Quality Characteristics and Storage Stability of Frying Steak Utilizing Wax-Based Korean Pine Seed Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fried Steak
2.2.1. Steak Making Process
2.2.2. Cooking Method
2.3. Determination of Food Quality
2.3.1. Loss of Cooking
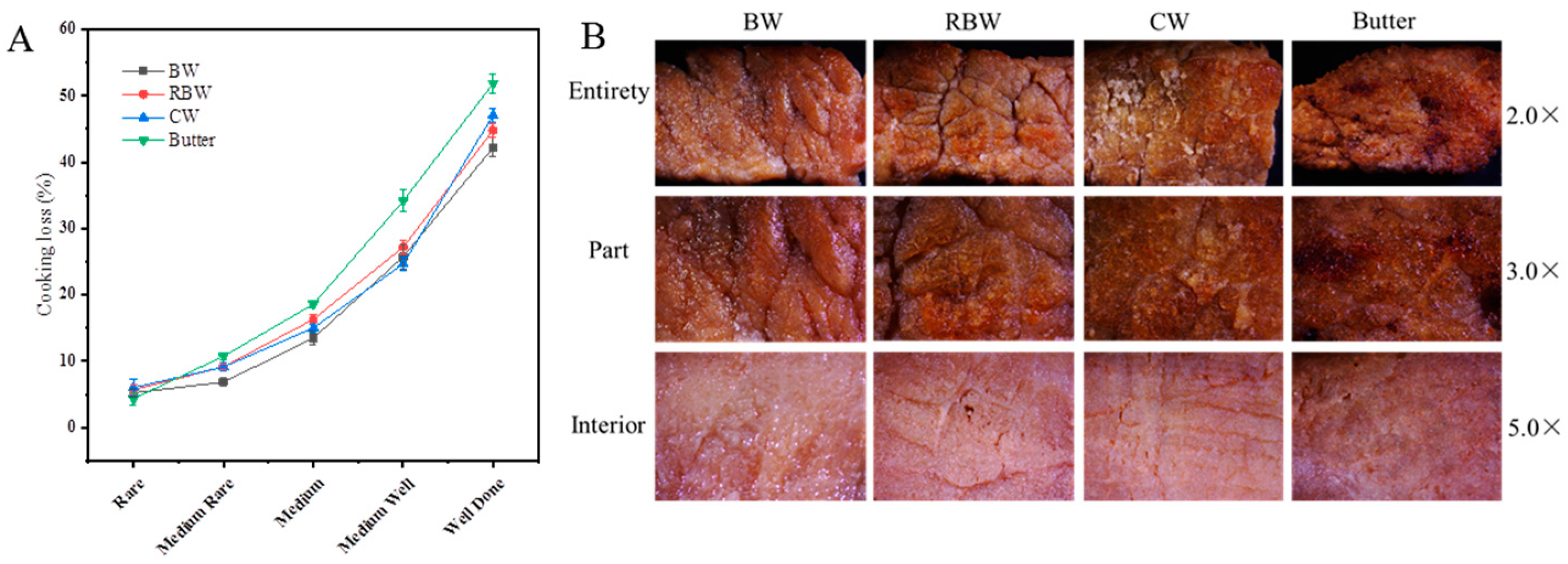
2.3.2. Microstructure
2.3.3. Color Determination
2.3.4. Texture Properties
2.3.5. Sensory Quality
2.3.6. Determination of Volatile Flavor Substances
2.4. Storage Stability Index
2.4.1. pH
2.4.2. Purge Loss
2.4.3. TVB-N
2.4.4. TBARS
2.5. Statistical Analysis
3. Results
3.1. Changes in Cooking Losses
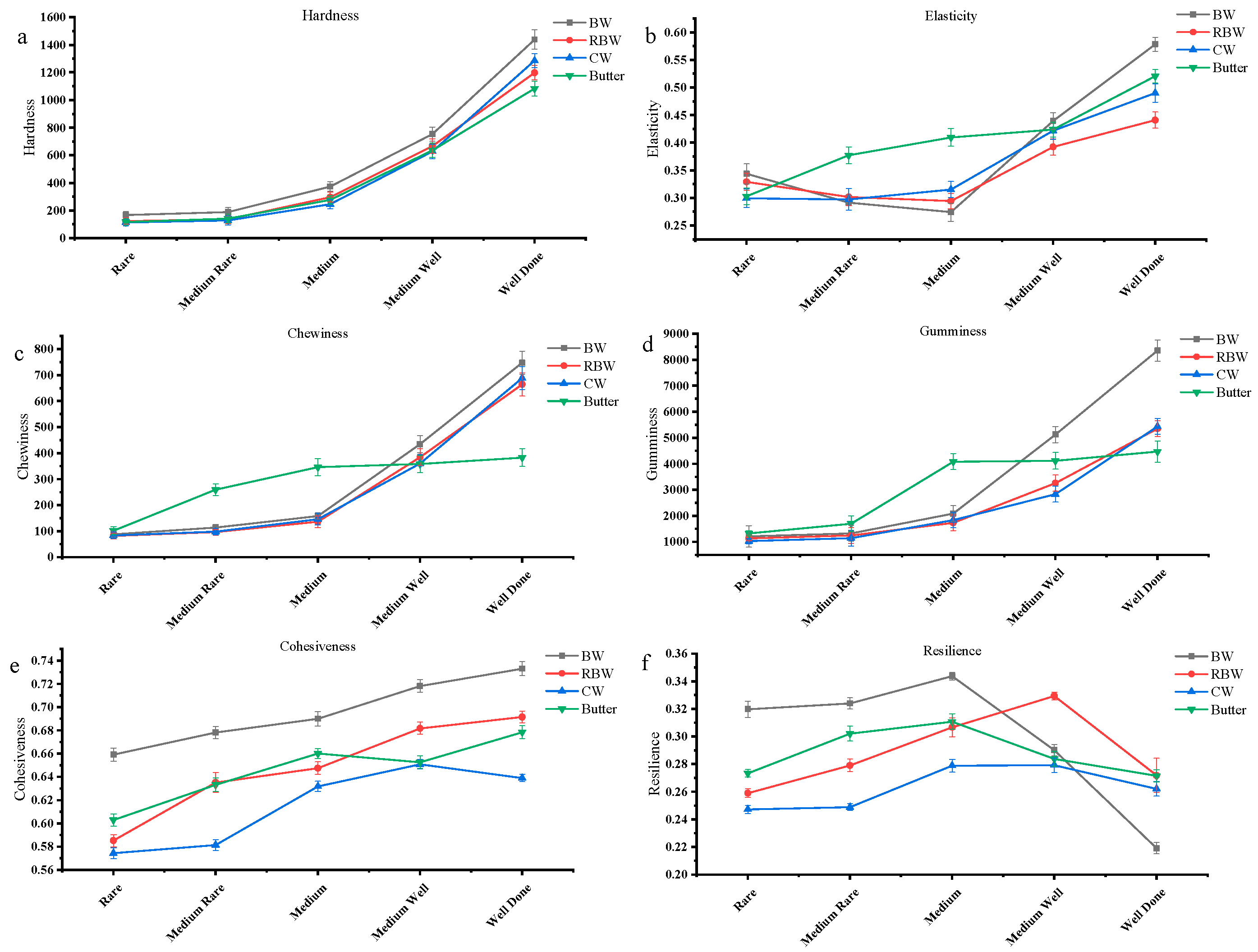
3.2. TPA
3.3. Color and Luster Change
3.4. Sensory Assessment
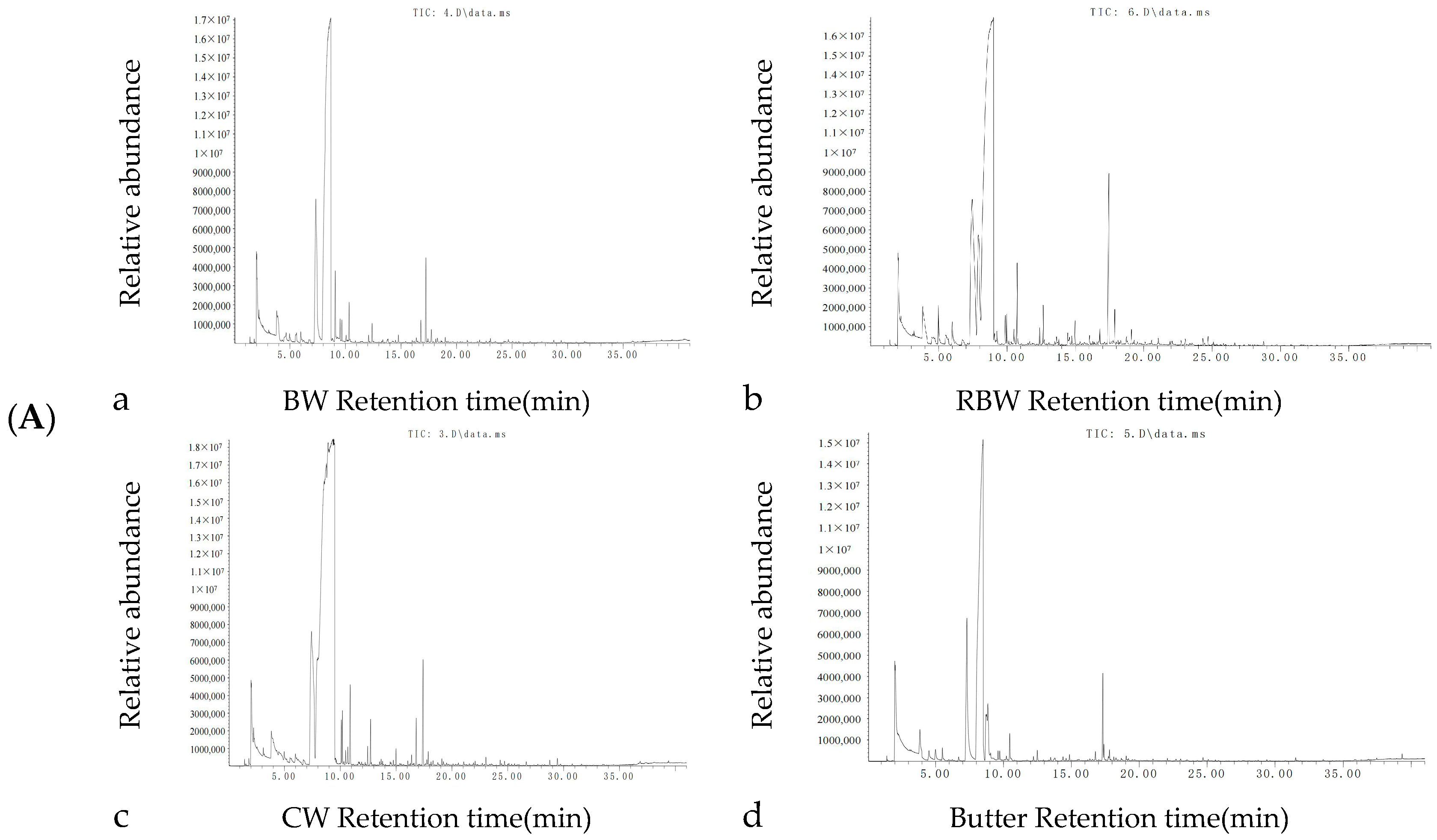
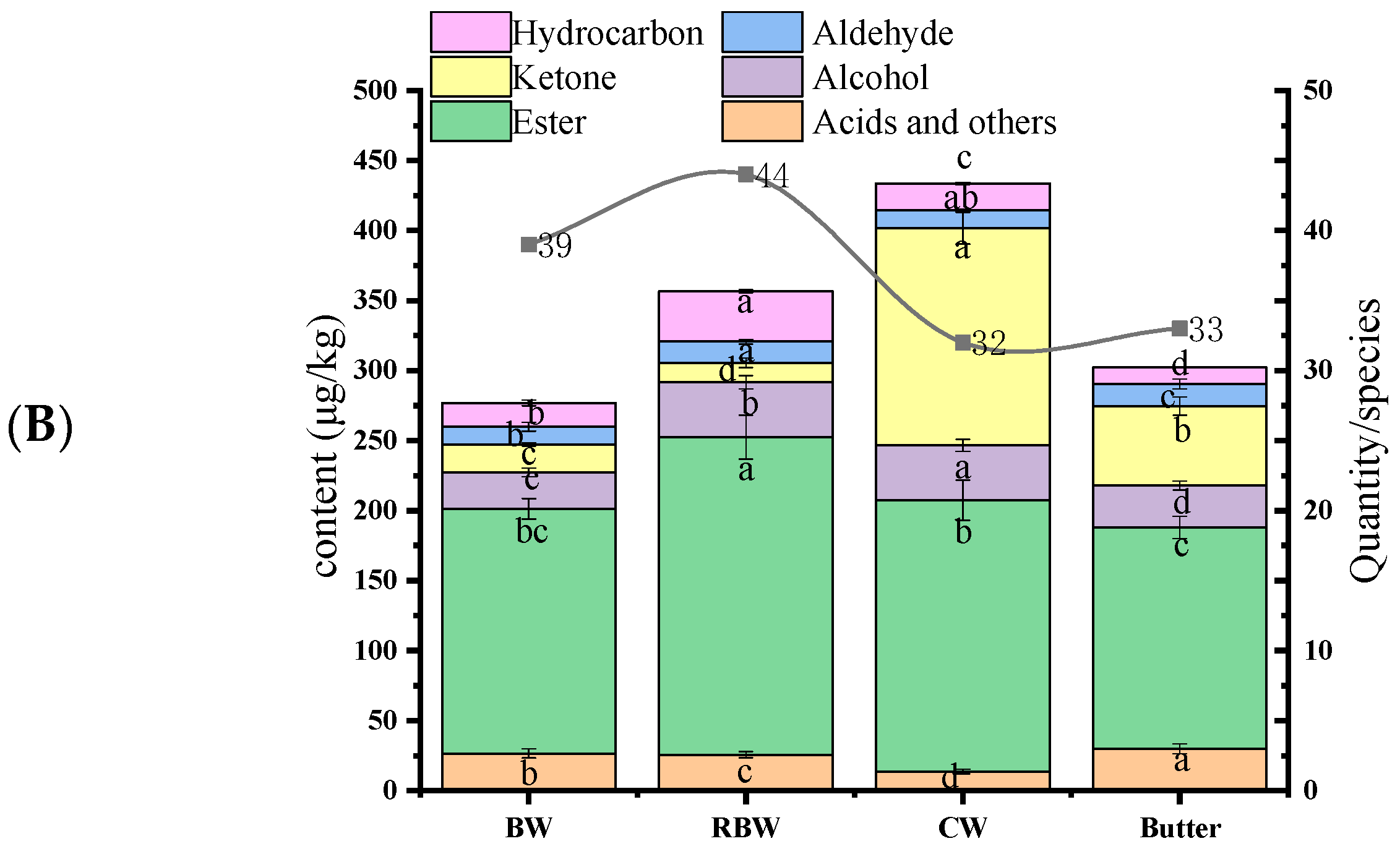
3.5. Volatile Flavor Compounds
| Category | Peak# | Compound | Molecular Formula | CAS | KI | R | Content/(μg·kg−1) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| BW | RBW | CW | Butter | |||||||
| Hydrocarbon (A) | A1 | n-Hexane | C6H14 | 110-54-3 | 618 | m,s,k | 1.67 ± 0.03 a | 1.54 ± 0.05 bc | 1.54 ± 0.01 c | ND |
| A2 | 2-ethyl-3-methyl-oxetane | C6H12O | 53778-62-4 | 724 | m | ND | 1.66 ± 0.05 b | 2.16 ± 0.18 a | ND | |
| A3 | Cyclohexane,(1,1-dimethylethyl)- | C10H20 | 3178-22-1 | 762 | m | ND | 2.81 ± 0.09 a | 2.70 ± 0.08 b | ND | |
| A4 | octylcyclopropane | C11H22 | 1472-09-9 | 732 | m | ND | ND | 2.19 ± 0.04 | ND | |
| A5 | 1,1,2-trimethylcyclopentane | C8H16 | 4259-00-1 | 1148 | m | ND | 4.67 ± 0.16 | ND | ND | |
| A6 | Tetradecane | C14H30 | 629-59-4 | 1448 | m,s,k | 1.31 ± 0.17d | 3.19 ± 0.21 a | 1.90 ± 0.05 c | 2.99 ± 0.05 b | |
| A7 | pentadecane | C15H32 | 629-62-9 | 1512 | m,s,k | ND | 1.75 ± 0.02 | ND | ND | |
| A8 | Hexadecane | C16H34 | 544-76-3 | 1612 | m,s,k | ND | 2.28 ± 0.09 a | ND | 1.72 ± 0.06 b | |
| A9 | 1-Iodononane | C9H19I | 4282-42-2 | 1224 | m | ND | 1.54 ± 0.06 | ND | ND | |
| A10 | Nonylcyclopropane | C12H24 | 74663-85-7 | 862 | m | 1.61 ± 0.17 a | 1.74 ± 0.02 b | ND | ND | |
| A11 | Heptane, 4,4-dimethyl- | C9H20 | 1068-19-5 | 1426 | m | 2.75 ± 0.47 a | 1.74 ± 0.02 b | ND | ND | |
| A12 | 2-di(propan-2-yl)phosphorylpropane | C9H21OP | 17513-58-5 | 1244 | m | 1.70 ± 0.13 c | 1.72 ± 0.13 b | 2.17 ± 0.14 a | 1.66 ± 0.03 d | |
| A13 | 2,4,4,6-tetramethyl-hept-2-ene | C11H22 | 103982-58-7 | 1140 | m,k | ND | 1.57 ± 0.09 b | 3.12 ± 0.18 a | ND | |
| A14 | 1-Tetradecene | C14H28 | 1120-36-1 | 1428 | m,s | 1.33 ± 0.12 b | ND | ND | 1.66 ± 0.01 a | |
| A15 | α-Copaene | C15H24 | 3856-25-5 | 1221 | m,s,k | ND | ND | ND | 1.88 ± 0.12 | |
| A16 | (+)-Limonene | C10H16 | 5989-27-5 | 1018 | m,s,k | ND | 3.27 ± 0.07 a | 1.51 ± 0.06 b | ND | |
| A17 | (1Z,3Z)-1,4-dimethoxy-1,3-butadiene | C6H10O2 | 83650-30-0 | 1140 | m | 1.86 ± 0.14 b | 4.49 ± 0.11 a | ND | ND | |
| A18 | trans-Caryophyllene | C15H24 | 87-44-5 | 1494 | m,s,k | 4.76 ± 1.11 a | 1.88 ± 0.05 c | 1.92 ± 0.14 b | 1.80 ± 0.08 d | |
| Aldehydes (B) | B1 | 1-hexadecanal | C16H32O | 629-80-1 | 1728 | m,k | ND | ND | 1.67 ± 0.14 | ND |
| B2 | Nonanal | C9H18O | 124-19-6 | 1104 | m,s,k | 4.30 ± 1.69 b | 3.53 ± 0.09 c | 1.46 ± 0.03 d | 5.07 ± 5.35 a | |
