Effect of Different Molecular Weight Hyaluronic Acids on Skim Milk Functional Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Experimental Design and Statistical Analysis
2.2.2. Skim Milk Analysis
2.2.3. Skim Milk Sample Preparation with Hyaluronic Acid
2.2.4. Rheological Behavior of Skim Milk
2.2.5. Protein Stability by Gravimetric Phase Separation
2.2.6. Heat Stability by Heat Coagulation Time Test (HCT)
2.2.7. Water Holding Capacity (WHC)
2.2.8. Oil Emulsifying Activity and Emulsion Stability
2.2.9. Foaming Capacity and Foaming Stability
2.2.10. Particle Size Analysis
2.2.11. Capillary Gel Electrophoresis (CGE)
2.2.12. Determination of Calcium Concentration Using the EDTA Complexometric Titration Method
2.2.13. Protein Microstructure Observation Using Confocal Laser Scanning Microscopy (CLSM)
3. Results and Discussions
3.1. Composition of Skim Milk
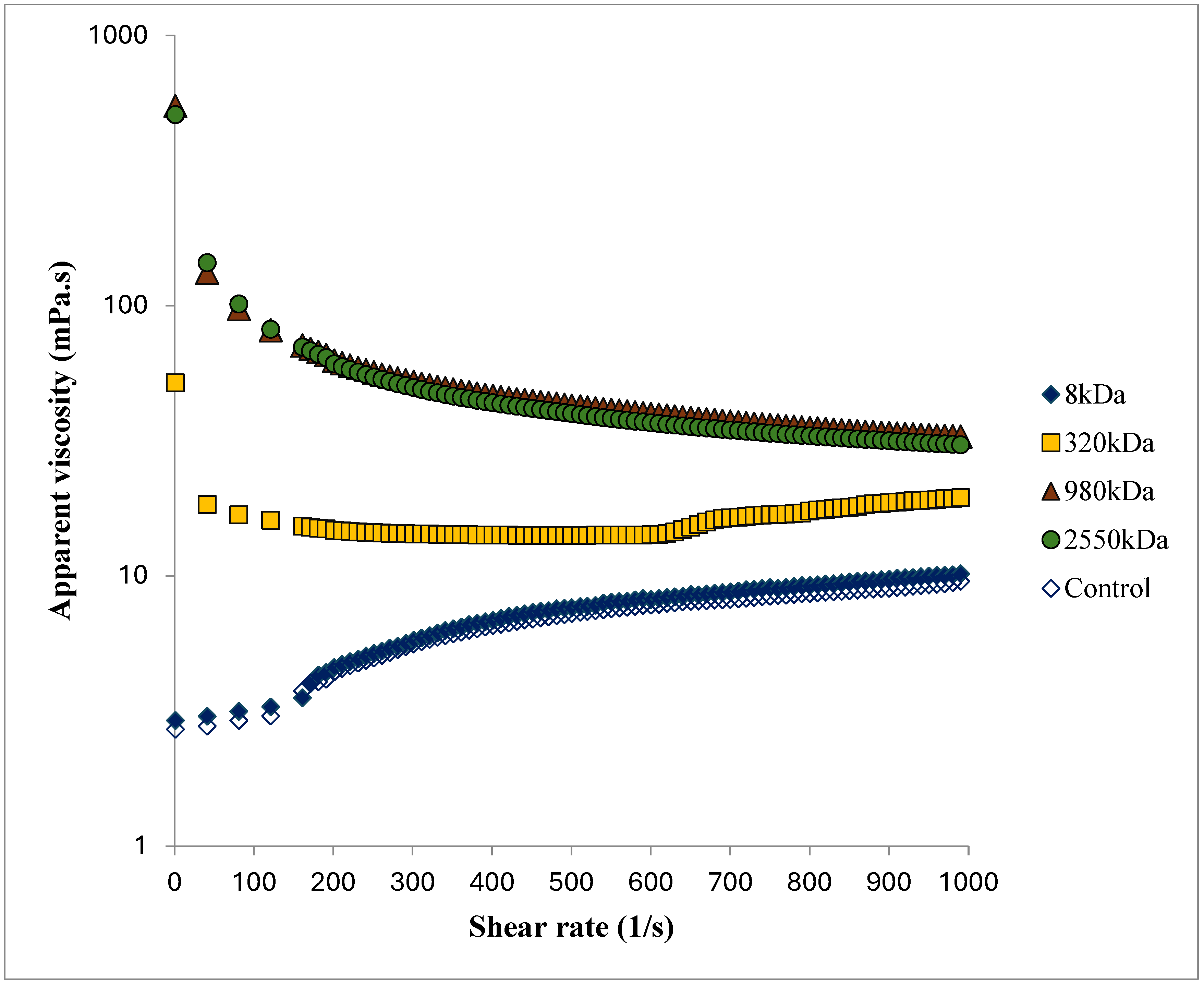
3.2. Rheological Behavior of Skim Milk
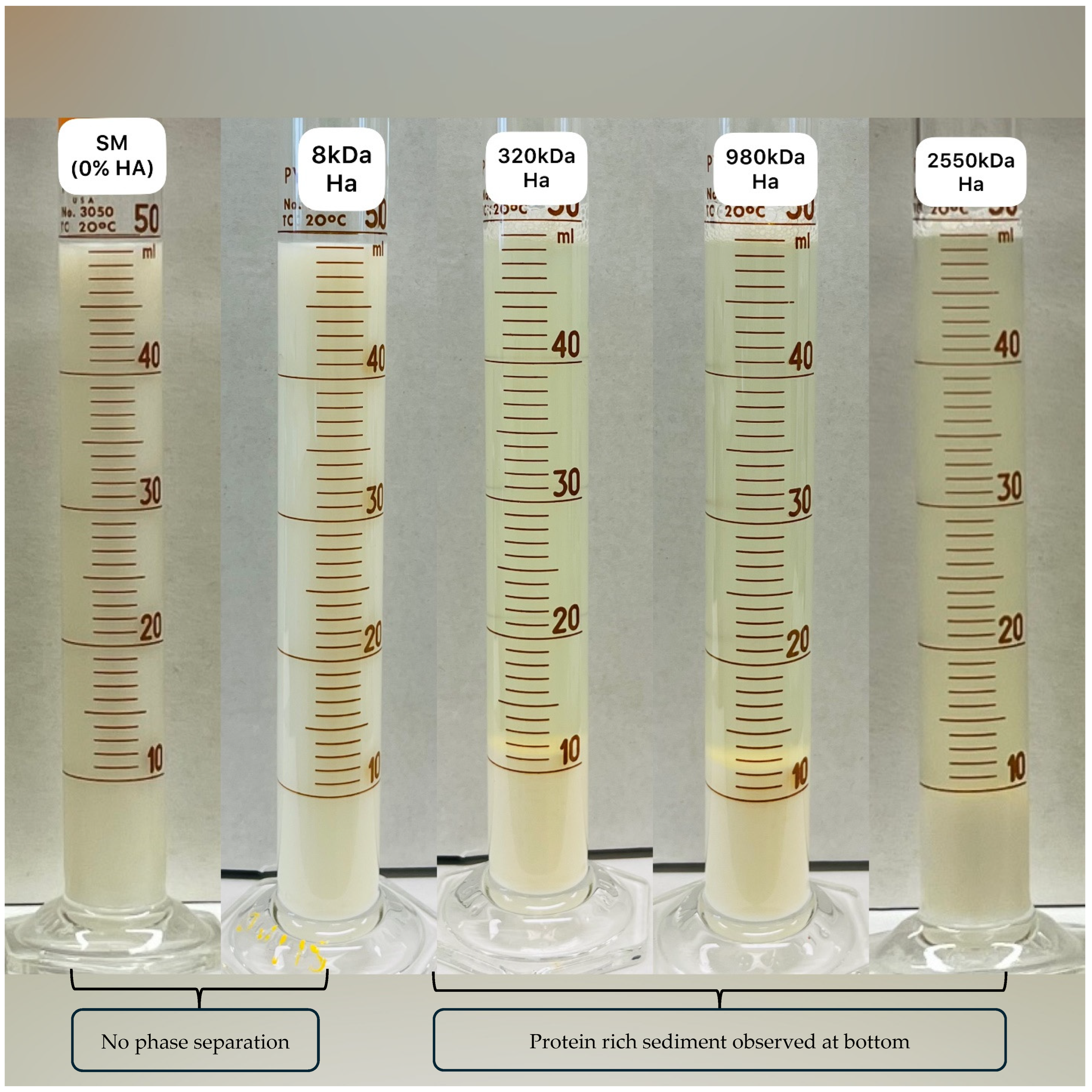
3.3. Protein Phase Separation
