The Browning Properties, Antioxidant Activity, and α-Glucosidase Inhibitory Improvement of Aged Oranges (Citrus sinensis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Black Oranges
2.3. Quality Characteristics of Black Oranges
2.3.1. Appearance
2.3.2. Color Analysis
2.3.3. Water Activity (Aw)
2.3.4. Total Weight and Weight Loss Ratio
2.3.5. Scanning Electron Microscope (SEM)
2.3.6. Fourier-Transform Infrared Spectroscopy (FTIR)
2.4. Physical Characteristics of Black Oranges
2.4.1. Browning Degree (A420)
2.4.2. pH
2.4.3. Total Soluble Solids (TSS)
2.5. Chemical Composition Analysis of Black Oranges
2.5.1. Total Reducing Sugars (TRS) and Sugar Analysis
2.5.2. Polyphenols Analysis
2.5.3. Ascorbic Acid Analysis
2.5.4. 5-Hydroxymethylfurfural Analysis
2.6. Functionality and Antioxidant Activity of Black Oranges
2.6.1. Total Phenol Content (TPC) Determination
2.6.2. Total Flavonoid Content (TFC) Determination
2.6.3. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical-Scavenging Capacity
2.6.4. Ferric-Reducing Antioxidant Power (FRAP)
2.7. In Vitro α-Glucosidase Inhibition Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Appearance and Color of Black Oranges
3.2. Scanning Electron Microscope of Black Oranges
3.3. FTIR Spectrum of Black Oranges
3.4. Physical Characteristics of Black Oranges
3.5. Chemical Composition of Black Orange
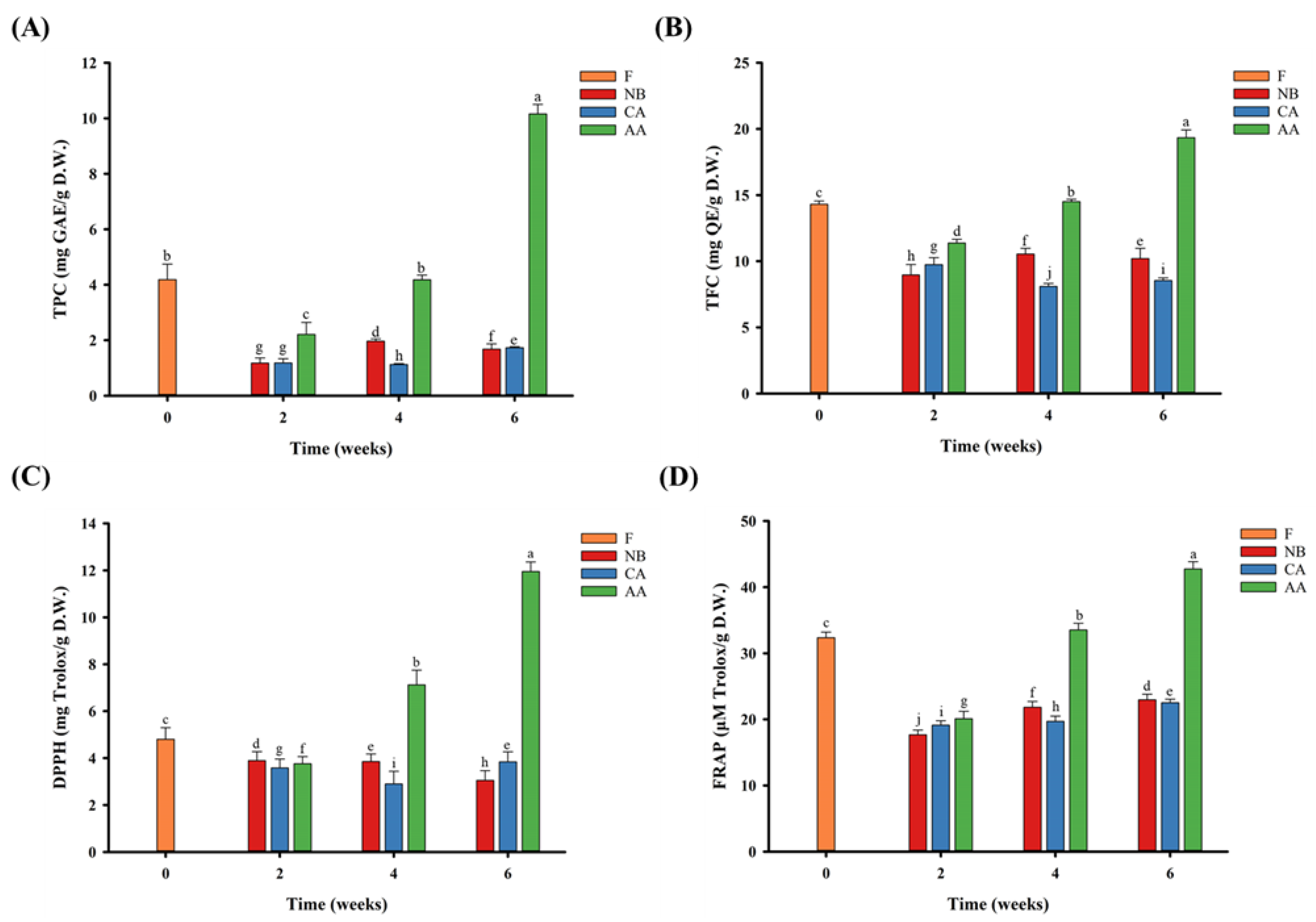
3.6. Functionality and Antioxidant Activity of Black Oranges
3.7. In Vitro α-Glucosidase Inhibitory Potential of Black Oranges
3.8. Principal Component Analysis of Black Oranges
3.9. Storage test of Black Oranges
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, M.L.; Yang, D.J.; Liu, S.C. Effects of drying temperature on the flavonoid, phenolic acid and antioxidative capacities of the methanol extract of citrus fruit (Citrus sinensis (L.) Osbeck) peels. Int. J. Food Sci. Technol. 2011, 46, 1179–1185. [Google Scholar] [CrossRef]
- Benavente-Garcia, O.; Castillo, J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Yi, L.; Ma, S.; Ren, D. Phytochemistry and bioactivity of Citrus flavonoids: A focus on antioxidant, anti-inflammatory, anticancer and cardiovascular protection activities. Phytochem. Rev. 2017, 16, 479–511. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-de-Montellano, C.G.S.; Samani, P.; van der Meer, Y. How can the circular economy support the advancement of the Sustainable Development Goals (SDGs)? A comprehensive analysis. Sustain. Prod. Consump. 2023, 40, 352–362. [Google Scholar] [CrossRef]
- Camargo, D.A.; Pereira, M.S.; dos Santos, A.G.; Fleuri, L.F. Isolated and fermented orange and grape wastes: Bromatological characterization and hytase, lipase and protease source. Innov. Food Sci. Emerg. Technol. 2022, 77, 102978. [Google Scholar] [CrossRef]
