High Dietary Folic Acid Supplementation Reduced the Composition of Fatty Acids and Amino Acids in Fortified Eggs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Use
2.2. Experimental Materials
2.3. Birds and Diets
2.4. Sample Collection
2.5. Performance and Egg Quality Measurement
2.6. Determination of Nutrients in the Egg Yolk
2.7. Quantitative PCR Analysis
2.8. Statistical Analysis
3. Results
3.1. Performance and Egg Quality
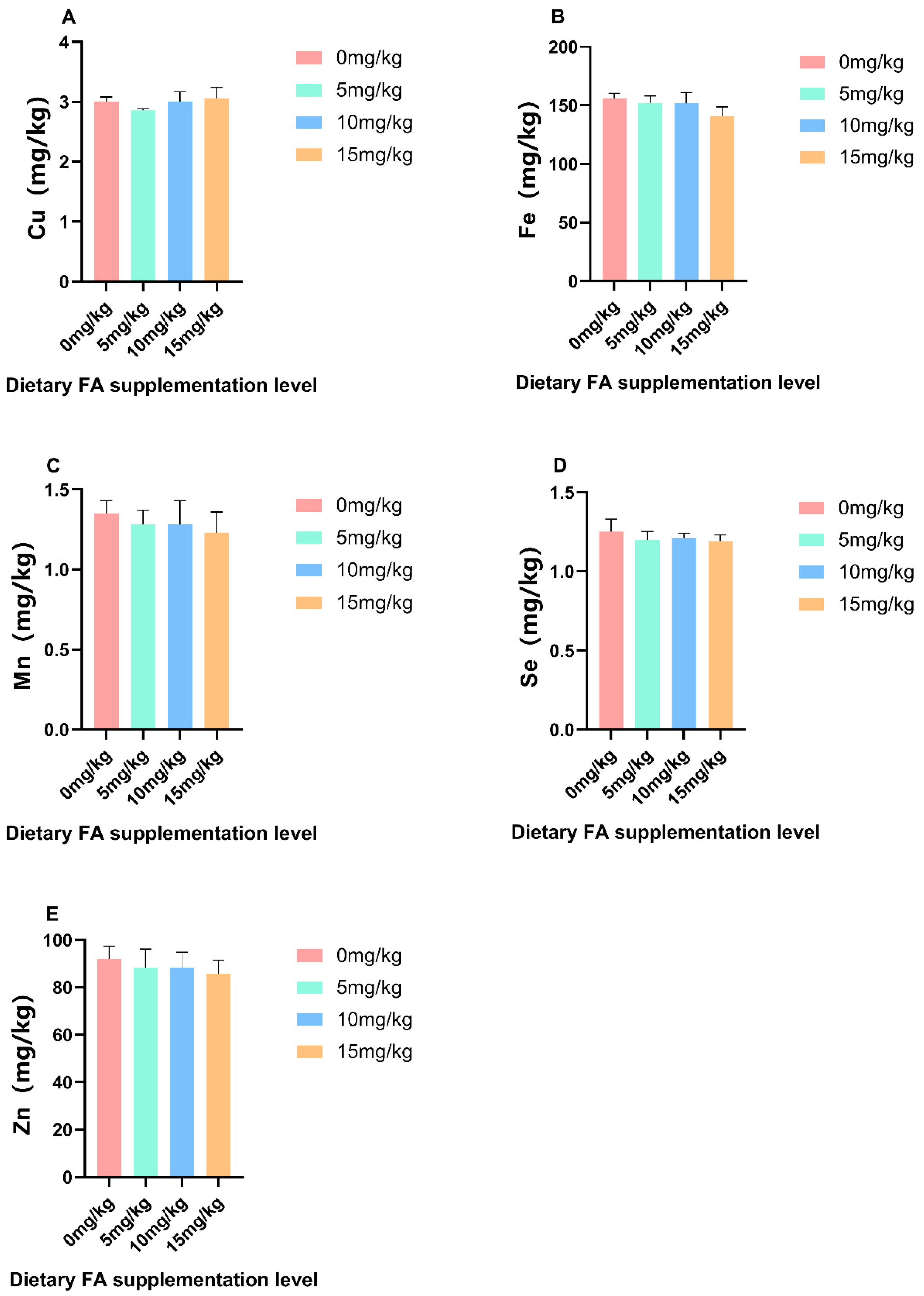
3.2. Nutrients in Egg Yolks
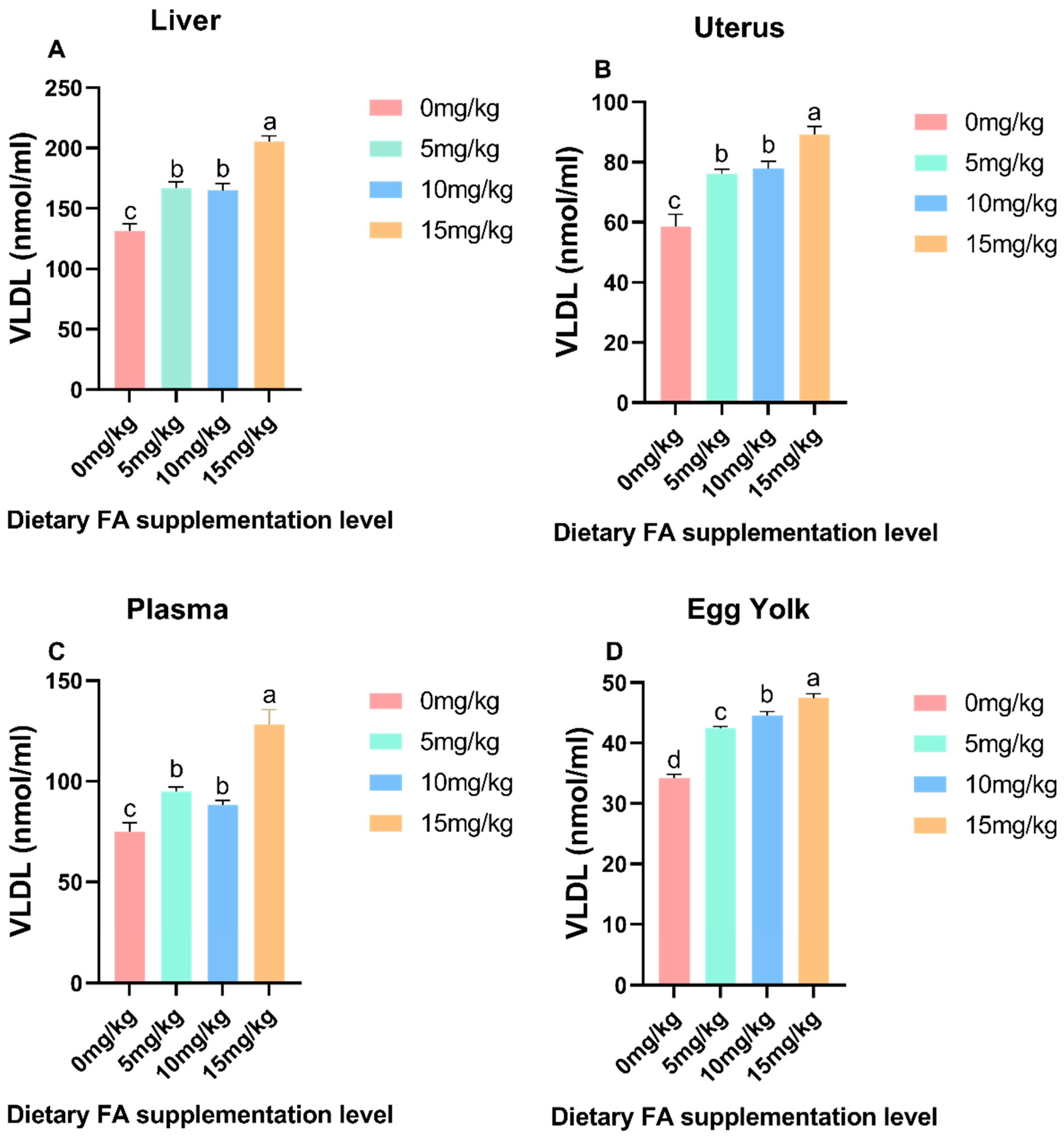
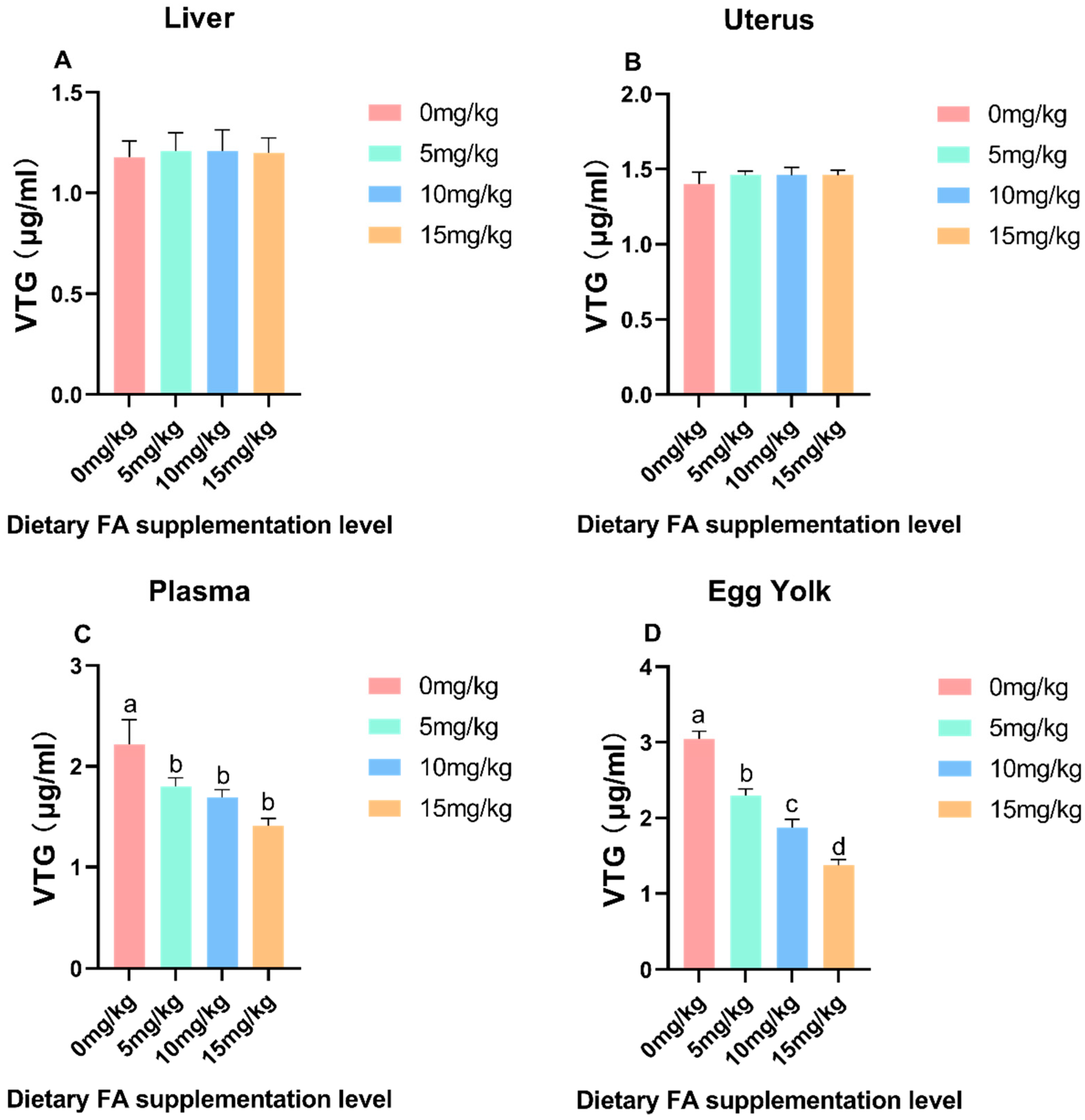
3.3. Determination of VLDL and VTG Content in Egg Yolk, Plasma, Liver, and Ovaries
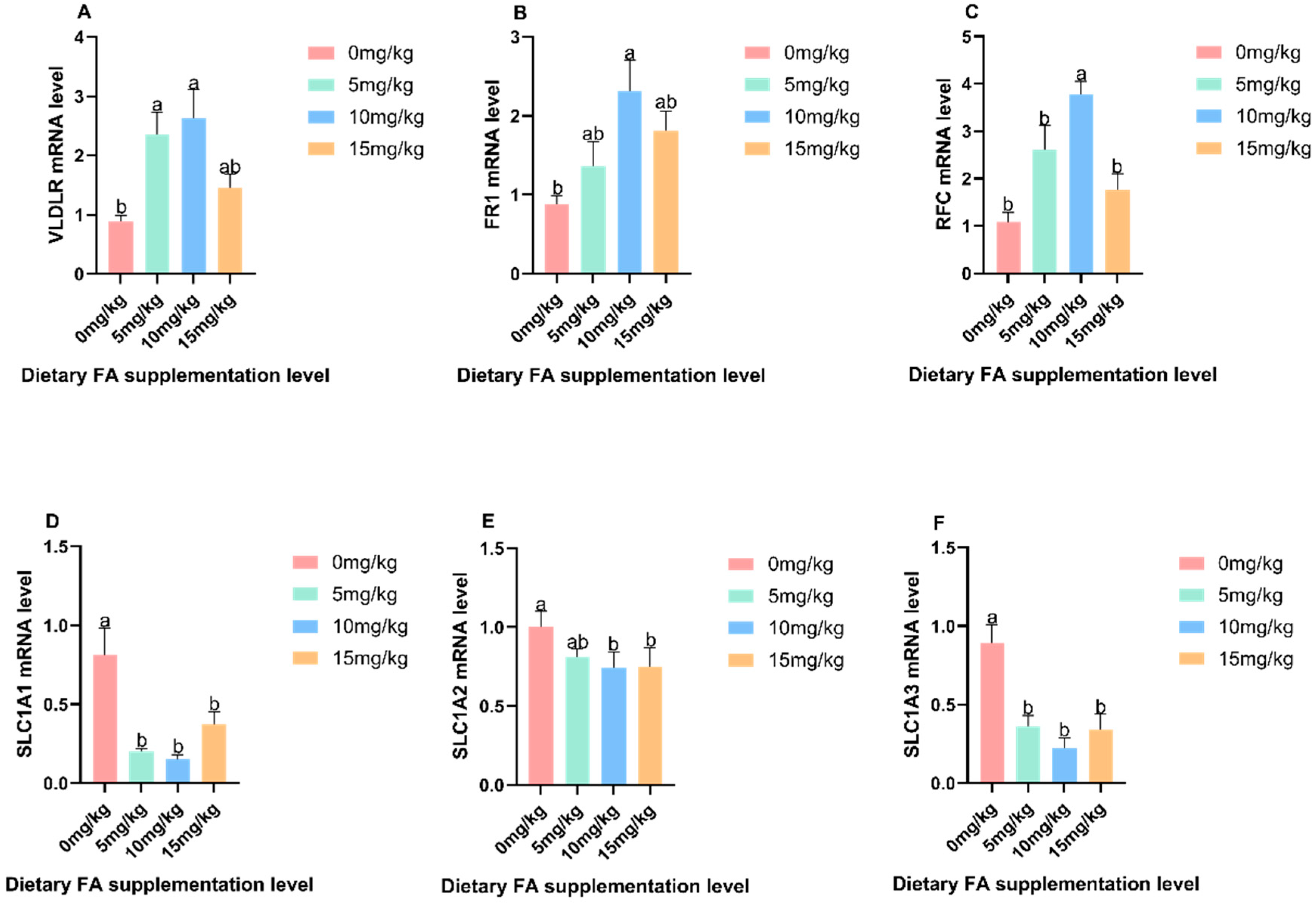
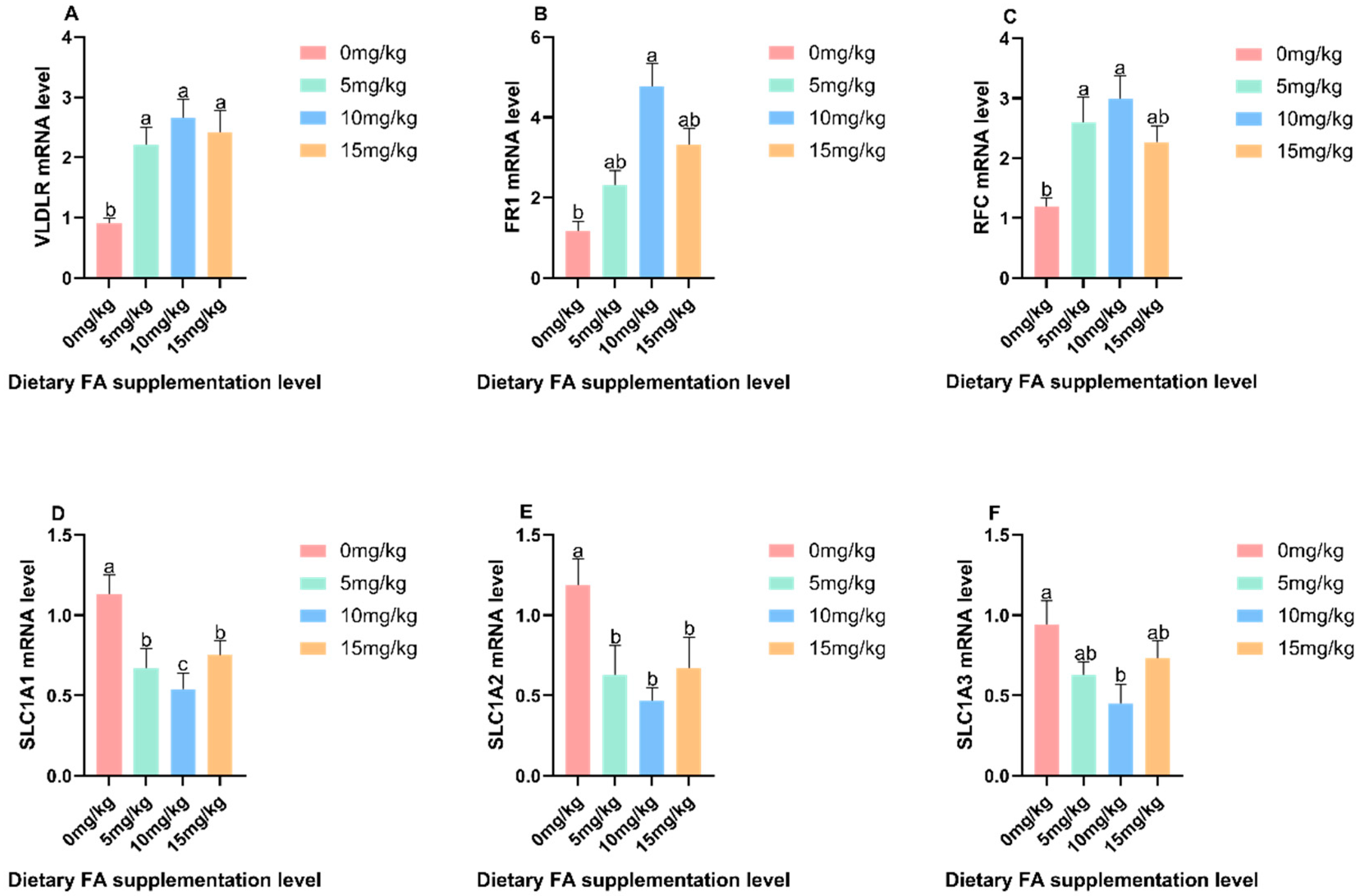
3.4. Mechanism of the Effect of FA on Nutrients in Egg Yolks
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lutter, C.K.; Iannotti, L.L.; Stewart, C.P. The potential of a simple egg to improve maternal and child nutrition. Matern. Child Nutr. 2018, 14, e12678. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.; Malan, L.; Kruger, H.S.; Asare, H.; Visser, M.; Mukwevho, T.; Ricci, C.; Smuts, C.M. Potential of Egg as Complementary Food to Improve Nutrient Intake and Dietary Diversity. Nutrients 2022, 14, 3396. