Volatile Metabolites to Assess the Onset of Chilling Injury in Fresh-Cut Nectarines
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Material, Processing and Storage Condition
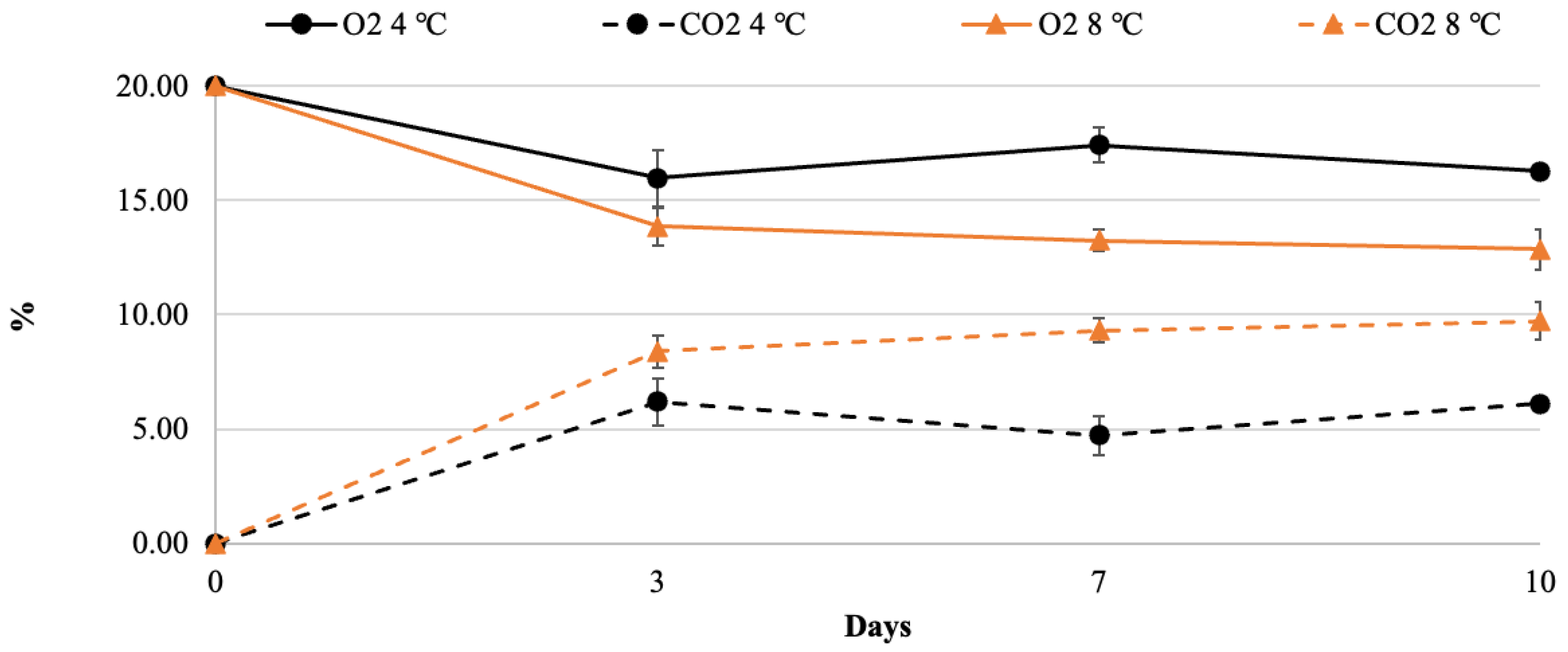
2.2. Headspace Gas Composition and Respiration Rate
2.3. Visual Quality and Physical Characteristics
2.4. Antioxidant Activity, O-Quinones, Total Carotenoids and Total Sugars
2.5. Polyphenol Oxidases (PPO) and Peroxidases (POD)
2.6. Phenylalanine Ammonia-Lyase (PAL)
2.7. Volatile Organic Compounds Analysis
2.7.1. Sample Preparation and HS-SPME Extraction
2.7.2. GC-MS Analysis
2.8. Extraction of Phenolic Compounds and HPLC-DAD Analysis
2.9. Statistical Data Analysis
3. Results and Discussion
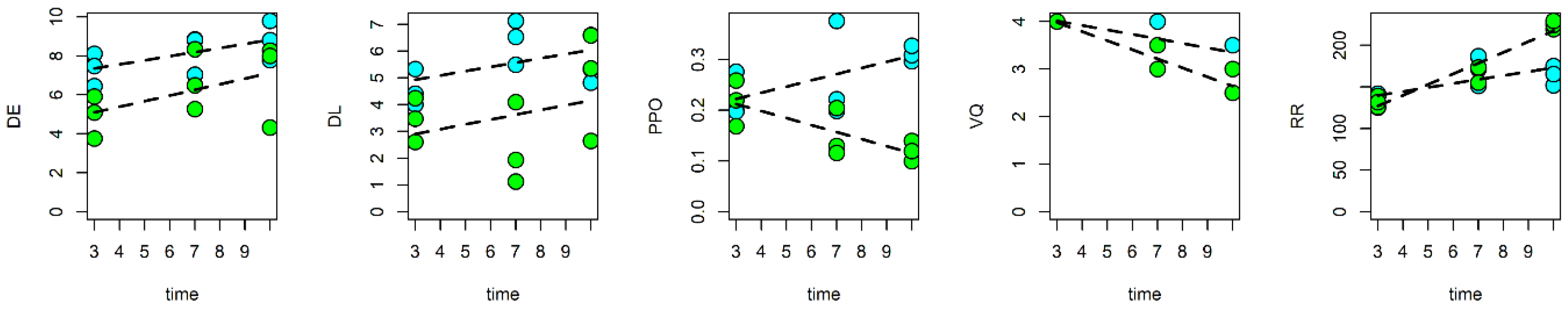
3.1. Quality Changes in Fresh-Cut Nectarines during Storage
3.2. Volatile Compounds of Fresh-Cut Nectarines during Storage
3.3. Phenolic Compounds of Fresh-Cut Nectarines during Storage
3.4. Analysis of Other Compounds in Fresh-Cut Nectarines during Storage
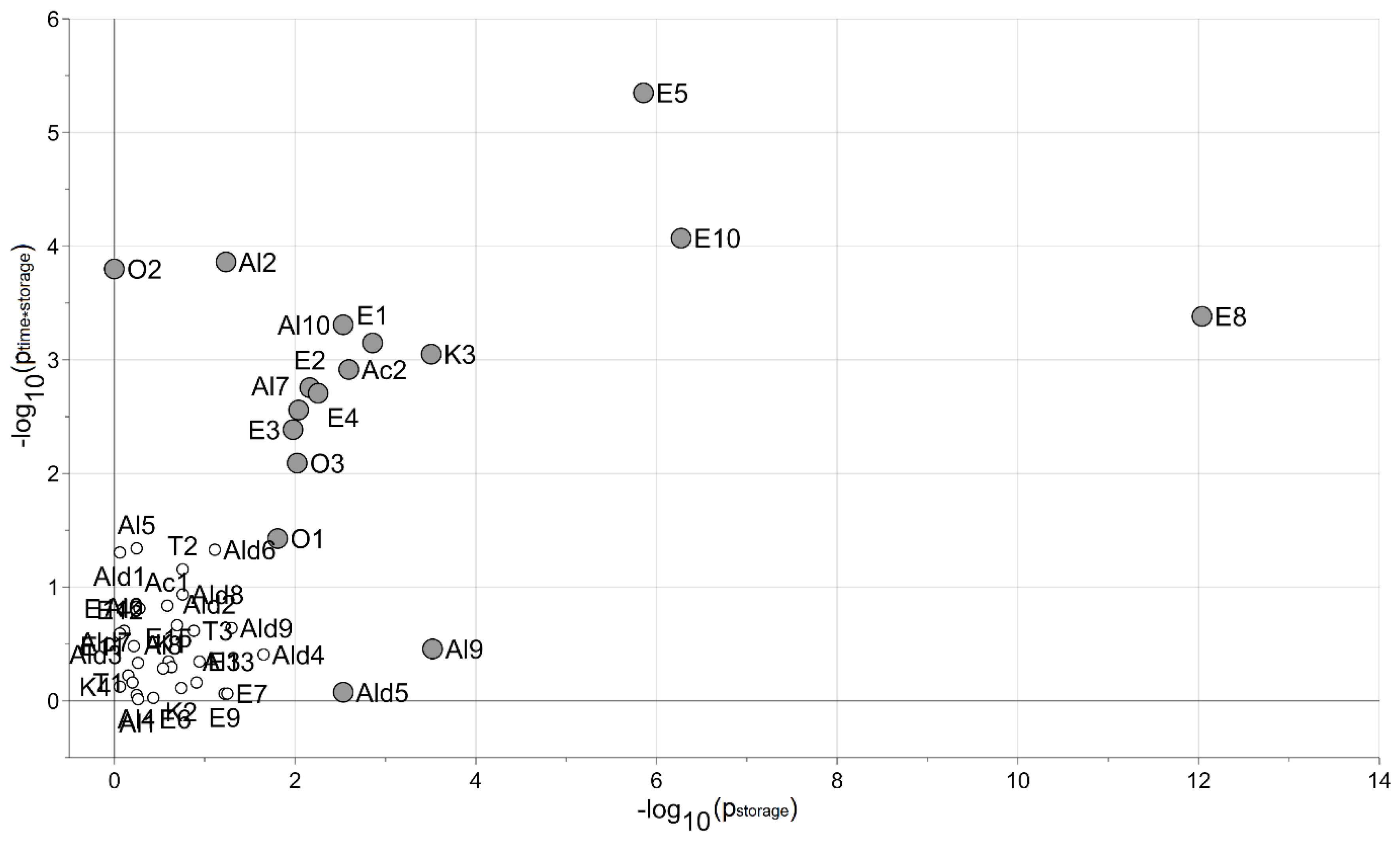
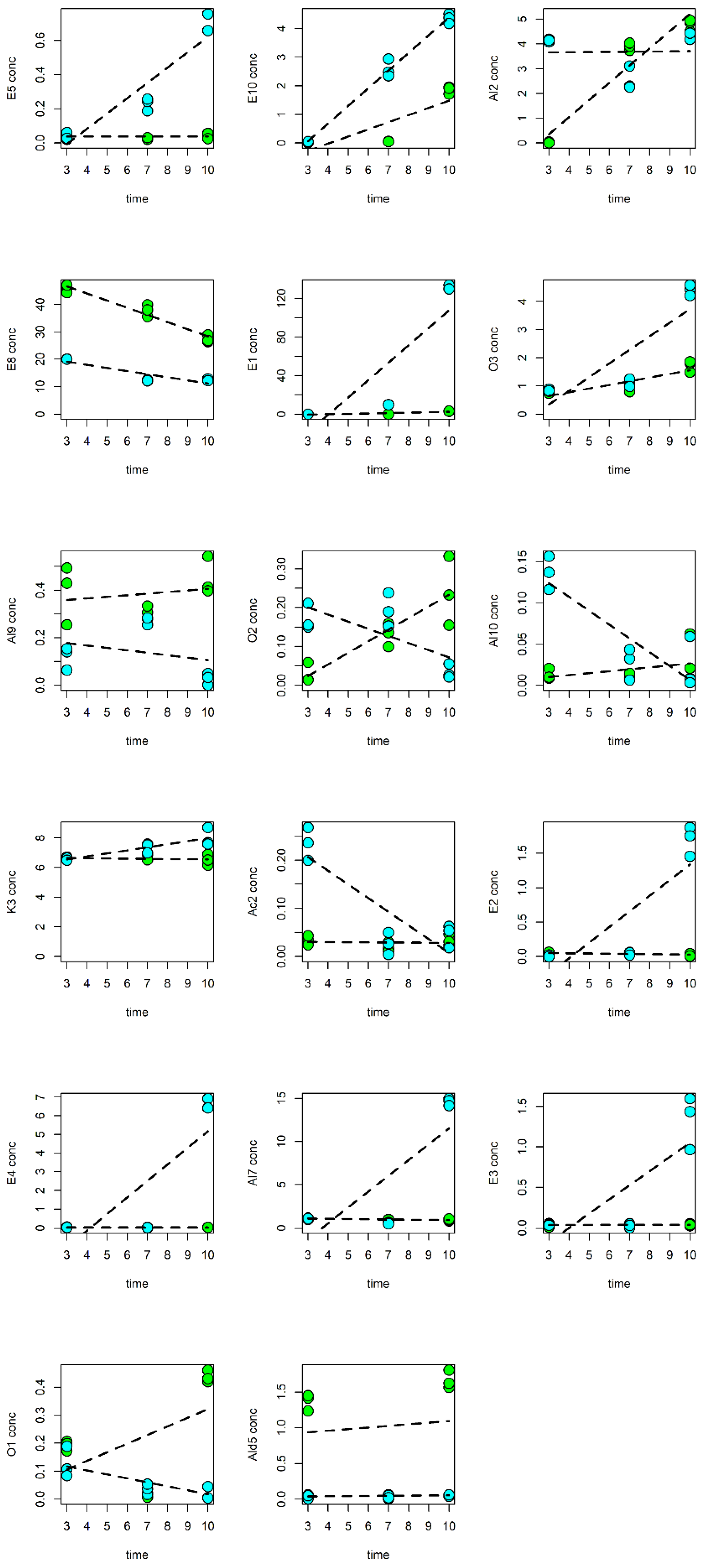
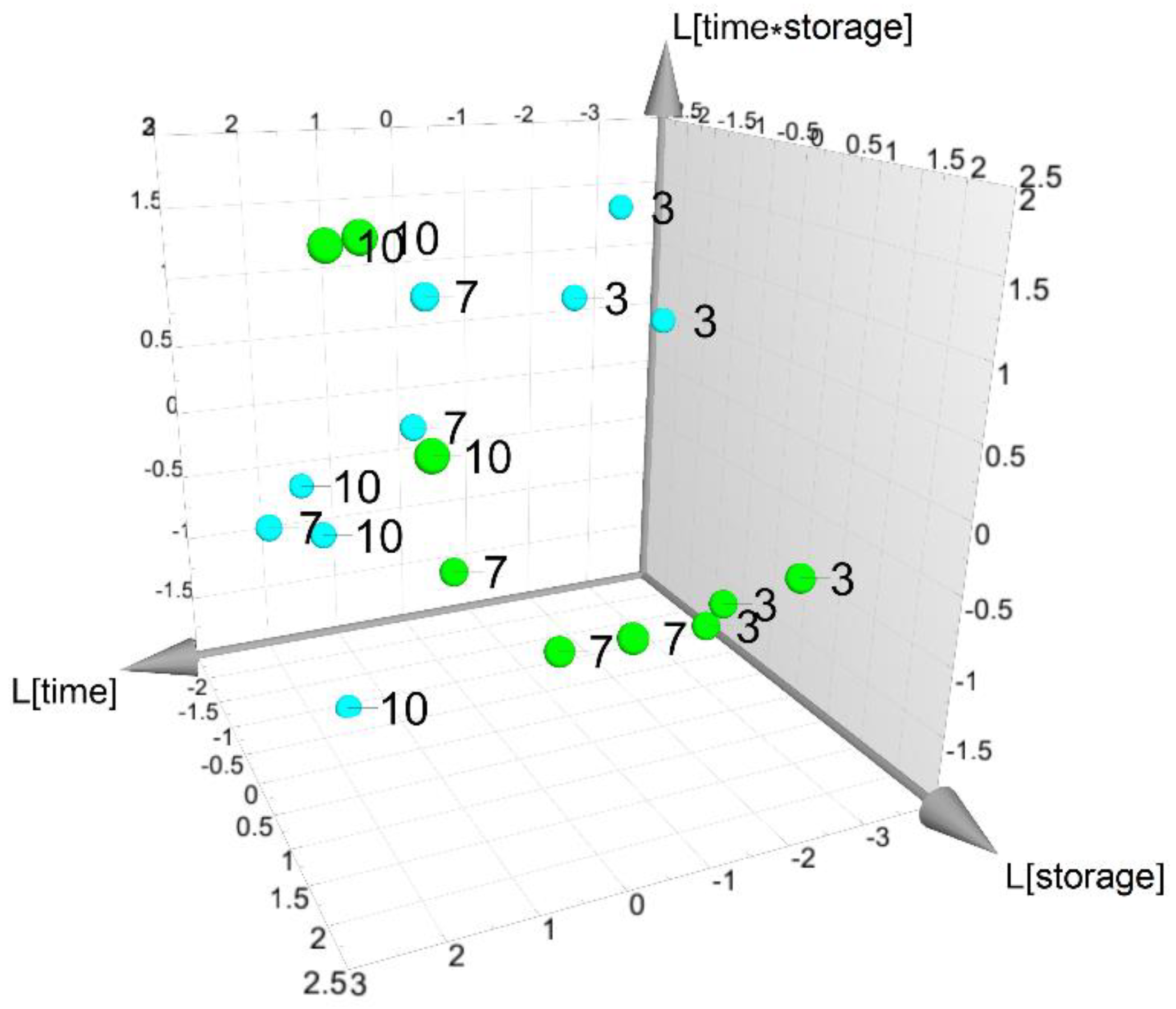
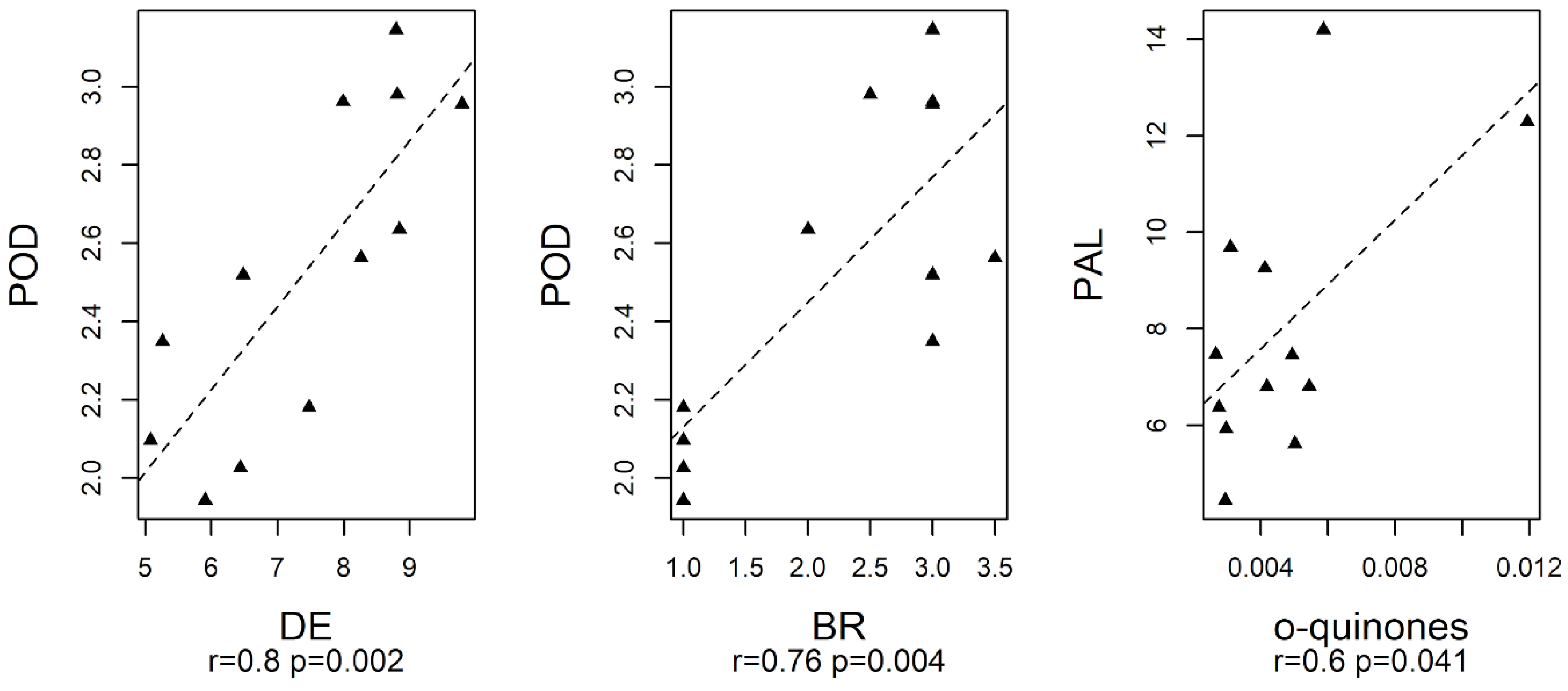
3.5. Data Analysis of the Responses of Fresh-Cut Nectarines during Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christofides, S.R.; Setarehnejad, A.; Fairchild, R.; Muzzalupo, I.; Bruno, L.; Muto, A.; Chiappetta, A.; Bitonti, M.B.; Müller, C.T.; Rogers, H.J.; et al. Cross-Cultural Differences between Italian and UK Consumer Preferences for ‘Big Top’ Nectarines in Relation to Cold Storage. Foods 2022, 11, 2424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, D.; Jiang, M.; Tu, K. Changes in Free and Glycosidically Bound Volatile Compounds of Nectarine Fruit during Low-Temperature Storage. Int. Food Res. J. 2022, 29, 843–856. [Google Scholar] [CrossRef]
- Ceccarelli, A.; Farneti, B.; Khomenko, I.; Cellini, A.; Donati, I.; Aprea, E.; Biasioli, F.; Spinelli, F. Nectarine Volatilome Response to Fresh-Cutting and Storage. Postharvest Biol. Technol. 2020, 159, 111020. [Google Scholar] [CrossRef]
- Gallotta, A.; Allegra, A.; Inglese, P.; Sortino, G. Fresh-Cut Storage of Fruit and Fresh-Cuts Affects the Behaviour of Minimally Processed Big Bang Nectarines (Prunus persica L. Batsch) during Shelf Life. Food Packag. Shelf 2018, 15, 62–68. [Google Scholar] [CrossRef]