- Badaoui, O.; Djebli, A.; Hanini, S. Solar drying of apple and orange waste: Evaluation of a new thermodynamic approach, and characterization analysis. Renew. Energy 2022, 199, 1593–1605. [Google Scholar] [CrossRef]
- Maia, G.D.; Horta, A.C.; Felizardo, M.P. From the conventional to the intermittent biodrying of orange solid waste biomass. Chem. Eng. Process. 2023, 188, 109361. [Google Scholar] [CrossRef]
- Rannou, C.; Laroque, D.; Renault, E.; Prost, C.; Sérot, T. Mitigation strategies of acrylamide, furans, heterocyclic amines and browning during the Maillard reaction in foods. Food Res. Int. 2016, 90, 154–176. [Google Scholar] [CrossRef]
- Nooshkam, M.; Varidi, M.; Bashash, M. The Maillard reaction products as food-born antioxidant and antibrowning agents in model and real food systems. Food Chem. 2019, 275, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Y.; Yu, A.N.; Wang, K. Effects of reaction parameters on self-degradation of L-ascorbic acid and self-degradation kinetics. Food Sci. Biotechnol. 2016, 25, 97–104. [Google Scholar] [CrossRef]
- Sun, Y.E.; Wang, W. Changes in nutritional and bio-functional compounds and antioxidant capacity during black garlic processing. J. Food Sci. Technol. 2018, 55, 479–488. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Wang, W.; Huang, Z.; Wang, J.; Li, X.; Sun, S. Structural characterisation and antioxidant activity of melanoidins from high-temperature fermented apple. Int. J. Food Sci. Technol. 2021, 56, 2471–2480. [Google Scholar] [CrossRef]
- Choi, I.S.; Cha, H.S.; Lee, Y.S. Physicochemical and antioxidant properties of black garlic. Molecules 2014, 19, 16811–16823. [Google Scholar] [CrossRef]
- González-Ramírez, P.J.; Pascual-Mathey, L.I.; García-Rodríguez, R.V.; Jiménez, M.; Beristain, C.I.; Sanchez-Medina, A.; Pascual-Pineda, L.A. Effect of relative humidity on the metabolite profiles, antioxidant activity and sensory acceptance of black garlic processing. Food Biosci. 2022, 48, 101827. [Google Scholar] [CrossRef]
- Wang, B.; Zhong, Y.; Wang, D.; Meng, F.; Li, Y.; Deng, Y. Formation, Evolution, and Antioxidant Activity of Melanoidins in Black Garlic under Different Storage Conditions. Foods 2023, 12, 3727. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, Y.; Wang, W.; Sun, S.; Wang, J.; Li, X.; Dai, F.; Jiang, Y. Changes in Physicochemical Properties, Volatile Profiles, and Antioxidant Activities of Black Apple during High-Temperature Fermentation Processing. Front. Nutr. 2022, 8, 794231. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.J.; Lin, J.A.; Chen, K.T.; Cheng, K.C.; Hsieh, C.W. Thermal treatment enhances the α-glucosidase inhibitory activity of bitter melon (Momordica charantia) by increasing the free form of phenolic compounds and the contents of Maillard reaction products. J. Food Sci. 2021, 86, 3109–3121. [Google Scholar] [CrossRef]