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, L.L.; Lutter, C.K.; Bunn, D.A.; Stewart, C.P. Eggs: The uncracked potential for improving maternal and young child nutrition among the world’s poor. Nutr. Rev. 2014, 72, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Galvis, Y.; Pineda, K.; Zapata, J.; Aristizabal, J.; Estrada, A.; Fernandez, M.L.; Barona-Acevedo, J. Consumption of Eggs Alone or Enriched with Annatto (Bixa orellana L.) Does Not Increase Cardiovascular Risk in Healthy Adults-A Randomized Clinical Trial, the Eggant Study. Nutrients 2023, 15, 369. [Google Scholar] [CrossRef] [PubMed]
- Šušnjara, P.; Kolobarić, N.; Matić, A.; Mihaljević, Z.; Stupin, A.; Marczi, S.; Drenjančević, I. Consumption of Hen Eggs Enriched with n-3 Polyunsaturated Fatty Acids, Selenium, Vitamin E and Lutein Incites Anti-Inflammatory Conditions in Young, Healthy Participants—A Randomized Study. Front. Biosci. (Landmark Ed.) 2022, 27, 332. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.M.; Seburg, S.; Flanagan, N.L. Enriched eggs as a source of N-3 polyunsaturated fatty acids for humans. Poult. Sci. 2000, 79, 971–974. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.; Rollins, M.; Cassidy, T.; Caffrey, A.; Marshall, B.; Dornan, J.; McLaughlin, M.; McNulty, B.A.; Ward, M.; Strain, J.J.; et al. Effect of continued folic acid supplementation beyond the first trimester of pregnancy on cognitive performance in the child: A follow-up study from a randomized controlled trial (FASSTT Offspring Trial). BMC Med. 2019, 17, 196. