Polyphenols and CRISPR as Quorum Quenching Agents in Antibiotic-Resistant Foodborne Human Pathogens (Salmonella Typhimurium, Campylobacter jejuni and Escherichia coli 0157:H7)
Abstract
1. Introduction
2. Polyphenols as Quorum Quenching Agents
3. Salmonella enterica Serovar Typhimurium Quorum Sensing Interference with Polyphenols
4. CRISPR and Quorum Quenching in S. Typhimurium
5. Polyphenols as QQ Agents in E. coli
6. CRISPR and QQ in E. coli 0157:H7
7. Polyphenols as QQ Agents in Campylobacter jejuni
8. CRISPR and QQ in Campylobacter jejuni
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AR | Antibiotic resistance |
| AI | Autoinducers |
| AI-1 | Autoinducers 1 |
| AI-2 | Autoinducers 2 |
| AI-3 | Autoinducers 3 |
| AHL | Acyl-L-homoserine-Lactone |
| AHLs | N-Acyl-Homoserine Lactones |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| CRISPS-dCas9 | “dead” Cas9, CRISPR interference |
| CRISPR Cas9-HDR | CRISPR Cas9-Direct Homologous Repair |
| CRISPRi | CRISPR interference |
| DT | Definitive Type |
| DSF | Diffusing Signaling Factors |
| EPS | Extracellular Polymeric Substance |
| ETEC | Enterotoxigenic E. coli |
| ETM | Electron Transmission Microscopy |
| NTS | Nontyphoidal Salmonella |
| PCR | Polymerase Chain Reaction |
| Quorum Quenching | |
| QQA | Quorum Quenching Agents |
| QS | Quorum Sensing |
| QS-Regulon | Genes that regulate the process |
| RAA | Recombinase-Aided Amplification |
| RASFF | Rapid Alert System for Food and Feed |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| SINAVE | National Epidemiological Surveillance System |
| SPI-1 | Salmonella pathogenicity island 1 |
| T3SS1 | Type III secretion system 1 |
| WHO | World Health Organization |
References
- Batz, M.B.; Batz, E.; Henke, B.; Kowalcyk, B. Long-Term Consequences of Foodborne Infections. Infect. Dis. Clin. N. Am. 2013, 27, 599–616. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Annual Report Alert and Cooperation Network. 2022. Available online: https://food.ec.europa.eu/system/files/2023-08/acn_annual-report_2022.pdf (accessed on 22 August 2023).
- Delahoy, M.J.; Shah, H.J.; Weller, D.L.; Ray, L.C.; Smith, K.; McGuire, S.; Trevejo, R.T.; Walter, E.S.; Wymore, K.; Rissman, T.; et al. Preliminary Incidence and Trends of Infections Caused by Pathogens Transmitted Commonly Through Food—Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Menkem, Z.E.; Ngangom, B.L.; Tamunjoh, S.S.A.; Boyom, F.F. Antibiotic Residues in Food Animals: Public health concern. Acta Ecol. Sin. 2019, 39, 411–415. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistance Infections Globally: Final Report and Recommendations. In The Review on Antimicrobial Resistance; Government of the United Kingdom: London, UK, 2016; Volume 81, p. 61. [Google Scholar]
- Mora, C.; McKenzie, T.; Graw, I.S.; Dean, J.M.; von Hammerstein, H.; Knudson, T.A. Over Half of Known Human Pathogenic Diseases Can Be Aggravated by Climate Change. Nat. Clim. Chang. 2022, 12, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial Biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum Sensing in Bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Wei, C.I. Biofilm Formation by Multidrug-resistant Salmonella enterica serotype Typhimurium Phage Type DT104 and Other Pathogens. J. Food Prot. 2007, 70, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Biofilms and Meat Safety: A Mini-Review. J. Food Prot. 2019, 82, 120–127. [Google Scholar] [CrossRef]
