Abstract
Nitric oxide (NO) is an inorganic radical produced by both the non-enzymatic nitrate (NO3−)—nitrite (NO2−)—NO pathway and enzymatic reactions catalyzed by nitric oxide synthase (NOS). Also, as nitrate and nitrite from dietary and other endogenous sources can be reduced back to nitric oxide in vivo, the endogenous NO level can be increased through the consumption of nitrate–rich vegetables. Ingestion of dietary NO3− has beneficial effects which have been attributed to a subsequent increase in NO: a signaling molecule that may regulate various systems, including the cardiovascular system. A diet rich in NO3− from green leafy and root vegetables has cardioprotective effects, with beetroot products being particularly good sources of NO3−. For example, various studies have demonstrated a significant increase in nitrite levels (regarded as markers of NO) in plasma after the intake of beetroot juice. The present review describes the current literature concerning the role of nitrate-rich vegetables (especially beetroot products) in the prophylaxis and treatment of cardiovascular diseases (CVDs). This review is based on studies identified in electronic databases, including PubMed, ScienceDirect, Web of Knowledge, Sci Finder, Web of Science, and SCOPUS.
1. Introduction
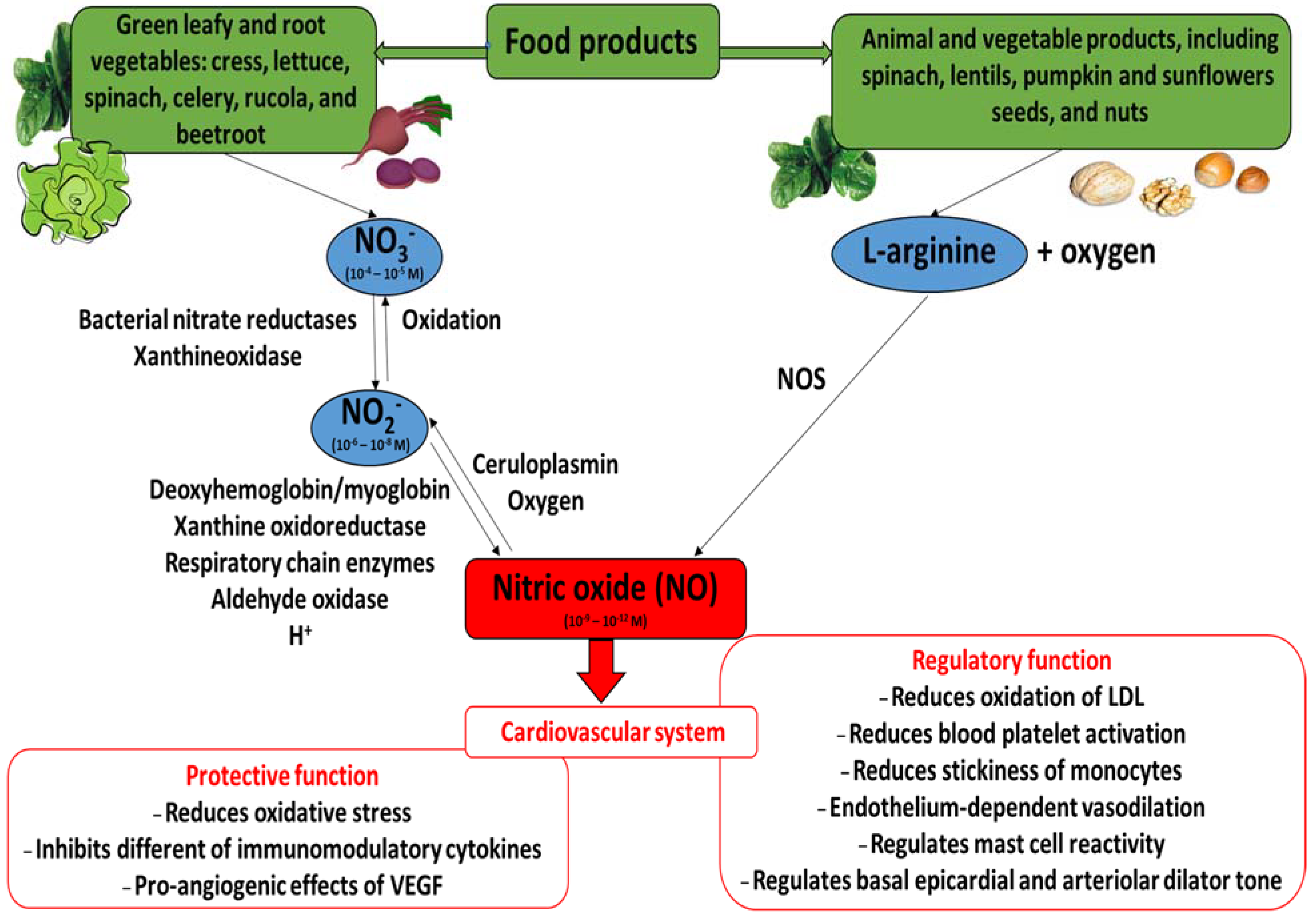
Nitric oxide (NO) is an inorganic radical produced by both non-enzymatic and enzymatic reactions catalyzed by nitric oxide synthase (NOS; EC 1.14.13.39) (Figure 1). This enzyme exists as three isoforms: inducible nitric oxide synthase (iNOS), neuronal nitric oxide synthase (nNOS, constitutive form), and endothelial nitric oxide synthase (eNOS, constitutive form). Various cells may produce NO, including endothelial cells, neutrophils and macrophages. The conversion of L-arginine to L-cytruline by NOS (L-arginine—NOS pathway) is the primary source of nitric oxide, yielding about 70%. Also, NO can be synthesized through the nitrate (NO3−)—nitrite (NO2−)—NO pathway. When blood pH falls and oxygen-dependent NOS activity is limited, during ischemia for example, the formation of NO by the non-enzymatic reduction of nitrite/nitrate from dietary and endogenous sources becomes important. There is evidence that mammalian tissues have the capacity to reduce NO3− to NO2− via xanthine oxidoreductase or by the oral microbiome. However, humans have a greater proportional dependence on NO3− reduction via the oral microbiome than by xanthine oxidoreductase compared with other mammals [1,2,3,4,5,6,7,8,9,10,11,12,13]. Interestingly, the reduction of nitrite by mitochondrial amidoxime reducing component (mARC) in the mitochondria may be an important signaling pathway for NADH-dependent hypoxic NO production [14,15].
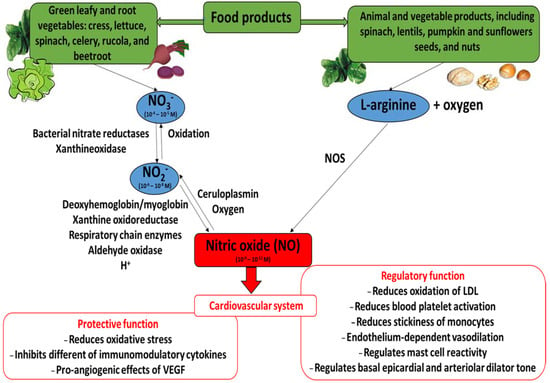
Figure 1.
The nitrate (NO3−)/nitrite (NO2−)/nitric oxide (NO) pathways and beneficial action of nitric oxide in cardiovascular system. Concentrations market for NO3−, NO2− and NO are those detected (NO3− and NO2−) or estimated (NO) in blood ([7], modified).
The cardioprotective action associated with the consumption of fruits and vegetables have been attributed to their constituents, including minerals, fiber, vitamins and secondary metabolites. These cardioprotective properties may be also associated with the consumption of nitrate-rich food products [16,17,18,19,20,21,22,23,24]. Although vegetables represent the primary source of dietary nitrate (about 70–80% of intake), the European Food Safety Authority [25] report that fruits are also important sources, contributing 50% to 75% to the overall dietary intake. For example, a traditional Japanese diet is very high in nitrate from vegetables, and Japan has lower rates of coronary heart disease than the United States. It is also important to note that dietary patterns associated with blood pressure lowering, such as the Dietary Approaches to Stop Hypertension (DASH) diet is based around combinations of vegetables supplying about 160 mg of nitrate/day [24].