3.4. Heat Coagulation Time Test (HCT)
3.5. Water Holding Capacity (WHC)
3.6. Oil Emulsifying Activity (EA) and Emulsion Stability (ES)
3.7. Foaming Capacity (FC) and Foaming Stability (FS)
3.8. Analyses of Individual Protein Fractions by Capillary Gel Electrophoresis (CGE)
3.9. Particle Size Analysis
3.10. Quhiantification of Soluble and Insoluble Calcium Concentrations
3.11. Protein Microstructure Observation Using Confocal Laser Scanning Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Springer, J.; Kooy, F.K.; van den Broek, L.A.; Eggink, G. Production methods for hyaluronan. Int. J. Carbohydr. Chem. 2013, 2013, 624967. [Google Scholar] [CrossRef]
- Cheng, F.; Gong, Q.; Yu, H.; Stephanopoulos, G. High-titer biosynthesis of hyaluronic acid by recombinant Corynebacterium glutamicum. Biotechnol. J. 2016, 11, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Ling, P.; Wang, F. Constructing a recombinant hyaluronic acid biosynthesis operon and producing food-grade hyaluronic acid in Lactococcus lactis. J. Ind. Microbiol. Biotechnol. 2015, 42, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Sze, J.; Brownlie, J.; Love, C. Biotechnological production of hyalu-ronic acid: A mini review. 3 Biotech 2016, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Zając, M.; Kulawik, P.; Tkaczewska, J.; Migdał, W.; Filipczak-Fiutak, M.; Fiutak, G. The effect of hyaluronic acid addition on the properties of smoked homogenised sausages. J. Sci. Food Agric. 2017, 97, 2316–2326. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, A. Hyaluronic acid: Evaluation of efficacy with different molecular weights. Int. J. Chem. Res 2019, 1, 13–18. [Google Scholar] [CrossRef]
- Food and Drug Administration. GRAS Notice: GRN 976: Intended for Use as an Ingredient in Fruit Drinks/Ades, Carbonated Soft Drinks, Candy, Milk Drinks, Yogurt, and Ready-to-Eat Cereals at Levels Ranging from 40–60 mg/Serving. 2020. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=976 (accessed on 7 January 2024).
