The Effects of Chia Defatted Flour as a Nutritional Supplement in C57BL/6 Mice Fed a Low-Quality Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Diet Preparation
2.3. Animals and Experimental Design
2.4. Metabolism of Carbohydrates: Determination of Glucose and Lactate Concentration
2.5. Analysis of General Redox State of Animals
2.5.1. Reactive Oxygen Species
Superoxide Determination
Hydroperoxide Determination
2.5.2. Analysis of the Antioxidant Enzyme Activity
2.5.3. Determination of Free Thiol Groups (Reduced Glutathione)
2.5.4. Determination of Advanced Oxidation Protein Products
2.6. Statistical Analysis
3. Results
3.1. Effects of Diets on Body Weight and Food and Water Intake
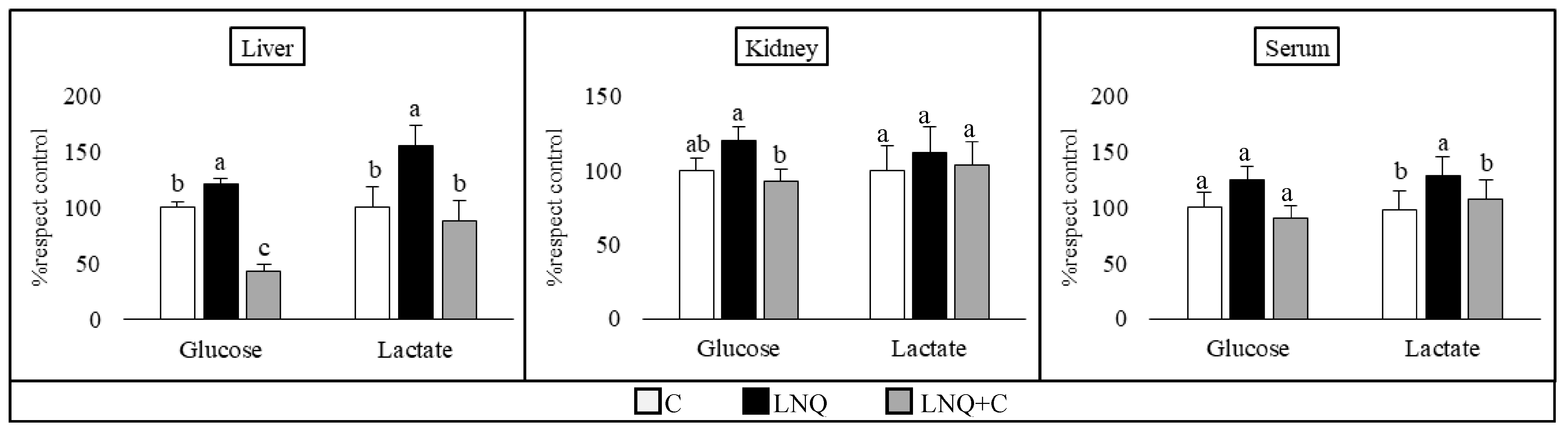
3.2. Effects of Diets on the Aerobic and Anaerobic Metabolism of Carbohydrates
3.3. Effects of the Nutritional Quality of the Diets in the General Redox State
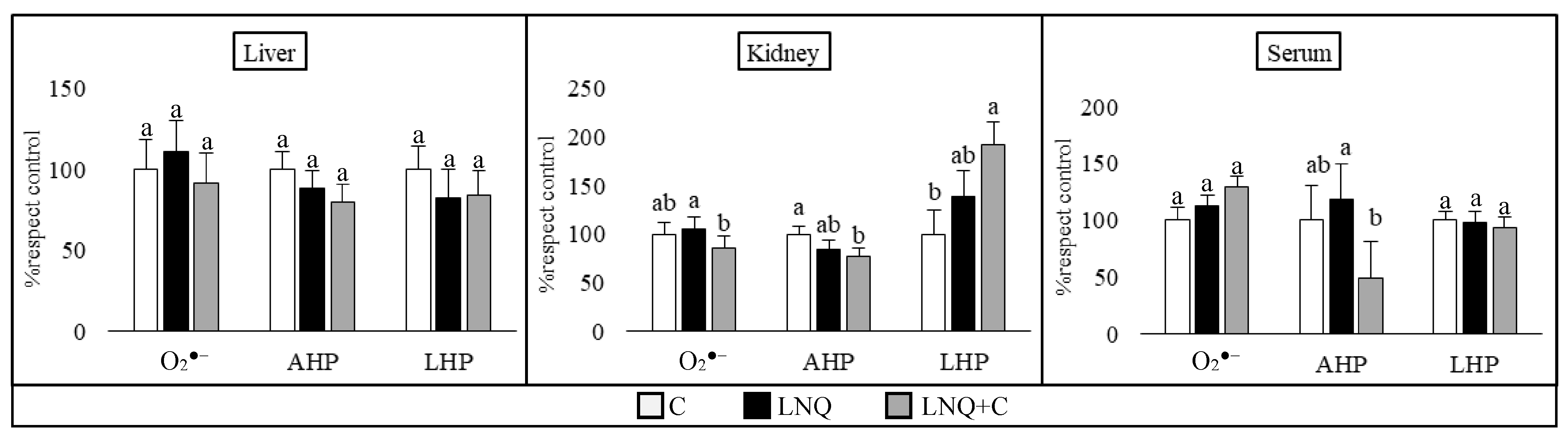
3.3.1. Levels of Reactive Oxygen Species
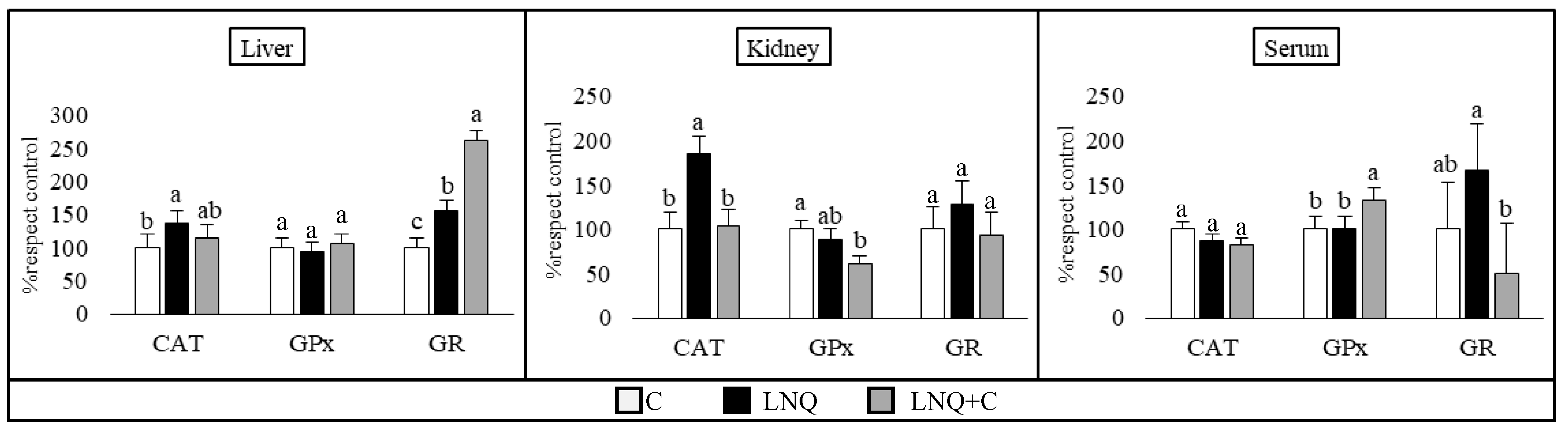
3.3.2. Antioxidant Enzyme Activity
3.3.3. Reduced Glutathione
3.3.4. Advanced Oxidation of Protein Products (AOPP)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef] [PubMed]
- Coronati, M.; Baratta, F.; Pastori, D.; Ferro, D.; Angelico, F.; Del Ben, M. Added Fructose in Non-Alcoholic Fatty Liver Disease and in Metabolic Syndrome: A Narrative Review. Nutrients 2022, 14, 1127. [Google Scholar] [CrossRef] [PubMed]
- Jalaba, S.; Trudeau, H.; Carlson, S. Obesity Prevention. Physician Assist. Clin. 2021, 7, 43–58. [Google Scholar] [CrossRef]
- Santa, K.; Kumazawa, Y.; Nagaoka, I. Prevention of Metabolic Syndrome by Phytochemicals and Vitamin D. Int. J. Mol. Sci. 2023, 24, 2627. [Google Scholar] [CrossRef]
- Din, Z.; Alam, M.; Ullah, H.; Shi, D.; Xu, B.; Li, H.; Xiao, C. Nutritional, Phytochemical and Therapeutic Potential of Chia Seed (Salvia hispanica L.). A Mini-Review. Food Hydrocoll. Health 2021, 1, 100010. [Google Scholar] [CrossRef]