- Lee, C.Y.; Kagan, V.; Jaworski, A.W.; Brown, S.K. Enzymic Browning in Relation to Phenolic Compounds and Polyphenoloxidase Activity among Various Peach Cultivars. J. Agric. Food Chem. 1990, 38, 99–101. [Google Scholar] [CrossRef]
- Allegra, A.; Barone, E.; Inglese, P.; Todaro, A.; Sortino, G. Variability of Sensory Profile and Quality Characteristics for ‘Pesca Di Bivona’ and ‘Pesca Di Leonforte’ Peach (Prunus persica Batsch) Fresh-Cut Slices during Storage. Postharvest Biol. Technol. 2015, 110, 61–69. [Google Scholar] [CrossRef]
- Li-Qin, Z.; Jie, Z.; Shu-Hua, Z.; Lai-Hui, G. Inhibition of Browning on the Surface of Peach Slices by Short-Term Exposure to Nitric Oxide and Ascorbic Acid. Food Chem. 2009, 114, 174–179. [Google Scholar] [CrossRef]
- Sortino, G.; Ingrassia, M.; Allegra, A.; Inglese, P. Sensory evaluation and suitability for fresh-cut produce of white peach [Prunus persica (L.) batsch] “Settembrina di Bivona”. Acta Hortic. 2015, 1084, 787–790. [Google Scholar] [CrossRef]
- Beaulieu, J. Factors Affecting Sensory Quality of Fresh-Cut Produce. In Advances in Fresh-Cut Fruits and Vegetables Processing; Food Preservation Technology; CRC Press: Boca Raton, FL, USA, 2010; Volume 20105958, pp. 115–143. [Google Scholar] [CrossRef]
- Mendoza-Enano, M.L.; Stanley, R.; Frank, D. Linking Consumer Sensory Acceptability to Volatile Composition for Improved Shelf-Life: A Case Study of Fresh-Cut Watermelon (Citrullus lanatus). Postharvest Biol. Technol. 2019, 154, 137–147. [Google Scholar] [CrossRef]
- Vela, G.; León, D.; García, H.; La Cruz, J.D. Polyphenoloxidase Activity during Ripening and Chilling Stress in ‘Manila’ Mangoes. J. Hortic. Sci. Biotechnol. 2003, 78, 104–107. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic Compounds and Related Enzymes as Determinants of Quality in Fruits and Vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Giné-Bordonaba, J.; Busatto, N.; Larrigaudière, C.; Lindo-García, V.; Echeverria, G.; Vrhovsek, U.; Farneti, B.; Biasioli, F.; De Quattro, C.; Rossato, M.; et al. Investigation of the Transcriptomic and Metabolic Changes Associated with Superficial Scald Physiology Impaired by Lovastatin and 1-Methylcyclopropene in Pear Fruit (Cv. “Blanquilla”). Hortic. Res. 2020, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Denoya, G.I.; Vaudagna, S.R.; Chamorro, V.C.; Godoy, M.F.; Budde, C.O.; Polenta, G.A. Suitability of Different Varieties of Peaches for Producing Minimally Processed Peaches Preserved by High Hydrostatic Pressure and Selection of Process Parameters. LWT 2017, 78, 367–372. [Google Scholar] [CrossRef]