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, Y.; Wang, D.; Chen, X.; Li, M.; Huang, X.; Jiang, Y.; Dou, Y.; Wang, Y.; Ma, X.; et al. Periconception Red Blood Cell Folate and Offspring Congenital Heart Disease: Nested Case-Control and Mendelian Randomization Studies. Ann. Intern. Med. 2022, 175, 1212–1220. [Google Scholar] [CrossRef]
- Elshahid, A.R.M.; Shahein, I.M.; Mohammed, Y.F.; Ismail, N.F.; Zakarria, H.B.A.E.; GamalEl Din, S.F. Folic acid supplementation improves erectile function in patients with idiopathic vasculogenic erectile dysfunction by lowering peripheral and penile homocysteine plasma levels: A case-control study. Andrology 2020, 8, 148–153. [Google Scholar] [CrossRef]
- Ebara, S. Nutritional role of folate. Congenit. Anom. 2017, 57, 138–141. [Google Scholar] [CrossRef]
- Blount, B.C.; Mack, M.M.; Wehr, C.M.; MacGregor, J.T.; Hiatt, R.A.; Wang, G.; Wickramasinghe, S.N.; Everson, R.B.; Ames, B.N. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: Implications for cancer and neuronal damage. Proc. Natl. Acad. Sci. USA 1997, 94, 3290–3295. [Google Scholar] [CrossRef] [PubMed]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The Concept of Folic Acid in Health and Disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef] [PubMed]