The present review describes the current literature concerning the role of nitrate-rich vegetables and their food products (especially beetroot products) in the prophylaxis and treatment of cardiovascular diseases (CVDs). The studies were identified in electronic databases, including PubMed, ScienceDirect, Web of Knowledge, Sci Finder, Web of Science, and SCOPUS. The last search was run on 12 February 2024, based on the following terms: “nitric oxide”; “nitrite”, “nitrate”, “beet”, “beetroot”, “cardiovascular system”, and “cardiovascular disease”.
2. Dietary Sources of Nitrate
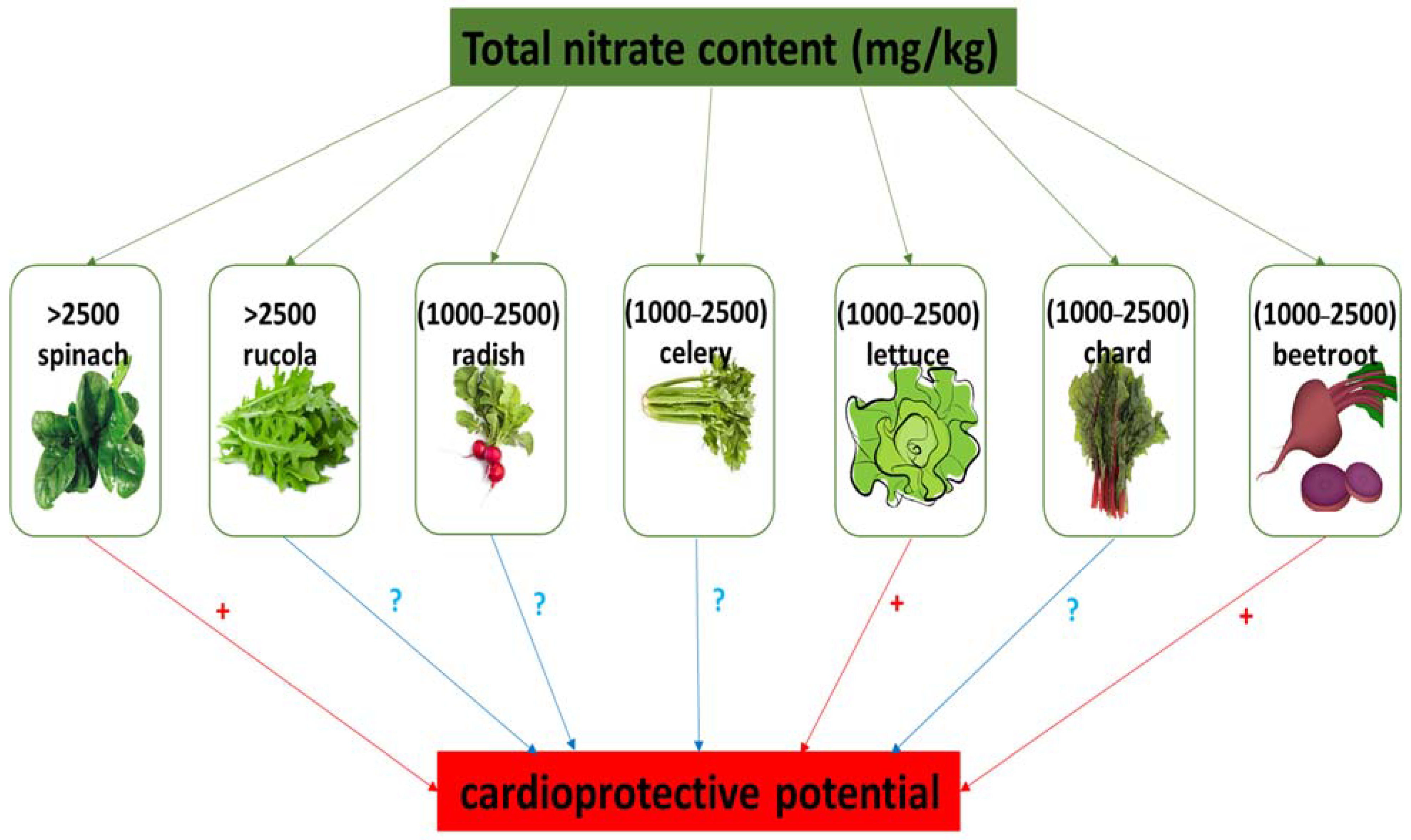
In humans, nitrate and nitrite reserves may be effectively increased through the consumption of green leafy vegetables and root vegetables, including beetroot, cress, lettuce, spinach, rucola, and celery, which may contain 1000 to 2000–2500 mg nitrate per kg−1 fresh weight [10,17,18,19]. In contrast, onions, peas, and potatoes contain low levels of NO3−. Also, leafy vegetables have higher levels of nitrate compared to tubers and seeds. Various dietary sources of nitrate and their classification based on nitrate content is presented in Table 1. In addition, Figure 2 shows the nitrate content (mg/kg) in selected vegetables and fruits as described by The European Food Safety Authority (EFSA) [25]. The level of NO3− in vegetables is dependent on different factors, including soil type, the intensity of sunlight, nitrate content in water and fertilizers, cooking procedures, transport methods, and storage conditions [10,17,19,26,27]. The results of Ding et al. [28] indicate that pickled vegetable (for example pickled beets) have a lower nitrate content compared to fresh vegetables.
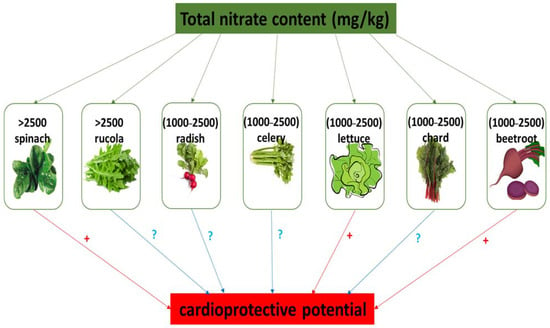
Figure 2.
Nitrate content in selected vegetables and their cardioprotective potential. (+) cardioprotective potential; (?) no data about cardioprotective properties.
After consumption, exogenous NO3− is absorbed by the gastrointestinal tract and enters the systemic circulation. Upon reaching the salivary glands, nitrate re-enters the oral cavity via protein transporters. About 25% is taken up by the salivary glands and concentrated in the saliva [29,30]. The results of Van Velzen et al. [31] report that nitrate from nitrate-rich vegetables has high bioavailability, and there are reports of close to 100% absorption following digestion [29,30].
NO3− administration may influence the efficacy of NO3−, given that NO3−-rich beetroot juice contains other bioactive compounds such as ascorbic acid, phenolics and betalains [26,32,33].
Recently, Cocksedge et al. [34] reported that independently increasing or lowering oral temperature or increasing oral pH significantly increased mean salivary NO2− after NO3− supplementation in healthy adults. In this experiment, seven healthy men consumed 70 mL/day of beetroot juice (which has about 6.2 nM NO3−) during six separate laboratory visits.

Table 1.
Dietary sources based on their nitrate content ([10,18,35], modified).
Table 1.
Dietary sources based on their nitrate content ([10,18,35], modified).
| Nitrate Content | Dietary Sources | References |
|---|---|---|
| Vegetables | ||
| Very high (>2500 mg/kg fw) | Celery, cress, chervil, lettuce, beetroot, spinach, rucola | [18] |
| High (1000 to 2500 mg/kg fw) | Celeriac, Chinese cabbage, endive, fennel, leek, parsley | [18] |
| Medium (500 to 1000 mg/kg fw) | Cabbage, dill, turnip | [18] |
| Low (200 to <500 mg/kg fw) | Broccoli, carrot, cauliflower, cucumber, pumpkin, chicory | [18] |
| Very Low (<200 mg/kg fw) | Artichoke, asparagus, garlic, onion, green bean, mushroom, pepper, potato, sweet potato, tomato, watermelon, apple, banana, grape, pear, orange, strawberry | [18,35] |
| Very low (<20 mg/kg fw) | Meat | [10] |
| Very low (5 mg per 100 g) | Water | [10] |
Fresh beetroot fresh juice is most commonly used for nitrate supplementation, but it has a lower concentration compared to other beetroot products (Table 2). Nevertheless, various studies have demonstrated a significant increase in nitrite levels (a marker of NO) in plasma after intake of beetroot juice [23,28,29,30,31,32,33,34,35,36,37,38,39].

Table 2.
Nitrate and nitrite contents of different beetroot products in 100 g of each products ([40], modified).