- Nam, S.H.; Han, Y.S.; Sim, K.H.; Yang, S.O.; Kim, M.H. Changes in the Physicochemical Properties, Antioxidant Activity and Metabolite Analysis of Black Elephant Garlic (Allium ampeloprasum L.) during Aging Period. Foods 2022, 12, 43. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O. Shaddock peels (Citrus maxima) phenolic extracts inhibit α-amylase, α-glucosidase and angiotensin I-converting enzyme activities: A nutraceutical approach to diabetes management. Diabetes Metab. Res. Rev. 2011, 5, 148–152. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Shpiro, F.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase. J. Agric. Food Chem. 2005, 53, 2760–2766. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Xu, Y.; Chen, Y.; Wu, J.; Yu, Y.; Zou, B.; An, K.; Xiao, G. Evaluation of bioactive flavonoids and antioxidant activity in Pericarpium Citri Reticulatae (Citrus reticulata ‘Chachi’) during storage. Food Chem. 2017, 230, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-C.; Kuo, C.-T. Hesperidin, nobiletin, and tangeretin are collectively responsible for the anti-neuroinflammatory capacity of tangerine peel (Citri reticulatae pericarpium). Food Chem. Toxicol. 2014, 71, 176–182. [Google Scholar] [CrossRef]
- Pari, L.; Karthikeyan, A.; Karthika, P.; Rathinam, A. Protective effects of hesperidin on oxidative stress, dyslipidaemia and histological changes in iron-induced hepatic and renal toxicity in rats. Toxicol. Rep. 2015, 2, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Chandan, A.; John, M.; Potdar, V. Achieving UN SDGs in Food Supply Chain Using Blockchain Technology. Sustainability 2023, 15, 2109. [Google Scholar] [CrossRef]
- Lanthong, P.; Nuisin, R.; Kiatkamjornwong, S. Graft copolymerization, characterization, and degradation of cassava starch-g-acrylamide/itaconic acid superabsorbents. Carbohydr. Polym. 2006, 66, 229–245. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, G.; Zhao, C.; Han, Y.; DiMarco-Crook, C.; Lu, C.; Bao, Y.; Li, C.; Xiao, H.; Zheng, J. Characterization of polymethoxyflavone demethylation during drying processes of citrus peels. Food Funct. 2019, 10, 5707–5717. [Google Scholar] [CrossRef]
- Scherer, R.; Rybka, A.C.P.; Ballus, C.A.; Meinhart, A.D.; Teixeira Filho, J.; Godoy, H.T. Validation of a HPLC method for simultaneous determination of main organic acids in fruits and juices. Food Chem. 2012, 135, 150–154. [Google Scholar] [CrossRef]
- Lee, C.H.; Chen, Y.T.; Hsieh, H.J.; Chen, K.T.; Chen, Y.A.; Wu, J.T.; Tsai, M.S.; Lin, J.A.; Hsieh, C.W. Exploring epigallocatechin gallate impregnation to inhibit 5-hydroxymethylfurfural formation and the effect on antioxidant ability of black garlic. LWT-Food Sci. Technol. 2020, 117, 108628. [Google Scholar] [CrossRef]