- House, J.D.; Braun, K.; Ballance, D.M.; O’Connor, C.P.; Guenter, W. The enrichment of eggs with folic acid through supplementation of the laying hen diet. Poult. Sci. 2002, 81, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Altic, L.; McNulty, H.; Hoey, L.; McAnena, L.; Pentieva, K. Validation of Folate-Enriched Eggs as a Functional Food for Improving Folate Intake in Consumers. Nutrients 2016, 8, 777. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.A.; Wang, T.; Xin, H.; Dolde, D. Supplementation of laying-hen feed with palm tocos and algae astaxanthin for egg yolk nutrient enrichment. J. Agric. Food Chem. 2012, 60, 1989–1999. [Google Scholar] [CrossRef]
- Leeson, S.; Caston, L. Enrichment of eggs with lutein. Poult. Sci. 2004, 83, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Qu, L.; Ma, M.; Li, Y.F.; Wang, X.G.; Yang, Z.; Wang, K.H. Efficacy evaluation of selenium-enriched yeast in laying hens: Effects on performance, egg quality, organ development, and selenium deposition. Poult. Sci. 2020, 99, 6267–6277. [Google Scholar] [CrossRef] [PubMed]
- GB/T 5916-2020; Formula Feeds for Layers and Broilers. Chinese Standard: Beijing, China, 2021.
