Supercritical CO2 Treatment to Modify Techno-Functional Properties of Proteins Extracted from Tomato Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Pomace and Tomato Seed Meals
2.2. Chemical Composition Analysis
2.3. Tomato Seed Meal Degreasing
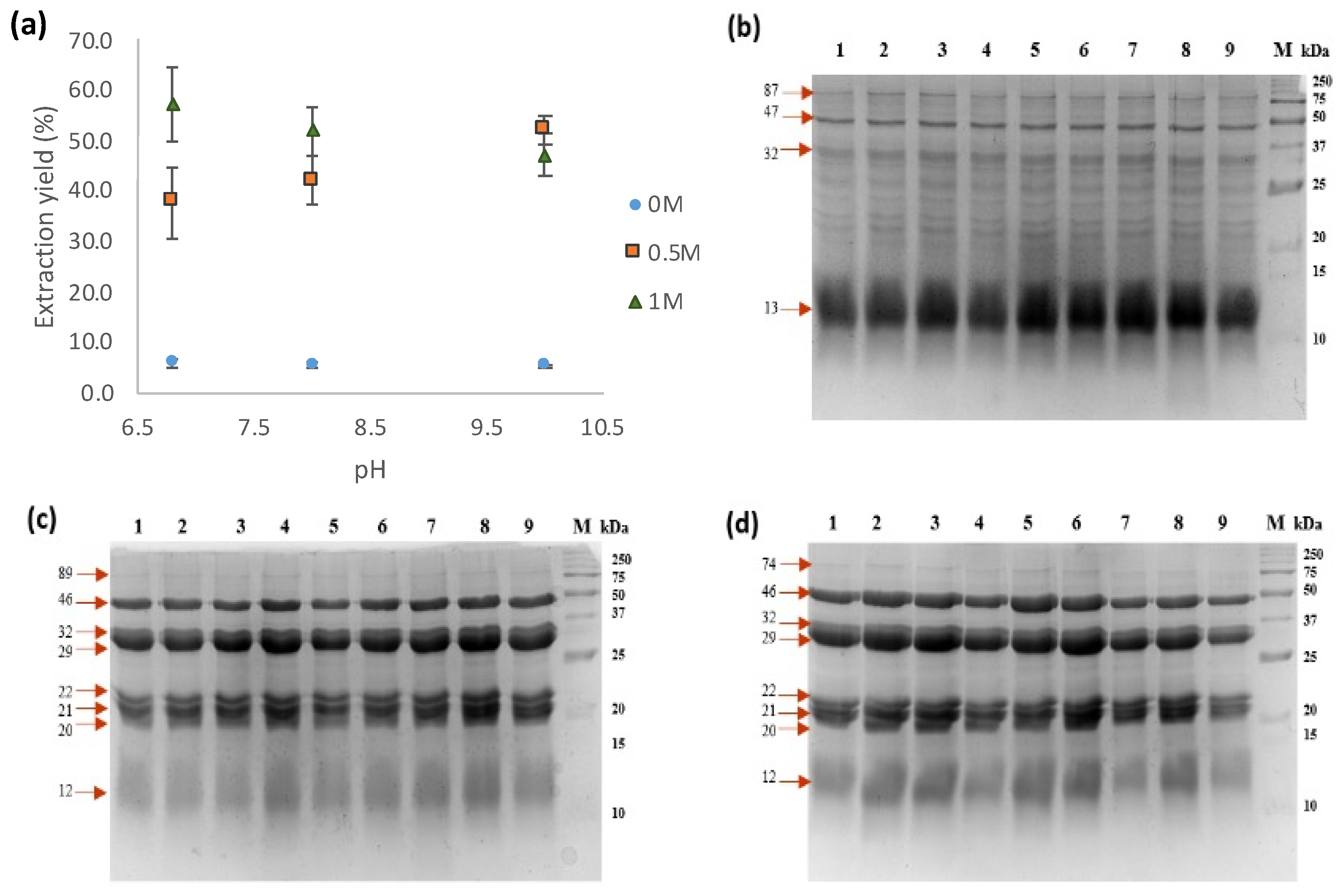
2.4. Protein Extraction, Yield and Quantitation
2.5. Electrophoretic Pattern: SDS-PAGE and 2D PAGE Analysis
2.6. Techno-Functional Properties
2.6.1. Water and Oil Absorption
2.6.2. Foaming Properties
2.6.3. Emulsifying Properties
2.7. Fourier Transform Infrared Spectroscopy (FT-IR)
2.8. Statistical Analysis
3. Results
3.1. Fresh Tomato Pomace Yield and Chemical Composition
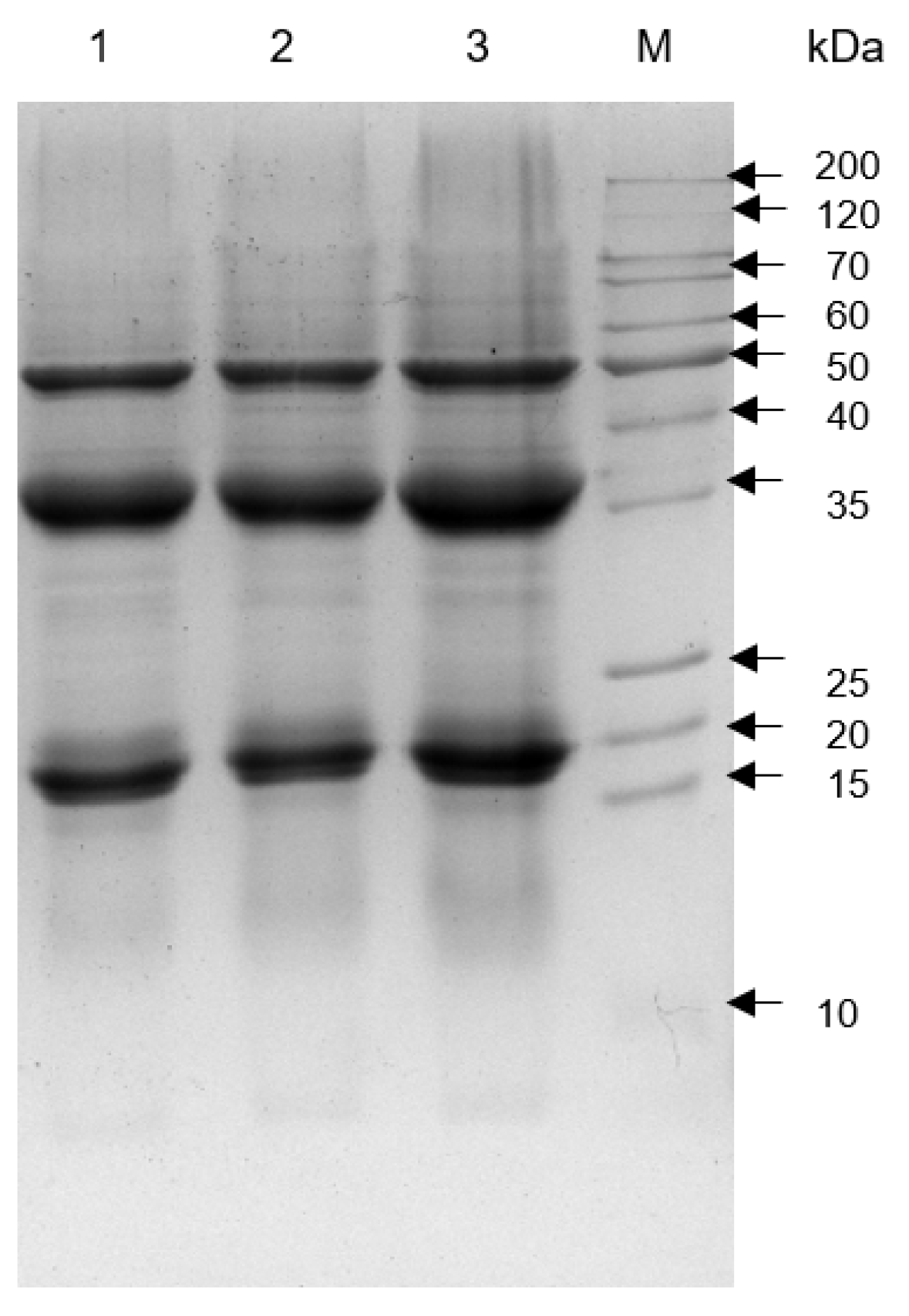
3.2. Protein Extracts and SDS-PAGE Assays
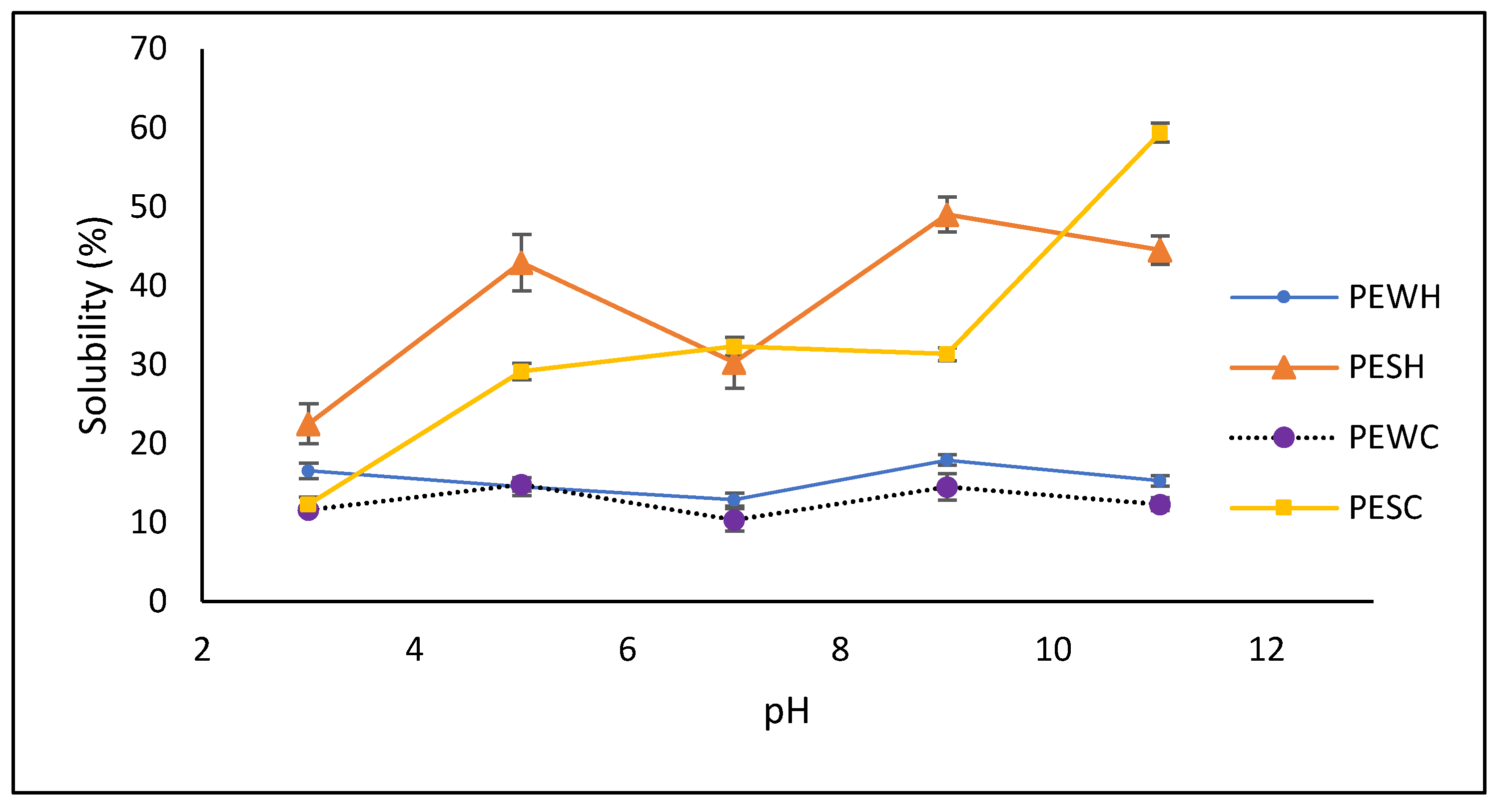
3.3. Techno-Functional Properties
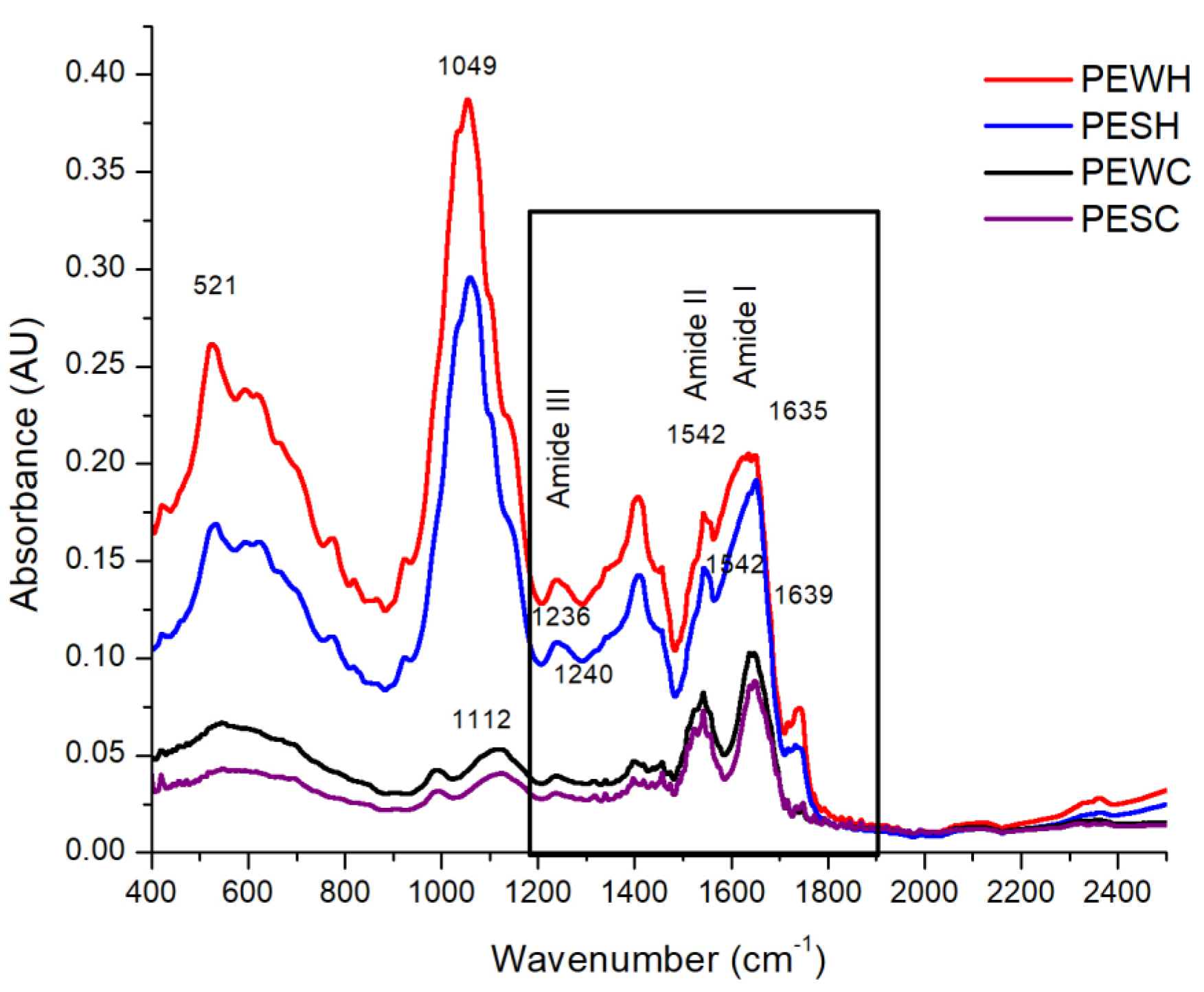
3.4. Structural Analysis
4. Discussion
4.1. Protein Extracts and SDS-PAGE Assays
4.2. Techno-Functional Properties and Structures of Some Proteins Were Affected by scCO2 Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, I.A. Foreword. In Sustainable Management of Arthropod Pests of Tomato; Waqas, W., Gerald, E.B., Thomas, M.P., Eds.; Academic Press: Cambridge, MA, USA, 2017; p. XV. [Google Scholar] [CrossRef]
- Maldonado-Torres, R.; Morales-Camacho, J.I.; López-Valdez, F.; Huerta-González, L.; Luna-Suárez, S. Assessment of techno-functional and nutraceutical potential of tomato (Solanum lycopersicum) seed meal. Molecules 2020, 25, 4235. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Chen, S.; Li, H.; Paengkoum, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Sinpru, B.; Sroichak, T.; Archa, P.; et al. Sustainable Valorization of Tomato Pomace (Lycopersicon esculentum) in Animal Nutrition: A Review. Animals 2022, 12, 3294. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Oberoi, H.S.; Dhillon, G.S. Fruit and vegetable processing waste: Renewable feed stocks for enzyme production. In Agro-Industrial Wastes as Feedstock for Enzyme Production; Academic Press: Cambridge, MA, USA, 2016; pp. 23–59. [Google Scholar] [CrossRef]
- Eslami, E.; Carpentieri, S.; Pataro, G.; Ferrari, G. A Comprehensive Overview of Tomato Processing By-Product Valorization by Conventional Methods versus Emerging Technologies. Foods 2023, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, A.H.Y. The chemical constituents of tomato seeds. Food Chem. 1982, 9, 315–318. [Google Scholar] [CrossRef]