| B3 | (2E)-2-Octenal | C8H14O2 | 2548-87-0 | 1013 | m,s,k | 1.93 ± 0.87 a | 1.87 ± 0.04 b | 1.73 ± 0.11 c | 1.86 ± 0.14 b | |
| B4 | (E)-2-Decenal | C10H18O | 3913-81-3 | 1242 | m | 1.70 ± 0.06 bc | 1.59 ± 0.23 c | 1.76 ± 0.14 a | 1.71 ± 0.16 b | |
| B5 | Benzaldehyde | C7H6O | 100-52-7 | 982 | m,s,k | ND | 2.25 ± 0.15 a | 2.14 ± 0.18 b | ND | |
| B6 | Tridecanal | C13H26O | 10486-19-8 | 1502 | m,s,k | ND | 1.99 ± 0.14 b | ND | 3.63 ± 2.71 a | |
| B7 | 2-Isopropylbutanal | C7H14O | 26254-92-2 | 1095 | m | 1.79 ± 0.13 b | ND | 2.03 ± 0.13 a | ND | |
| B8 | 1-Nonana | C9H18O | 124-19-6 | 1104 | m | 1.36 ± 0.06 b | ND | ND | 1.76 ± 0.16 a | |
| B9 | (2E,4E)-Deca-2,4-dienal | C10H16O | 25152-84-5 | 1195 | m | 1.57 ± 0.36 c | 3.90 ± 0.48 a | 1.82 ± 0.11 b | 1.51 ± 0.08 d | |
| Ketone (C) | C1 | Butyl 2-methyl-1-propenyl ketone | C9H16O | 19860-71-0 | 847 | m | 1.43 ± 0.02 b | ND | 1.71 ± 0.14 a | ND |
| C2 | Isobutyrylacetone | C6H10O2 | 7307-03-1 | 728 | m | 1.43 ± 0.06 c | ND | 146.73 ± 11.57 a | 39.61 ± 7.48 b | |
| C3 | 1-(6-methyl-3,4-dihydro-2H-pyran-2-yl)propan-1-one | C9H14O2 | 62255-24-7 | 764 | m | 2.88 ± 0.18 b | ND | ND | 5.65 ± 1.21 a | |
| C4 | 1-(2-Hydroxy-6-methoxyphenyl)ethanone | C9H10O3 | 703-23-1 | 837 | m | ND | 2.77 ± 0.19 a | ND | 2.64 ± 0.61 b | |
| C5 | 2,2,7-trimethyl-3,5-octanedione | C11H20O2 | 69725-37-7 | 984 | m | 6.90 ± 0.78 a | 2.02 ± 0.19 c | 1.49 ± 0.06 d | 2.89 ± 0.35 b | |
| C6 | 2,5-Piperazinedione,3-methyl-(9CI) | C5H8N2O2 | 6062-46-0 | 652 | m | ND | 3.28 ± 2.49 | ND | ND | |
| C7 | 3-methyl-Cyclohexanone | C7H12O | 591-24-2 | 717 | m | 1.70 ± 0.06 a | ND | ND | 1.44 ± 0.12 b | |
| C8 | 4-Octanone | C8H16O | 589-63-9 | 952 | m,k | 4.22 ± 0.12 cd | 5.87 ± 0.54 a | 5.12 ± 2.91 b | 4.30 ± 0.15 c | |
| C9 | 3-Nonen-2-one | C9H16O | 14309-57-0 | 1188 | m | 1.34 ± 0.03 | ND | ND | ND | |
| Alcohol (D) | D1 | 4-chloro-2-methylbutan-2-ol | C5H11ClO | 1985-88-2 | 890 | m,k | ND | ND | 1.95 ± 0.04 | ND |
| D2 | 4-Hydroxy-4-methyl-2-pentanone | C6H12O2 | 123-42-2 | 874 | m | ND | ND | ND | 1.52 ± 0.01 | |
| D3 | 2-Hexyl-1-decanol | C16H34O | 2425-77-6 | 1159 | m | 1.39 ± 0.05 b | 1.60 ± 0.06 a | ND | ND | |
| D4 | 2-methyl-2-tetradecanol | C15H32O | 27570-83-8 | 1854 | m | ND | 1.89 ± 0.11 | ND | ND | |
| D5 | 2-Dodecen-1-ol | C12H24O | 22104-81-0 | 1107 | m | ND | 2.00 ± 0.13 | ND | ND | |
| D6 | 1-Octen-3-ol | C8H16O | 3391-86-4 | 969 | m,s,k | 1.45 ± 0.11 d | 2.71 ± 0.04 a | 1.92 ± 0.14 b | 1.73 ± 0.18 c | |
| D7 | 3-Octanol | C8H18O | 589-98-0 | 1064 | m,k | 1.75 ± 0.19 c | 1.85 ± 0.09 a | 1.72 ± 0.06 d | 1.80 ± 0.11 b | |
| D8 | 4-Isopropoxybutanol | C7H16O2 | 31600-69-8 | 928 | m | 3.35 ± 0.82 d | 5.93 ± 0.59 a | 3.80 ± 0.17 c | 4.38 ± 0.04 b | |
| D9 | 2-(2-Ethoxyethoxy)ethanol | C6H14O3 | 111-90-0 | 842 | m | 1.52 ± 0.05 d | 2.07 ± 0.61 b | 1.81 ± 0.06 c | 2.66 ± 0.12 a | |
| D10 | 1-Hexanol | C6H14O | 111-27-3 | 860 | m,s,k | 16.53 ± 3.89 d | 20.16 ± 3.44 b | 28.16 ± 6.82 a | 18.53 ± 4.06 c | |
| Esters (E) | E1 | Octanoic acid, 2-propyl-, methyl ester, (2R) | C12H24O2 | 946516-84-3 | 973 | m,s,k | 1.46 ± 0.08 b | ND | 5.34 ± 1.24 a | ND |