- Oliva, M.E.; Ferreira, M.d.R.; Joubert, M.B.V.; D’Alessandro, M.E. Salvia hispanica L. (chia) Seed Promotes Body Fat Depletion and Modulates Adipocyte Lipid Handling in Sucrose-Rich Diet-Fed Rats. Food Res. Int. 2021, 139, 109842. [Google Scholar] [CrossRef]
- Enes, B.N.; Moreira, L.d.P.D.; Lopes Toledo, R.C.; Aguiar Moraes, É.; de Castro Moreira, M.E.; Miranda Hermsdorff, H.H.; Noratto, G.; Mertens-Talcott, S.U.; Talcott, S.; Duarte Martino, H.S. Effect of Different Fractions of Chia (Salvia hispanica L.) on Glucose Metabolism, in Vivo and in Vitro. J. Funct. Foods 2020, 71, 104026. [Google Scholar] [CrossRef]
- da Silva Marineli, R.; Alves Lenquiste, S.; Aguiar Moraes, É.; Maróstica, M.R. Antiozidant Potential of Dietary Chia Seed and Oil (Salvia hispanica L.) in Diet-Induced Obese Rats. Food Res. Int. 2015, 76, 666–674. [Google Scholar] [CrossRef]
- Alarcon, G.; Valoy, A.; Rossi, A.; Jerez, S. Study of the Residue from Salvia hispanica (Chia) Seed Oil Extraction by Cold Pressing for Repurposing as Functional Food to Prevent Metabolic Syndrome. Biol. Life Sci. Forum 2022, 17, 13. [Google Scholar] [CrossRef]
- Mishima, M.D.V.; Da Silva, B.P.; Gomes, M.J.C.; Toledo, R.C.L.; Mantovani, H.C.; de Sao José, V.P.B.; Costa, N.M.B.; Tako, E.; Marino, H.S.D. Effect of Chia (Salvia hispanica L.) Associated with High-Fat Diet on the Intestinal Health of Wistar Rats. Nutrients 2022, 14, 4924. [Google Scholar] [CrossRef]
- Mas, A.L.; Brigante, F.I.; Salvucci, E.; Pigni, N.B.; Martinez, M.L.; Ribotta, P.; Wunderlin, D.A.; Baroni, M.V. Defatted Chia Flour as Functional Ingredient in Sweet Cookies. How Do Processing, Simulated Gastrointestinal Digestion and Colonic Fermentation Affect Its Antioxidant Properties? Food Chem. 2020, 316, 126279. [Google Scholar] [CrossRef]
- Mármol, I.; Quero, J.; Ibarz, R.; Ferreira-Santos, P.; Teixeira, J.A.; Rocha, C.M.R.; Pérez-Fernández, M.; García-Juiz, S.; Osada, J.; Martín-Belloso, O.; et al. Valorization of Agro-Food by-Products and Their Potential Therapeutic Applications. Food Bioprod. Process. 2021, 128, 247–258. [Google Scholar] [CrossRef]