- Rossini, G.; Toscano, G.; Duca, D.; Corinaldesi, F.; Foppa Pedretti, E.; Riva, G. Analysis of the characteristics of the tomato manufacturing residues finalized to the energy recovery. Biomass Bioenergy 2013, 51, 177–182. [Google Scholar] [CrossRef]
- Sogi, D.S.; Bhullar, J.K. Shelf life studies and refining of tomato seed oil. J. Food Sci. Technol. 2000, 37, 542–544. [Google Scholar]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J.C. Structural and functional characterization of kidney bean and field pea protein extracts: A comparative study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Madia, V.N.; De Vita, D.; Ialongo, D.; Tudino, V.; De Leo, A.; Scipione, L.; Di Santo, R.; Costi, R.; Messore, A. Recent advances in recovery of lycopene from tomato waste: A potent antioxidant with endless benefits. Molecules 2021, 26, 4495. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Jun Xue, S.; Jiang, Y.; Ye, X. Supercritical-fluid extraction of lycopene from tomatoes. In Separation, Extraction and Concentration Processes in the Food, Beverage and Nutraceutical Industries; Rizvi, S.S.H., Ed.; Woodhead Publishing: Philadelphia, PA, USA, 2010; pp. 619–640. [Google Scholar]
- Yu, T.; Niu, L.; Iwahashi, H. High-pressure carbon dioxide used for pasteurization in food industry. Food Eng. Rev. 2020, 12, 364–380. [Google Scholar] [CrossRef]
- Roche, J.; Royer, C.A. Lessons from pressure denaturation of proteins. J. R. Soc. Interface 2018, 15, 20180244. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International; AOAC: Rockville, MD, USA, 2000. [Google Scholar]
- Conde-Hernández, L.A.; Espinosa-Victoria, J.R.; Trejo, A.; Guerrero-Beltrán, J.A. CO2-supercritical extraction, hydrodistillation and steam distillation of essential oil of rosemary (Rosmarinus officinalis). J. Food Eng. 2017, 200, 81–86. [Google Scholar] [CrossRef]
- Delgado-García, Y.I.; Luna-Suárez, S.; López-Malo, A.; Morales-Camacho, J.I. Effect of supercritical carbon dioxide on physicochemical and techno-functional properties of amaranth flour. Chem. Eng. Process 2022, 178, 109031. [Google Scholar] [CrossRef]