| E2 | Butyl Valerate | C9H18O2 | 591-68-4 | 1381 | m,s,k | 1.66 ± 0.17 d | 3.05 ± 1.054 b | 2.83 ± 0.01 c | 4.29 ± 0.21 a | |
| E3 | heptyl 2,2,2-trifluoroacetate | C9H15F3O2 | 2710-89-6 | 1164 | m | ND | ND | 1.52 ± 0.19 b | 2.96 ± 0.51 a | |
| E4 | Carbonic acid, butylethyl ester | C7H14O3 | 30714-78-4 | 972 | m | 15.32 ± 2.51 | ND | ND | ND | |
| E5 | Pentanoic acid,1-methylethyl ester | C8H16O2 | 18362-97-5 | 1481 | m | 124.1 ± 1.85 d | 202.94 ± 14.37 a | 157.92 ± 13.47 b | 141.17 ± 6.47 c | |
| E6 | Pentanoic acid, propylester | C8H16O2 | 141-06-0 | 1481 | m | 6.43 ± 0.084 a | 5.30 ± 0.22 c | 5.86 ± 0.23 b | 3.88 ± 0.43 d | |
| E7 | Hexyl valerate | C11H22O2 | 1117-59-5 | 1780 | m | 3.51 ± 0.16 c | 4.20 ± 0.13 a | 3.06 ± 0.13 d | 3.75 ± 0.14 b | |
| E8 | 2,4-dimethylpentan-3-yl 2-methylpropanoate | C11H22O2 | 87386-67-2 | 1914 | m | 1.51 ± 0.11 c | 3.10 ± 0.34 a | 1.79 ± 0.14 b | ND | |
| E9 | Pentanoic acid, pentyl ester | C10H20O2 | 2173-56-0 | 1814 | m | 2.65 ± 0.32 c | 4.77 ± 0.28 a | 3.20 ± 0.32 b | ND | |
| E10 | 4-Heptanolide | C7H12O2 | 105-21-5 | 1582 | m | 19.00 ± 3.24 a | ND | 13.37 ± 1.05 b | 2.09 ± 0.04 c | |
| E11 | cyclobutanecarboxylic acid cyclobutyl ester | C9H14O2 | 42392-30-3 | 1282 | m | 1.68 ± 0.33 b | 3.51 ± 0.67 a | ND | ND | |
| Acids and other classes (F) | F1 | Pentanoic acid | C5H10O2 | 109-52-4 | 1126 | m,s,k | ND | ND | 2.00 ± 0.25 b | 2.68 ± 0.09 a |
| F2 | Lauric acid | C12H24O2 | 143-07-7 | 958 | m | ND | ND | 1.63 ± 0.23 | ND | |
| F3 | Eugenol | C10H12O2 | 97-53-0 | 1392 | m,s,k | ND | ND | ND | 2.11 ± 0.17 | |
| F4 | Nonanoic acid | C9H18O2 | 112-05-0 | 1272 | m | ND | ND | ND | 1.82 ± 0.07 | |
| F5 | 1,2-Benzenedicarboxylic acid | C16H22O4 | 84-74-2 | 1482 | m | ND | ND | ND | 3.11 ± 0.24 | |
| F6 | Palmitic acid | C16H32O2 | 57-10-3 | 1479 | m,s,k | 1.28 ± 0.05 b | ND | ND | 1.89 ± 0.21 a | |
| F7 | Toluene | C7H8 | 108-88-3 | 786 | m | 2.83 ± 0.19 d | 11.16 ± 1.15 a | 4.25 ± 0.38 c | 7.60 ± 0.58 b | |
| F8 | n-Valeric anhydride | C10H18O3 | 2082-59-9 | 1108 | m | ND | 2.66 ± 0.08 a | 1.66 ± 0.21 b | ND | |
| F9 | 2-Amylfuran | C9H14O | 3777-69-3 | 983 | m | 1.45 ± 0.11 c | 1.96 ± 0.11 a | 1.54 ± 0.13 b | 1.72 ± 0.12 ab | |
| F10 | Pyridine | C5H5N | 110-86-1 | 852 | m | 16.84 ± 4.37 a | 4.04 ± 0.56 c | ND | 3.59 ± 0.77 b | |
| F11 | Naphthalene | C10H8 | 91-20-3 | 1122 | m | 1.56 ± 0.13 c | 2.92 ± 0.02 a | ND | 2.90 ± 0.03 b | |
| F12 | cis-Anethol | C10H12O | 104-46-1 | 1190 | m,s,k | 1.39 ± 0.19 c | 2.54 ± 0.58 ab | 2.16 ± 0.37 b | 2.93 ± 0.38 a | |
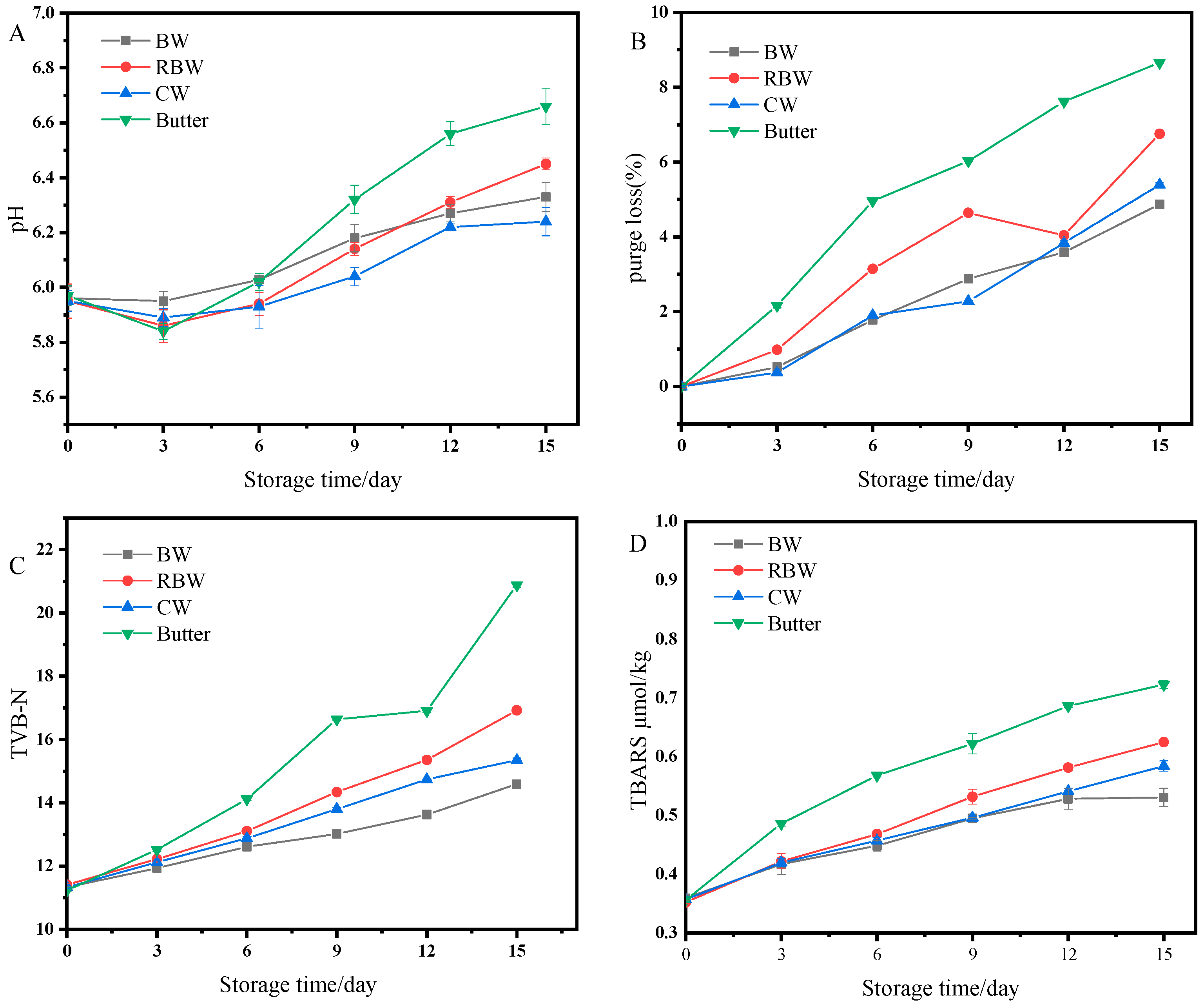
3.6. Storage Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Korean pine seed oil | KPSO |
| Beeswax | BW |
| Rice bran wax | RBW |
| Carnauba wax | CW |
References
- Wang, F.; Xia, X.; Zhang, N.; Fu, Q.; Guo, Q. Kinetic model, fatty acid composition and antioxidant activity of Korean pine seed oil extracted by decompression assisted ultrasonic. J. Cent. South Univ. For. Technol. 2019, 39, 110–117. [Google Scholar]
- An, J.Y.; Adelina, N.M.; Zhang, L.G.; Zhao, Y.H. Effect of roasting pre-treatment of two grafted pine nuts (Pinus koraiensis) on yield, color, chemical compositions, antioxidant activity, and oxidative stability of the oil. J. Food Process Preserv. 2022, 46, 14. [Google Scholar] [CrossRef]