The stability of NO3− in vegetables and their food products is important when considering their functional activity. Corleto et al. [41] studied the stability of NO3− in beetroot juice and arugula juice for 32 days at different temperatures (25, and 4 °C). They observed that NO3− degradation starts within 24 h at 25 °C.
3. Regulatory Limits of Dietary Nitrate and Nitrite
The World Health Organization (WHO) and European Food Safety Authority have established the Acceptable Daily Intake of nitrate as 3.7 mg/kg of body weight, and nitrite as 0.06 mg/kg of body weight [42,43]. These limits translate into about 222 mg/day (NO3−) and 3.6 mg/day (NO2−) for a 60 kg person. In line with these recommendations, the consumption of 400 g of various vegetables and fruits per day, assuming median nitrate concentrations, would provide about 157 mg NO3− per day [17,19].
It is important to note that NO3−/NO2− can also be used as additives in foods. Nitrates (potassium nitrate—E252, sodium nitrate—E251), and nitrites (potassium nitrite—E250, sodium nitrite—E249) are authorized as food additives in the European Union under Commission Regulation (EU) No 1129/2011. They are used to stabilize processed cheese and meat. For example, the maximum concentration of nitrite is 150 mg/kg in cheese and in uncooked meat [44]. The maximum level for nitrate in vegetables (including spinach, lettuce and rocket) is also laid down in regulation (EC) No. 1258/2011 (set in the EU), expressed as mg nitrate/kg fresh weights [44].
Nitrates also act as anti-nutrient compounds [45,46], which have direct and indirect effects ranging from mild reactions to death. For example, the main anti-nutrients in lettuce include not only nitrates, but also tannins, phytates, and oxalates; these are described in a review by Shi et al. [45]. A number of papers have studied the conversion of NO3− and NO2− into nitrosamines, which have carcinogenetic potential [46,47]. Interestingly, the ecological risks connected with high nitrate consumption indicate that high nitrate content in vegetable is beneficial due to high content of natural antioxidants (polyphenols, betalain pigments, vitamins, and other) that prevent the formation of nitrosamines [48].
4. Nitric Oxide and Cardiovascular System
Nitric oxide acts as a gasomediator in various biological systems [49]. In the cardiovascular system, it induces vasorelaxation and promotes cardioprotection [3]. Moreover, different papers describe the fact that nitrate supplementation (including green leafy and root vegetables) may have an effect on cardiovascular health. They may help regulate blood pressure, limit a the progression of atherosclerosis, and improve myocardial contractility in healthy subjects and patients with cardiovascular diseases [8,10,23,36,37,39]. Figure 1 presents the beneficial action of nitric oxide (its regulatory and protective functions) in the cardiovascular system, together with the nitrate/nitrite/nitric oxide pathways.
Obtaining NO from natural food products is a better option for avoiding certain side effects than supplementation (for example with L-arginine and L-citruline), which may have mild to moderate side-effects, including gastrointestinal disturbances, heartburn, headache, and palpitations [50].
5. Role of Vegetable Nitrate in CVDs
Diabetes, obesity, hyperlipidemia and hypertension are considered risk factors for CVDs, including coronary heart, peripheral arterial disease, and cerebrovascular disease. In addition, CVDs become increasingly prevalent with age [51]; this has been attributed to vascular dysfunction, including the stiffening of the large elastic arteries (for example, carotid arteries and aorta). The development of endothelial dysfunction is the major clinical antecedent to atherosclerotic diseases such as occlusive stroke, coronary artery disease, and peripheral artery disease [52,53,54].
Various clinical studies and epidemiological evidence indicate an intake of diet enriched in vegetables and fruits has a significant effect on the prophylaxis and treatment of CVDs. Consuming a healthy diet may be also a good strategy for preserving vascular function with aging, and some papers suggest that sodium nitrite and nitrate supplementation may also be effective [18,55,56,57,58,59,60,61]. For example, Sindler et al. [55,56], Flennor et al. [57] and Woodward et al. [61] found that three-week supplementation of drinking water with sodium nitrite (80 mg/day) reduced systemic vascular resistance in C57BL/6 mice. In addition, many of the biologically active phytochemicals (including NO3−) present in green leafy and root vegetables may bestow cardioprotective benefits by various mechanisms.
In humans, consumption of nitrate (as a meal or supplement) induces increased circulating NO3− and NO2−. Moreover, chronic consumption of whole food NO3− sources elevates plasma NO3− and NO2− concentrations which are comparable to beetroot juice [62,63]. A review by Karwowska and Kononiuk [44] indicate that NO3− intake has a number of beneficial effects on the cardiovascular system such as triglyceride reduction, blood pressure regulation, and stroke and atherosclerosis prevention. Moreover, dietary NO3− may also play an important role in improving cardiovascular risk factors, with beneficial effects observed on a reduction in blood platelet activation, including platelet aggregation [10]. These properties were observed in both healthy subjects and patients with obesity and hypertension. The effects were particularly visible when dietary NO3− intake (between 68 mg/day and 1395 mg/day) was in the form of beetroot juice and breads, arugula juice, and spinach leaves and juice administered between two hours and 42 days [10]. For example, Ashworth et al. [58] observed that the consumption of two portions of high-nitrate vegetables daily resulted in a reduction in blood pressure in normotensive women.
Various dietary components may interact with nitrate and/or nitrite, interfering with their antihypertensive action. For example, thiocyanate competes with NO3− for absorption by the salivary glands. Cigarette smoke may also impair the metabolism of NO3−. On the other hand, the consumption of foods rich in phenolic compounds may enhance nitrite reduction in the stomach, which, in turn, may boost the effect of NO3− on blood pressure [64].
6. Beetroot Products
Beetroot (Beta vulgaris L.) belongs to the Chenopodiaceae family. It is grown in various countries, and is consumed as part of the normal diet. In addition, it is used in manufacturing as a food coloring agent (known as E162) [39,65]. It is a source of various important bioactive compounds: dietary fiber, minerals (sodium, potassium, copper, iron, zinc, phosphorus, calcium, and magnesium), phenolic compounds (for example, phenolic acids, and flavonoids), ascorbic acid, carotenoids and betalains, including betanin [39,40,66,67]. Most importantly, it is also considered as a valuable source of nitrate. However, traditional beetroot formulations, including cooked vegetables and fresh juice must be offered in large amounts to reach pharmacological nitrate concentrations [40,66]. Despite this, consumption of concentrated beetroot juice significantly increases NO3− and NO2− with peak concentration occurring one to three hours post-consumption [62,63]. Beetroot juice contains a high concentration of nitrate (up to 11.4 g/L) as compared to drinking water (<45 mg/L in European countries) [68]. Webb et al. [2] noted a significant increase in NO3− and NO2− concentration in plasma: NO3− up to 182 ± 55 µM after one to two hours (equivalent to 550%), and NO2−—up to 373 ± 211 µM after two to three hours (equivalent to 400%).
Recently, Brzezinska-Rojek et al. [69] reported that a serving of fresh beetroot provides significantly more nitrates and nitrites than most daily portions of beetroot-based dietary supplements.
Other studies have examined the effects of other beetroot products, including fermented juice, powder, bread, chips, crunchy slices, gel, and cereal bars, as supplements in healthy subjects or patients [40,66,70,71,72].
Beetroot and its products provide a variety of health advantages and may help prevent and manage various diseases, including CVDs. In addition, taking 8 g of dried beetroot for 20 days has an effect on hematological parameters. For example, these results showed a mild increase in hemoglobin readings, a decrease in the total iron binding capacity, decrease in transferrin and increase in ferritin [40,66,70,71,72].
Other potential positive effects of beetroot supplementation on cardiovascular health include reduced blood pressure, increased blood flow, improved endothelial function, a reduced renal resistance index and others [73]. Various systematic reviews about the effects of beetroot juice on blood pressure indicate that its consumption may have a beneficial role in the prevention and treatment of hypertension [36,73,74]. For example, a meta-regression by Siervo et al. [75] demonstrated an association between a daily dose of inorganic nitrate and changes in systolic blood pressure. Beetroot juice supplementation was also associated with a significant reduction in systolic blood pressure in adults. This study included 254 participants [75].