- Walker, J.M. The Protein Protocols Handbook, 3rd ed.; Humana Press: Totowa, NJ, USA, 2009. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Morán, Y.; Morales-Camacho, J.I.; Delgado-Macuil, R.; Rosas-Cárdenas, F.D.F.; Luna-Suárez, S. Improvement of techno-functional properties of acidic subunit from amaranth 11S globulin modified by bioactive peptide insertions. Electron. J. Biotechnol. 2023, 61, 45–53. [Google Scholar] [CrossRef]
- Fidantsi, A.; Doxastakis, G. Emulsifying and foaming properties of amaranth seed protein extracts. Colloids Surf. B 2001, 21, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mingyu, H.; Na, W.; Mingda, L.; Changling, W.; Yang, L.; Fei, T.L. Spectroscopic analysis of the effect of vitamin B12-soy protein isolate on the soy protein isolate structure. J. Mol. Liq. 2021, 325, 115148. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Puray, J.J.; Villaber, R.A. Extraction, characterization, and vascular response of proteins from catfish (Clarias batrachus L.) mucus. Food Chem. 2023, 3, 100444. [Google Scholar] [CrossRef]
- Hu, T.G.; Feng-Xiang, T.; Lu, L.; Ke-Jing, A.; Bo, Z.; Jin, W.; Ji-Jun, W.; Geng-Sheng, X.; Yuan-Shan, Y.; Yu-Juan, X. Structural elucidation and physicochemical properties of litchi polysaccharide with the promoting effect on exopolysaccharide production by Weissella confusa. Int. J. Biol. Macromol. 2023, 253, 126944. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Gautam, J.; Mohd, A.; Mohd, M.; Abdullah, A.; Aysha, F.; Nazia, S. Quantum Computational, Spectroscopic (FT-IR, FT-Raman, NMR, and UV–Vis) Hirshfeld Surface and Molecular Docking-Dynamics Studies on 5-Hydroxymethyluracil (Monomer and Trimer). Molecules 2023, 28, 2116. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chicheste, UK, 2006. [Google Scholar] [CrossRef]
- Ganim, Z.; Chung, H.S.; Smith, A.W.; Deflores, L.P.; Jones, K.C.; Tokmakoff, A. Amide I two-dimensional infrared spectroscopy of proteins. Acc. Chem. Res. 2008, 41, 432–441. [Google Scholar] [CrossRef] [PubMed]
- González, M.; Cid, M.C.; Lobo, M.G. Usage of tomato (Lycopersicum esculentum Mill.) seeds in Health. In Nuts and Seeds in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2011; pp. 1123–1132. [Google Scholar] [CrossRef]
- Sarkar, A.; Kaul, P. Evaluation of tomato processing by-products: A comparative study in a pilot scale setup. J. Food Process Eng. 2014, 37, 299–307. [Google Scholar] [CrossRef]