- Magro, A.E.A.; de Castro, R.J.S. Effects of solid-state fermentation and extraction solvents on the antioxidant properties of lentils. Biocatal. Agric. Biotechnol. 2020, 28, 101753. [Google Scholar] [CrossRef]
- Fakhri, L.A.; Ghanbarzadeh, B.; Falcone, P.M. New Healthy Low-Sugar and Carotenoid-Enriched/High-Antioxidant Beverage: Study of Optimization and Physicochemical Properties. Foods 2023, 12, 3265. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Lin, C.; Chen, M.H.; Chiang, P.Y. Stability and quality of anthocyanin in purple sweet potato extracts. Foods 2019, 8, 393. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, G.; Mo, L.; Li, M.; Luo, J.; Shen, Q.; Quan, W. Citrus Peel Extracts: Effective Inhibitors of Heterocyclic Amines and Advanced Glycation End Products in Grilled Pork Meat Patties. Foods 2024, 13, 114. [Google Scholar] [CrossRef]
- Saleh, M.; Amro, L.; Barakat, H.; Baker, R.; Reyash, A.A.; Amro, R.; Qasem, J. Fruit by-product processing and bioactive compounds. J. Food Qual. 2021, 2021, 5513358. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Lu, X.; Liu, P.; Qiao, X. Effects of temperature on the quality of black garlic. J. Sci. Food Agric. 2016, 6, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.; Barbero-Barrera, M.D.M.; Cascone, S.M. Thermal, Physical and Mechanical Performance of Orange Peel Boards: A New Recycled Material for Building Application. Sustainability 2021, 13, 7945. [Google Scholar] [CrossRef]
- Michael-Igolima, U.; Abbey, S.J.; Ifelebuegu, A.O.; Eyo, E.U. Modified Orange Peel Waste as a Sustainable Material for Adsorption of Contaminants. Materials 2023, 16, 1092. [Google Scholar] [CrossRef]
- Mengstie, M.A.; Habtu, N.G. Synthesis and characterization of 5-hydroxymethylfurfural from corncob using solid sulfonated carbon catalyst. Int. J. Chem. Eng. 2020, 2020, 8886361. [Google Scholar] [CrossRef]
- Lopez-Velazquez, M.A.; Santes, V.; Balmaseda, J.; Torres-Garcia, E. Pyrolysis of orange waste: A thermo-kinetic study. J. Anal. Appl. Pyrolysis. 2013, 99, 170–177. [Google Scholar] [CrossRef]
- Chauhan, R.; Kinney, K.; Akalkotkar, A.; Nunn, B.M.; Keynton, R.S.; Soucy, P.A.; O’Toole, M.G. Radiation-induced curcumin release from curcumin–chitosan polymer films. RSC Adv. 2020, 10, 16110–16117. [Google Scholar] [CrossRef] [PubMed]
- Pittia, P.; Antonello, P. Safety by control of water activity: Drying, smoking, and salt or sugar addition. In Regulating Safety of Traditional and Ethnic Foods; Prakash, V., Martín-Belloso, O., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 1, pp. 7–28. ISBN 978-0-12-800605-4. [Google Scholar]