A systematic review of 11 studies by Ocampo et al. [36] also showed that beetroot juice supplementation is an effective strategy that may reduce blood pressure in populations of healthy and hypertensive patients, probably through the nitrate/nitrite/nitric oxide pathway and secondary metabolites found in beetroot. However, this effect depends on age, sex, baseline blood pressure, body composition, and body weight. Even so, beetroot juice supplementation seems to improve blood pressure control throughout adult life (45+). There is also a better response after this supplementation in the population with body mass index (BMI) > 25 and when there is a high baseline blood pressure. A study by Kim et al. on postmenopausal women [76] found blood pressure to be reduced after consumption of beetroot juice. Another meta-analysis showed a two-week supplementation with 500 mL/day of beetroot juice to have a particularly beneficial effect on blood pressure, and that the effect of this supplementation generated better results, compared to those with a duration of one week [60,77]. For example, reduced blood pressure was achieved with 250 mL intake of beetroot juice daily over four weeks.
On the other hand, other studies do not demonstrate any such reduction after nitrate-rich beetroot supplementation [78,79,80,81]. For example, Perez et al. [81] observed no significant changes in blood pressure in during handgrip exercise after a single shot of beetroot juice versus placebo, and neither did Craig et al. [78].
Oxidative stress is known to be an important factor for the development of CVDs, and plant antioxidants often have cardioprotective effects. Clifford et al. [39] report that beetroot supplementation may serve as a useful strategy for protecting cellular components from oxidative stress (in vitro and in vivo), and that beetroot juice demonstrates comparable, or higher, antioxidant capacity compared to carrot or tomato juice, and to pineapple or orange juice. Only pomegranate juice had a higher antioxidant capacity based on in ferric reducing antioxidant power (FRAP) assay. The study tested ten commercially available vegetable and fruit beverages in the UK [32,39,82].
A few papers have suggested that nitrate supplementation increases vasodilatation in human skin following heat stress, but that NOS-dependent vasodilatation was not affected by nitrate supplementation [83,84,85,86,87,88]. Other research groups observed that beetroot, as a natural NO donor, may preserve or restore endothelial function. For example, Webb et al. [2] demonstrated that beetroot supplementation (500 mL/day) preserves brachial artery endothelial function in healthy participants. Joris and Mensik [83] also observed that beetroot juice (140 mL/day) improves postprandial endothelial function in overweight and slightly obese men. A systematic review and meta-analysis also demonstrated that inorganic nitrate and beetroot supplementation, including beetroot juice, was associated with beneficial effects on endothelial function. In addition, these effects appear to be reduced in older subjects and in subjects with greater cardiometabolic risk [89]. On the other hand, some papers indicate that beetroot products have no influence on endothelial function; for example, Kenjale et al. [90] indicated that beetroot juice supplementation (500 mL/day) has no effect on endothelial function in peripheral arterial disease patients.
A pivotal mechanism in the pathogenesis of various CVDs, including stroke and acute coronary syndrome, is blood platelet activation. McKnight et al. [91] reported that oral nitrate inhibits platelet activation in healthy volunteers, and that the formation of S-nitrosothiols may be involved in the inhibition of platelet activation. Richardson et al. [92] also indicated that nitrate (0.5 mmol and 2 mmol) increases gastric S-nitrosothiol concentrations and inhibits blood platelet activation in healthy subjects. Other authors also found dietary nitrate from beetroot juice to have an inhibitory effect on platelet activation induced by ADP and collagen treatment [2,77].
Beetroot may be consumed not only in juice form, but also a whole food, gel form, and incorporated into bread [12,71,93]. Capper et al. [12] suggest that beetroot in its whole form may be a source of nitrate, but NO3− concentrations vary depending on various factors, including the time of harvest. In addition, they examined whether beetroot food is a good form of dietary nitrate that can reduce blood pressure and improve blood flow in both young and older adults. In this experiment, 24 healthy, non-smoking participants, i.e., 12 young (age: 27 ± 4 years) and 12 older (age: 64 ± 5 years), consumed whole cooked beetroot in three portions: 100, 200, and 300 g, on four separate occasions over a four-week period. The main finding was that while incremental doses of dietary nitrate reduced systolic and diastolic blood pressure in young participants, significant decreases were only observed with the highest dose in the older group. Moreover, none of the interventions modified microvascular blood flow in either tested group; however, all interventions increased plasma nitrate and nitrite levels in both groups.
In addition, Hobbs et al. [71] noted that intake of a beetroot-enriched bread (100 g/day) can augment marked improvements in intravascular function in young healthy men.
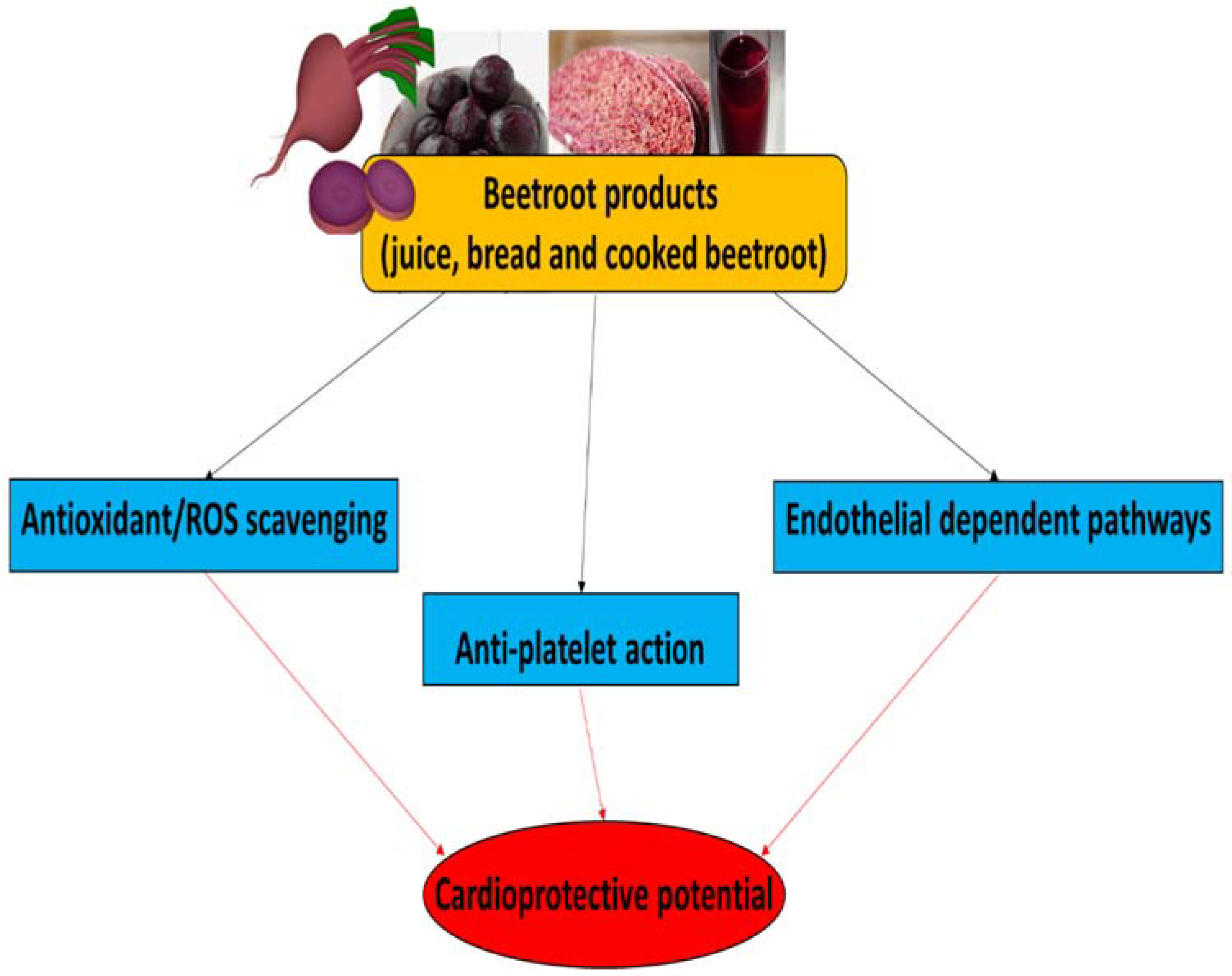
More information about the functional properties of beetroot, especially beetroot juice, in the management of cardio-metabolic diseases, including hypertension, insulin resistance, diabetes and kidney dysfunction, have been described in a review paper by Mirmiran et al. [94]. The therapeutic potential of beetroot products for CVDs, and their main biological properties, are summarized in Figure 3.