- Shao, D.; Atungulo, G.G.; Pan, Z.; Yue, T.; Zhang, A.; Chen, X. Separation methods and chemical and nutritional characteristics of tomato pomace. Trans. ASABE 2013, 56, 261–268. [Google Scholar] [CrossRef]
- Liadakis, G.N.; Tzia, C.; Oreopoulou, V.; Thomopoulos, C.D. Protein isolation from tomato seed meal, extraction optimization. J. Food Sci. 1995, 60, 477–482. [Google Scholar] [CrossRef]
- Saldivar, S.O.S. CEREALS | Dietary Importance. In Encyclopedia of Food Sciences and Nutrition; Academic Press: Cambridge, MA, USA, 2003; pp. 1027–1033. [Google Scholar] [CrossRef]
- Allen, L.H. Legumes. In Encyclopedia of Human Nutrition, 3rd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 3–4, pp. 74–79. [Google Scholar] [CrossRef]
- Salunkhe, D.; Kadam, S.; Chavan, J. Postharvest Biotechnology of Food Legumes; CRC Press: Boca Raton, FL, USA, 1985; pp. 1–35. [Google Scholar]
- Sogi, D.S.; Arora, M.S.; Garg, S.K.; Bawa, A.S. Fractionation and electrophoresis of tomato waste seed proteins. Food Chem. 2002, 76, 449–454. [Google Scholar] [CrossRef]
- Zayas, J.F. Solubility of Proteins. In Functionality of Proteins in Food; Zayas, J.F., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–21. [Google Scholar] [CrossRef]
- Seena, S.; Sridhar, K.R. Physicochemical, functional and cooking properties of under explored legumes, Canavalia of the southwest coast of India. Food Res. Int. 2005, 38, 803–814. [Google Scholar] [CrossRef]
- Shao, D.; Atungulu, G.G.; Pan, Z.; Yue, T.; Zhang, A.; Fan, Z. Characteristics of isolation and functionality of protein from tomato pomace produced with different industrial processing methods. Food Bioprocess. Technol. 2014, 7, 532–541. [Google Scholar] [CrossRef]
- Mechmeche, M.; Kachouri, F.; Ksontini, H.; Hamdi, M. Production of bioactive peptides from tomato seed isolate by Lactobacillus plantarum fermentation and enhancement of antioxidant activity. Food Biotechnol. 2017, 31, 94–113. [Google Scholar] [CrossRef]
- Sarkar, A.; Kamaruddin, H.; Bentley, A.; Wang, S. Emulsion stabilization by tomato seed protein isolate: Influence of pH, ionic strength and thermal treatment. Food Hydrocoll. 2016, 57, 160–168. [Google Scholar] [CrossRef]