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. An analysis of the changes on intermediate products during the thermal processing of black garlic. Food Chem. 2018, 39, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Garau, M.C.; Simal, S.; Rossello, C.; Femenia, A. Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chem. 2007, 104, 1014–1024. [Google Scholar] [CrossRef]
- Santos, K.L.; Bragança, V.A.; Pacheco, L.V.; Ota, S.S.; Aguiar, C.P.; Borges, R.S. Essential features for antioxidant capacity of ascorbic acid (vitamin C). J. Mol. Model. 2022, 28, 1. [Google Scholar] [CrossRef]
- Burdurlu, H.S.; Koca, N.; Karadeniz, F. Degradation of vitamin C in citrus juice concentrates during storage. J. Food Eng. 2006, 74, 211–216. [Google Scholar] [CrossRef]
- Chang, W.C.; Lin, W.C.; Wu, S.C. Optimization of the Black Garlic Processing Method and Development of Black Garlic Jam Using High-Pressure Processing. Foods 2023, 12, 1584. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, S.; Lukasiewicz, M.; Duda-Chodak, A.; Ziec, G. 5-Hydroxymethyl-2-furfural (HMF)–heat-induced formation, occurrence in food and biotransformation-a review. Pol. J. Food Nutr. Sci. 2013, 63, 207–225. [Google Scholar] [CrossRef]
- Sasaki, M.; Manalu, H.T.; Kamogawa, R.; Issasi, C.S.C.; Quitain, A.T.; Kida, T. Fast and selective production of quercetin and saccharides from rutin using microwave-assisted hydrothermal treatment in the presence of graphene oxide. Food Chem. 2023, 405, 134808. [Google Scholar] [CrossRef] [PubMed]
- Mohdaly, A.A.A.; Roby, M.H.; Sultan, S.A.R.; Groß, E.; Smetanska, I. Potential of low cost agro-industrial wastes as a natural antioxidant on carcinogenic acrylamide formation in potato fried chips. Molecules 2022, 27, 7516. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89. [Google Scholar] [CrossRef]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant activity of Citrus fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Assefa, S.T.; Yang, E.Y.; Chae, S.Y.; Song, M.; Lee, J.; Cho, M.C.; Jang, S. Alpha glucosidase inhibitory activities of plants with focus on common vegetables. Plants 2019, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Beattie, J.R.; Esmonde-White, F.W. Exploration of principal component analysis: Deriving principal component analysis visually using spectra. Appl. Spectrosc. 2021, 75, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Li, L.; Xue, S.; Yang, D.; Wang, S. Stability of flavonoid, carotenoid, soluble sugar and vitamin C in ‘Cara Cara’juice during storage. Foods 2019, 8, 417. [Google Scholar] [CrossRef]
- Yang, Z.S.; Song, H.Y.; Yang, K.M.; Chiang, P.Y. The physicochemical properties and the release of sodium caseinate/polysaccharide gum chlorophyll multiple-layer particles by rotary side-spray fluid bed technology. Food Chem. 2022, 394, 133442. [Google Scholar] [CrossRef]





| Group | Blanching | Hot Air Drying | Aging |
|---|---|---|---|