- Koukounaras, A.; Diamantidis, G.; Sfakiotakis, E. The Effect of Heat Treatment on Quality Retention of Fresh-Cut Peach. Postharvest Biol. Technol. 2008, 48, 30–36. [Google Scholar] [CrossRef]
- Pizato, S.; Cortez-Vega, W.R.; De Souza, J.T.A.; Prentice-Hernández, C.; Borges, C.D. Effects of Different Edible Coatings in Physical, Chemical and Microbiological Characteristics of Minimally Processed Peaches (Prunus persica L. B Atsch). J. Food Saf. 2013, 33, 30–39. [Google Scholar] [CrossRef]
- Cefola, M.; Pace, B.; Sergio, L.; Baruzzi, F.; Gatto, M.A.; Carito, A.; Linsalata, V.; Cascarano, N.A.; Di Venere, D. Postharvest Performance of Fresh-cut ‘Big Top’ Nectarine as Affected by Dipping in Chemical Preservatives and Packaging in Modified Atmosphere. Int. J. Food Sci. Technol. 2014, 49, 1184–1195. [Google Scholar] [CrossRef]
- Denoya, G.I.; Polenta, G.A.; Apóstolo, N.M.; Budde, C.O.; Sancho, A.M.; Vaudagna, S.R. Optimization of High Hydrostatic Pressure Processing for the Preservation of Minimally Processed Peach Pieces. Innov. Food Sci. Emerg. Technol. 2016, 33, 84–93. [Google Scholar] [CrossRef]
- Cozzolino, R.; Cefola, M.; Pace, B.; Malorni, L.; Martignetti, A.; Montemurro, N.; Pellicano, M.P. Quality, Sensory and Volatile Profiles of Fresh-cut Big Top Nectarines Cold Stored in Air or Modified Atmosphere Packaging. Int. J. Food Sci. Technol. 2018, 53, 1736–1743. [Google Scholar] [CrossRef]
- Drogoudi, P.; Pantelidis, G.E.; Goulas, V.; Manganaris, G.A.; Ziogas, V.; Manganaris, A. The Appraisal of Qualitative Parameters and Antioxidant Contents during Postharvest Peach Fruit Ripening Underlines the Genotype Significance. Postharvest Biol. Technol. 2016, 115, 142–150. [Google Scholar] [CrossRef]
- Kader, A.A. (Ed.) Methods of gas mixing, sampling and analysis. In Postharvest Technology of Horticultural Crops; University of California Agriculture and Natural Resources: Oakland, CA, USA, 2002; pp. 145–148. [Google Scholar]