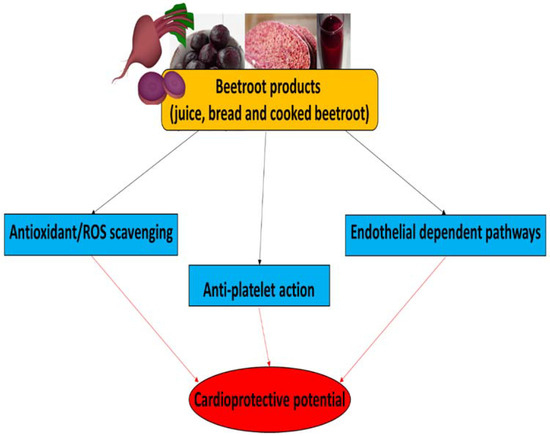
Figure 3.
The therapeutic potential of beetroot products for CVDs and their main biological properties.
7. Spinach Products
Spinach (Spinacia oleracea L.) is a member of the family Amaranthaceae and is known for its dark green leaves. The family also includes chard and beets. About 82 unique varieties of spinach exist. Moreover, it is classified into three types according to the leaf texture: (I) savoy, (II) semi-savoy, and (III) smooth-leaf. Savoy and semi-savoy are used for cooking (for example soups, casseroles, and steaming), whereas smooth-leaf spinach is the preferred leaf type for salads, smoothies, and processing. In America, spinach is often consumed with other green leafy vegetables, such as cabbage (Brassica oleracea L. var. capitate L.), lettuce (Lactuca sativa L.), and broccoli (Brassica oleracea var. italica Plenck) [95].
Spinach is primarily composed of water (91.4%) and contains small amounts of lipids (0.4%, mainly mono- and polysaturated fatty acids), carbohydrates (3.6%), and proteins (2.9%). It also contains 2.2 g fiber, different vitamins and phenolic acids [95].
Various in vitro and in vivo studies on animals and humans indicate that spinach may protect against chronic diseases, including CVDs [95,96]. It is known to have various antioxidant, hypoglycemic, lipid-lowering, anti-obesity, antiproliferative, and anti-inflammatory properties [95,97]. For example, a recent study by Panda et al. [96] in which rats were treated with spinach mixture (400 and 800 mg/kg daily for 30 days), found spinach to demonstrate cardioprotective activity against isoproterenol-induced myocardial infarction in rats. In addition, Jovanovski et al. [98] studied the effect of 500 mL of spinach soup (845 mg nitrate/day for seven days) on arterial stiffness and blood pressure on 27 healthy participants. Their findings demonstrate that dietary NO3− has promising potential to improve vascular health by decreasing arterial stiffness and blood pressure.
8. Lettuce and Other Nitrate-Rich Vegetable Products
Lettuce (Lactuca sativa L.), belonging to the Asteracea family, is a successful and diverse plant distributed worldwide. Lettuce is considered a particularly important leafy vegetable. It is rich in water (94–95% content) and low in calories. It is also a good source of minerals, vitamins, phenolic compounds and chlorophyll, but the phytochemical contents differ between the types of lettuce; for example, red lettuce has higher phenolic compound level than green [45,99,100,101,102].
Lettuce has a number of interesting impacts on CVD factors by different mechanisms owing to its fiber content and antioxidant availability [45,100,101,103]. For example, Nicolle et al. [103] noted a beneficial effect of lettuce consumption on lipid metabolism and on tissue oxidation in rats. Moreover, Abdalla et al. [104] observed that 20 mg/mL methanol extract from red lettuce has stronger radical scavenging activity than green lettuce based on free radical 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) assay in vitro. Rolnik et al. [100] also indicated that preparations from green lettuce and red lettuce (L. sativa var. crispa) leaves have antioxidant properties in human plasma treated with a hydroxyl radical donor (in vitro model). The red lettuce leaf preparation also demonstrated anti-platelet potential in vitro, inhibiting blood platelet adhesion to collagen and fibrinogen. In addition, neither preparation was found to cause blood platelet lysis [101].
Kammoun et al. [105] report that Ulva lactuce ethanolic extract has hypolipidemic and cardioprotective actions in hypercholesterolemic mice. The tested extract alleviated cardiotoxicity, as shown by cell viability, heart oxidative stress, index of atherogenesis and plasma biochemical parameters. In addition, hypercholesterolemic mice supplemented with U. lactuca decreased expression of proinflammatory cytokines (tumor necrosis factor-α (TNF-α), intereleukins (IL-1β and IL-6)). Moreover, used extract had antioxidant activity in vitro.
However, none of the authors described the concentration of NO3− in preparations and extracts from lettuce [100,101,103,105]. In addition, no studies have examined the relationships between cardioprotective action and NO3− concentration among other NO3− rich vegetables such as rucola, radish, celery and chard. For example, a review by Kooti and Daraei [106] only demonstrates that the antioxidant activity of celery is associated with various phenolic compounds, including apigenin, luteolin, and kaempferol.
9. Conclusions
Vegetable nitrate, especially that found in beetroot products (including juice, bread, and cooked beetroot) should be promoted as a key component of a healthy lifestyle aimed at controlling cardiovascular system function; however, there is also a need to study other factors related to supplementation with nitrate-rich vegetables, including secondary metabolites more deeply [107]. In addition, the cardioprotective mechanism by which NO3−-rich vegetable consumption remains unclear and poorly defined in the scientific literature.
The supplementation of beetroot products (especially, beetroot juice, bread and cooked beetroot) has been reported to reduce blood pressure, attenuate inflammation, decrease oxidative stress and inhibit blood platelet activation. However, there is a need for further clinical trials examining the cardioprotective potential and safety of nitrate-rich vegetables (not only beetroot, but also spinach, rucola, radish, celery, lettuce, and chard) and their products.
Like other vegetables, beetroot also contains a number of phenolic compounds which may have cardioprotective activity themselves as well as various synergistic effects.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
BMI—body mass index; CVDs—cardiovascular diseases; DPPH—2,2-diphenyl-1-picryl-hydrazyl-hydrate; EFSA—The European Food Safety Authority; FRAP—ferric-reducing antioxidant power; IL-interleukin; NOS—nitric oxide synthase; eNOS—endothelial nitric oxide synthase; iNOS—inducible nitric oxide synthase; nNOS—neuronal nitric oxide synthase; NO—nitric oxide; NO3−—nitrate; NO2−—nitrite; TNF-α—tumor necrosis factor-α; WHO—World Health Organization.