- Pou, S.A.; del Pilar Díaz, M.; De La Quintana, A.G.; Forte, C.A.; Aballay, L.R. Identification of Dietary Patterns in Urban Population of Argentina: Study on Diet-Obesity Relation in Population-Based Prevalence Study. Nutr. Res. Pract. 2016, 10, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Canalis, A.M.; Cittadini, M.C.; Albrecht, C.; Soria, E.A. In Vivo Redox Effects of Aspidosperma Quebracho-Blanco Schltdl., Lantana Grisebachii Stuck and Ilex Paraguariensis A. St.-Hil. on Blood, Thymus and Spleen of Mice. Indian J. Exp. Biol. 2014, 52, 882–889. [Google Scholar] [PubMed]
- National Institutes of Health. National Academy of Sciences Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies: Washington, DC, USA, 2011. [Google Scholar]
- Maughan, R. Carbohydrate Metabolism. Surgery 2013, 31, 273–277. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beutler, E. Catalase. In Red Cell Metabolism, a Manual of Biochemical Methods; Beutler, E., Ed.; Grune and Stratton: New York, NY, USA, 1982; pp. 105–106. [Google Scholar]
- Drotar, A.; Phelps, P.; Fall, R. Evidence for Glutathione Peroxidase Activities in Cultured Plant Cells. Plant Sci. 1985, 42, 35–40. [Google Scholar] [CrossRef]
- Tanaka, K.; Sano, T.; Ishizuka, K.; Kitta, K.; Kawamura, Y. Comparison of Properties of Leaf and Root Glutathione Reductases from Spinach. Physiol. Plant. 1994, 91, 353–358. [Google Scholar] [CrossRef]
- Boyne, A.F.; Ellman, G.L. A Methodology for Analysis of Tissue Sulfhydryl Components. Anal. Biochem. 1972, 46, 639–653. [Google Scholar] [CrossRef]
- Matteucci, E.; Biasci, E.; Giampietro, O. Advanced Oxidation Protein Products in Plasma: Stability during Storage and Correlation with Other Clinical Characteristics. Acta Diabetol. 2001, 38, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2020. Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. Available online: http://www.infostat.com.ar (accessed on 10 September 2023).