- Wang, C.; Ye, F.; Kumar, V.; Gao, Y.-G.; Zhang, L.-H. BswR Controls Bacterial Motility and Biofilm Formation in Pseudomonas aeruginosa Through Modulation of The Small RNA rsmZ. Nucleic Acids Res. 2014, 42, 4563–4576. [Google Scholar] [CrossRef]
- Wang, R.; Bono, J.L.; Kalchayanand, N.; Shackelford, S.; Harhay, D.M. Biofilm Formation by Shiga Toxin-producing Escherichia coli O157:H7 and Non-O157 Strains in Their Tolerance to Sanitizers Commonly Used in the Food Processing Environment. J. Food Prot. 2012, 75, 1418–1428. [Google Scholar] [CrossRef]
- Santos, C.A.; França, L.E.M.; Gombossy de Melo, M.B.D.; Pinto, U.M. Exploring Phenolic Compounds as Quorum Sensing Inhibitors in Foodborne Bacteria. Front. Microbiol. 2021, 12, 735931. [Google Scholar] [CrossRef] [PubMed]
- Raju, D.V.; Nagarajan, A.; Pandit, S.; Nag, M.; Lahiri, D.; Upadhye, V. Effect of Bacterial Quorum Sensing and Mechanism of Antimicrobial Resistance. Biocatal. Agric. Biotechnol. 2022, 43, 102409. [Google Scholar] [CrossRef]
- Otero-Casal, A.M.; Muñoz-Crego, A.; Bernárdez-Hermida, M.I.; Fábregas-Casal, J. Quorum Sensing: El lenguaje de las Bacterias; Editorial Acribia, S.A.: Zaragoza, Spain, 2005; ISBN 84-200-1046-4. [Google Scholar]
- Li, J.; Zhao, X. Effects of Quorum Sensing on The Biofilm Formation and Viable but Non-culturable State. Food Res. Int. 2020, 137, 109742. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; Coppola, R. Quorum Sensing and Phytochemicals. Int. J. Mol. Sci. 2013, 14, 12607–12619. [Google Scholar] [CrossRef]
- Godínez-Oviedo, A.; Tamplin, M.L.; Bowman, J.P.; Hernández, M. Salmonella Enterica in México 2000–2017: Epidemiology, Antimicrobial Resistance and Prevalence in Food. Foodborne Pathog. Dis. 2020, 17, 98–118. [Google Scholar] [CrossRef] [PubMed]
- SINAVE. Distribución de Casos Nuevos de Enfermedades Por Fuente de Notificación. 2020. Available online: https://epidemiologia.salud.gob.mx/anuario/2020/morbilidad/nacional/distribucion_casos_nuevos_enfermedad_fuente_notificacion.pdf (accessed on 22 August 2023).
- Fan, Q.; Zuo, J.; Wang, H.; Greiner, D.; Yi, L.; Wang, Y. Contribution of Quorum Sensing to Virulence and Antibiotic Resistance in Zoonotic Bacteria. Biotechnol. Adv. 2022, 59, 107965. [Google Scholar] [CrossRef]
- Michael, B.; Smith, J.N.; Swift, S.; Heffron, F.; Ahmer, B.M.M. SdiA of Salmonella Enterica is a LuxR Homolog That Detects Mixed Microbial Communities. J. Bacteriol. 2001, 183, 5733–5742. [Google Scholar] [CrossRef]
- Sabag-Daigle, A.; Soares, J.A.; Smith, J.N.; Elmasry, M.E.; Ahmer, B.M. The Acyl Homoserine Lactone Receptor, SdiA, of Escherichia coli and Salmonella enterica Serovar Typhimurium Does Not Respond to Indole. Appl. Environ. Microbiol. 2012, 78, 5424–5431. [Google Scholar] [CrossRef]
- Grandclément, C.; Tannières, M.; Moréra, S.; Dessaux, Y.; Faure, D. Quorum Quenching: Role in Nature and Applied Developments. FEMS Microbiol. Rev. 2016, 40, 86–116. [Google Scholar] [CrossRef]