References
- Govoni, M.; Jansson, E.A.; Weitzberg, E.; Lundberg, J.O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 2008, 19, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N.; et al. Acute blood pressure lowering, vsoprotective, and antiplatelet properties of dietry nitrate via bioconversion to nitrite. Hypertension 2008, 51, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Gasomediators (NO, CO, and H2S) and their role in hemostasis and thrombosis. Clin. Chim. Acta 2015, 445, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Khatri, J.; Mills, C.E.; Maskell, P.; Odongerel, C.; Webb, A.J. It is rocket science—Why dietary nitrate is hard to “beet”! Part I—twists and turns in the realization of the nitrate-nitrite-NO pathway. Br. J. Clin. Pharmacol. 2017, 83, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.E.; Khatri, J.; Maskell, P.; Odongerel, C.; Webb, A.J. It is rocket science—Why dietary nitrate is hard to “beet”! Part II: Further mechanisms and therapeutic potential of the nitrate-nitrite-NO pathway. Br. J. Clin. Pharmacol. 2017, 83, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Malone-Povolny, M.; Maloney, S.E.; Schoenfisch, M.H. Nitric oxide therapy for diabetic wound healing. Adv. Healthc. Mater. 2019, 8, e1801210. [Google Scholar] [CrossRef]
- Jones, A.M.; Vanhatalo, A.; Seals, D.R.; Rossman, M.J.; Piknova, B.; Jonvik, K.L. Dietary nitrate and nitric oxide metabolism: Mouth, circulation, skeletal muscle, and exercise performance. Med. Sci. Sports Exerc. 2021, 1, 280–294. [Google Scholar] [CrossRef]
- Dudhe, R.; Dudhe, A.C.; Raut, S.D. Significance of inorganic nitrate supplement in cardiovascular health. Cardiovasc. Hematol. Agents Med. Chem. 2022, 20, 83–89. [Google Scholar] [CrossRef]
- Kiani, A.K.; Bonetti, G.; Medori, M.C.; Caruso, P.; Manganotti, P.; Fioretti, F.; Nodari, S.; Connelly, S.T.; Bertelli, M. Dietary supplements for improving nitric-oxide synthesis. J. Prev. Med. Hyg. 2022, 63, E239–E245. [Google Scholar]
- Rajendra, A.; Bondonno, N.P.; Rainey-Smith, S.R.; Gardener, S.L.; Hodgson, J.M.; Bondonno, C.P. Potential role of dietary nitrate in relation to cardiovascular and cerebrovascular health, cognition, cognitive decline and dementia: A review. Food Funct. 2022, 13, 12572–12589. [Google Scholar] [CrossRef]
- Capper, T.E.; Siervo, M.; Clifford, T.; Taylor, G.; Iqbal, W.; West, D.; Stevenson, E.J. Pharmacokinetic profile of incremental oral doses of dietary nitrate in young and older adults: A crossover randomized trial. J. Nutr. 2022, 152, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Capper, T.; Clifford, T.; Taylor, G.; Iqbal, W.; West, D.; Stevenson, E.; Siervo, M. Ageing modifies acute resting blood pressure responses to incremental consumption of dietary nitrate: A randomized, croos-over clinical trial. Br. J. Nutr. 2023, 129, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.M.; Townsend, J.R.; Pinzone, A.G.; Hoffman, J.R. Supplementation with nitric oxide precursors for strength performance: A review of the current literature. Nutrients 2023, 15, 660. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Jimenez, M.; Chamizo-Ampudia, A.; Calatrava, V.; Galvan, A.; Fernandez, E.; Liamas, A. From the eukaryotic molybdenum cofactor biosynthesis to the moonlighting enzyme mARC. Molecules 2018, 23, 3287. [Google Scholar] [CrossRef] [PubMed]
- Sparacino-Watkins, C.E.; Tejero, J.; Sun, B.; Gauthier, M.C.; Thomas, J.; Ragireddy, V.; Merchant, B.A.; Wang, J.; Azarov, I.; Basu, P.; et al. Nitrite reductase and nitric-oxide synthase activity of the mitochondrial molybdopterin enzymes mARC1 and mARC2. J. Biol. Chem. 2014, 289, 10345–10358. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S. Cardioproetctive actions of nitrite therapy and dietary considerations. Front. Biosci. 2009, 14, 4793–4808. [Google Scholar] [CrossRef] [PubMed]
- Hord, N.G.; Ghannam, J.S.; Garg, H.K.; Berens, P.D.; Bryan, N.S. Nitrate and nitrite content of human, formula, bovine, and soy milks: Implications for dietary nitrite and nitrate recommendations. Breastfeed Med. 2011, 6, 393–399. [Google Scholar] [CrossRef]
- Hord, N.G. Dietary nitrates, nitrites, and cardiovascular disease. Curr. Atheroscler. Rep. 2011, 13, 484–492. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 2. [Google Scholar] [CrossRef]
- Verma, T.; Sinha, M.; Bansal, N.; Raj Yadav, S.; Shah, K.; Chauhan, N.G. Plants used as antihypertensive. Nat. Prod. Bioprosp. 2021, 11, 155–184. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. Vegetables from Cucurbitaceae family anf their products: Positive effect on human health. Nutrition 2020, 78, 110788. [Google Scholar] [CrossRef] [PubMed]
- Rolnik, A.; Olas, B. The plants of the Asteraceae family as agents in the protection of human health. Intern. J. Molec. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef]
- Benjamin, C.J.R.; Sousa, Y.B.A.; Porto, A.A.; de Moraes Pontes, Y.M.; Tavares, S.S.; da Silva Rodrigues, G.; da Silva, L.S.L.; da Silva Goncalves, L.; Uimaraes, C.S.; Rebelo, M.A.; et al. Nitrate-rich beet juice intake on cardiovascular performance in response to exercise in postmenopausal women with arterial hypertension: Study protocol for randomized controlled trial. Trials 2023, 24, 94. [Google Scholar] [CrossRef]
- Ahluwalia, A.; Gladwin, M.; Coleman, G.D.; Hord, N.; Howard, G.; Kim-Shapiro, D.B.; Lajous, M.; Larsen, F.J.; Lefer, D.J.; McClure, L.A.; et al. Dietary nitrate and the epidemiology of cardiovascular disease: Report from a National Heart, lung, and blood institute workshop. J. Am. Heart Assoc. 2016, 5, e003402. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Nitrate in vegetables. Scientific opinion of the panel on contaminants in the food chain. EFSA J. 2008, 689, 1–79. [Google Scholar]
- Rahimi, P.; Mesbah-Namin, A.S.; Ostadrahimi, A.; Abedimanesh, S.; Separham, A.; Jafarabadi, M.A. Effects of betalains on atherogenic risk factors in patients with atheroserotic caradiovascular disease. Food Funct. 2019, 10, 8286–8297. [Google Scholar] [CrossRef]
- Anjana, A.; Umar, S.; Iqbal, M.; Abrol, Y.P. Are nitrate concentrations in leafy vegetables within safe limits? Curr. Sci. 2007, 92, 355–360. [Google Scholar]
- Ding, Z.; Johanningsmeir, S.D.; Price, R.; Reynolds, R.; Troung, V.D.; Payton, S.C.; Breidt, F. Evaluation of nitrate and nitrite content in pickled fruit and vegetable products. Food Control. 2018, 90, 304–311. [Google Scholar] [CrossRef]
- Jones, A.M. Dietary nitrate supplementation and exercise performance. Sports Med. 2014, 44, S35–S45. [Google Scholar] [CrossRef]
- Tan, R.; Cano, L.; Lago-Rodriguez, A.; Dominguez, R. The effects of dietary nitrate supplementation on explosive exercise performance: A systemiatic review. Int. J. Environ. Res. Public Health 2022, 19, 762. [Google Scholar] [CrossRef]
- Van Velzen, A.G.; Sips, A.J.; Schothorst, R.C.; Lambers, A.C.; Meulenbelt, J. The oral bioavailability of nitrate from nitrate-rich vegetables in human. Toxicol. Lett. 2008, 181, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Ryan, L. Combined use of multiple methodologies for the measurement of total antioxidant capacity in UK commercially available vegetable juices. Plant Foods Hum. Nutr. 2012, 67, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Cocksedge, S.P.; Causer, A.J.; Winyard, P.G.; Jones, A.M.; Bailey, S.J. Oral temperature and pH influence dietary nitrate metabolism in healthy adults. Nutrients 2023, 15, 784. [Google Scholar] [CrossRef]
- Uddin, R.; Thakur, M.U.; Uddin, M.Z.; Islam, G.M.R. Study of nitrate levels in fruits and vegetables to assess the potential health risks in Bangladesh. Sci. Rep. 2021, 1, 4704. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, D.A.B.; Paipilla, A.F.; Marin, E.; Vargas-Molina, S.; Petro, J.L.; Perez-Idarraga, A. Dietary nitrate from beetroot juice for hypertension: A systematic review. Biomolecules 2018, 2, 1–11. [Google Scholar]
- Zamani, P.; Rawat, D.; Shiva-Kumar, P.; Geraci, S.; Bhuva, R.; Konda, P.; Doulias, P.T.; Ischiropoulos, H.; Townsend, R.R.; Margulies, K.B.; et al. Effect of inorganicnitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation 2015, 131, 371–380. [Google Scholar] [CrossRef]