- Barbosa, P.O.; Souza, M.O.; Silva, M.P.S.; Santos, G.T.; Silva, M.E.; Bermano, G.; Freitas, R.N. Açaí (Euterpe Oleracea Martius) Supplementation Improves Oxidative Stress Biomarkers in Liver Tissue of Dams Fed a High-Fat Diet and Increases Antioxidant Enzymes’ Gene Expression in Offspring. Biomed. Pharmacother. 2021, 139, 111627. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Song, X.; Chen, Y.; Li, L.; Sun, J.; Huang, C.; Ou, S.; Zhang, H. Effects of Sorghum, Purple Rice and Rhubarb Rice on Lipids Status and Antioxidant Capacity in Mice Fed a High-Fat Diet. J. Funct. Foods 2017, 39, 103–111. [Google Scholar] [CrossRef]
- la Paz, S.M.-D.; Pérez-Pérez, A.; Vilariño-García, T.; Jiménez-Cortegana, C.; Muriana, F.J.G.; Millán-Linares, M.C.; Sánchez-Margalet, V. Nutritional Modulation of Leptin Expression and Leptin Action in Obesity and Obesity-Associated Complications. J. Nutr. Biochem. 2021, 89, 108561. [Google Scholar] [CrossRef] [PubMed]
- Gentilcore, D.; Chaikomin, R.; Jones, K.L.; Russo, A.; Feinle-Bisset, C.; Wishart, J.M.; Rayner, C.K.; Horowitz, M. Effects of Fat on Gastric Emptying of and the Glycemic, Insulin, and Incretin Responses to a Carbohydrate Meal in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 2062–2067. [Google Scholar] [CrossRef]
- Koska, J.; Ozias, M.K.; Deer, J.; Kurtz, J.; Salbe, A.D.; Harman, S.M.; Reaven, P.D. A Human Model of Dietary Saturated Fatty Acid Induced Insulin Resistance. Metabolism 2016, 65, 1621–1628. [Google Scholar] [CrossRef]
- Jayanthy, G.; Subramanian, S. Rosmarinic Acid, a Polyphenol, Ameliorates Hyperglycemia by Regulating the Key Enzymes of Carbohydrate Metabolism in High Fat Diet-STZ Induced Experimental Diabetes Mellitus. Biomed. Prev. Nutr. 2014, 4, 431–437. [Google Scholar] [CrossRef]
- Kehrer, J.P.; Robertson, J.D.; Smith, C.V. Free Radicals and Reactive Oxygen Species. Compr. Toxicol. Second Ed. 2010, 14, 277–307. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative Stress Mitigation by Antioxidants-An Overview on Their Chemistry and Influences on Health Status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef]
- Feillet-Coudray, C.; Sutra, T.; Fouret, G.; Ramos, J.; Wrutniak-Cabello, C.; Cabello, G.; Cristol, J.P.; Coudray, C. Oxidative Stress in Rats Fed a High-Fat High-Sucrose Diet and Preventive Effect of Polyphenols: Involvement of Mitochondrial and NAD(P)H Oxidase Systems. Free Radic. Biol. Med. 2009, 46, 624–632. [Google Scholar] [CrossRef]
