisation. Int. J. Food Sci. Technol. 2021, 56, 3157–3165. [Google Scholar] [CrossRef]
- Wang, X.; Choi, S.G.; Kerr, W.L. Water dynamics in white bread and starch gels as affected by water and gluten content. LWT 2004, 37, 377–384. [Google Scholar] [CrossRef]
- Iwaki, S.; Fu, B.X.; Hayakawa, K. Behavior of protein aggregates via electrostatic interactions or hydrogen boynds during dough formation. J. Cereal Sci. 2023, 111, 103683. [Google Scholar] [CrossRef]
- Bock, J.E.; Damodaran, S. Bran-induced changes in water structure and gluten conformation in model gluten dough studied by fourier transform infrared spectroscopy. Food Hydrocoll. 2013, 31, 146–155. [Google Scholar] [CrossRef]
- Anjum, F.M.; Khan, M.R.; Din, A.; Saeed, M.; Pasha, I.; Arshad, M.U. Wheat gluten: High molecular weight glutenin subunits—Structure, genetics, and relation to dough elasticity. J. Food Sci. 2007, 72, R56–R63. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ehmke, L.; Miller, R.; Faa, P.; Smith, G.; Li, Y. Effect of sodium chloride and sodium bicarbonate on the physicochemical properties of soft wheat flour doughs and gluten polymerization. J. Agr. Food Chem. 2018, 66, 6840–6850. [Google Scholar] [CrossRef] [PubMed]
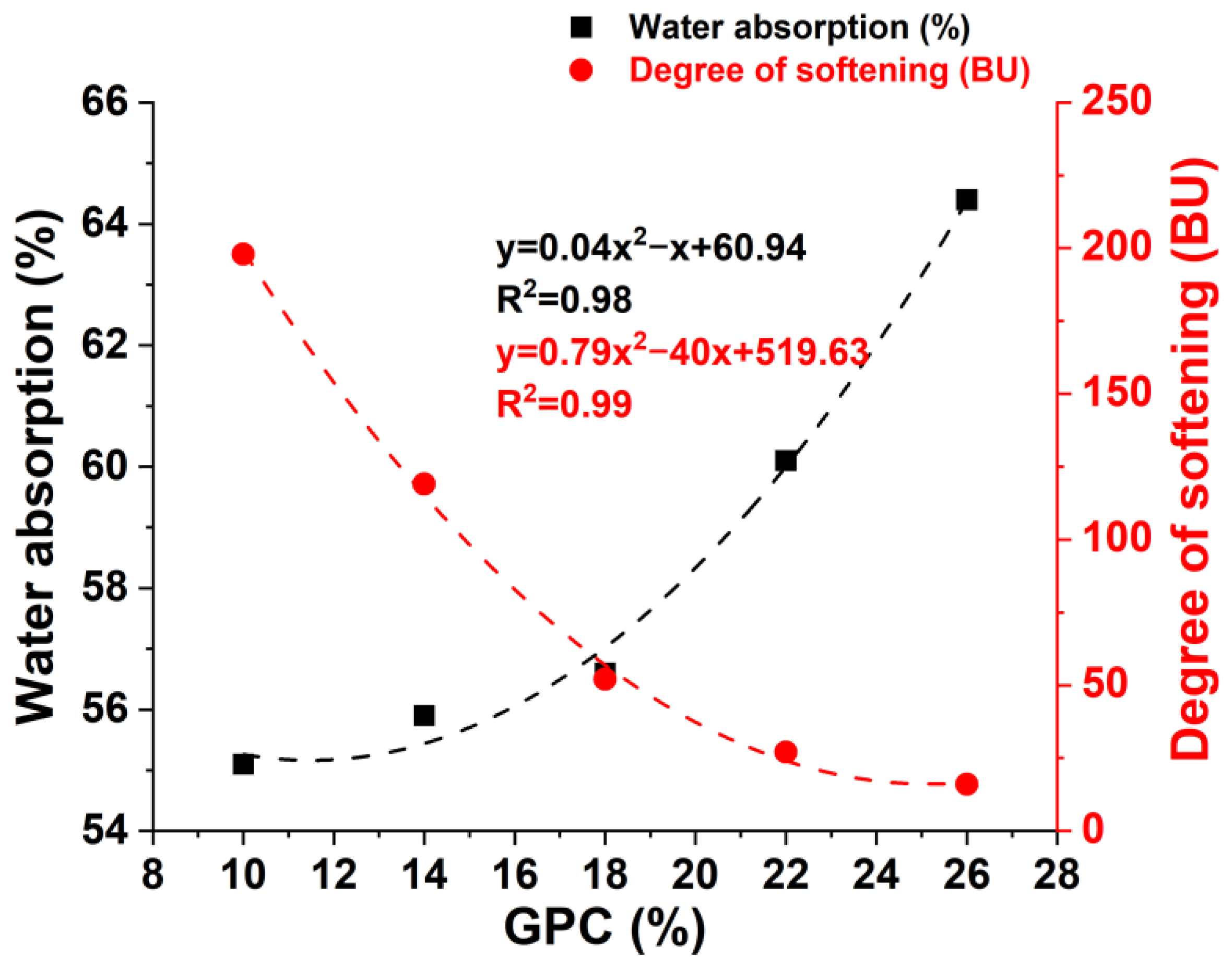



| GPC (%) | Water Absorption (%) | Development Time (min) | Stability Time (min) | The Degree of Softening (BU) | Farinograph Index (mm) |
|---|---|---|---|---|---|
| 10 | 55.1 | 0.5 | 0.5 | 198 | 7 |
| 14 | 55.9 | 0.5 | 0.8 | 119 | 8 |
| 18 | 56.6 | 0.9 | 8.1 | 52 | 18 |
| 22 | 60.1 | 3.5 | 21.5 | 27 | 111 |
| 26 | 64.4 | 6.0 | 36.1 | 16 | 165 |