- Stotani, S.; Gatta, V.; Medarametla, P.; Padmanaban, M.; Karawajczyk, A.; Giordanetto, F.; Tammela, P.; Laitinen, T.; Poso, A.; Tzalis, D.; et al. DPD-Inspired Discovery of Novel LsrK Kinase Inhibitors: An Opportunity to Fight Antimicrobial Resistance. J. Med. Chem. 2019, 62, 2720–2737. [Google Scholar] [CrossRef]
- Nazir, A.; Malik, K.; Qamar, H.; Hamza, M.B.; Liaqat, A.; Shahid, M.; Islam, K.M.; Fatima, A.; Irshad, A.; Sadia, H. A Review: Use of Plant Extracts and Their Phytochemical Constituents to Control Antibiotic Resistance in S. aureus. Pure Appl. Biol. 2020, 9, 720–727. [Google Scholar] [CrossRef]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Suppression of Bacterial Cell-cell Signalling, Biofilm Formation and Type III Secretion System by Citrus Flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Taganna, J.C.; Quanico, J.P.; Perono, R.M.G.; Amor, E.C.; Rivera, W.L. Tanning-rich Fraction From Terminalia catappa Inhibits Quorum Sensing (QS) in Chromobacterium violaceum and the QS-controlled Biofilm Maturation and LasA Staphylolytic Activity in Pseudomonas aeruginosa. J. Ethnopharmacol. 2011, 134, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Nazareth, M.S.; Shreelakshmi, S.V.; Shetty, N.P. Identification and Characterization of Polyphenols from Carissa spinarum Fruit and Evaluation of Their Antioxidant and Anti-quorum Sensing Activity. Curr. Microbiol. 2021, 78, 1277–1285. [Google Scholar] [CrossRef]
- Huber, B.; Eberl, L.; Feucht, W.; Polster, J. Influence of Polyphenols on Bacterial Biofilm Formation and Quorum-sensing. Z. Naturoforsch. C J. Biosci. 2003, 58, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; d’Acierno, A.; De Feo, V.; Ayala-Zavala, F.J.; Gomes-Cruz, A.; Granato, D.; Coppola, R. Chapter 8: Effect of Polyphenols on Microbial Cell-Cell Communications; Tommonaro, G., Ed.; Quorum Sensing; Academic Press: Cambridge, MA, USA, 2019; pp. 195–223. [Google Scholar]
- Al-Hussaini, R.; Mahasneh, A.M. Microbial Growth and Quorum Sensing Antagonist Activities of Herbal Plants Extracts. Molecules 2009, 14, 3425–3435. [Google Scholar] [CrossRef]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rajaonson, S.; Diallo, B.; Mol, A.; El Jaziri, M.; Baucher, M. Identification of Catechin as One of the Flavonoids from Combretum albiflorum Bark Extract That Reduces The Production of Quorum-sensing-controlled Virulence Factors in Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2010, 76, 243–253. [Google Scholar] [CrossRef]
- Lima, E.M.F.; Winans, S.C.; Manoel-Pinto, U. Quorum Sensing Interference by Phenolic Compounds—A Matter of Bacterial Misunderstanding. Heliyon 2023, 9, e17657. [Google Scholar] [CrossRef]
- Ahmer, B.M.; van Reeuwijk, J.; Timmers, C.D.; Valentine, P.J.; Heffron, F. Salmonella typhimurium Encodes an SDIA Homolog, a Putative Quorum Sensor of the LuxR Family, That Regulates Genes on the Virulence Plasmid. J. Bacteriol. 1998, 180, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.A.; Vargas, E.L.; Carneiro, D.G.; Pinto, U.M.; Vanetti, M.C. Virtual Screening of Plant Compounds and Nonsteroidal Anti-inflammatory Drugs for Inhibition of Quorum Sensing and Biofilm Formation in Salmonella. Microb. Pathog. 2018, 121, 369–388. [Google Scholar] [CrossRef] [PubMed]