| Condition | 95 ± 2 °C, 5 min | 55 °C, 1 day | 55 °C, 75% RH, 6 weeks |
| F | – | – | – |
| NB | – | – | ● |
| CA | ● | – | ● |
| AA | – | ● | ● |
| Week | Group | L* | a* | b* | ΔE | A420 |
|---|---|---|---|---|---|---|
| 0 | F | 60.37 ± 0.01 a | 22.67 ± 0.02 a | 37.03 ± 0.01 a | – | 0.56 ± 0.01 d |
| 2 | NB | 38.85 ± 0.01 b | 11.63 ± 0.05 b | 11.60 ± 0.02 c | 36.46 ± 0.02 h | 0.53 ± 0.01 e |
| CA | 36.65 ± 0.01 c | 6.30 ± 0.03 d | 14.30 ± 0.01 b | 29.79 ± 0.02 i | 0.43 ± 0.00 g | |
| AA | 23.68 ± 0.02 g | 4.98 ± 0.04 f | 5.62 ± 0.01 g | 51.37 ± 0.04 e | 0.38 ± 0.00 h | |
| 4 | NB | 30.79 ± 0.03 d | 9.36 ± 0.04 c | 11.35 ± 0.02 d | 46.89 ± 0.01 f | 0.45 ± 0.01 f |
| CA | 25.78 ± 0.01 f | 4.90 ± 0.00 g | 9.35 ± 0.00 e | 40.90 ± 0.01 g | 0.38 ± 0.01 h | |
| AA | 16.58 ± 0.00 h | 0.75 ± 0.06 i | 0.82 ± 0.02 i | 60.83 ± 0.03 b | 1.39 ± 0.03 b | |
| 6 | NB | 26.85 ± 0.05 e | 5.57 ± 0.01 e | 7.57 ± 0.01 f | 53.05 ± 0.02 c | 0.54 ± 0.02 e |
| CA | 14.39 ± 0.01 j | 3.22 ± 0.01 h | 4.38 ± 0.02 h | 52.89 ± 0.03 d | 0.61 ± 0.01 c | |
| AA | 15.01 ± 0.02 i | 0.28 ± 0.00 j | 0.80 ± 0.02 j | 62.11 ± 0.01 a | 3.44 ± 0.07 a |
| Week | Group | Aw | Total Weight (g) | Weight Loss Ratio (%) | pH | TSS (°Brix) | TRS (mg/g D.W.) |
|---|---|---|---|---|---|---|---|
| 0 | F | 0.97 ± 0.01 a | 544.21 ± 0.00 a | 0.00 ± 0.00 j | 4.73 ± 0.01 a | 2.62 ± 0.00 g | 278.28 ± 0.82 j |
| 2 | NB | 0.96 ± 0.00 ab | 415.14 ± 0.00 d | 23.72 ± 0.00 g | 4.08 ± 0.00 b | 2.99 ± 0.01 f | 473.95 ± 3.25 h |
| CA | 0.96 ± 0.01 ab | 450.75 ± 0.00 b | 17.17 ± 0.00 i | 4.07 ± 0.00 b | 3.11 ± 0.00 d | 460.74 ± 2.61 i | |
| AA | 0.95 ± 0.00 bc | 267.15 ± 0.00 h | 50.91 ± 0.00 c | 3.88 ± 0.00 c | 3.05 ± 0.00 e | 524.04 ± 2.45 e | |
| 4 | NB | 0.95 ± 0.01 bc | 409.34 ± 0.00 e | 24.78 ± 0.00 f | 3.85 ± 0.00 d | 3.14 ± 0.01 c | 545.86 ± 4.84 d |
| CA | 0.94 ± 0.01 c | 442.54 ± 0.01 c | 18.68 ± 0.01 h | 3.89 ± 0.00 c | 3.21 ± 0.01 b | 555.34 ± 5.63 b | |
| AA | 0.71 ± 0.00 d | 114.61 ± 0.01 i | 78.94 ± 0.01 b | 3.74 ± 0.00 g | 3.24 ± 0.01 a | 517.58 ± 4.47 f | |
| 6 | NB | 0.96 ± 0.00 ab | 355.31 ± 0.01 f | 34.71 ± 0.02 e | 3.78 ± 0.01 f | 3.15 ± 0.00 c | 563.47 ± 4.04 a |
| CA | 0.96 ±0.00 ab | 348.27 ± 0.00 g | 36.00 ± 0.01 d | 3.80 ± 0.01 e | 3.10 ± 0.01 d | 547.45 ± 3.73 c | |
| AA | 0.54 ± 0.01 e | 100.51 ± 0.01 j | 81.53 ± 0.01 a | 3.69 ± 0.02 h | 3.14 ± 0.01 c | 477.94 ± 3.41 g |
| Week | Group | Hesperidin (mg/g D.W.) | Nobiletin (mg/g D.W.) | Ascorbic Acid (mg/g D.W.) | Sucrose (mg/g D.W.) | Glucose (mg/g D.W.) | Fructose (mg/g D.W.) | 5-HMF (mg/g D.W.) |
|---|---|---|---|---|---|---|---|---|
| 0 | F | 114.34 ± 0.13 a | 0.47 ± 0.02 a | 0.66 ± 0.01 a | 96.96 ± 0.14 a | 91.44 ± 0.10 j | 106.07 ± 0.23 j | – |
| 2 | NB | 47.53 ± 0.13 f | 0.30 ± 0.00 e | 0.24 ± 0.02 h | 64.41 ± 0.11 b | 153.81 ± 0.14 g | 199.86 ± 0.14 h | – |