- GB 5009.211-2014; Determination of Folic Acid in Food. National Health Commission of China: Beijing, China, 2016.
- Li, J.; Zhou, D.; Li, H.; Luo, Q.; Wang, X.; Qin, J.; Xu, Y.; Lu, Q.; Tian, X. Effect of purple corn extract on performance, antioxidant activity, egg quality, egg amino acid, and fatty acid profiles of laying hen. Front. Vet. Sci. 2022, 9, 1083842. [Google Scholar] [CrossRef]
- Luo, Q.; Li, J.; Li, H.; Zhou, D.; Wang, X.; Tian, Y.; Qin, J.; Tian, X.; Lu, Q. The Effects of Purple Corn Pigment on Growth Performance, Blood Biochemical Indices, Meat Quality, Muscle Amino Acids, and Fatty Acids of Growing Chickens. Foods 2022, 11, 1870. [Google Scholar] [CrossRef]
- Chen, X.; Ma, X.; Yang, C.; Jiang, S.; Huang, L.; Li, Y.; Zhang, F.; Jiao, N.; Yang, W. Low Level of Dietary Organic Trace Elements Improve the Eggshell Strength, Trace Element Utilization, and Intestinal Function in Late-Phase Laying Hens. Front. Vet. Sci. 2022, 9, 903615. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Wang, M.; Chen, L.; Long, C.; Guo, Y.; Sheng, X.; Wang, X.; Xing, K.; Xiao, L.; Ni, H.; et al. Effects of dietary pretreated Chinese herbal medicine supplementation on production performance, egg quality, uterine histopathological changes, and antioxidant capacity in late-phase laying hens. Front. Physiol. 2023, 14, 1110301. [Google Scholar] [CrossRef] [PubMed]
- Bunchasak, C.; Kachana, S. Dietary folate and vitamin B12 supplementation and consequent vitamin deposition in chicken eggs. Trop. Anim. Health Prod. 2009, 41, 1583–1589. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Janmohammadi, H.; Maleki, R.; Ostadrahimi, A.; Kianfar, R. Laying hen performance, egg quality improved and yolk 5-methyltetrahydrofolate content increased by dietary supplementation of folic acid. Anim. Nutr. 2019, 5, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Rohner, F.; Wirth, J.P.; Zeng, W.; Petry, N.; Donkor, W.E.S.; Neufeld, L.M.; Mkambula, P.; Groll, S.; Mbuya, M.N.; Friesen, V.M. Global Coverage of Mandatory Large-Scale Food Fortification Programs: A Systematic Review and Meta-Analysis. Adv. Nutr. 2023, 14, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Keats, E.C.; Neufeld, L.M.; Garrett, G.S.; Mbuya, M.N.N.; Bhutta, Z.A. Improved micronutrient status and health outcomes in low- and middle-income countries following large-scale fortification: Evidence from a systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 109, 1696–1708. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.H.; Nawijn, E.L.; Verkaik-Kloosterman, J. Contribution of voluntary fortified foods to micronutrient intake in The Netherlands. Eur. J. Nutr. 2022, 61, 1649–1663. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, A.; McNulty, H.; Rollins, M.; Prasad, G.; Gaur, P.; Talcott, J.B.; Witton, C.; Cassidy, T.; Marshall, B.; Dornan, J.; et al. Effects of maternal folic acid supplementation during the second and third trimesters of pregnancy on neurocognitive development in the child: An 11-year follow-up from a randomised controlled trial. BMC Med. 2021, 19, 73. [Google Scholar] [CrossRef]
- Engevik, M.A.; Morra, C.N.; Röth, D.; Engevik, K.; Spinler, J.K.; Devaraj, S.; Crawford, S.E.; Estes, M.K.; Kalkum, M.; Versalovic, J. Microbial Metabolic Capacity for Intestinal Folate Production and Modulation of Host Folate Receptors. Front. Microbiol. 2019, 10, 2305. [Google Scholar] [CrossRef]
- Sherwood, T.A.; Alphin, R.L.; Saylor, W.W.; White, H.B.R. Folate metabolism and deposition in eggs by laying hens. Arch. Biochem. Biophys. 1993, 307, 66–72. [Google Scholar] [CrossRef]