- Zamani, H.; de Joode, M.E.J.R.; Hossein, I.J.; Henckens, N.F.T.; Guggeis, M.A.; Berends, J.E.; de Kok, T.M.C.M.; van Breda, S.G.J. The benefits and risks of beetrrot juice consumption: A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 61, 788–804. [Google Scholar] [CrossRef]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef] [PubMed]
- dos Baiao, D.S.; da Silva, D.V.T.; Paschoalin, V.M.F. Beetroot, a remarkable vegetable: Its nitrate and phytochemical contents can be adjusted in novel formulations to benefit health and support cardiovascular disease therapies. Antioxidants 2020, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Corleto, K.A.; Singh, J.; Jayaprakasha, G.K.; Patil, B.S. Storage stability of dietary nitrate and phenolic compounds in beetrrot (Beta vulgaris) and arugula (Eruca sativa) juices. J. Food Sci. 2018, 83, 1237–1248. [Google Scholar] [CrossRef]
- WHO. Nitrate and Nitrite. In WHO Food Additive Series; WHO: Geneva, Switzerland, 2014; Volume 50, pp. 1–14. [Google Scholar]
- EFSA. EFSA Confirms Safe Levels for Nitrites and Nitrtes to Food; EFSA: Parma, Italy, 2017. [Google Scholar]
- Karwowska, M.; Kononiuk, A. Nitrates/nitrites in food—Risk for nitrative stress and benefits. Antioxidant 2020, 9, 241. [Google Scholar] [CrossRef]
- Shi, M.; Gu, J.; Wu, H.; Rauf, A.; Emran, T.B.; Khan, Z.; Mitra, S.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Al-Awthan, Y.S.; et al. Phytochemicals, nutrition, metabolism, bioavailability, and health benefits in lettuce—A comprehensive review. Antioxidants 2022, 11, 1158. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Carlstrom, M.; Weitzberg, E. Metabolic effects of dietary nitrate in health and disease. Cell Metab. 2018, 28, 9–22. [Google Scholar] [CrossRef]
- Tricker, A.R.; Preussmann, R. Carcinogenic N-nitrosamines in the diet: Occurrence, formation, mechanism and carcinogenesis potential. Mut. Res. 1991, 259, 277–289. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.; Hu, Z.; Wu, S.; Jin, C. Beetroot as a functional food with huge health benefits: Antioxidant, antitumor, physical function, and chronic metabolomics activity. Food Sci. Nutr. 2021, 9, 6406–6420. [Google Scholar] [CrossRef] [PubMed]
- Bescos, R.; Sureda, A.; Tur, J.A.; Pons, A. The effect of nitric-oxide-related supplements on human performance. Sports Med. 2012, 42, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Grimble, G.K. Adverse gastrointestinal effects of arginine and related amino acids. J. Nutr. 2007, 137, 1693S–1701S. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.-D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the global burden of cardiovascular disease. Part 1: The epidemiology and risk factors. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Lakatta, E.G. Arterial and cardiac aging major shereholders in cardiovascular disease enterpriss. Part III: Cellular and molecular clues to heart and arterial aging. Circulation 2003, 107, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Berglund, L.; Larsson, A.; Sundstrom, J. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation 2011, 123, 1545–1551. [Google Scholar] [CrossRef]
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and vascular endothelial function in humans. Clin. Sci. 2011, 120, 357–375. [Google Scholar] [CrossRef]
- Sindler, A.L.; Fleenor, B.S.; Calvert, J.W.; Marshall, K.D.; Zigler, M.L.; Lefer, D.J.; Seals, D.R. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 2011, 10, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Sindler, A.L.; Devan, A.E.; Fleenor, B.S.; Seals, D.R. Inorganic nitrite supplementation for healthy arterial aging. J. Appl. Physiol. 2014, 116, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Fleenor, B.S.; Sindler, A.L.; Eng, J.S.; Nair, D.P.; Dodson, R.B.; Seals, D.R. Sodium nitrite de-stiffening of large elastic arteries with aging: Role of normalization of advanced glycation end-products. Exp. Gerontol. 2012, 47, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, A.; Mitechell, K.; Blackwell, J.R.; Vanhatalo, A.; Jones, A.M. High-nitrate vegetable diet increases plasma nitrate and nitrite concentrations and reduces blood pressure in healthy women. Public Health Nutr. 2015, 18, 2669–2678. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.J.; Justus, N.W.; Hauser, J.I.; Case, A.H.; Helms, C.C.; Basu, S.; Miller, G.D. Dietary nitrate supplementation improves exercise performance and decreases blood pressure in COPD patients. Nitric Oxide 2015, 48, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Khambata, R.S.; Robertson, A.; Caulfield, M.J.; Ahluwalia, A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients. A randomized, phase 2, double-blind, placebo-controlled study. Hypertension 2015, 65, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Woodward, K.A.; Santos-Parker, J.R.; Lubieniecki, K.L.; Nagy, E.; Bryan, N.S.; Chonchol, M.; Justice, J.N.; Seals, D.R.; Rossman, M.J. Sodium nitrite supplementation improves vascular endothelial function but not motor or cognitive function in middle-aged and older adults. FASEB J. 2019, 33, 833.13. [Google Scholar] [CrossRef]
- Bailey, S.J.; Winyard, P.; Vanhatalo, A.; Blockwell, J.R.; Di Menna, F.J.; Wilkerson, D.P.; Tarr, J.; Benjmanin, N.; Jones, A.M. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009, 107, 1144–1155. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; DiMenna, F.J.; Pavey, T.G.; Wilkerson, D.P.; Benjamin, N.; Winyard, P.G.; Jones, A.M. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1121–R1131. [Google Scholar] [CrossRef]
- Milton-Laskibar, I.; Martinez, J.A.; Portillo, M.P. Current knowledge on beetrrot bioactive compounds: Role of nitrate and betalains in health and disease. Foods 2021, 10, 1314. [Google Scholar] [CrossRef]
- Ninfali, P.; Angelino, D. Nutritional and functional potential of Beta vulgaris cicla and rubra. Fitoterapia 2013, 89, 188–199. [Google Scholar] [CrossRef] [PubMed]
- dos Baiao, D.S.; da Silva, D.V.T.; Del Aguila, E.M.; Paschoalin, V.M.F. Nutritional, bioactive and physicochemical characteristics of different beetroot formulations. In Food Additivies; Karunaratne, D.N., Pamunuowa, G., Eds.; Intech Open: London, UK, 2017; Chapter 2; pp. 21–44. [Google Scholar]
- da Silva, D.V.T.; dos Santo Baiao, D.; Ferreira, V.F.; Paschoalin, V.M.F. Betanin as amultipath oxidative stress and inflammation modulator: A beetroot pigment with protective effects on cardiovascular disease pathogenesis. Crit. Rev. Food Sci. Nutr. 2022, 62, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Drinks, J.W. Nitrate 3000 Concentrate. 2019. Available online: https://www.beet-it.com/buy-now/beet-it-sport-nitrate-3000 (accessed on 4 June 2019).
- Brzezinska-Rojek, J.; Sagatovych, S.; Malinowska, P.; Gadaj, P.; Prokopowicz, M.; Grembecka, M. Antioxidant capacity, nitrate and nitrite content in beetroot-based dietary supplements. Foods 2023, 12, 1017. [Google Scholar] [CrossRef]
- Hobbs, D.A.; Goulding, M.G.; Nguyen, A.; Malaver, T.; Walker, C.F.; George, T.W.; Lovegrove, J.A. Acute ingestion of beetroot bread increases endothelium-independent vasodilation and lowers diastolic blood pressure in healthy men: A randomized controlled trial. J. Nutr. 2013, 143, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, D.A.; Kaffa, N.; George, T.W.; Methven, L.; Lovegrove, J.B. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. Br. J. Nutr. 2012, 108, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Czyżewska, A.; Klewicka, E.; Libudzisz, Z. The influence of lactic acid fermentation proces of red beet juice on the stability of biologically colorants. Eur. Food Res. Technol. 2006, 223, 110–116. [Google Scholar] [CrossRef]
- Stanaway, L.; Rutherfurd-Markwick, K.; Page, R.; Ali, A. Performance and health benefits of dietary nitrate supplementation in older adults: A systematic review. Nutrients 2017, 9, 1171. [Google Scholar] [CrossRef] [PubMed]
- Remington, J.; Winters, K. Effectiveness of dietary inorganic nitrate for lowering blood pressure in hypertensive adults: A systematic review. JBI Database Syst. Rev. Imploment. Rep. 2019, 17, 365–389. [Google Scholar] [CrossRef]