| GPC (%) | Sampling Point | Intermolecular β-Sheet (%) | Intramolecular β-Sheet (%) | α-Helix (%) | Ramdon Coil (%) | β-Turn (%) |
|---|---|---|---|---|---|---|
| 10 | F500 | 6.03 ± 0.08 Bb | 31.23 ± 0.17 Aa | 20.74 ± 0.06 Aa | 18.55 ± 0.18 Bb | 23.46 ± 0.09 Bb |
| Peak | 5.88 ± 0.04 Bb | 30.93 ± 0.08 Aa | 20.79 ± 0.05 Aa | 18.72 ± 0.06 Bb | 23.67 ± 0.07 Bb | |
| D500 | 5.66 ± 0.03 Bb | 30.66 ± 0.15 Aa | 20.8 ± 0.18 Aa | 18.7 ± 0.14 Bb | 24.19 ± 0.15 Bb | |
| Peak12 | 7.66 ± 0.19 Aa | 13.89 ± 0.3 Ba | 19.93 ± 0.15 Ba | 23.7 ± 0.3 Aa | 34.82 ± 0.09 Aa | |
| 14 | F500 | 6.01 ± 0.06 Bb | 30.85 ± 0.46 Aa | 20.53 ± 0.32 Aa | 18.72 ± 0.32 Bb | 23.9 ± 0.55 Bb |
| Peak | 5.99 ± 0.05 Bb | 30.38 ± 0.02 Aa | 20.61 ± 0.05 Aa | 18.8 ± 0.09 Bb | 24.21 ± 0.1 Bb | |
| D500 | 8.22 ± 0.15 Aa | 14.44 ± 0.22 Bb | 19.72 ± 0.12 Ba | 23.45 ± 0.18 Aa | 34.17 ± 0.12 Aa | |
| Peak12 | 7.99 ± 0.03 Aa | 14.46 ± 0.03 Ba | 19.64 ± 0.04 Ba | 23.35 ± 0.04 Aa | 34.57 ± 0.06 Aa | |
| 18 | F500 | 5.46 ± 0.07 Bb | 30.09 ± 0.08 Aa | 20.74 ± 0.08 Aa | 18.83 ± 0.09 Bb | 24.87 ± 0.05 Bb |
| Peak | 5.98 ± 0.13 Bb | 30.3 ± 0.11 Aa | 20.42 ± 0.07 Aa | 18.7 ± 0.12 Bb | 24.61 ± 0.07 Bb | |
| D500 | 7.89 ± 0.04 Aa | 13.61 ± 0.08 Bb | 19.51 ± 0.07 Ba | 23.67 ± 0.04 Aa | 35.31 ± 0.16 Aa | |
| Peak12 | 8.01 ± 0.23 Aa | 13.8 ± 0.22 Ba | 19.68 ± 0.09 Ba | 23.55 ± 0.22 Aa | 34.96 ± 0.16 Aa | |
| 22 | F500 | 8.19 ± 0.2 Aa | 14.11 ± 0.25 Ab | 19.63 ± 0.04 Aa | 23.61 ± 0.16 Aa | 34.46 ± 0.32 Aa |
| Peak | 8.29 ± 0.04 Aa | 14.11 ± 0.06 Ab | 19.33 ± 0.03 Aa | 23.49 ± 0.04 Aa | 34.77 ± 0.11 Aa | |
| D500 | 8.1 ± 0.13 Aa | 13.89 ± 0.2 Ab | 19.61 ± 0.08 Aa | 23.65 ± 0.05 Aa | 34.75 ± 0.43 Aa | |