- Sabharanjak, S.; Mayor, S. Folate receptor endocytosis and trafficking. Adv. Drug Deliver. Rev. 2004, 56, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Fernandez, C.; Perez-Arnillas, Q.; Ruiz-Echeverria, L.; Rodriguez-Rubi, D.; Sanchez-Lorenzo, L.; Li-Torres, W.; Izquierdo-Manuel, M.; Berros, J.P.; Luque-Cabal, M.; Jimenez-Fonseca, P.; et al. Reduced folate carrier (RFC) as a predictive marker for response to pemetrexed in advanced non-small cell lung cancer (NSCLC). Investig. New Drug. 2014, 32, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Matherly, L.H.; Hou, Z.; Deng, Y. Human reduced folate carrier: Translation of basic biology to cancer etiology and therapy. Cancer Metastasis Rev. 2007, 26, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Saremi, B.; Gilbert, E.R.; Wong, E.A. Physiological and biochemical aspects of methionine isomers and a methionine analogue in broilers. Poult. Sci. 2017, 96, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; Miao, S.; Liu, Y.; Li, H.; Zhou, W.; Wang, X.; Dong, X.; Zou, X. Effects of Dietary Valine Levels on Production Performance, Egg Quality, Antioxidant Capacity, Immunity, and Intestinal Amino Acid Absorption of Laying Hens during the Peak Lay Period. Animals 2021, 11, 1972. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Xu, J.; Tang, S.; An, N.; Jiang, L.; Zhang, Y.; Zhang, S.; Zhang, Q.; Shen, Y.; et al. SLC1A1-mediated cellular and mitochondrial influx of R-2-hydroxyglutarate in vascular endothelial cells promotes tumor angiogenesis in IDH1-mutant solid tumors. Cell Res. 2022, 32, 638–658. [Google Scholar] [CrossRef]
- Tajan, M.; Hock, A.K.; Blagih, J.; Robertson, N.A.; Labuschagne, C.F.; Kruiswijk, F.; Humpton, T.J.; Adams, P.D.; Vousden, K.H. A Role for p53 in the Adaptation to Glutamine Starvation through the Expression of SLC1A3. Cell Metab. 2018, 28, 721–736. [Google Scholar] [CrossRef]
- Poitelon, Y.; Kopec, A.M.; Belin, S. Myelin Fat Facts: An Overview of Lipids and Fatty Acid Metabolism. Cells 2020, 9, 812. [Google Scholar] [CrossRef]
- Custers; Emma, E.M.; Kiliaan; Amanda, J. Dietary lipids from body to brain. Prog. Lipid Res. 2022, 85, 101144. [Google Scholar] [CrossRef]
- Krauss, R.M.; Eckel, R.H.; Howard, B.; Appel, L.J.; Daniels, S.R.; Deckelbaum, R.J.; Erdman, J.J.; Kris-Etherton, P.; Goldberg, I.J.; Kotchen, T.A.; et al. AHA Dietary Guidelines: Revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 2000, 102, 2284–2299. [Google Scholar] [CrossRef]
- Krishnan, S.; Cooper, J.A. Effect of dietary fatty acid composition on substrate utilization and body weight maintenance in humans. Eur. J. Nutr. 2014, 53, 691–710. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, E.; Muszyński, S.; Arczewska-Włosek, A.; Domaradzki, P.; Pyz-Łukasik, R.; Donaldson, J.; Świątkiewicz, S. Cholesterol Content, Fatty Acid Profile and Health Lipid Indices in the Egg Yolk of Eggs from Hens at the End of the Laying Cycle, Following Alpha-Ketoglutarate Supplementation. Foods 2021, 10, 596. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, H.; Li, S.; Jiang, Y.; Kang, L.; Deng, J.; Yang, C.; Zhao, X.; Zhao, J.; Jiang, L.; et al. The effects of fermented feedstuff derived from Citri Sarcodactylis Fructus by-products on growth performance, intestinal digestive enzyme activity, nutrient utilization, meat quality, gut microbiota, and metabolites of broiler chicken. Front. Vet. Sci. 2023, 10, 1231996. [Google Scholar] [CrossRef] [PubMed]
- Yair, R.; Uni, Z. Content and uptake of minerals in the yolk of broiler embryos during incubation and effect of nutrient enrichment. Poult. Sci. 2011, 90, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Charoensiriwatana, W.; Srijantr, P.; Teeyapant, P.; Wongvilairattana, J. Consuming iodine enriched eggs to solve the iodine deficiency endemic for remote areas in Thailand. Nutr. J. 2010, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Bourre, J.M.; Galea, F. An important source of omega-3 fatty acids, vitamins D and E, carotenoids, iodine and selenium: A new natural multi-enriched egg. J. Nutr. Health Aging 2006, 10, 371–376. [Google Scholar] [PubMed]
- Mattioli, S.; Cartoni Mancinelli, A.; Bravi, E.; Angelucci, E.; Falcinelli, B.; Benincasa, P.; Castellini, C.; Sileoni, V.; Marconi, O.; Dal Bosco, A. Dietary Freeze-Dried Flaxseed and Alfalfa Sprouts as Additional Ingredients to Improve the Bioactive Compounds and Reduce the Cholesterol Content of Hen Eggs. Antioxidants 2022, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Walzem, R.L.; Hansen, R.J.; Williams, D.L.; Hamilton, R.L. Estrogen induction of VLDLy assembly in egg-laying hens. J. Nutr. 1999, 129, 467S–472S. [Google Scholar] [CrossRef]