- Pace, B.; Cefola, M.; Renna, F.; Attolico, G. Relationship between visual appearance and browning as evaluated by image analysis and chemical traits in fresh-cut nectarines. Postharvest Biol. Technol. 2011, 61, 178–183. [Google Scholar] [CrossRef]
- Jin, P.; Zhu, H.; Wang, L.; Shan, T.; Zheng, Y. Oxalic Acid Alleviates Chilling Injury in Peach Fruit by Regulating Energy Metabolism and Fatty Acid Contents. Food Chem. 2014, 161, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Fadda, A.; Pace, B.; Angioni, A.; Barberis, A.; Cefola, M. Suitability for Ready-to-Eat Processing and Preservation of Six Green and Red Baby Leaves Cultivars and Evaluation of Their Antioxidant Value during Storage and after the Expiration Date: Ready-to-Eat Baby Leaves Cultivars. J. Food Process. Preserv. 2016, 40, 550–558. [Google Scholar] [CrossRef]
- Degl’Innocenti, E.; Pardossi, A.; Tognoni, F.; Guidi, L. Physiological Basis of Sensitivity to Enzymatic Browning in ‘Lettuce’, ‘Escarole’ and ‘Rocket Salad’ When Stored as Fresh-Cut Products. Food Chem. 2007, 104, 209–215. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Buysse, J.A.N.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Cefola, M.; D’Antuono, I.; Pace, B.; Calabrese, N.; Carito, A.; Linsalata, V.; Cardinali, A. Biochemical Relationships and Browning Index for Assessing the Storage Suitability of Artichoke Genotypes. Food Res. Int. 2012, 48, 397–403. [Google Scholar] [CrossRef]
- Gao, H.; Wu, S.; Zeng, Q.; Li, P.; Guan, W. Effects of Exogenous γ-Aminobutyric Acid Treatment on Browning and Food-Borne Pathogens in Fresh-Cut Apples. Postharvest Biol. Technol. 2018, 146, 1–8. [Google Scholar] [CrossRef]
- Benjamini, Y. Discovering the False Discovery Rate. J. R. Stat. Soc. B Stat. Methodol. 2010, 72, 405–416. [Google Scholar] [CrossRef]
- Stocchero, M. PLS for designed experiments. Chemom. Intell. Lab. Syst. 2023, 240, 104928. [Google Scholar] [CrossRef]
- Lurie, S.; Crisosto, C.H. Chilling Injury in Peach and Nectarine. Postharvest Biol. Technol. 2005, 37, 195–208. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Flath, R.A.; Guentert, M.; Jennings, W. Nectarine Volatiles: Vacuum Steam Distillation versus Headspace Sampling. J. Agric. Food Chem. 1988, 36, 553–560. [Google Scholar] [CrossRef]
- Agozzino, P.; Avellone, G.; Filizzola, F.; Farina, V.; Bianco, R.L. Changes in quality parameters and volatile aroma compounds in ‘fairtime’ peach during fruit development and ripening. Ital. J. Food Sci. 2007, 19, 3. [Google Scholar]