| CA | 45.81 ± 0.08 g | 0.31 ± 0.01 e | 0.24 ± 0.00 h | 60.12 ± 0.07 c | 154.57 ± 0.09 f | 205.17 ± 0.09 f | – | |
| AA | 53.90 ± 0.15 b | 0.35 ± 0.02 d | 0.31 ± 0.00 g | 18.66 ± 0.10 d | 167.93 ± 0.12 e | 231.34 ± 0.17 c | – | |
| 4 | NB | 43.16 ± 0.13 i | 0.31 ± 0.01 e | 0.39 ± 0.01 e | 4.68 ± 0.14 f | 177.97 ± 0.10 b | 225.68 ± 0.15 d | – |
| CA | 47.75 ± 0.05 e | 0.26 ± 0.01 g | 0.35 ± 0.02 f | 11.72 ± 0.11 e | 176.51 ± 0.16 c | 235.90 ± 0.23 b | – | |
| AA | 51.06 ± 0.09 c | 0.41 ± 0.00 c | 0.39 ± 0.01 e | 1.66 ± 0.11 i | 149.94 ± 0.08 h | 202.52 ± 0.17 g | 0.02 ± 0.02 b | |
| 6 | NB | 45.48 ± 0.09 h | 0.28 ± 0.00 f | 0.47 ± 0.10 d | 4.67 ± 0.10 f | 168.84 ± 0.10 d | 223.26 ± 0.22 e | – |
| CA | 49.65 ± 0.11 d | 0.28 ± 0.01 f | 0.51 ± 0.02 c | 3.91 ± 0.16 g | 178.26 ± 0.13 a | 237.85 ± 0.19 a | – | |
| AA | 40.10 ±0.01 j | 0.43 ±0.00 b | 0.57 ± 0.07 b | 3.88 ± 0.09 h | 110.92 ± 0.14 i | 169.61 ± 0.24 i | 0.09 ± 0.01 a |
| Day | Environment | Group | Aw | L* | a* | b* | ΔE | Hesperidin (mg/g D.W.) | Nobiletin (mg/g D.W.) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | Control | NB | 25 °C | 0.95 ± 0.01 a | 29.55 ± 0.02 a | 8.22 ± 0.08 a | 7.94 ± 0.06 a | – | 45.12 ± 0.13 a | 0.25 ± 0.02 h |
| AA | 25 °C | 0.45 ± 0.01 e | 23.75 ± 0.01 f | 5.53 ± 0.04 e | 5.03 ± 0.03 e | – | 40.21 ± 0.13 b | 0.41 ± 0.01 a | ||
| 30 | Light | NB | 4 °C | 0.89 ± 0.00 b | 23.70 ± 0.01 f | 6.51 ± 0.05 c | 5.19 ± 0.02 e | 1.89 ± 0.03 k | 30.52 ± 0.09 f | 0.26 ± 0.00 g |
| 25 °C | 0.90 ± 0.01 b | 24.58 ± 0.02 e | 6.89 ± 0.03 b | 6.42 ± 0.01 c | 5.37 ± 0.01 c | 30.14 ± 0.13 f | 0.23 ± 0.02 i | |||
| 55 °C | 0.90 ± 0.00 b | 17.75 ± 0.01 k | 0.71± 0.01 l | 0.42 ± 0.03 k | 13.55 ± 0.02 b | 30.27 ± 0.13 f | 0.25 ± 0.02 h | |||
| AA | 4 °C | 0.42 ± 0.01 f | 20.36 ± 0.01 i | 4.09 ± 0.05 j | 3.22 ± 0.02 h | 2.77 ± 0.02 g | 31.20 ± 0.01 e | 0.39 ± 0.01 b | ||
| 25 °C | 0.41 ± 0.00 f | 22.07 ± 0.01 g | 5.32 ± 0.04 f | 4.45 ± 0.01 f | 1.79 ± 0.01 l | 31.75 ± 0.13 d | 0.37 ± 0.02 c | |||
| 55 °C | 0.36 ± 0.01 h | 23.75 ± 0.01 f | 5.02 ± 0.01 g | 4.72 ± 0.01 f | 2.97 ± 0.02 f | 30.56 ± 0.13 f | 0.36 ± 0.02 c | |||
| Dark | NB | 4 °C | 0.90 ± 0.00 b | 23.94 ± 0.01 e | 6.86 ± 0.04 b | 5.51 ± 0.02 d | 1.75 ± 0.03 l | 32.15 ± 0.09 d | 0.27 ± 0.01 f | |
| 25 °C | 0.93 ± 0.01 b | 25.67 ± 0.02 b | 6.95 ± 0.03 b | 6.84 ± 0.01 b | 5.26 ± 0.01 c | 31.95 ± 0.13 d | 0.26 ± 0.03 g | |||
| 55 °C | 0.92 ± 0.00 b | 18.02 ± 0.01 j | 2.58 ± 0.02 k | 2.71 ± 0.03 i | 13.41 ± 0.03 b | 32.04 ± 0.13 d | 0.26 ± 0.02 g | |||
| AA | 4 °C | 0.44 ± 0.01 e | 20.36 ± 0.02 i | 4.72 ± 0.05 h | 3.58 ± 0.02 h | 2.58 ± 0.02 h | 33.74 ± 0.01 c | 0.40 ± 0.01 b | ||
| 25 °C | 0.42 ± 0.00 f | 22.41 ± 0.01 g | 5.38 ± 0.03 f | 4.65 ± 0.01 f | 1.64 ± 0.01 m | 33.85 ± 0.13 c | 0.39 ± 0.03 b | |||