- Vieira, P.M.; Vieira, A.V.; Sanders, E.J.; Steyrer, E.; Nimpf, J.; Schneider, W.J. Chicken yolk contains bona fide high density lipoprotein particles. J. Lipid Res. 1995, 36, 601–610. [Google Scholar] [CrossRef]
- Bujo, H.; Hermann, M.; Kaderli, M.O.; Jacobsen, L.; Sugawara, S.; Nimpf, J.; Yamamoto, T.; Schneider, W.J. Chicken oocyte growth is mediated by an eight ligand binding repeat member of the LDL receptor family. EMBO J. 1994, 13, 5165–5175. [Google Scholar] [CrossRef]
- Schneider, W.J. Lipid transport to avian oocytes and to the developing embryo. J. Biomed. Res. 2016, 30, 174–180. [Google Scholar] [PubMed]


| Ingredients | Content | Nutrient Level | Content |
|---|---|---|---|
| Corn | 63.55 | Metabolizable energy, MJ·kg−1 | 11.15 |
| Soybean meal | 26 | Crude protein (%) | 16.7 |
| Stone powder | 8.8 | Calcium (%) | 3.38 |
| Calcium hydrogen phosphate | 0.96 | Available phosphorus (%) | 0.31 |
| Salt | 0.3 | dl-Methionine (%) | 0.40 |
| Choline chloride | 0.12 | l-Lysine (%) | 0.79 |
| Phytase | 0.02 | ||
| DL-met | 0.122 | ||
| Premix a | 0.128 | ||
| Total b | 100 |
| Genes | Primer Sequence (5′-3′) | Fragment Length (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| SLC1A1 | F: AGACATTGGTAGTGGTACGGAGAC | 116 | 58 |
| R: CAAAGCAGCAACTCCTGTGAT | |||
| SLC1A2 | F: CCGAAGCAAGTAGAAGTGAGGATG | 121 | 58 |
| R: CACCCAGGATGACACCAAATACAG | |||
| SLC1A3 | F: TGGTCATTGTGCTTACATCAGTAGG | 108 | 58 |
| R: AGACATTGGTAGTGGTACGGAGAC | |||
| FR1 | F: AGGTGCTGCTGGTGCTGTTG | 98 | 58 |
| R: GGCTTGGTTTTGTGGTGCTTGG | |||
| RFC | F: GCCTGTTCCTCACCCTCTTC | 92 | 58 |
| R: CTGTCCATCTTGTCCCCACC | |||
| VTG II | F: GTCGACGGAAGGGTGAGAAG | 95 | 58 |
| R: GGTCCTTACGATGCCTGAGC | |||
| VLDLR | F: TGAGGATGGGTCTGACGAGAG | 101 | 58 |
| R: CACACTCCAACTCATCAC | |||
| β-actin | F: GCCAACAGAGAGAAGATGACAC | 118 | 58 |
| R: GTAACACCATCACCAGAGTCCA |
| Item | Time (Week) | FA (mg/kg) | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | ANOVA | Linear | Quadratic | |||
| Laying rate (%) | 1–2 | 86.40 | 86.01 | 86.21 | 85.87 | 0.01 | 0.72 | 0.37 | 0.94 |
| 3–4 | 86.02 | 85.63 | 85.02 | 85.80 | 0.01 | 0.10 | 0.33 | 0.05 | |
| 5–6 | 85.43 | 85.61 | 86.19 | 85.02 | 0.01 | 0.27 | 0.72 | 0.11 | |
| 1–6 | 85.95 | 85.75 | 85.80 | 85.77 | 0.69 | 0.59 | 0.23 | 0.92 | |
| Feed efficiency (kg/kg) | 1–2 | 2.16 | 2.11 | 2.07 | 2.10 | 0.17 | 0.39 | 0.21 | 0.26 |
| 3–4 | 2.05 | 2.09 | 1.98 | 2.02 | 0.03 | 0.14 | 0.19 | 0.88 | |
| 5–6 | 2.02 | 2.14 | 2.13 | 2.07 | 0.04 | 0.17 | 0.47 | 0.04 | |
| 1–6 | 2.08 | 2.11 | 2.06 | 2.07 | 0.01 | 0.29 | 0.38 | 0.39 | |
| Egg weight (g/egg) | 1–2 | 61.69 | 61.27 | 62.11 | 61.09 | 0.01 | 0.31 | 0.59 | 0.46 |
| 3–4 | 61.86 | 62.34 | 62.39 | 61.36 | 0.03 | 0.46 | 0.53 | 0.16 | |
| 5–6 | 62.43 | 61.35 | 61.69 | 62.17 | 0.01 | 0.42 | 0.84 | 0.12 | |
| 1–6 | 62.00 | 61.66 | 62.07 | 61.54 | 0.01 | 0.40 | 0.41 | 0.72 | |
| Item | Time (Week) | FA (mg/kg) | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | ANOVA | Linear | Quadratic | |||
| Haugh unit | 1–6 | 83.99 | 82.86 | 85.42 | 83.71 | 0.79 | 0.73 | 0.81 | 0.86 |
| Yolk color 1 | 1–6 | 6.27 | 6.70 | 6.49 | 6.10 | 0.13 | 0.40 | 0.55 | 0.13 |
| Albumen height | 1–6 | 7.15 | 7.25 | 7.64 | 7.35 | 0.10 | 0.43 | 0.32 | 0.37 |
| Eggshell strength (N/cm2) | 1–6 | 39.19 | 39.27 | 40.96 | 40.55 | 0.70 | 0.78 | 0.39 | 0.87 |
| Shell thickness (μm) | 1–6 | 0.45 | 0.47 | 0.47 | 0.48 | 0.01 | 0.24 | 0.063 | 0.54 |
| Shape index | 1–6 | 1.24 | 1.25 | 1.25 | 1.24 | 0.01 | 0.90 | 0.63 | 0.58 |
| Item | FA (mg/kg) | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | ANOVA | Linear | Quadratic | ||
| Asp | 3.00 a | 2.99 a | 2.93 ab | 2.90 b | 0.01 | 0.01 | 0.00 | 0.65 |
| Thr | 1.62 | 1.62 | 1.60 | 1.58 | <0.01 | 0.12 | 0.03 | 0.52 |
| Ser | 2.64 | 2.63 | 2.62 | 2.58 | 0.01 | 0.12 | 0.03 | 0.43 |
| Glu | 3.92 a | 3.91 a | 3.77 b | 3.75 b | 0.02 | 0.008 | 0.00 | 0.79 |
| Pro | 1.24 | 1.24 | 1.24 | 1.22 | <0.01 | 0.30 | 0.17 | 0.19 |
| Gly | 0.97 | 0.97 | 0.97 | 0.96 | <0.01 | 0.27 | 0.09 | 0.37 |
| Ala | 0.17 | 0.17 | 0.16 | 0.16 | <0.01 | 0.15 | 0.03 | 0.56 |
| Val | 1.85 | 1.86 | 1.84 | 1.82 | <0.01 | 0.14 | 0.06 | 0.23 |
| Ile | 1.63 ab | 1.64 a | 1.60 ab | 1.58 b | <0.01 | 0.03 | 0.01 | 0.22 |