- Mihaylova, D.; Popova, A.; Vrancheva, R.; Dincheva, I. HS-SPME-GC–MS Volatile Profile Characterization of Peach (Prunus persica L. Batsch) Varieties Grown in the Eastern Balkan Peninsula. Plants 2022, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Farcuh, M.; Hopfer, H. Aroma Volatiles as Predictors of Chilling Injury Development during Peach (Prunus persica (L) Batsch) Cold Storage and Subsequent Shelf-Life. Postharvest Biol. Technol. 2023, 195, 112137. [Google Scholar] [CrossRef]
- Aprea, E.; Corollaro, M.L.; Betta, E.; Endrizzi, I.; Demattè, M.L.; Biasioli, F.; Gasperi, F. Sensory and Instrumental Profiling of 18 Apple Cultivars to Investigate the Relation between Perceived Quality and Odour and Flavour. Food Res. Int. 2012, 49, 677–686. [Google Scholar] [CrossRef]
- Deza-Durand, K.M.; Petersen, M.A. The Effect of Cutting Direction on Aroma Compounds and Respiration Rate of Fresh-Cut Iceberg Lettuce (Lactuca sativa L.). Postharvest Biol. Technol. 2011, 61, 83–90. [Google Scholar] [CrossRef]
- Cellini, A.; Buriani, G.; Rocchi, L.; Rondelli, E.; Savioli, S.; Rodriguez Estrada, M.T.; Cristescu, S.M.; Costa, G.; Spinelli, F. Biological Relevance of Volatile Organic Compounds Emitted during the Pathogenic Interactions between Apple Plants and Erwinia Amylovora. Mol. Plant Pathol. 2018, 19, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Raffo, A.; Nardo, N.; Tabilio, M.R.; Paoletti, F. Effects of Cold Storage on Aroma Compounds of White- and Yellow-Fleshed Peaches. Eur. Food Res. Technol. 2008, 226, 1503–1512. [Google Scholar] [CrossRef]
- Aubert, C.; Bony, P.; Chalot, G.; Landry, P.; Lurol, S. Effects of Storage Temperature, Storage Duration, and Subsequent Ripening on the Physicochemical Characteristics, Volatile Compounds, and Phytochemicals of Western Red Nectarine (Prunus persica L. Batsch). J. Agric. Food Chem. 2014, 62, 4707–4724. [Google Scholar] [CrossRef]
- Dong, X.; He, Y.; Yuan, C.; Cheng, X.; Li, G.; Shan, Y.; Zhu, X. Controlled Atmosphere Improves the Quality, Antioxidant Activity and Phenolic Content of Yellow Peach during the Shelf Life. Antioxidants 2022, 11, 2278. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Wu, X.; Boon-Ek, Y.; Xu, L.; Pan, H.; Xu, P.; Wu, Y.; Supapvanich, S. Effect of Honey and Calcium Dips on Quality of Fresh-Cut Nectarine (Prunus persica L. Batsch). Agric. Nat. Resour. 2018, 52, 140–145. [Google Scholar] [CrossRef]