- Martinez, E.; Pardo, J.E.; Rabadan, A.; Alvarez-Orti, M. Effects of Animal Fat Replacement by Emulsified Melon and Pumpkin Seed Oils in Deer Burgers. Foods 2023, 12, 1279. [Google Scholar] [CrossRef] [PubMed]
- Moghtadaei, M.; Soltanizadeh, N.; Goli, S.A.H. Production of sesame oil oleogels based on beeswax and application as partial substitutes of animal fat in beef burger. Food Res. Int. 2018, 108, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Tarté, R.; Paulus, J.S.; Acevedo, N.C.; Prusa, K.J.; Lee, S.L. High-oleic and conventional soybean oil oleogels structured with rice bran wax as alternatives to pork fat in mechanically separated chicken-based bologna sausage. LWT Food Sci. Technol. 2020, 131, 109659. [Google Scholar] [CrossRef]
- Wolfer, T.L.; Acevedo, N.C.; Prusa, K.J.; Sebranek, J.G.; Tarté, R. Replacement of pork fat in frankfurter-type sausages by soybean oil oleogels structured with rice bran wax. Meat Sci. 2018, 145, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Çakir, M.; Özer, C.O.; Var, G.B. Utilization of Sunflower Oil-based Oleogel for Deep-Fried Coated Chicken Products. J. Oleo Sci. 2023, 72, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Adrah, K.; Adegoke, S.C.; Tahergorabi, R. Physicochemical and microbial quality of coated raw and oleogel-fried chicken. LWT-Food Sci. Technol. 2022, 154, 112589. [Google Scholar] [CrossRef]
- Vote, D.J.; Platter, W.J.; Tatum, J.D.; Schmidt, G.R.; Belk, K.E.; Smith, G.C.; Speer, N.C. Injection of beef strip loins with solutions containing sodium tripolyphosphate, sodium lactate, and sodium chloride to enhance palatability. J. Anim. Sci. 2000, 78, 952–957. [Google Scholar] [CrossRef]
- Agbeniga, B.; Webb, E.C.; O’Neill, H.A. Influence of Kosher (Shechita) and conventional slaughter techniques on shear force, drip and cooking loss of beef. S. Afr. J. Anim. Sci. 2013, 43, 98–102. [Google Scholar] [CrossRef]
- Gil, M.; Rudy, M.; Stanisławczyk, R.; Duma-Kocan, P. Effect of Traditional Cooking and Sous Vide Heat Treatment, Cold Storage Time and Muscle on Physicochemical and Sensory Properties of Beef Meat. Molecules 2022, 27, 7307. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Xie, P.; Li, J.; Li, H.; Sun, B. Effect of cooking final temperature and cutting method on eating quality of pan-fried steak. Trans. Chin. Soc. Agric. Eng. 2015, 31, 317–325. [Google Scholar]
- Borela, V.L.; de Alencar, E.R.; Mendonça, M.A.; Han, H.; Raposo, A.; Ariza-Montes, A.; Araya-Castillo, L.; Zandonadi, R.P. Influence of Different Cooking Methods on Fillet Steak Physicochemical Characteristics. Int. J. Environ. Res. Public Health 2022, 19, 606. [Google Scholar] [CrossRef] [PubMed]
- Lucherk, L.W.; O’Quinn, T.G.; Legako, J.F.; Rathmann, R.J.; Brooks, J.C.; Miller, M.F. Consumer and trained panel evaluation of beef strip steaks of varying marbling and enhancement levels cooked to three degrees of doneness. Meat Sci. 2016, 122, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Tuell, J.R.; Nondorf, M.J.; Kim, B. Post-Harvest Strategies to Improve Tenderness of Underutilized Mature Beef: A Review. Food Sci. Anim. Resour. 2022, 42, 723–743. [Google Scholar] [CrossRef] [PubMed]