- Smith, D.M. Protein separation and characterization procedures. In Food Analysis; Nielsen, S.S., Ed.; Food Science Text Series; Springer: Cham, Switzerland, 2017; pp. 431–453. [Google Scholar] [CrossRef]
- Farrell, H.M.; Qi, P.X.; Brown, E.M.; Cooke, P.H.; Tunick, M.H.; Wickham, E.D.; Unruh, J.J. Molten globule structures in milk proteins: Implications for potential new structure-function relationships. J. Dairy Sci. 2002, 85, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Jin, M. Enhanced functionalities of whey proteins treated with supercritical carbon dioxide. J. Dairy Sci. 2008, 91, 490–499. [Google Scholar] [CrossRef]
- Awolu, O.O.; Osemeke, R.O.; Ifesan, B.O.T. Antioxidant, functional and rheological properties of optimized composite flour, consisting wheat and amaranth seed, brewers’ spent grain and apple pomace. J. Food Sci. Technol. 2016, 53, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Özyurt, V.H.; Tetik, I.; Ötleş, S. Influence of process conditions on ultrasound-assisted protein extraction from cold pressed tomato seed waste. J. Food Process. Preserv. 2021, 45, e16079. [Google Scholar] [CrossRef]
- Gratacós-Cubarsí, M.; Lametsch, R. Determination of changes in protein conformation caused by pH and temperature. Meat Sci. 2008, 80, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hao, J.; Xie, Q.; Pi, X.; Peng, Z.; Sun, Y.; Cheng, J. pH-induced physiochemical and structural changes of milk proteins mixtures and its effect on foaming behavior. Int. J. Biol. Macromol. 2024, 254, 127838. [Google Scholar] [CrossRef] [PubMed]
- Aderinola, T.A.; Alashi, A.M.; Nwachukwu, I.D.; Fagbemi, T.N.; Enujiugha, V.N.; Aluko, R.E. In vitro digestibility, structural and functional properties of Moringa oleifera seed proteins. Food Hydrocoll. 2020, 101, 105574. [Google Scholar] [CrossRef]
- Kheto, A.; Sehrawat, R.; Gul, K.; Kumar, L. Effect of extraction pH on amino acids, nutritional, in-vitro protein digestibility, intermolecular interactions, and functional properties of guar germ proteins. Food Chem. 2024, 444, 138628. [Google Scholar] [CrossRef] [PubMed]
- Chandi, K.; Sogi, D. Functional properties of rice bran protein concentrates. J. Food Eng. 2007, 79, 592–597. [Google Scholar] [CrossRef]