- Trejo-Márquez, M.A.; Ramírez-Villatoro, G.; Camacho De La Rosa, N.A. Polyphenol oxidase and peroxidase activities in mangoes stored at chilling temperature. Acta Hortic. 2010, 864, 395–402. [Google Scholar] [CrossRef]
- Rodrigues, C.; Gaspar, P.D.; Simões, M.P.; Silva, P.D.; Andrade, L.P. Review on Techniques and Treatments toward the Mitigation of the Chilling Injury of Peaches. J. Food Process. Preserv. 2022, 46, e14358. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G. Effects of Edible Coatings on Quality Maintenance of Fresh-Cut Nectarines. Emir. J. Food Agric. 2016, 28, 201. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, W.; Xiong, Z.; Zou, L.; Chen, J.; Liu, J.; Zhong, J. Different Modes of Inhibition for Organic Acids on Polyphenoloxidase. Food Chem. 2016, 199, 439–446. [Google Scholar] [CrossRef]


| VOCs | Code | FRESH | 4 °C | 8 °C | ||||
|---|---|---|---|---|---|---|---|---|
| 3 d | 7 d | 10 d | 3 d | 7 d | 10 d | |||
| Ethyl acetate | E1 | nd | nd (ns) | nd (ns) | 3.1 (s) | nd (ns) | 9.9 (s) | 132.6 (s) |
| Ethyl propanoate | E2 | nd | nd (ns) | nd (ns) | nd (ns) | nd (ns) | nd (ns) | 1.7 (s) |
| Propyl acetate | E3 | nd | nd (ns) | nd (ns) | nd (ns) | nd (ns) | nd (ns) | 1.3 (s) |
| 2-Methylpropyl acetate | E4 | nd | nd (ns) | nd (ns) | nd (ns) | nd (ns) | nd (ns) | 6.6 (s) |
| Ethyl butyrate | E5 | nd | nd (ns) | nd (ns) | nd (ns) | nd (ns) | 0.2 (s) | 0.7 (s) |
| Methyl caproate | E6 | nd | 0.8 (s) | 0.6 (s) | nd (ns) | 0.8 (s) | 0.8 (s) | nd (ns) |
| Hexyl acetate | E7 | 64.3 | 18.5 (s) | 176.7 (s) | 6.6 (s) | 10.6 (s) | 6 (s) | 2.7 (s) |
| 3-Hexen-1-ol acetate | E8 | 124.2 | 45.8 (s) | 37.8 (s) | 27.4 (s) | 20.1 (s) | 12.3 (s) | 12.5 (s) |
| 2-Hexen-1-ol acetate | E9 | 66.8 | 28.1 (s) | 173.1 (s) | 30.5 (s) | 24.5 (s) | 21.5 (s) | 29.6 (s) |
| Ethyl octanoate | E10 | 3.9 | nd (s) | nd (s) | 1.9 (s) | nd (s) | 2.6 (s) | 4.4 (ns) |
| cis-3-Hexenyl isobutyrate | E11 | 1.1 | nd (s) | nd (s) | nd (s) | nd (s) | nd (s) | nd (s) |
| 2-Hexenyl butanoate | E12 | 2.4 | 0.5 (s) | 1.6 (s) | nd (s) | 1.2 (s) | 1.0 (s) | nd (s) |
| Hexyl caproate | E13 | 1.7 | nd (s) | nd (s) | nd (s) | nd (s) | nd (s) | nd (s) |