- Sutariya, S.G.; Salunke, P. Effect of hyaluronic acid on milk properties: Rheology, protein stability, acid and rennet gelation properties. Food Hydrocoll. 2022, 131, 107740. [Google Scholar] [CrossRef]
- Oe, M.; Mitsugi, K.; Odanaka, W.; Yoshida, H.; Matsuoka, R.; Seino, S.; Kanemitsu, T.; Masuda, Y. Dietary hyaluronic acid migrates into the skin of rats. Sci. World J. 2014, 2014, 378024. [Google Scholar] [CrossRef]
- Tan, C.; Yao, X.; Jafari, S.M.; Sun, B.; Wang, J. Hyaluronic acid-based nanodelivery systems for food bioactive compounds. Trends Food Sci. Technol. 2023, 141, 104163. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, J.; Jiang, Y.; Meng, Y.; Zhang, K.; Ban, Q.; Wang, X. Emulsions stabilised by casein and hyaluronic acid: Effects of high intensity ultrasound on the stability and vitamin E digestive characteristics. Ultrason. Sonochem. 2023, 94, 106314. [Google Scholar] [CrossRef] [PubMed]
- Sutariya, S.G.; Salunke, P. Effect of Hyaluronic Acid and Kappa-Carrageenan on Milk Properties: Rheology, Protein Stability, Foaming, Water-Holding, and Emulsification Properties. Foods 2023, 12, 913. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Li, C.; Diao, M.; Yan, M.; Wang, C.; Zhang, T. Characterization of interactions between whey protein isolate and hyaluronic acid in aqueous solution: Effects of pH and mixing ratio. Colloids Surf. B Biointerfaces 2021, 203, 111758. [Google Scholar] [CrossRef]
- Chon, J.-W.; Kim, B.; Seo, K.-H.; Bae, D.; Jeong, D.; Song, K.-Y. Physiochemical and organoleptic properties of kefir containing different concentrations of hyaluronic acid: A preliminary study. J. Dairy Sci. Biotechnol. 2020, 38, 146–153. [Google Scholar] [CrossRef]
- Allison, D.D.; Grande-Allen, K.J. Hyaluronan: A powerful tissue engineering tool. Tissue Eng. 2006, 12, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Fagien, S.; Cassuto, D. Reconstituted injectable hyaluronic acid: Expanded applications in facial aesthetics and additional thoughts on the mechanism of action in cosmetic medicine. Plast. Reconstr. Surg. 2012, 130, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Kogan, G.; Šoltés, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef]
- Jagannath, S.; Ramachandran, K. Influence of competing metabolic processes on the molecular weight of hyaluronic acid synthesized by Streptococcus zooepidemicus. Biochem. Eng. J. 2010, 48, 148–158. [Google Scholar] [CrossRef]
- Tammi, R.H.; Kultti, A.; Kosma, V.-M.; Pirinen, R.; Auvinen, P.; Tammi, M.I. Hyaluronan in human tumors: Pathobiological and prognostic messages from cell-associated and stromal hyaluronan. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2008; Volume 18, pp. 288–295. [Google Scholar]
- Hooi, R.; Barbano, D.; Bradley, R.; Budde, D.; Bulthaus, M.; Chettiar, M.; Lynch, J.; Reddy, R. Chapter 15 chemical and physical methods. In Standard Methods for the examination of dairy products, 17th ed.; American Public Health Association: Washington DC, USA, 2004; pp. 480–510. [Google Scholar]