- Olawuni, I.A.; Uruakpa, F.O.; Uzoma, A. Unripe Plantain Flours. In Therapeutic, Probiotic, and Unconventional Foods; Alexandru, M.G., Alina, M.H., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 341–366. [Google Scholar] [CrossRef]
- Tang, Q.; Roos, Y.H.; Miao, S. Plant Protein versus Dairy Proteins: A pH-Dependency Investigation on Their Structure and Functional Properties. Foods 2023, 12, 368. [Google Scholar] [CrossRef]
- Zhang, R.; Fang, X.; Feng, Z.; Chen, M.; Qiu, X.; Sun, J.; Wu, M.; He, J. Protein from rapeseed for food applications: Extraction, sensory quality, functional and nutritional properties. Food Chem. 2024, 439, 138109. [Google Scholar] [CrossRef] [PubMed]
- Batish, I.; Brits, D.; Valencia, P.; Miyai, C.; Rafeeq, S.; Xu, Y.; Galanopoulos, M.; Sismour, E.; Ovissipour, R. Effects of enzymatic hydrolysis on the functional properties, antioxidant activity and protein structure of black soldier fly (Hermetia illucens) protein. Insects 2020, 11, 876. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Faber, I.; Berton-Carabin, C.C.; Nikiforidis, C.V.; Linden, E.V.; Sagis, L.M.C. Foams and air-water interfaces stabilised by mildly purified rapeseed proteins after defatting. Food Hydrocoll. 2021, 112, 106270. [Google Scholar] [CrossRef]
- Mao, X.; Hua, Y. Composition, structure and functional properties of protein concentrates and extracts produced from walnut (Juglans regia L.). Int. Mol. Sci. 2012, 13, 1561–1581. [Google Scholar] [CrossRef] [PubMed]
- Dunford, N.T.; Temelli, F.; Leblanc, E.L. Supercritical CO2 extraction of oil and residual roteins from atlantic mackerel (Scomber scombrus) as affected by moisture content. J. Food Sci. 1997, 62, 289–294. [Google Scholar] [CrossRef]
- Morais, A.R.C.; Costa Lopes, A.M.; Bogel-Łukasik, R. Carbon dioxide in biomass processing: Contributions to the green biorefinery concept. Chem. Rev. 2015, 115, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Rogalinski, T.; Liu, K.; Albrecht, T.; Brunner, G. Hydrolysis kinetics of biopolymers in subcritical water. J. Supercrit. Fluids 2008, 46, 335–341. [Google Scholar] [CrossRef]
- Long, S.; Linlin, Z.; Meihu, M. Study of high pressure carbon dioxide on the physicochemical, interfacial and rheological properties of liquid whole egg. Food Chem. 2021, 337, 127989. [Google Scholar] [CrossRef]