- Abed, N.; Grepinet, O.; Canepa, S.; Hurtado-Escobar, G.A.; Guichard, N.; Wiedemann, A.; Velge, P.; Virlogeux-Payant, I. Direct Regulation of the pefI-srgC Operon Encoding the Rck Invasin by the Quorum-sensing Regulator SdiA in Salmonella typhimurium. Mol. Microbiol. 2014, 94, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Moreira, G.C.; Weinshenker, D.; Sperandio, V. QseC Mediates Salmonella enterica Serovar Typhimurium Virulence In Vitro and In Vivo. Infect. Immun. 2010, 78, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Dyszel, J.L.; Smith, J.N.; Lucas, D.E.; Soares, J.A.; Swearingen, M.C.; Vross, M.A.; Young, G.M.; Ahmer, B.M. Salmonella enterica Serovar Typhimurium Can Detect Acyl Homoserine Lactone Production by Yersinia enterocolitica in Mice. J. Bacteriol. 2010, 192, 29–37. [Google Scholar] [CrossRef]
- de Freitas, L.L.; Dos Santos, C.I.A.; Carneiro, D.G.; Vanetti, M.C.D. Nisin and Acid Resistance in Salmonella is Enhanced by N-dodecanoylhomoserine Lactone. Microb. Pathog. 2020, 147, 104320. [Google Scholar] [CrossRef]
- Gao, R.; Huang, H.; Hamel, J.; Levesque, R.C.; Goodridge, L.D.; Ogunremi, D. Application of a High-Throughput Targeted Sequence AmpliSeq Procedure to Assess the Presence and Variants of Virulence Genes in Salmonella. Microorganisms 2022, 10, 369. [Google Scholar] [CrossRef]
- Puig-Peña, Y.; Espino-Hernández, M.; Leyva-Castillo, V. Resistencia Antimicrobiana en Salmonella y E. coli Aisladas de Alimentos: Revisión de la literatura. Panor. Cuba Salud 2011, 6, 30–38. [Google Scholar]
- Zaidi, M.B.; León, V.; Canché, C.; Pérez, C.; Zhao, S.; Hubert, S.K.; Abbott, J.; Blickenstaff, K.; McDermott, P.F. Rapid and Widespread Dissemination of Multi-Drug Resistance blaCMY-2 Salmonella typhimurium in Mexico. J. Antimicrob. Chemother. 2007, 60, 398–401. [Google Scholar] [CrossRef]
- Guzman-Hernandez, R.; Contreras-Rodriguez, A.; Hernandez-Velez, R.; Perez-Martinez, I.; Lopez-Merino, A.; Zaidi, M.B.; Estrada-Garcia, T. Mexican Unpasteurized Fresh Cheeses Are Contaminated with Salmonella spp., non-O157 Shiga Toxin Producing Escherichia coli and Potential Uropathogenic E. coli strains: A public Health Risk. Int. J. Food Microbiol. 2016, 237, 10–16. [Google Scholar] [CrossRef]
- Cloeckaert, A.; Schwarz, S. Molecular Characterization, Spread and Evolution of Multidrug Resistance in Salmonella Enterica Typhimurium DT104. Vet. Res. 2001, 32, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Nitiema, L.W.; Savadogo, A.; Simpore, J.; Dianou, D.; Traore, A.S. In Vitro Antimicrobial Activity of Some Phenolic Compounds (Coumarin and Quercetin) Against Gastroenteritis Bacterial Strains. Int. J. Microbiol. Res. 2012, 3, 183–187. [Google Scholar]