- Unal, K.; Babaoglu, A.S.; Karakaya, M. Improving the textural and microstructural quality of cow meat by black chokeberry, grape, and hawthorn vinegar-based marination. Food Sci. Nutr. 2023, 11, 6260–6270. [Google Scholar] [CrossRef] [PubMed]
- Bagdatli, A.; Kayaardi, S. Influence of storage period and packaging methods on quality attributes of fresh beef steaks. Cyta-J. Food 2015, 13, 124–133. [Google Scholar] [CrossRef]
- Kang, N.M.; Panzone, L.; Kuznesof, S. The role of cooking in consumers’ quality formation: An exploratory study of beef steaks. Meat Sci. 2022, 186, 108730. [Google Scholar] [CrossRef]
- Legako, J.F.; Brooks, J.C.; O’Quinn, T.G.; Hagan, T.D.J.; Polkinghorne, R.; Farmer, L.J.; Miller, M.F. Consumer palatability scores and volatile beef flavor compounds of five USDA quality grades and four muscles. Meat Sci. 2015, 100, 291–300. [Google Scholar] [CrossRef]
- Wall, K.R.; Kerth, C.R.; Miller, R.K.; Alvarado, C. Grilling temperature effects on tenderness, juiciness, flavor and volatile aroma compounds of aged ribeye, strip loin, and top sirloin steaks. Meat Sci. 2019, 150, 141–148. [Google Scholar] [CrossRef]
- Yoo, J.H.; Kim, J.W.; Yong, H.I.; Baek, K.H.; Lee, H.J.; Jo, C. Effects of Searing Cooking on Sensory and Physicochemical Properties of Beef Steak. Food Sci. Anim. Resour. 2020, 40, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, H.L.; Hou, Y.L.; Wang, J.Y.; Fan, Y.; Zhang, N.; Guo, Q.Q. Formation and In Vitro Simulated Digestion Study of Gelatinized Korean Pine Seed Oil Encapsulated with Calcified Wax. Molecules 2023, 28, 7334. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, H.; Li, S.; Zhu, N.; Zhang, S.; Zhao, B.; Pan, X.; Qiao, X.; Chen, W.; Qu, C. Changes in Volatile Flavour Compounds during Storage and Analysis of Off-Flavour Substances in Air-Dried Sausage. Food Sci. 2019, 40, 208–216. [Google Scholar]
- Wang, C.; Sun, B.; Luo, X.; Zhang, S.; Li, H.; Xie, P.; Zhang, L.; Sha, K.; Lei, Y. Study on quality properties of prepared beef. Sci. Technol. Food Ind. 2014, 35, 77. [Google Scholar]
- Ma, Y.; Han, L.; Yu, Y.; Jin, X.; Han, G.; Yu, Q.; Xie, Q. Effect of different processing methods on eating quality of pan-fried restructured steak. Sci. Technol. Food Ind. 2016, 37, 302. [Google Scholar]
- GB/T 22210-2008; Specification for Sensory Evaluation of Meat and Meat Products. Standardization Administration of China: Beijing, China, 2008.
- Meng, X.; Wang, H.; Yang, Z. Study on quality changes of different degrees of cooked beef. Sci. Technol. Food Ind. 2015, 36, 101. [Google Scholar]
- Meilgaard, M.C.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques, 4th ed.; CRC Press LLC: New York, NY, USA, 2007. [Google Scholar]
- Gong, H.; Yang, Z.; Shi, Z.; Liu, M.; Tao, R.; Qu, C.; Xu, D. Effect of Different Cooking Times on Volatile Flavor Components in Beijing Spiced Beef. Food Sci. 2017, 38, 183–190. [Google Scholar]
- GB 5009.237-2016; Food Safety National Standard Food pH Value Determination. Standardization Administration of China: Beijing, China, 2016.