- Siervo, M.; Lara, J.; Ogbonmwan, I.; Mathers, J.C. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in dults: A systematic review and meta-analysis. J. Nutr. 2013, 143, 818–826. [Google Scholar] [CrossRef]
- Kim, D.J.; Roe, C.A.; Somani, Y.B.; Moore, D.J.; Barrett, M.A.; Flanagan, M.; Kim-Shapiro, D.B.; Basu, S.; Muller, M.D.; Proctor, D.N. Effects of acute dietary nitrate supplementation on aortic blood pressures and pulse wave characteristics in post-menopausal women. Nitric Oxide 2019, 85, 10–16. [Google Scholar] [CrossRef]
- Velmurugan, S.; Kapil, V.; Ghosh, S.M.; Davies, S.; McKnight, A.; Aboud, Z.; Khambata, R.S.; Webb, A.J.; Poole, A.; Ahluwalia, A. Antiplatelet effects of dietary nitrate in healthy volunteers: Involvement of cGMP and influence of sex. Free Radic. Biol. Med. 2013, 65, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.C.; Broxterman, R.M.; Smith, J.R.; Allen, J.D.; Barstow, T.J. Effect of dietry nitrate supplementation on conduit artery blood flow, muscle oxygenation, and metabolic rate during handgrip exercise. J. Appl. Physiol. 2018, 125, 254–262. [Google Scholar] [CrossRef] [PubMed]
- de Vries, C.J.; DeLorey, D.S. Effect of acute dietary nitrate supplementation on sympathetic vasoconstriction at rest and during exercise. J. Appl. Physiol. 2019, 127, 81–88. [Google Scholar] [CrossRef]
- Notay, K.; Incognito, A.V.; Millar, P.J. Acute beetroot juice supplementation on systemaitic nerve activity: A randomized, double-blind, placebo-controlled proof-of-concept study. Am. J. Physiol. 2017, 313, H59–H65. [Google Scholar]
- Perez, J.M.; Dobson, J.L.; Ryan, G.A.; Riggs, A.J. The effects of beetroot juice on VO2max and blood pressure during submaximal exercise. Intern. J. Exerc. Sci. 2019, 12, 332–342. [Google Scholar]
- Ryan, L.; Prescott, S.L. Stability of the antioxidant capacity of twenty-five commercially available fruit juices subjected to an in vitro digestion. Int. J. Food Sci. Technol. 2010, 45, 1191–1197. [Google Scholar] [CrossRef]
- Joris, P.J.; Mensink, R.P. Beetroot juice improves in overweight and slightly obese men postprandial endothelial function after consumption of a mixed meal. Aterosclerosis 2013, 231, 78–83. [Google Scholar] [CrossRef]
- Lidder, S.; Webb, A.J. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate-nitrite-nitric oxide pathway. Br. J. Clin. Pharmacol. 2013, 75, 677–696. [Google Scholar] [CrossRef]
- Bakker, E.; Engan, H.; Patrician, A.; Schagatay, E.; Karlsen, T.; Wisloff, U.; Gaustad, S.E. Acute dietary nitrate supplementation improves arterial endothelial function at high altitude: A double –blinded randomized controlled cross over study. Nitric Oxide 2015, 50, 58–64. [Google Scholar] [CrossRef]
- Keen, J.T.; Levitt, E.L.; Hodges, G.J.; Wong, B.J. Short-term dietary nitrate supplementation augments cutaneous vasodilatation and reduces mean arterial pressure in healthy humans. Microvasc. Res. 2015, 98, 48–53. [Google Scholar] [CrossRef]
- Lee, J.S.; Stebbins, C.L.; Jung, E.; Nho, H.; Kim, J.K.; Chang, M.J.; Choi, H.M. Effects of chronic dietary nitrate supplementation on the hemodynamic response to dynamic exercise. Am. J. Physiol. 2015, 309, R459–R466. [Google Scholar] [CrossRef]
- Levitt, E.L.; Keen, J.T.; Wong, B.J. Augmented reflex cutaneous vasodilatation following short-term dietary nitrate supplementation in humans. Exp. Physiol. 2015, 100, 708–718. [Google Scholar] [CrossRef]
- Lara, J.; Ashor, A.W.; Oggioni, C.; Ahluwalia, A.; Mathers, J.C. Effects of inorganic nitrate and beetroot supplementation on endothelial function: A systemiatic review and meta-analysis. Eur. J. Nutr. 2016, 55, 451–459. [Google Scholar] [CrossRef]
- Kenjale, A.A.; Ham, K.L.; Stabler, T.; Robbins, J.L.; Johnson, J.L.; Vanbruggen, M.; Privette, G.; Yim, E.; Kraus, W.E.; Allen, J.D. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J. Appl. Physiol. 2011, 110, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- McKnight, G.M.; Duncan, C.W.; Leifert, C.; Golden, M.H. Dietary nitrate in man: Frein or foe? Br. J. Nutr. 1999, 81, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Richardson, G.; Hicks, S.L.; O’Byrne, S.; Frost, M.T.; Moore, K.; Benjamin, N.; McKnight, G.M. The ingestion of inorganic nitrate increases gastric S-nitrosothiol levels and inhibits platelet function in humans. Nitric Oxide 2002, 7, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Morgado, M.; de Oliveira, G.V.; Vasconcellos, J.; Monteiro, M.L.; Conte-Junior, C.; Pierucci, A.P.T.R.; Alvares, T.S. Development of a beetroot-based nutritional gel containing high content of bioaccessible dietary nitrate and antioxidants. Int. Food Sci. Nutr. 2016, 67, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Houshialsadat, Z.; Gaeini, Z.; Bahadoran, Z.; Azizi, F. Functional properties of beetroot (Beta vulgaris) in management of cardio-metabolic diseases. Nutr. Metab. 2020, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.L.; Moreau, R. Functionl properties of spinach (Spinacia oleracea L.) phytochemicals and bioactives. Food Funct. 2016, 7, 3337–3353. [Google Scholar] [CrossRef]
- Panda, V.; Bhandare, N.; Mistry, K.; Sudhamani, S.; Dande, P. Cardioprotective potential of Spinacia oleracea (Spinach) against isoproterenol-induced myocardial infraction in rats. Arch. Physiol. Biochem. 2022, 128, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Arru, L.; Mussi, F.; Forti, L.; Buschini, A. Biological effect of different spinach extracts in comparison with the individual components of the phytocomplex. Foods 2021, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Jovanovski, E.; Bosco, L.; Khan, K.; Au-Yeung, F.; Ho, H.; Zurbau, A.; Jenkins, A.L.; Vuksan, V. Effect of spinach, a high dietary nitrate source, on arterial stiffness and related homodynamic measures: A randomized, controlled trial in helthy adults. Clin. Nutr. Res. 2015, 4, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.; Morrison, S.; Hedges, L.; Kerkhofs, N.; Lister, C. Phenolics contribute significantly to higher antioxidant activity of red lettuce compared to green lettuce. In Proceedings of the XXII International Conference on Polyphenols, Helsinki, Finland, 25–28 August 2004; pp. 273–274. [Google Scholar]
- Rolnik, A.; Soluch, A.; Kowalska, I.; Olas, B. Antioxidant and hemostatic properties of preparations from Asteraceae family and their chemical composition—Comparative studies. Biomed. Pharmacol. 2021, 142, 111982. [Google Scholar] [CrossRef] [PubMed]
- Rolnik, A.; Stochmal, A.; Olas, B. The in vitro anti-platelet activities of plant extracts from the Asterceae family. Biomed. Pharmacol. 2022, 149, 112809. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gil, M.I.; Yang, Q.; Tomas-Barberan, F.A. Bioactive compounds inlettuce: Highlighting the benefits to human health and impacts of preharvest and postharvest practices. Comp. Rev. Food Sci. Food Saf. 2022, 21, 4–45. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, C.; Cardinault, N.; Gueux, E.; Jaffrelo, L.; Rock, E.; Mazur, A.; Amoroux, P.; Remesy, C. Health effect of vegetable-based diet: Lettuce consumption improves cholesterol metabolism and antioxidant status in the rat. Clin. Nutr. 2004, 23, 605–614. [Google Scholar] [CrossRef]
- Abdalla, M.A.; Wenzel-Storjohann, A.; Sulieman, S.; Tasdemir, D.; Muhling, K.H. Comparative metabolite profile, biological activity and overall quality of three lettuce (Lactuca sativa L.; Asteracea) cultivars in response to sulfur nutrition. Pharmaceutics 2021, 13, 713. [Google Scholar] [CrossRef]
- Kammoun, I.; Salah, H.B.; Saad, H.B.; Cherif, B.; Droguet, M.; Magne, C.; Kallel, C.; Boudawara, O.; Hakim, A.; Gharsallah, N.; et al. Hypolipidemic and cardioprotective effects of Ulva lactuca ethanolic extract in hypochoelsterolemic mice. Arch. Physiol. Biochem. 2018, 124, 313–325. [Google Scholar] [CrossRef]
- Kooti, W.; Daraei, N. A review of the antioxidant activity of celery (Apium graveolens L). Top. Rev. Artic. 2017, 22, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Bagar, S.P.; Sharma, N.; Sanwal, N.; Lorenzo, J.M.; Sahu, J.K. Bioactive potential of beetroot (Beta vulgaris). Food Res. Int. 2022, 158, 111556. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).