- Johny, A.K.; Hoagland, T.; Venkitanarayanan, K. Effect of Subinhibitory Concentrations of Plant-derived Molecules in Increasing the Sensitivity of Multidrug-resistant Salmonella enterica Serovar Typhimurium DT104 to Antibiotics. Foodborne Pathog. Dis. 2010, 7, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Gnanendra, S.; Mohamed, S.; Natarjan, J. Identification of Potent Inhibitors for Salmonella typhimurium Quorum Sensing Via Virtual Screening and Pharmacophore Modeling. Comb. Chem. High Throughput Screen. 2013, 16, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Martinez, Z.; Bravo, P.; Kennedy, N.F.; Krishna, M.; Hussain, S.; Young, A.C.; Biswas, D. Antimicrobial and Antivirulence Impacts of Phenolics on Salmonella Enterica Serovar Typhimurium. Antibiotics 2020, 9, 668. [Google Scholar] [CrossRef]
- Birhanu, B.T.; Lee, E.B.; Lee, S.J.; Park, S.C. Targeting Salmonella typhimurium Invasion and Intracellular Survival Using Pyrogallol. Front. Microbiol. 2021, 12, 631426. [Google Scholar] [CrossRef]
- Giovagnoni, G.; Rossi, B.; Tugnoli, B.; Ghiselli, F.; Bonetti, A.; Piva, A.; Grilli, E. Thymol and Carvacrol Downregulate the Expresion of Salmonella typhimurium Virulence Genes During and In Vitro Infection on Caco-2 Cells. Microorganisms 2020, 8, 862. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Luo, J.; Jie, J.; Deng, X.; Song, L. The Herbal Compound Thymol Targets Multiple Salmonella typhimurium Virulence Factors for Lon Protease Degradation. Front. Pharmacol. 2021, 12, 674955. [Google Scholar] [CrossRef]
- Tokam-Kuaté, C.R.; Bisso-Ndezo, B.; Dzoyem, J.P. Synergistic Antibiofilm Effect of Thymol and Piperine in Combination with Aminoglycosides Antibiotics Against Four Salmonella Enterica Serovars. Evid.-Based Complement. Altern. Med. 2021, 2021, 1567017. [Google Scholar] [CrossRef]
- Almuzaini, A.M. Phytochemicals: Potential Alternative Strategy to Fight Salmonella Enterica Serovar Typhimurium. Front. Vet. Sci. 2023, 10, 1188752. [Google Scholar] [CrossRef]
- Sakarikou, C.; Kostoglou, D.; Simoes, M.; Giaouris, E. Exploitation of Plant Extracts and Phytochemicals Against Resistant Salmonella spp. in Biofilms. Food Res. Int. 2020, 128, 108806. [Google Scholar] [CrossRef]
- Kiga, K.; Tan, X.E.; Ibarra-Chávez, R.; Watanabe, S.; Aiba, Y.; Sato, Y.; Li, F.Y.; Sasahara, T.; Cui, B.; Kawauchi, M.; et al. Development of CRISPR-Cas13a-based Antimicrobials Capable of Sequence-specific Killing of Target Bacteria. Nat. Commun. 2020, 11, 2934. [Google Scholar] [CrossRef]
- Abavisani, M.; Khayami, R.; Hoseinzadeh, M.; Kodori, M.; Kesharwani, P.; Sahebkar, A. CRISPR-Cas System as a Promising Player Against Bacterial Infection and Antibiotic Resistance. Drug Resist. Updates 2023, 68, 100948. [Google Scholar] [CrossRef]
- Sharma, N.; Das, A.; Raja, P.; Marathe, S.A. The CRISPR-Cas System Differentially Regulates Surface-attached and Pellicle Biofilm in Salmonella enterica Serovar Typhimurium. Microbiol. Spectr. 2022, 10, e00202-22. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step Inactivation of Chromosomal Genes in Escherichia coli K-12 Using PCR Products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Li, Y.; Liao, D.; Zhang, W.; Han, X.; Man, S. A ratiometric fluorescent biosensing platform for ultrasensitive detection of Salmonella typhimurium via CRISPR/Cas12a and silver nanoclusters. J. Hazard. Mater. 2023, 443, 130234. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Z.; Bai, L.; Zhang, X.; Feng, J.; Qian, C.; Wang, Y.; Wang, R. Next-generation CRISPR-based diagnostic tools for human diseases. TrAC Trends Anal. Chem. 2023, 168, 117328. [Google Scholar] [CrossRef]