- Liu, J.; Yang, B.; Luo, R.; Li, Z.; You, L. Mechanism Underlying Changes in the Water Retention of Tan Sheep Meat during Cold Storage. Food Sci. 2022, 43, 191–198. [Google Scholar]
- GB 5009.228-2016; Determination of Volatile Base Total Nitrogen in Food of National Standard for Food Safety Products. Standardization Administration of China: Beijing, China, 2016.
- Etemadian, Y.; Shabanpour, B.; Mahoonak, A.R.S.; Shabani, A.; Alami, M. Cryoprotective effects of polyphosphates on Rutilus frisii kutum fillets. Food Chem. 2011, 129, 1544–1551. [Google Scholar] [CrossRef]
- Aaslyng, M.D.; Bejerholm, C.; Ertbjerg, P.; Bertram, H.C.; Andersen, H.J. Cooking loss and juiciness of pork in relation to raw meat quality and cooking procedure. Food Qual. Prefer. 2003, 14, 277–288. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Xie, J. Analysis of aroma compounds in roast beef patty. Food Ferment. Ind. 2022, 48, 259–267. [Google Scholar]
- Caine, W.R.; Aalhus, J.L.; Best, D.R.; Dugan, M.E.R.; Jeremiah, L.E. Relationship of texture profile analysis and Warner-Bratzler shear force with sensory characteristics of beef rib steaks. Meat Sci. 2003, 64, 333–339. [Google Scholar] [CrossRef]
- Roldán, M.; Antequera, T.; Pérez-Palacios, T.; Ruiz, J. Effect of added phosphate and type of cooking method on physico-chemical and sensory features of cooked lamb loins. Meat Sci. 2014, 97, 69–75. [Google Scholar] [CrossRef]
- Huang, F.; Huang, M.; Xu, X.L.; Zhou, G.H. Influence of heat on protein degradation, ultrastructure and eating quality indicators of pork. J. Sci. Food Agric. 2011, 91, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Yun, J.; Zhao, F.; Ai, D.; Zhang, W.; He, K.; Wang, R.; Wu, S. Effect of Second Inoculation Time on the Volatile Flavor of Fermented Dry-Cured Meat. Food Sci. 2022, 43, 285–293. [Google Scholar]
- Zhang, W.; Song, Q.Q.; Wu, F.; Zhang, J.Z.; Xu, M.S.; Li, H.H.; Han, Z.J.; Gao, H.X.; Xu, N.Y. Evaluation of the four breeds in synthetic line of Jiaxing Black Pigs and Berkshire for meat quality traits, carcass characteristics, and flavor substances. Anim. Sci. J. 2019, 90, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Jiang, W.; Wang, Q.; Chen, J.; Ye, X.; Liu, D. Process Optimization and Analysis of Volatile Flavor Compounds of Braised Pork. J. Chin. Inst. Food Sci. Technol. 2017, 17, 208–216. [Google Scholar]
- Liu, M.; Zhang, S.; Zang, M.; Zhao, B.; Huang, H.; Zhu, N.; Li, S.; Wu, Q.; Liu, B.; Zhao, Y.; et al. Dynamic Changes of Volatile Compounds of Beef during the Dry-aging Process. Food Sci. 2022, 43, 279–284. [Google Scholar]
- Dos Santos-Donado, P.R.; Donado-Pestana, C.M.; Tanaka, F.A.O.; Venturini, A.C.; Delgado, E.F.; Contreras-Castillo, C.J. Effects of high-oxygen, carbon monoxide modified atmospheres and vacuum packaging on quality of Longissimus thoracis et lumborum steaks from Nellore cows during ageing. Food Res. Int. 2021, 143, 110226. [Google Scholar] [CrossRef]
- Watanabe, A.; Kamada, G.; Imanari, M.; Shiba, N.; Yonai, M.; Muramoto, T. Effect of aging on volatile compounds in cooked beef. Meat Sci. 2015, 107, 12–19. [Google Scholar] [CrossRef]