- Cittadini, M.C.; Canalis, A.M.; Albrecht, C.; Soria, E.A. Effects of Oral Phytoextract Intake on Phenolic Concentration and Redox Homeostasis in Murine Encephalic Regions. Nutr. Neurosci. 2015, 18, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mateos, R.; Goya, L.; Amigo-Benavent, M.; Sarriá, B.; Bravo, L. A Phenolic Extract from Grape by-Products and Its Main Hydroxybenzoic Acids Protect Caco-2 Cells against pro-Oxidant Induced Toxicity. Food Chem. Toxicol. 2016, 88, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Yoo, J.H.; Lee, Y.S.; Park, E.J.; Lee, H.J. Ameliorative Effects of Black Ginseng on Nonalcoholic Fatty Liver Disease in Free Fatty Acid–induced HepG2 Cells and High-Fat/high-Fructose Diet-Fed Mice. J. Ginseng Res. 2020, 44, 350–361. [Google Scholar] [CrossRef]
- Yorimitsu, T.; Nair, U.; Yang, Z.; Klionsky, D.J. Endoplasmic Reticulum Stress Triggers Autophagy. J. Biol. Chem. 2006, 281, 30299–30304. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Hazama, M.; Urata, Y.; Goto, S.; Horiuchi, S.; Sumikawa, K.; Kondo, T. Protective Role of Glutathione Synthesis in Response to Oxidized Low Density Lipoprotein in Human Vascular Endothelial Cells. Free. Radic. Biol. Med. 1999, 26, 589–602. [Google Scholar] [CrossRef]
- Cai, J.; Huang, Z.Z.; Lu, S. Differential Regulation of γ-Glutamylcysteine Synthetase Heavy and Light Subunit Gene Expression. Biochem. J. 1997, 326, 167–172. [Google Scholar] [CrossRef]
- Sabir, U.; Irfan, H.M.; Asim, M.H. Reduction of Hepatic Steatosis, Oxidative Stress, Inflammation, Ballooning and Insulin Resistance After Therapy with Safranal in NAFLD Animal Model: A New Approach. J. Inflamm. Res. 2022, 15, 1293–1316. [Google Scholar] [CrossRef]
- Miah, P.; Mohona, S.B.S.; Rahman, M.M.; Subhan, N.; Khan, F.; Hossain, H.; Sharker, S.M.; Alam, M.A. Supplementation of Cumin Seed Powder Prevents Oxidative Stress, Hyperlipidemia and Non-Alcoholic Fatty Liver in High Fat Diet Fed Rats. Biomed. Pharmacother. 2021, 141, 111908. [Google Scholar] [CrossRef]
- Li, L.; Zhai, S.; Wang, R.; Kong, F.; Yang, A.; Wang, C.; Yu, H.; Li, Y.; Wang, D. Anti-Obesity Effect of Auricularia delicate Involves Intestinal-Microbiota-Mediated Oxidative Stress Regulation in High-Fat-Diet-Fed Mice. Nutrients 2023, 15, 872. [Google Scholar] [CrossRef]
- Rodriguez-Sojo, M.J.; Ruiz-Malagón, A.J.; Hidalgo-García, L.; Molina-Tijeras, J.A.; Diez-Echave, O.; López-Escanez, L.; Rosati, L.; González-Lozano, E.; Cenis-Cifuentes, L.; García-García, J.; et al. The Prebiotic Effects of an Extract with Antioxidant Properties from Morus alba L. Contribute to Ameliorate Hihj-Fat Diet-Induced Obestiy in Mice. Antioxidants 2023, 12, 978. [Google Scholar] [CrossRef]
- Rosas-Villegas, A.; Sánchez-Tapia, M.; Avila-Nava, A.; Ramírez, V.; Tovar, A.R.; Torres, N. Differential Effect of Sucrose and Fructose in Combination with High Fat Diet on Intestinal Microbiota and Kidney Oxidative Stress. Nutrients 2017, 9, 393. [Google Scholar] [CrossRef] [PubMed]
| Component | C | LNQ | LNQ+C |
|---|---|---|---|
| Proteins (casein) | 160 | 180 | 152.3 |
| Carbohydrates | |||
| Cornstarch | 400 | 350 | 329.35 |
| Sucrose | 300 | - | - |