- Melgosa, R.; Trigueros, E.; Sanz, M.T.; Cardeira, M.; Rodrigues, L.; Fernández, N.; Matias, A.; Bronze, M.R.; Marques, M.; Paiva, A.; et al. Supercritical CO2 and subcritical water technologies for the production of bioactive extracts from sardine (Sardina pilchardus) waste. J. Supercrit. Fluids 2020, 164, 104943. [Google Scholar] [CrossRef]
- Rivas-Vela, C.I.; Castaño-Tostado, E.; Cardador-Martínez, A.; Amaya-Llano, S.L.; Castillo-Herrera, G. Subcritical water hydrolysis for the obtention of bioactive peptides from a grasshopper Sphenarium purpurascens protein concentrate. J. Supercrit. Fluids 2023, 197, 105893. [Google Scholar] [CrossRef]
- Striolo, A.; Favaro, A.; Elvassore, N.; Bertucco, A.; Di Noto, V. Evidence of conformational changes for protein films exposed to high -pressure CO2 by FT-IR spectorscopy. J. Supercrit. Fluids 2003, 27, 283–295. [Google Scholar] [CrossRef]
- Ma, M.; Ren, Y.; Xie, W.; Zhou, D.; Tang, S.; Kuang, M.; Wang, Y.; Du, S. Physicochemical and functional properties of protein isolate obtained from cottonseed meal. Food Chem. 2018, 240, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.C.; Seixas, F.A.V.; Coimbra, J.S.R.; Pimentel, T.C.; Barão, C.E.; Cardozo-Filho, L. Continuous fractionation of whey protein extracts by using supercritical carbon dioxide. J. CO2 Util. 2019, 30, 112–122. [Google Scholar] [CrossRef]

| Component | g/100 g Tomato Seed Meal |
|---|---|
| Protein | 28.44 ± 0.19 |
| Fat | 18.34 ± 0.21 |
| Moisture | 8.18 ± 0.26 |
| Crude fiber | 26.19 ± 1.36 |
| Ash | 3.9 ± 0.14 |
| * Carbohydrates | 14.95 * |
| Factor | FC | EC | WHC | OHC | ES |
|---|---|---|---|---|---|
| Defatting | |||||
| CO2 | 21.0 ± 9.0 A | 27.0 ± 5.9 A | 0.9 ± 0.2 A | 3.6 ± 0.6 A | 43.7 ± 10.8 A |
| Hex | 5.4 ± 1.7 B | 5.1 ± 2.2 B | 0.8 ± 0.3 A | 2.9 ± 0.5 B | 0.9 ± 0.02 B |
| Protein extraction | |||||
| Salt | 0.9 ± 0.02 B | 8.4 ± 1.9 B | 1.2 ± 0.3 A | 3.8 ± 0.7 A | 38.7 ± 12.3 A |
| Water | 25.6 ± 8.2 A | 23.8 ± 6.9 A | 0.5 ± 0.1 B | 2.8 ± 0.5 B | 6.0 ± 1.5 B |
| pH | |||||
| pH 5 | 6.3 ± 1.8 B | 14.6 ± 5.7 B | 0.2 ± 0.02 B | 1.4 ± 0.05 B | 17.4 ± 7.1 B |
| pH 7 | 20.1 ± 9.1 A | 17.6 ± 5.4 A | 1.5 ± 0.2 A | 5.1 ± 0.4 A | 27.2 ± 12.1 A |
| Significance | |||||
| Defatting | ** | ** | NS | ** | ** |
| Protein extraction | ** | ** | ** | ** | ** |
| pH | ** | ** | ** | ** | ** |
| Property | TSMH | TSMC | ||||||
|---|---|---|---|---|---|---|---|---|