- Sutariya, S.G.; Huppertz, T.; Patel, H.A. Influence of milk pre-heating conditions on casein–whey protein interactions and skim milk concentrate viscosity. Int. Dairy J. 2017, 69, 19–22. [Google Scholar] [CrossRef]
- Kasinos, M.; Karbakhsh, R.R.; Van der Meeren, P. Sensitivity analysis of a small-volume objective heat stability evaluation test for recombined concentrated milk. Int. J. Dairy Technol. 2015, 68, 38–43. [Google Scholar] [CrossRef]
- Sutariya, S.; Patel, H. Effect of hydrogen peroxide on improving the heat stability of whey protein isolate solutions. Food Chem. 2017, 223, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Czaja, T.P.; Glover, Z.J.; Ipsen, R.; Jæger, T.C.; Rovers, T.A.; Simonsen, A.C.; Svensson, B.; van den Berg, F.; Hougaard, A.B. Water mobility and microstructure of acidified milk model gels with added whey protein ingredients. Food Hydrocoll. 2022, 127, 107548. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Subcritical water extraction (SWE) modified by deep eutectic solvent (DES) for pectin recovery from a Brazilian berry by-product. J. Supercrit. Fluids 2022, 189, 105729. [Google Scholar] [CrossRef]
- Cano-Medina, A.; Jiménez-Islas, H.; Dendooven, L.; Herrera, R.P.; González-Alatorre, G.; Escamilla-Silva, E.M. Emulsifying and foaming capacity and emulsion and foam stability of sesame protein concentrates. Food Res. Int. 2011, 44, 684–692. [Google Scholar] [CrossRef]
- Salunke, P.; Marella, C.; Metzger, L.E. Microfiltration and ultrafiltration process to produce micellar casein and milk protein concentrates with 80% crude protein content: Partitioning of various protein fractions and constituents. Dairy 2021, 2, 367–384. [Google Scholar] [CrossRef]
- Lin, L.; Wong, M.; Deeth, H.; Oh, H. Calcium-induced skim milk gels using different calcium salts. Food Chem. 2018, 245, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.; Reeder, W. Method for Titrating Calcium. U.S. Patent 2,910,349, 27 October 1959. [Google Scholar]
- Pearce, K. The complexometric determination of calcium in dairy products. N. Z. J. Dairy Sci. Technol. 1977, 12, 113–115. [Google Scholar]
- Zhang, Y.; Xu, X.; Xu, J.; Zhang, L. Dynamic viscoelastic behavior of triple helical Lentinan in water: Effects of concentration and molecular weight. Polymer 2007, 48, 6681–6690. [Google Scholar] [CrossRef]
- de Bont, P.W.; van Kempen, G.M.; Vreeker, R. Phase separation in milk protein and amylopectin mixtures. Food Hydrocoll. 2002, 16, 127–138. [Google Scholar] [CrossRef]
- Nayak, S.; Makrariya, A.; Singh, R.; Patel, A.; Sindhu, J.; Patil, G.; Tomar, P. Heat stability and flow behaviour of buffalo milk added with corn starch. Food Hydrocoll. 2004, 18, 379–386. [Google Scholar] [CrossRef]
- Tziboula, A.; Muir, D.D. Milk protein-carbohydrate interactions. Int. Dairy J. 1993, 3, 209–223. [Google Scholar] [CrossRef]
- Singh, H.; Creamer, L. Heat stability of milk. In Advanced Dairy Chemistry-1: Proteins; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1992; pp. 621–656. [Google Scholar]