| Fructose | - | 350 | 329.35 |
| Fiber (wheat bran) | 40 | - | - |
| Corn oil | 60 | - | - |
| Palm oil | - | 80 | 66.4 |
| Chia defatted flour | - | - | 100 |
| Proteins from chia | - | - | 27.70 |
| Fiber from chia | 41.30 | ||
| Lipids from chia | 13.60 | ||
| Vitamins | |||
| Choline chloride | 1 | 1 | 1 |
| Inositol | 0.2 | 0.2 | 0.2 |
| Folic acid | 0.005 | 0.005 | 0.005 |
| Vitamin D3 (cholecalciferol) | 1.13 × 10−4 | 1.13 × 10−4 | 1.13 × 10−4 |
| Vitamin E (α-tocopherol) | 0.1 | 0.1 | 0.1 |
| Vitamin K3 (menadione) | 2.5 × 10−3 | 2.5 × 10−3 | 2.5 × 10−3 |
| † Supradyn® | 5 pills | 5 pills | 5 pills |
| Minerals | |||
| CaCO3 | 13.15 | 13.15 | 13.15 |
| KH2PO4 | 5.3 | 5.3 | 5.3 |
| FeSO4 | 0.27 | 0.27 | 0.27 |
| KCl | 2.8 | 2.8 | 2.8 |
| NaCl | 1.72 | 1.72 | 1.72 |
| MgSO4 | 0.17 | 0.17 | 0.17 |
| AlKSO4 | 0.042 | 0.042 | 0.042 |
| Body Weight Gain (%) | Food Intake (g/Week/Animal) | Water Intake (mL/Week/Animal) | Liver Weight (g) | Kidney Weight (g) | |
|---|---|---|---|---|---|
| C | 80.56 ± 12.56 a | 22.4 ± 0.33 b | 26.83 ± 1.64 a | 1.1197 ± 0.0250 a | 0.2117 ± 0.0217 a |
| LNQ | 79.77 ± 15.38 a | 20.84 ± 0.62 c | 23.07 ± 2.10 a | 1.1485 ± 0.1550 a | 0.2658 ± 0.0559 a |
| LNQ+C | 122.63 ± 12.56 a | 24.06 ± 0.45 a | 24.97 ± 1.54 a | 1.1502 ± 0.0983 a | 0.2164 ± 0.0496 a |
| GSH | AOPP | ||||
|---|---|---|---|---|---|
| Liver | Kidney | Serum | Liver | Kidney | |
| C | 100.00 ± 13.79 ab | 100.00 ± 10.98 a | 100.00 ± 8.52 a | 100.00 ± 11.82 b | 100.00 ± 12.01 a |
| LNQ | 114.20 ± 14.15 a | 113.06 ± 11.03 a | 118.86 ± 8.19 a | 112.82 ± 11.85 a | 103.95 ± 12.05 a |
| LNQ+C | 84.28 ± 13.70 b | 102.34 ± 10.83 a | 115.44 ± 7.62 a | 84.37 ± 11.97 b | 103.36 ± 11.97 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucini Mas, A.; Canalis, A.M.; Pasqualini, M.E.; Wunderlin, D.A.; Baroni, M.V. The Effects of Chia Defatted Flour as a Nutritional Supplement in C57BL/6 Mice Fed a Low-Quality Diet. Foods 2024, 13, 678. https://doi.org/10.3390/foods13050678
Lucini Mas A, Canalis AM, Pasqualini ME, Wunderlin DA, Baroni MV. The Effects of Chia Defatted Flour as a Nutritional Supplement in C57BL/6 Mice Fed a Low-Quality Diet. Foods. 2024; 13(5):678. https://doi.org/10.3390/foods13050678
Chicago/Turabian StyleLucini Mas, Agustin, Alejandra Mariel Canalis, María Eugenia Pasqualini, Daniel Alberto Wunderlin, and María Verónica Baroni. 2024. "The Effects of Chia Defatted Flour as a Nutritional Supplement in C57BL/6 Mice Fed a Low-Quality Diet" Foods 13, no. 5: 678. https://doi.org/10.3390/foods13050678
APA StyleLucini Mas, A., Canalis, A. M., Pasqualini, M. E., Wunderlin, D. A., & Baroni, M. V. (2024). The Effects of Chia Defatted Flour as a Nutritional Supplement in C57BL/6 Mice Fed a Low-Quality Diet. Foods, 13(5), 678. https://doi.org/10.3390/foods13050678