- Lorenzo, J.M. Changes on physico-chemical, textural, lipolysis and volatile compounds during the manufacture of dry-cured foal “Cecina”. Meat Sci. 2014, 96, 256–263. [Google Scholar] [CrossRef]
- Wang, R.; Hu, X.; Agyekumwaa, A.K.; Li, X.; Xiao, X.; Yu, Y. Synergistic effect of kojic acid and tea polyphenols on bacterial inhibition and quality maintenance of refrigerated sea bass (Lateolabrax japonicus) fillets. LWT 2021, 137, 110452. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, G.; Zhang, W.; Xu, X.; Zhou, G. Comparison of Chicken Meat Quality with Different Water-holding Capacity and Correlation Analysis. Food Sci. 2013, 34, 45–49. [Google Scholar]
- Zhang, J.; Zhao, Y.; Zhang, M.; Ding, Y.; Lu, F. Quality Changes and Predictive Modeling of Shelf Life of Sauced Duck Stored at Different Temperatures. Food Sci. 2019, 40, 250–257. [Google Scholar]
- Zhu, Y.; Ma, L.; Dang, X.; Wang, Y. Effect of packaging and storage temperature on water holding capacity of catfish fillets during storage based on low field NMR. Trans. Chin. Soc. Agric. Eng. 2016, 32, 281–289. [Google Scholar]
- Chen, J.; Yang, R.; Wang, Y.; Koseki, S.; Fu, L.; Wang, Y. Inhibitory effect of D-Tryptophan on the spoilage potential of Shewanella baltica and Pseudomonas fluorescens and its potential application in salmon fillet preservation. Food Microbiol. 2022, 108, 104104. [Google Scholar] [CrossRef]
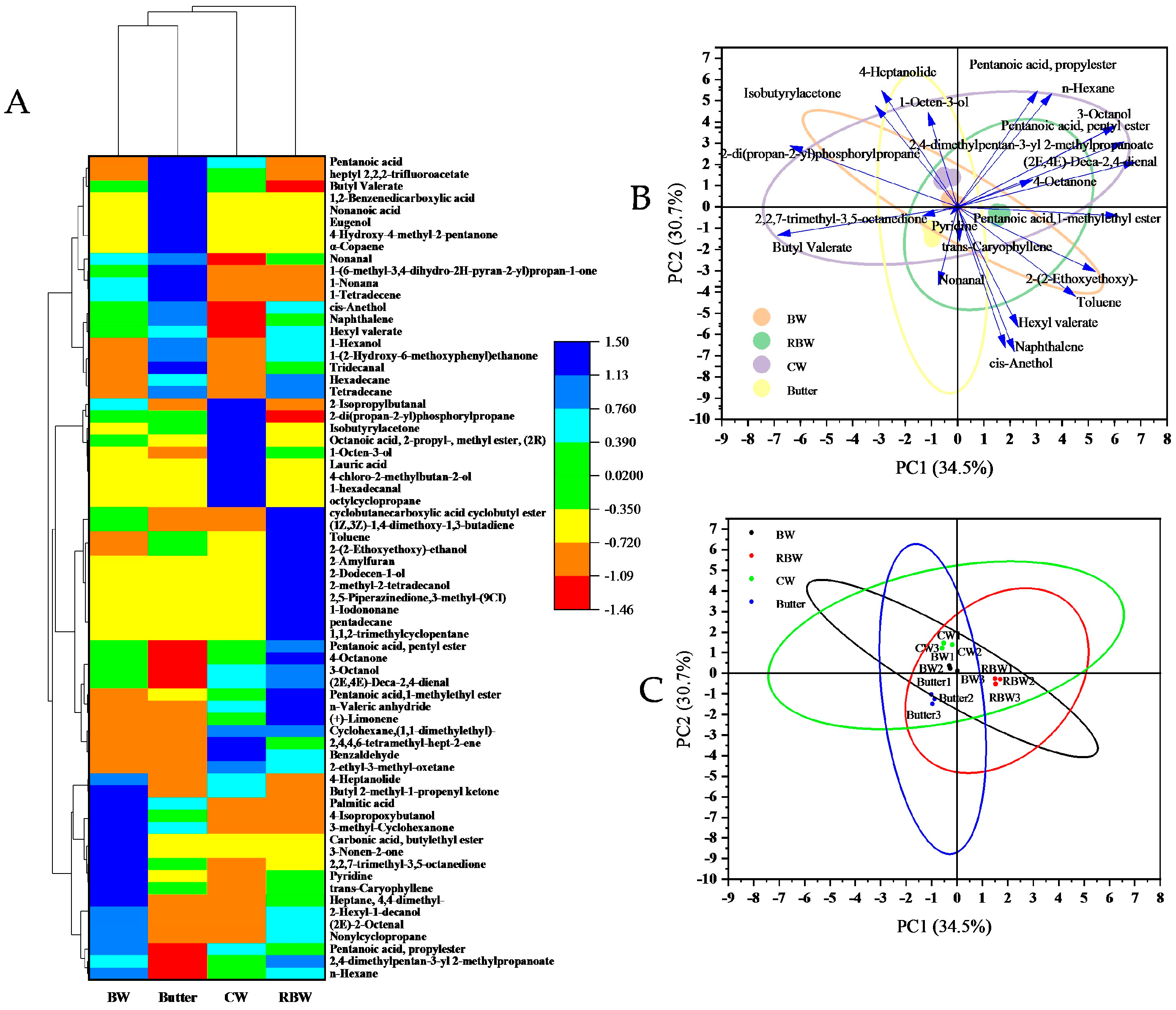
| Degree of Sophistication | Rare | Medium Rare | Medium | Medium Well | Well Done | |
|---|---|---|---|---|---|---|
| L* | BW | 31.77 ± 0.48 c | 32.71 ± 0.47 b | 32.58 ± 0.06 bc | 34.26 ± 0.63 a | 35.04 ± 0.64 a |
| RBW | 32.29 ± 0.23 c | 32.83 ± 0.25 bc | 33.45 ± 0.28 a | 33.67 ± 0.45 a | 33.38 ± 0.31 ab | |
| CW | 30.67 ± 0.21 d | 31.46 ± 0.32 c | 31.69 ± 0.26 bc | 31.99 ± 0.22 ab | 32.16 ± 0.09 a | |
| Butter | 32.38 ± 0.32 c | 32.99 ± 0.59 c | 34.32 ± 0.34 b | 35.37 ± 0.22 a | 35.67 ± 0.22 a | |
| a* | BW | 7.50 ± 0.17 c | 7.85 ± 0.19 b | 8.17 ± 0.05 a | 8.45 ± 0.22 a | 7.63 ± 0.10 bc |
| RBW | 7.70 ± 0.34 c | 9.55 ± 0.31 a | 8.92 ± 0.30 b | 9.53 ± 0.20 a | 7.62 ± 0.35 c | |
| CW | 8.29 ± 0.17 d | 9.34 ± 0.35 c | 10.40 ± 0.22 a | 9.77 ± 0.11 b | 9.76 ± 0.08 b | |
| Butter | 7.43 ± 0.21 c | 8.32 ± 0.35 b | 8.74 ± 0.16 ab | 8.82 ± 0.13 a | 7.54 ± 0.37 c | |
| b* | BW | 10.90 ± 0.40 d | 11.91 ± 0.25 c | 13.09 ± 0.15 b | 13.45 ± 0.34 b | 14.21 ± 0.29 a |
| RBW | 12.83 ± 0.63 d | 12.83 ± 0.30 d | 13.85 ± 0.20 c | 14.52 ± 0.15 b | 15.75 ± 0.46 a | |
| CW | 10.62 ± 0.31 c | 11.74 ± 0.25 b | 12.03 ± 0.55 bc | 11.94 ± 0.12 b | 12.54 ± 0.29 a | |
| Butter | 12.81 ± 0.26 b | 11.79 ± 0.13 c | 12.55 ± 0.24 b | 13.62 ± 0.34 a | 13.84 ± 0.25 a | |
| Sensory Attributes | Oil Sample | Rare | Medium Rare | Medium | Medium Well | Well Done |
|---|---|---|---|---|---|---|
| Color | BW | 6.98 ± 0.33 a | 6.57 ± 0.35 a | 7.56 ± 0.13 a | 8.73 ± 0.28 a | 7.76 ± 0.25 a |
| RBW | 6.24 ± 0.46 b | 6.54 ± 0.19 a | 7.51 ± 0.37 a | 8.49 ± 0.29 a | 7.59 ± 0.37 a | |
| CW | 6.28 ± 0.17 b | 6.11 ± 0.16 b | 7.62 ± 0.34 a | 8.24 ± 0.24 ab | 7.67 ± 0.26 a | |
| Butter | 6.34 ± 0.34 b | 6.62 ± 0.26 a | 7.39 ± 0.11 b | 8.12 ± 0.18 b | 7.12 ± 0.37 b | |