| 55 °C | 0.38 ± 0.01 g | 23.86 ± 0.02 f | 5.81 ± 0.01 e | 4.92 ± 0.02 f | 2.81 ± 0.02 f | 32.14 ± 0.13 d | 0.37 ± 0.02 c | |||
| 60 | Light | NB | 4 °C | 0.84 ± 0.00 c | 22.73 ± 0.01 g | 5.85 ± 0.01 e | 4.68 ± 0.01 f | 3.16 ± 0.01 e | 27.25 ± 0.09 i | 0.22 ± 0.01 j |
| 25 °C | 0.80 ± 0.01 c | 24.93 ± 0.01 d | 6.90 ± 0.05 b | 6.59 ± 0.01 c | 4.99 ± 0.01 d | 27.38 ± 0.13 i | 0.20 ± 0.01 k | |||
| 55 °C | 0.72 ± 0.01 d | 15.75 ± 0.01 m | 0.32 ± 0.01 l | 0.57 ± 0.01 k | 15.10 ± 0.01 a | 27.24 ± 0.13 i | 0.21 ± 0.02 j | |||
| AA | 4 °C | 0.40 ± 0.01 f | 20.30 ± 0.07 i | 4.09 ± 0.01 j | 3.25 ± 0.02 h | 2.81 ± 0.02 f | 28.18 ± 0.01 h | 0.32 ± 0.00 e | ||
| 25 °C | 0.36 ± 0.00 h | 21.05 ± 0.01 h | 4.42 ± 0.05 i | 3.88 ± 0.02 g | 3.14 ± 0.01 e | 28.27 ± 0.13 h | 0.31 ± 0.01 e | |||
| 55 °C | 0.27 ± 0.01 j | 24.18 ± 0.06 e | 5.69 ± 0.01 e | 5.59 ± 0.01 d | 2.04 ± 0.03 j | 27.15 ± 0.13 i | 0.30 ± 0.01 e | |||
| Dark | NB | 4 °C | 0.86 ± 0.00 c | 22.89 ± 0.01 g | 6.19 ± 0.01 d | 4.94 ± 0.01 f | 3.05 ± 0.01 e | 28.31 ± 0.09 h | 0.25 ± 0.02 h | |
| 25 °C | 0.85 ± 0.01 c | 25.27 ± 0.01 c | 6.92 ± 0.04 b | 6.65 ± 0.01 b | 4.85 ± 0.01 d | 28.41 ± 0.13 h | 0.24 ± 0.00 i | |||
| 55 °C | 0.76 ± 0.01 d | 16.45 ± 0.02 l | 2.17 ± 0.01 k | 1.98 ± 0.02 j | 15.01 ± 0.01 a | 28.15 ± 0.13 h | 0.24 ± 0.02 i | |||
| AA | 4 °C | 0.42 ± 0.01 f | 20.32 ± 0.07 i | 4.27 ± 0.01 i | 3.41 ± 0.02 h | 2.77 ± 0.02 g | 29.34 ± 0.01 g | 0.36 ± 0.01 c | ||
| 25 °C | 0.39 ± 0.00 g | 21.81 ± 0.01 h | 4.94 ± 0.04 g | 4.12 ± 0.02 g | 3.12 ± 0.01 e | 29.51 ± 0.13 g | 0.35 ± 0.02 d | |||
| 55 °C | 0.31 ± 0.01 i | 24.20 ± 0.06 e | 5.72 ± 0.01 e | 5.38 ± 0.02 d | 2.21 ± 0.03 i | 29.24 ± 0.13 g | 0.34 ± 0.02 d | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, T.-Y.; Yang, K.-M.; Chiang, Y.-C.; Lin, L.-Y.; Chiang, P.-Y. The Browning Properties, Antioxidant Activity, and α-Glucosidase Inhibitory Improvement of Aged Oranges (Citrus sinensis). Foods 2024, 13, 1093. https://doi.org/10.3390/foods13071093
Hsu T-Y, Yang K-M, Chiang Y-C, Lin L-Y, Chiang P-Y. The Browning Properties, Antioxidant Activity, and α-Glucosidase Inhibitory Improvement of Aged Oranges (Citrus sinensis). Foods. 2024; 13(7):1093. https://doi.org/10.3390/foods13071093
Chicago/Turabian StyleHsu, Ting-Yu, Kai-Min Yang, Yi-Chan Chiang, Li-Yun Lin, and Po-Yuan Chiang. 2024. "The Browning Properties, Antioxidant Activity, and α-Glucosidase Inhibitory Improvement of Aged Oranges (Citrus sinensis)" Foods 13, no. 7: 1093. https://doi.org/10.3390/foods13071093
APA StyleHsu, T.-Y., Yang, K.-M., Chiang, Y.-C., Lin, L.-Y., & Chiang, P.-Y. (2024). The Browning Properties, Antioxidant Activity, and α-Glucosidase Inhibitory Improvement of Aged Oranges (Citrus sinensis). Foods, 13(7), 1093. https://doi.org/10.3390/foods13071093