| PEWHpH5 | PEWHpH7 | PESHpH5 | PESHpH7 | PEWCpH5 | PEWCpH7 | PESCpH5 | PESCpH7 | |
| WHC * | 0.3 ± 0.0C | 0.5 ± 0.1C | 0.2 ± 0.03C | 2.4 ± 0.3A | 0.3 ± 0.0C | 1.2 ± 0.07B | 0.2 ± 0.0C | 2.0 ± 0.1A |
| OHC + | 1.3 ± 0.05C | 3.2 ± 0.5B | 1.5 ± 0.05C | 5.9 ± 0.5A | 1.3 ± 0.0C | 5.2 ± 0.4A | 1.6 ± 0.1BC | 6.2 ± 0.3A |
| FC (%) « | 13.3 ± 3.3B | 6.7 ± 1.6BC | 0.9 ± 0.06C | 0.9 ± 0.05C | 10.0 ± 0.0B | 72.2 ± 2.7A | 0.9 ± 0.05C | 0.9 ± 0.03C |
| EC (%) α | 0.9 ± 0.03D | 0.9 ± 0.05D | 0.9 ± 0.03D | 17.8 ± 1.1B | 46.7 ± 3.3A | 46.7 ± 1.6A | 10.0 ± 0.0C | 4.8 ± 0.2CD |
| ES (%) β | 0.9 ± 0.02C | 0.9 ± 0.05C | 0.9 ± 0.05C | 0.9 ± 0.05C | 11.3 ± 1.2C | 10.7 ± 0.3C | 56.7 ± 6.6B | 96.3 ± 3.7A |
| Secondary Structure | TSMH | TSMC | ||
|---|---|---|---|---|
| PEWH | PESH | PEWC | PESC | |
| Helix (%) | 19.2 ± 1.3 | 8.0 ± 1.2 | 24.4 ± 1.8 | NS |
| Sheet (%) | 33.2 ± 1.5 | 48.7 ± 1.4 | 33.5 ± 1.1 | 48.5 ± 1.4 |
| Turns (%) | 31.7 ± 1.9 | 32.0 ± 2.1 | 25.3 ± 1.6 | 30.4 ± 1.3 |
| Unordered (%) | 15.9 ± 1.6 | 11.3 ± 1.7 | 16.8 ± 1.1 | 21.1 ± 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateo-Roque, P.; Morales-Camacho, J.I.; Jara-Romero, G.J.; Rosas-Cárdenas, F.d.F.; Huerta-González, L.; Luna-Suárez, S. Supercritical CO2 Treatment to Modify Techno-Functional Properties of Proteins Extracted from Tomato Seeds. Foods 2024, 13, 1045. https://doi.org/10.3390/foods13071045
Mateo-Roque P, Morales-Camacho JI, Jara-Romero GJ, Rosas-Cárdenas FdF, Huerta-González L, Luna-Suárez S. Supercritical CO2 Treatment to Modify Techno-Functional Properties of Proteins Extracted from Tomato Seeds. Foods. 2024; 13(7):1045. https://doi.org/10.3390/foods13071045
Chicago/Turabian StyleMateo-Roque, Paola, Jocksan I. Morales-Camacho, Guadalupe Janet Jara-Romero, Flor de Fátima Rosas-Cárdenas, Luis Huerta-González, and Silvia Luna-Suárez. 2024. "Supercritical CO2 Treatment to Modify Techno-Functional Properties of Proteins Extracted from Tomato Seeds" Foods 13, no. 7: 1045. https://doi.org/10.3390/foods13071045
APA StyleMateo-Roque, P., Morales-Camacho, J. I., Jara-Romero, G. J., Rosas-Cárdenas, F. d. F., Huerta-González, L., & Luna-Suárez, S. (2024). Supercritical CO2 Treatment to Modify Techno-Functional Properties of Proteins Extracted from Tomato Seeds. Foods, 13(7), 1045. https://doi.org/10.3390/foods13071045