- Tziboula, A.; Muir, D.D. Effect of starches on the heat stability of milk. Int. J. Food Sci. Technol. 1993, 28, 13–24. [Google Scholar] [CrossRef]
- Pang, Z.; Deeth, H.; Bansal, N. Effect of polysaccharides with different ionic charge on the rheological, microstructural and textural properties of acid milk gels. Food Res. Int. 2015, 72, 62–73. [Google Scholar] [CrossRef]
- Wilde, P.; Mackie, A.; Husband, F.; Gunning, P.; Morris, V. Proteins and emulsifiers at liquid interfaces. Adv. Colloid Interface Sci. 2004, 108, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Huang, G. Improving method, properties and application of polysaccharide as emulsifier. Food Chem. 2022, 376, 131937. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Medronho, B.; Filipe, A.; Mira, I.; Lindman, B.; Edlund, H.; Norgren, M. Emulsion formation and stabilization by biomolecules: The leading role of cellulose. Polymers 2019, 11, 1570. [Google Scholar] [CrossRef] [PubMed]
- Bureiko, A.; Trybala, A.; Kovalchuk, N.; Starov, V. Current applications of foams formed from mixed surfactant–polymer solutions. Adv. Colloid Interface Sci. 2015, 222, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Walstra, P.; Jenness, R. Dairy Chemistry & Physics; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Fox, P.F.; Mcsweeney, P.L.; Paul, L. Dairy Chemistry and Biochemistry; Springer: Cham, Switzerland, 1998. [Google Scholar]
- Farrell Jr, H.; Jimenez-Flores, R.; Bleck, G.; Brown, E.; Butler, J.; Creamer, L.; Hicks, C.; Hollar, C.; Ng-Kwai-Hang, K.; Swaisgood, H. Nomenclature of the proteins of cows’ milk—Sixth revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef]
- Sinaga, H.; Bansal, N.; Bhandari, B. Effects of milk pH alteration on casein micelle size and gelation properties of milk. Int. J. Food Prop. 2017, 20, 179–197. [Google Scholar] [CrossRef]





| % Composition | ||||
|---|---|---|---|---|
| Fat | Total Protein | Lactose | Total Solids | pH@ 25 °C |
| 0.06 ± 0.04 | 3.74 ± 0.03 | 4.98 ± 0.01 | 9.50 ± 0.03 | 6.65 ± 0.05 |
| Parameter | Treatment | ||||
|---|---|---|---|---|---|
| Control | 8 kDa | 320 kDa | 980 kDa | 2550 kDa | |
| log K (Pa sn) | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.02 ± 0.00 b | 0.92 ± 0.33 a | 0.71 ± 0.01 a |
| n (-) | 1.33 ± 0.04 a | 1.33 ± 0.03 a | 0.96 ± 0.02 b | 0.57 ± 0.04 c | 0.54 ± 0.02 c |
| Treatment | |||||
|---|---|---|---|---|---|
| Parameter | Control | 8 kDa | 320 kDa | 980 kDa | 2550 kDa |
| WHC | 0.00 ± 0.00 c | 0.15 ± 0.03 c | 15.00 ± 0.37 b | 17.08 ± 1.00 a | 14.60 ± 0.22 b |
| EA | 40.00 ± 0.00 c | 39.58 ± 0.42 c | 55.83 ± 3.52 b | 89.17 ± 1.54 a | 95.83 ± 0.83 a |
| ES | 38.00 ± 1.23 c | 37.50 ± 1.71 c | 45.00 ± 3.16 c | 81.00 ± 4.40 b | 95.00 ± 0.00 a |
| PPS | 5.43 ± 2.58 b | 4.50 ± 0.00 b | 61.54 ± 1.51 a | 66.74 ± 5.79 a | 66.04 ± 7.40 a |
| Parameter | Time Points | Treatment | ||||
|---|---|---|---|---|---|---|
| Control | 8 kDa | 320 kDa | 980 kDa | 2550 kDa | ||
| % Foam capacity | 0 h | 100.00 ± 0.00 abA | 103.33 ± 13.33 aA | 96.66 ± 3.33 ab | 63.33 ± 6.66 bc | 53.33 ± 3.33 cA |