| Odor | BW | 6.57 ± 0.27 b | 7.72 ± 0.35 a | 8.58 ± 0.38 a | 8.23 ± 0.13 b | 8.68 ± 0.26 a |
| RBW | 7.55 ± 0.23 a | 7.73 ± 0.16 a | 8.63 ± 0.33 a | 8.37 ± 0.16 ab | 8.12 ± 0.19 b | |
| CW | 6.66 ± 0.14 b | 6.43 ± 0.32 c | 8.55 ± 0.32 a | 8.38 ± 0.27 ab | 8.31 ± 0.22 ab | |
| Butter | 7.78 ± 0.12 a | 7.27 ± 0.28 b | 8.14 ± 0.36 b | 8.45 ± 0.23 a | 8.52 ± 0.26 a | |
| Flavor | BW | 6.34 ± 0.10 ab | 6.61 ± 0.07 b | 7.30 ± 0.02 c | 8.37 ± 0.07 b | 7.62 ± 0.04 a |
| RBW | 6.21 ± 0.07 b | 6.85 ± 0.08 a | 7.46 ± 0.06 b | 8.28 ± 0.04 b | 7.08 ± 0.05 b | |
| CW | 6.40 ± 0.08 a | 6.38 ± 0.05 c | 7.35 ± 0.03 c | 7.75 ± 0.10 c | 7.17 ± 0.05 b | |
| Butter | 6.34 ± 0.10 ab | 6.51 ± 0.08 b | 7.67 ± 0.07 a | 8.58 ± 0.05 a | 6.43 ± 0.09 c | |
| Tenderness | BW | 6.20 ± 0.05 d | 6.26 ± 0.02 b | 6.89 ± 0.08 c | 8.45 ± 0.18 a | 7.54 ± 0.04 b |
| RBW | 6.26 ± 0.23 b | 6.25 ± 0.10 c | 7.13 ± 0.08 b | 8.22 ± 0.06 b | 7.64 ± 0.09 a | |
| CW | 6.23 ± 0.09 c | 6.30 ± 0.04 a | 7.12 ± 0.06 b | 7.83 ± 0.04 b | 7.06 ± 0.03 c | |
| Butter | 6.29 ± 0.08 a | 6.22 ± 0.05 d | 7.41 ± 0.04 a | 8.22 ± 0.05 c | 6.48 ± 0.05 d | |
| Juiciness | BW | 6.34 ± 0.08 a | 6.31 ± 0.03 b | 7.27 ± 0.05 ab | 8.60 ± 0.06 a | 7.39 ± 0.04 a |
| RBW | 6.21 ± 0.10 a | 6.38 ± 0.04 b | 7.38 ± 0.06 a | 8.70 ± 0.02 a | 7.10 ± 0.07 b | |
| CW | 5.71 ± 0.23 b | 6.12 ± 0.06 c | 7.21 ± 0.05 b | 8.09 ± 0.05 c | 6.87 ± 0.05 c | |
| Butter | 5.70 ± 0.32 b | 6.69 ± 0.04 a | 7.17 ± 0.10 b | 8.35 ± 0.11 b | 6.29 ± 0.03 d | |
| Chewiness | BW | 6.48 ± 0.15 ab | 6.95 ± 0.29 a | 7.27 ± 0.31 ab | 8.03 ± 0.22 c | 8.03 ± 0.39 a |
| RBW | 6.65 ± 0.25 a | 6.88 ± 0.32 a | 7.58 ± 0.23 a | 8.29 ± 0.17 a | 8.37 ± 0.19 a | |
| CW | 6.17 ± 0.18 c | 6.51 ± 0.11 b | 7.24 ± 0.11 b | 8.48 ± 0.16 a | 8.06 ± 0.33 a | |
| Butter | 6.34 ± 0.13 b | 6.38 ± 0.24 b | 7.37 ± 0.32 ab | 8.31 ± 0.22 a | 7.63 ± 0.21 b | |
| Overall Impression | BW | 6.67 ± 0.43 b | 6.56 ± 0.16 b | 7.28 ± 0.36 c | 8.47 ± 0.31 a | 8.22 ± 0.24 ab |
| RBW | 6.15 ± 0.48 c | 6.32 ± 0.25 bc | 7.92 ± 0.25 a | 8.76 ± 0.28 a | 8.34 ± 0.17 a | |
| CW | 6.73 ± 0.53 ab | 6.21 ± 0.18 c | 7.59 ± 0.16 b | 8.25 ± 0.14 b | 8.51 ± 0.37 a | |
| Butter | 6.83 ± 0.13 a | 6.96 ± 0.27 a | 7.04 ± 0.15 d | 8.38 ± 0.23 ab | 7.69 ± 0.43 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Wang, J.; Fan, Y.; Zhang, N.; Guo, Q. Quality Characteristics and Storage Stability of Frying Steak Utilizing Wax-Based Korean Pine Seed Oil. Foods 2024, 13, 1099. https://doi.org/10.3390/foods13071099
Wang P, Wang J, Fan Y, Zhang N, Guo Q. Quality Characteristics and Storage Stability of Frying Steak Utilizing Wax-Based Korean Pine Seed Oil. Foods. 2024; 13(7):1099. https://doi.org/10.3390/foods13071099
Chicago/Turabian StyleWang, Peng, Jingyi Wang, Yue Fan, Na Zhang, and Qingqi Guo. 2024. "Quality Characteristics and Storage Stability of Frying Steak Utilizing Wax-Based Korean Pine Seed Oil" Foods 13, no. 7: 1099. https://doi.org/10.3390/foods13071099
APA StyleWang, P., Wang, J., Fan, Y., Zhang, N., & Guo, Q. (2024). Quality Characteristics and Storage Stability of Frying Steak Utilizing Wax-Based Korean Pine Seed Oil. Foods, 13(7), 1099. https://doi.org/10.3390/foods13071099