| cis-3-Hexenyl hexanoate | E14 | 0.7 | nd (s) | nd (s) | nd (s) | nd (s) | nd (s) | nd (s) |
| cis-2-Hexenyl hexanoate | E15 | 1.5 | nd (s) | nd (s) | nd (s) | nd (s) | nd (s) | nd (s) |
| 3-Pentanone | K1 | 7.7 | 2.7 (s) | 2.5 (s) | 4.4 (s) | 3.1 (s) | 2.4 (s) | 5.6 (s) |
| 1-Penten-3-one | K2 | 1.0 | 1.6 (ns) | 1.1 (ns) | 1.7 (s) | 2.1 (s) | 0.9 (ns) | 2.5 (s) |
| 3-Octanone | K3 | 5.3 | 6.6 (s) | 6.6 (s) | 6.5 (s) | 6.6 (s) | 7.4 (s) | 8.0 (s) |
| 1-Octen-3-one | K4 | nd | 0.8 (s) | nd (ns) | nd (ns) | 0.8 (s) | nd (ns) | nd (ns) |
| Hexanal | Ald1 | 75.8 | 46.1 (s) | 29.5 (s) | 61.4 (s) | 63.1 (s) | 26.3 (s) | 44.3 (s) |
| 3-Hexenal | Ald2 | 3.0 | 3.6 (ns) | 2.5 (ns) | 4.7 (s) | 3.5 (ns) | 3 (ns) | 6.1 (s) |
| Heptanal | Ald3 | nd | nd (ns) | nd (ns) | 1.1 (s) | nd (ns) | nd (ns) | 1.4 (s) |
| 2-Hexenal | Ald4 | 156.7 | 325.3 (s) | 235.1 (s) | 352.1 (s) | 459.1 (s) | 259.3 (s) | 626.2 (s) |
| Octanal | Ald5 | 3.4 | 1.4 (s) | nd (s) | 1.7 (s) | nd (s) | nd (s) | nd (s) |
| trans-2-Heptenal | Ald6 | 4.5 | 2.4 (s) | nd (s) | nd (s) | 2.5 (s) | nd (s) | 3.1 (s) |
| 2-Octenal | Ald7 | nd | 1.4 (s) | nd (ns) | nd (ns) | 1.7 (s) | nd (ns) | nd (ns) |
| Decanal | Ald8 | nd | nd (ns) | nd (ns) | 0.8 (s) | nd (ns) | nd (ns) | 1.6 (s) |
| Benzaldehyde | Ald9 | nd | 0.6 (s) | 1.4 (s) | 1.2 (s) | 1.5 (s) | 0.7 (s) | 3.4 (s) |
| 1-Penten-3-ol | Al1 | 16.8 | 5.7 (s) | 5.8 (s) | 9.2 (s) | 5.4 (s) | 5.3 (s) | 9.0 (s) |
| cis-2-Penten-1-ol | Al2 | nd | nd (ns) | 3.9 (s) | 4.8 (s) | 4.1 (s) | 2.6 (s) | 4.4 (s) |
| 1-Hexanol | Al3 | 353.0 | 110 (s) | 155 (s) | 57.4 (s) | 128.1 (s) | 41.5 (s) | 71.6 (s) |
| cis3-Hexenol | Al4 | 11.4 | 3.6 (s) | 5.9 (s) | 4.9 (s) | 4.4 (s) | 2.6 (s) | 6.4 (s) |
| trans-3-Hexenol | Al5 | 153.3 | 50.7 (s) | 14.2 (s) | 24.4 (s) | 32.5 (s) | 13.5 (s) | 34.1 (s) |
| 2-Hexen-1-ol | Al6 | 335.7 | 257.8 (s) | 230.7 (s) | 207.6 (s) | 251.4 (s) | 122.4 (s) | 299.5 (s) |
| 1-Octen-3-ol | Al7 | 1.7 | 1.1 (s) | 0.9 (s) | 0.9 (s) | 1.0 (s) | 0.6 (s) | 14.6 (s) |
| 2-Ethylhexanol | Al8 | nd | nd (ns) | 1.2 (s) | 1.2 (s) | nd (ns) | nd (ns) | 1.7 (s) |
| 1-Octanol | Al9 | 1.1 | 0.4 (s) | 0.3 (s) | 0.5 (s) | 0.1 (s) | 0.3 (s) | nd (s) |
| 2-Furanmethanol | Al10 | nd | nd (ns) | nd (ns) | nd (ns) | 0.1 (s) | nd (ns) | nd (ns) |
| Limonene | T1 | 1.8 | nd (s) | nd (s) | nd (s) | nd (s) | nd (s) | nd (s) |