| Leu | 2.75 | 2.75 | 2.73 | 2.70 | <0.01 | 0.14 | 0.04 | 0.31 |
| Tyr | 1.39 a | 1.39 ab | 1.37 ab | 1.35 b | <0.01 | 0.02 | 0.00 | 0.55 |
| Phe | 1.38 a | 1.36 ab | 1.34 b | 1.33 b | <0.01 | 0.008 | 0.00 | 0.64 |
| His | 0.80 | 0.79 | 0.79 | 0.78 | <0.01 | 0.11 | 0.02 | 0.65 |
| Lys | 2.47 | 2.47 | 2.45 | 2.42 | <0.01 | 0.12 | 0.03 | 0.37 |
| Arg | 2.29 | 2.3 | 2.29 | 2.25 | <0.01 | 0.14 | 0.05 | 0.21 |
| Cys | 0.54 a | 0.54 a | 0.54 a | 0.53 b | 0.73 | 0.007 | 0.34 | 0.75 |
| Met | 0.78 a | 0.75 ab | 0.77 ab | 0.75 b | <0.01 | 0.03 | 0.03 | 0.72 |
| Trp | 0.41 | 0.40 | 0.39 | 0.39 | <0.01 | 0.75 | 0.38 | 0.57 |
| Item | FA (mg/kg) | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | ANOVA | Linear | Quadratic | ||
| C4:0 | 0.0074 | 0.0073 | 0.0076 | 0.0073 | <0.01 | 0.64 | 0.93 | 0.58 |
| C8:0 | 0.0058 b | 0.0067 a | 0.0060 ab | 0.0059 b | <0.01 | <0.01 | 0.82 | 0.00 |
| C10:0 | 0.013 | 0.013 | 0.014 | 0.013 | <0.01 | 0.41 | 0.65 | 0.13 |
| C12:0 | 0.018 ab | 0.018 ab | 0.018 a | 0.017 b | <0.01 | 0.05 | 0.11 | 0.10 |
| C13:0 | 0.0031 | 0.0031 | 0.0030 | 0.0030 | <0.01 | 0.78 | 0.34 | 0.98 |
| C14:0 | 0.23 | 0.21 | 0.22 | 0.21 | <0.01 | 0.17 | 0.17 | 0.43 |
| C15:0 | 0.032 | 0.035 | 0.033 | 0.031 | <0.01 | 0.70 | 0.58 | 0.46 |
| C16:0 | 16.04 | 15.36 | 15.87 | 15.14 | 0.20 | 0.38 | 0.24 | 0.96 |
| C17:0 | 0.064 | 0.072 | 0.061 | 0.070 | <0.01 | 0.35 | 0.77 | 0.84 |
| C18:0 | 10.77 | 10.75 | 10.92 | 10.70 | 0.16 | 0.97 | 0.99 | 0.76 |
| C14:1 | 0.073 a | 0.062 b | 0.073 a | 0.061 b | <0.01 | 0.02 | 0.11 | 0.94 |
| C15:1 | 0.0053 | 0.0054 | 0.0055 | 0.005 | <0.01 | 0.92 | 0.56 | 0.79 |
| C16:1 | 2.76 | 2.49 | 2.74 | 2.42 | 0.06 | 0.09 | 0.13 | 0.77 |
| C17:1 | 0.056 | 0.057 | 0.057 | 0.052 | <0.01 | 0.98 | 0.82 | 0.76 |
| C18:1n9t | 4.58 b | 5.9 a | 6.21 a | 4.56 b | 0.32 | 0.02 | 0.87 | <0.05 |
| C18:1n9c | 12.6 | 13.03 | 12.61 | 11.75 | 0.18 | 0.08 | 0.06 | 0.07 |
| C20:1n9 | 0.14 | 0.14 | 0.14 | 0.13 | <0.01 | 0.28 | 0.50 | 0.11 |
| C18:2n6t | 0.59 | 0.76 | 0.97 | 0.75 | 0.066 | 0.25 | 0.24 | 0.15 |
| C20:2 | 0.2 | 0.19 | 0.2 | 0.2 | 0.003 | 0.35 | 0.63 | 0.73 |
| C18:2n6c | 5.88 a | 4.99 b | 5.34 ab | 5.61 ab | 0.11 | 0.02 | 0.57 | <0.01 |
| C18:3n6 | 0.061 | 0.081 | 0.098 | 0.096 | 0.02 | <0.01 | <0.01 | <0.01 |
| C18:3n3 | 0.12 | 0.12 | 0.12 | 0.13 | 0.00 | 0.1 | 0.06 | 0.39 |
| C20:3, n6 | 0.24 | 0.25 | 0.26 | 0.27 | 0.01 | 0.85 | 0.41 | 0.77 |
| C20:4 n6 | 1.18 | 1.19 | 1.17 | 1.18 | 0.02 | 0.99 | 0.96 | 0.99 |
| C20:3n3 | 0.045 | 0.046 | 0.046 | 0.045 | 0.00 | 0.66 | 0.66 | 0.25 |
| C20:5n3 EPA | 0.02 | 0.02 | 0.03 | 0.03 | 0.001 | 0.31 | 0.08 | 0.51 |
| C22:6 n3 DHA | 0.34 | 0.36 | 0.34 | 0.35 | 0.005 | 0.86 | 0.86 | 0.90 |
| SFA | 27.18 | 26.48 | 27.15 | 26.21 | 0.33 | 0.67 | 0.47 | 0.86 |
| MUFA | 20.20 b | 21.69 a | 21.84 a | 18.97 b | 0.29 | <0.01 | 0.03 | <0.01 |
| PUFA | 8.70 | 8.02 | 8.57 | 8.65 | 0.15 | 0.39 | 0.78 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, A.-C.; Deng, Y.-H.; Long, C.; Sheng, X.-H.; Wang, X.-G.; Xiao, L.-F.; Lv, X.-Z.; Chen, X.-N.; Chen, L.; Qi, X.-L. High Dietary Folic Acid Supplementation Reduced the Composition of Fatty Acids and Amino Acids in Fortified Eggs. Foods 2024, 13, 1048. https://doi.org/10.3390/foods13071048
Yu A-C, Deng Y-H, Long C, Sheng X-H, Wang X-G, Xiao L-F, Lv X-Z, Chen X-N, Chen L, Qi X-L. High Dietary Folic Acid Supplementation Reduced the Composition of Fatty Acids and Amino Acids in Fortified Eggs. Foods. 2024; 13(7):1048. https://doi.org/10.3390/foods13071048
Chicago/Turabian StyleYu, Ao-Chuan, Yu-Han Deng, Cheng Long, Xi-Hui Sheng, Xiang-Guo Wang, Long-Fei Xiao, Xue-Ze Lv, Xiang-Ning Chen, Li Chen, and Xiao-Long Qi. 2024. "High Dietary Folic Acid Supplementation Reduced the Composition of Fatty Acids and Amino Acids in Fortified Eggs" Foods 13, no. 7: 1048. https://doi.org/10.3390/foods13071048
APA StyleYu, A.-C., Deng, Y.-H., Long, C., Sheng, X.-H., Wang, X.-G., Xiao, L.-F., Lv, X.-Z., Chen, X.-N., Chen, L., & Qi, X.-L. (2024). High Dietary Folic Acid Supplementation Reduced the Composition of Fatty Acids and Amino Acids in Fortified Eggs. Foods, 13(7), 1048. https://doi.org/10.3390/foods13071048