| Peak12 | 7.85 ± 0.11 Aa | 13.6 ± 0.06 Aa | 19.6 ± 0.15 Aa | 23.7 ± 0.09 Aa | 35.24 ± 0.1 Aa | |
| 26 | F500 | 8.12 ± 0.16 Aa | 13.85 ± 0.28 Ab | 19.46 ± 0.13 Aa | 23.51 ± 0.12 Aa | 35.05 ± 0.35 Aa |
| Peak | 8.21 ± 0.21 Aa | 14.07 ± 0.22 Ab | 19.52 ± 0.15 Aa | 23.59 ± 0.17 Aa | 34.61 ± 0.26 Aa | |
| D500 | 8.14 ± 0.22 Aa | 13.95 ± 0.24 Ab | 19.52 ± 0.15 Aa | 23.61 ± 0.1 Aa | 34.78 ± 0.32 Aa | |
| Peak12 | 8.15 ± 0.26 Aa | 13.97 ± 0.34 Aa | 19.49 ± 0.13 Aa | 23.57 ± 0.11 Aa | 34.82 ± 0.51 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, H.; Zhang, Y.; Wang, L.; Ban, J.; Wei, Y.; Fan, F.; Guo, B. Dynamic Study on Water State and Water Migration during Gluten–Starch Model Dough Development under Different Gluten Protein Contents. Foods 2024, 13, 996. https://doi.org/10.3390/foods13070996
Ye H, Zhang Y, Wang L, Ban J, Wei Y, Fan F, Guo B. Dynamic Study on Water State and Water Migration during Gluten–Starch Model Dough Development under Different Gluten Protein Contents. Foods. 2024; 13(7):996. https://doi.org/10.3390/foods13070996
Chicago/Turabian StyleYe, Haoxuan, Yingquan Zhang, Lei Wang, Jinfu Ban, Yimin Wei, Fanghui Fan, and Boli Guo. 2024. "Dynamic Study on Water State and Water Migration during Gluten–Starch Model Dough Development under Different Gluten Protein Contents" Foods 13, no. 7: 996. https://doi.org/10.3390/foods13070996
APA StyleYe, H., Zhang, Y., Wang, L., Ban, J., Wei, Y., Fan, F., & Guo, B. (2024). Dynamic Study on Water State and Water Migration during Gluten–Starch Model Dough Development under Different Gluten Protein Contents. Foods, 13(7), 996. https://doi.org/10.3390/foods13070996