| Linalool | T2 | 1.9 | 0.5 (s) | nd (s) | nd (s) | 0.3 (s) | nd (s) | nd (s) |
| α-Farnesene | T3 | 1.5 | 0.3 (s) | 0.3 (s) | nd (s) | 0.3 (s) | 0.2 (s) | nd (s) |
| Pentanoic acid | Ac1 | nd | 0.7 (ns) | 0.7 (ns) | 0.5 (ns) | 0.6 (ns) | 0.9 (ns) | 2.5 (ns) |
| Hexanoic acid | Ac2 | nd | nd (ns) | nd (ns) | nd (ns) | 0.2 (s) | nd (ns) | nd (ns) |
| 2-Ethylfuran | O1 | nd | 0.2 (s) | nd (ns) | 0.4 (s) | 0.1 (s) | nd (ns) | nd (ns) |
| Heptadecane | O2 | nd | nd (ns) | 0.1 (ns) | 0.2 (s) | 0.2 (s) | 0.2 (s) | nd (ns) |
| γ-Caprolactone | O3 | nd | 0.8 (s) | 0.9 (s) | 1.7 (s) | 0.8 (s) | 1.1 (s) | 4.4 (s) |
| Phenolic Compounds | Code | FRESH | 4 °C | 8 °C | ||||
|---|---|---|---|---|---|---|---|---|
| 3 d | 7 d | 10 d | 3 d | 7 d | 10 d | |||
| Neochlorogenic acid | P1 | 12.7 | 10.6 (ns) | 33.7 (s) | 13.1 (ns) | 23.5 (s) | 17.9 (s) | 20.8 (s) |
| Cryptochlorogenic acid | P2 | 8.8 | 9.3 (ns) | 7.9 (ns) | 10.4 (ns) | 5.0 (s) | 10.9 (s) | 7.8 (ns) |
| Chlorogenic acid | P3 | 33.3 | 37.5 (s) | 49.9 (s) | 24.4 (s) | 41.2 (s) | 26.3 (s) | 35.5 (ns) |
| Cyanidin-3-O-glucoside | P4 | 45.6 | 22.8 (s) | 41.3 (s) | 22.1 (s) | 38.2 (s) | 17.2 (s) | 21.8 (s) |
| Rutin | P5 | 25.6 | 31.9 (s) | 22.2 (s) | 30.9 (s) | 16.8 (s) | 26.8 (ns) | 19.3 (s) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palumbo, M.; Cefola, M.; Pace, B.; Ricci, I.; Siano, F.; Amato, G.; Stocchero, M.; Cozzolino, R. Volatile Metabolites to Assess the Onset of Chilling Injury in Fresh-Cut Nectarines. Foods 2024, 13, 1047. https://doi.org/10.3390/foods13071047
Palumbo M, Cefola M, Pace B, Ricci I, Siano F, Amato G, Stocchero M, Cozzolino R. Volatile Metabolites to Assess the Onset of Chilling Injury in Fresh-Cut Nectarines. Foods. 2024; 13(7):1047. https://doi.org/10.3390/foods13071047
Chicago/Turabian StylePalumbo, Michela, Maria Cefola, Bernardo Pace, Ilde Ricci, Francesco Siano, Giuseppe Amato, Matteo Stocchero, and Rosaria Cozzolino. 2024. "Volatile Metabolites to Assess the Onset of Chilling Injury in Fresh-Cut Nectarines" Foods 13, no. 7: 1047. https://doi.org/10.3390/foods13071047
APA StylePalumbo, M., Cefola, M., Pace, B., Ricci, I., Siano, F., Amato, G., Stocchero, M., & Cozzolino, R. (2024). Volatile Metabolites to Assess the Onset of Chilling Injury in Fresh-Cut Nectarines. Foods, 13(7), 1047. https://doi.org/10.3390/foods13071047