| % Foam stability (Foam retention) | 0.5 h | 45.00 ± 5.00 bAB | 76.66 ± 13.33 abA | 96.66 ± 3.33 a | 66.66 ± 0.00 ab | 50.00 ± 8.82 bAB |
| 1 h | 40.00 ± 10.00 bAB | 63.33 ± 8.82 abABC | 90.00 ± 5.77 a | 63.33 ± 6.67 ab | 46.66 ± 3.33 bAB | |
| 1.5 h | 35.00 ± 15.00 bAB | 56.66 ± 3.33 bABC | 90.00 ± 5.77 a | 63.33 ± 6.67 ab | 46.66 ± 3.33 bAB | |
| 2 h | 25.00 ± 15.00 cAB | 43.33 ± 6.66 bcABC | 90.00 ± 5.77 a | 63.33 ± 6.66 ab | 46.66 ± 3.33 bcAB | |
| 4 h | 10.00 ± 10.00 cAB | 30.00 ± 11.55 bcBC | 90.00 ± 5.77 a | 63.33 ± 6.67 ab | 43.33 ± 3.33 bcAB | |
| 6 h | 0.00 ± 0.00 cB | 23.33 ± 14.53 cBC | 90.00 ± 5.77 a | 63.33 ± 6.67 ab | 40.00 ± 0.00 bcB | |
| 18 h | 0.00 ± 0.00 cB | 6.66 ± 6.67 cC | 83.33 ± 12.02 a | 60.00 ± 10.00 ab | 40.00 ± 0.00 bcB | |
| 24 h | 0.00 ± 0.00 bcB | 0.00 ± 0.00 cC | 66.66 ± 14.53 a | 60.00 ± 10.00 a | 40.00 ± 0.00 abB | |
| Parameters | Control | 8 kDa | 320 kDa | 980 kDa | 2550 kDa | |
|---|---|---|---|---|---|---|
| Particle size (nm) | 188.11 ± 1.76 | 190.28 ± 1.77 | 199.28 ± 0.51 | 194.56 ± 3.49 | 190.70 ± 4.35 | |
| %Area | β-CN | 33.36 ± 0.00 | 26.63 ± 1.85 | 27.31 ± 2.58 | 26.29 ± 1.51 | 28.52 ± 1.30 |
| αS1-CN | 37.50 ± 0.00 | 36.50 ± 0.97 | 38.20 ± 2.06 | 39.29 ± 1.39 | 36.05 ± 1.48 | |
| αS2-CN | 6.57 ± 0.00 | 9.98 ± 0.52 | 8.87 ± 1.45 | 10.23 ± 1.42 | 7.96 ± 0.61 | |
| k-CN | 6.05 ± 0.00 | 8.29 ± 1.30 | 7.99 ± 1.64 | 5.03 ± 1.43 | 5.81 ± 1.35 | |
| γ-CN | 1.08 ± 0.00 | 1.66 ± 0.34 | 1.80 ± 0.52 | 2.50 ± 0.66 | 2.36 ± 0.50 | |
| α-LA | 4.24 ± 0.00 | 3.64 ± 0.28 | 3.12 ± 0.23 | 3.76 ± 0.39 | 4.13 ± 0.17 | |
| β-LG | 11.20 ± 0.00 | 13.24 ± 0.72 | 12.59 ± 1.71 | 12.85 ± 1.72 | 15.15 ± 0.28 | |
| %Total CN | 84.56 ± 0.00 | 83.06 ± 0.96 | 84.18 ± 1.97 | 83.34 ± 2.09 | 80.71 ± 0.40 | |
| % Total WP | 15.40 ± 0.00 | 16.88 ± 0.96 | 15.71 ± 1.92 | 16.61 ± 2.09 | 19.27 ± 0.42 | |
| Treatments | Mass of Soluble Calcium in Serum (mmol/L) | Mass of Insoluble Calcium in Sediment (Calculated) (mmol/L) |
|---|---|---|
| Control | 11.25 ± 0.48 | 22.00 ± 1.47 |
| 8 kDa | 11.33 ± 0.84 | 22.50 ± 0.99 |
| 320 kDa | 10.50 ± 0.76 | 23.33 ± 0.80 |
| 980 kDa | 10.83 ± 0.48 | 23.33 ± 0.80 |
| 2550 kDa | 10.66 ± 0.42 | 23.33 ± 1.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, R.; Sutariya, S.G.; Salunke, P. Effect of Different Molecular Weight Hyaluronic Acids on Skim Milk Functional Properties. Foods 2024, 13, 690. https://doi.org/10.3390/foods13050690
Joshi R, Sutariya SG, Salunke P. Effect of Different Molecular Weight Hyaluronic Acids on Skim Milk Functional Properties. Foods. 2024; 13(5):690. https://doi.org/10.3390/foods13050690
Chicago/Turabian StyleJoshi, Rutvi, Suresh G. Sutariya, and Prafulla Salunke. 2024. "Effect of Different Molecular Weight Hyaluronic Acids on Skim Milk Functional Properties" Foods 13, no. 5: 690. https://doi.org/10.3390/foods13050690
APA StyleJoshi, R., Sutariya, S. G., & Salunke, P. (2024). Effect of Different Molecular Weight Hyaluronic Acids on Skim Milk Functional Properties. Foods, 13(5), 690. https://doi.org/10.3390/foods13050690