- Rohatgi, A.; Gupta, P. Natural and Synthetic Plant Compounds as Anti-biofilm Agents Against Escherichia coli O157:H7 biofilm. Infect. Genet. Evol. 2021, 95, 105055. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, S.; Sharma, P.; Chandola, D.; Dang, S.; Gupta, S.; Gabrani, R. Escherichia coli Biofilm: Development and Therapeutic Strategies. J. Appl. Microbiol. 2016, 121, 9–319. [Google Scholar] [CrossRef]
- Zambrano, C.; Kerekes, E.B.; Kotogán, A.; Papp, T.; Vágvölgyi, C.; Krisch, J.; Takó, M. Antimicrobial Activity of Grape, Apple and Pitahaya Residue Extracts After Carbohydrase Treatment Against Food-related Bacteria. LWT Food Sci. Technol. 2019, 100, 416–425. [Google Scholar] [CrossRef]
- Lee, J.H.; Regmi, S.C.; Kim, J.A.; Cho, M.H.; Yun, H.; Lee, C.S.; Lee, J. Apple Flavonoid Phloretin Inhibits Escherichia coli O157: H7 Biofilm Formation and Ameliorates Colon Inflammation in Rats. Infect. Immun. 2011, 79, 4819–4827. [Google Scholar] [CrossRef]
- Girennavar, B.; Cepeda, M.L.; Soni, K.A.; Vikram, A.; Jesudhasan, P.; Jayaprakasha, G.K.; Pillai, S.D.; Patil, B.S. Grapefruit Juice and Its Furocoumarins Inhibits Autoinducer Signaling and Biofilm Formation in Bacteria. Int. J. Food Microbiol. 2008, 125, 204–208. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, H.S.; Joo, S.W.; Regmi, S.C.; Kim, J.A.; Ryu, C.M.; Ryu, S.Y.; Cho, M.H.; Lee, J. Diverse Plant Extracts and Trans-Resveratrol Inhibit Biofilm Formation and Swarming of Escherichia coli O157:H7. Biofouling 2013, 29, 1189–1203. [Google Scholar] [CrossRef]
- Song, Y.J.; Yu, H.H.; Kim, Y.J.; Lee, N.K.; Paik, H.D. Anti-Biofilm Activity of Grapefruit Seed Extract against Staphylococcus aureus and Escherichia coli. J. Microbiol. Biotechnol. 2019, 29, 1177–1183. [Google Scholar] [CrossRef]
- Sheng, L.; Olsen, S.A.; Hu, J.; Yue, W.; Means, W.J.; Zhu, M.J. Inhibitory Effects of Grape Seed Extract on Growth, Quorum Sensing, and Virulence Factors of CDC “Top-six” Non-O157 Shiga Toxin Producing E. coli. Int. J. Food Microbiol. 2016, 16, 24–32. [Google Scholar] [CrossRef]
- Wood, T.K. Insights on Escherichia coli Biofilm Formation and Inhibition from Whole-transcriptome Profiling. Environ. Microbiol. 2009, 11, 1–15. [Google Scholar] [CrossRef]
- Cegelski, L.; Pinkner, J.S.; Hammer, N.D.; Cusumano, C.K.; Hung, C.S.; Chorell, E.; Aberg, V.; Walker, J.N.; Seed, P.C.; Almqvist, F.; et al. Small-molecule Inhibitors Target Escherichia coli Amyloid Biogenesis and Biofilm Formation. Nat. Chem. Biol. 2009, 5, 913–919. [Google Scholar] [CrossRef]
- Lo, A.W.; Van de Water, K.; Gane, P.J.; Chan, A.W.E.; Steadman, D.; Stevens, K.; Selwood, D.L.; Waksman, G.; Remaut, H. SupPression of Type 1 Pilus Assembly in Uropathogenic Escherichia coli by Chemical Inhibition of Subunit Polymerization. J. Antimicrob. Chemother. 2014, 69, 1017–1026. [Google Scholar] [CrossRef]
- Andersson, E.K.; Bengtsson, C.; Evans, M.L.; Chorell, E.; Sellstedt, M.; Lindgren, A.E.; Hufnagel, D.A.; Bhattacharya, M.; Tessier, P.M.; Whittung-Stafshede, P.; et al. Modulation of Curli Assembly and Pellicle Biofilm Formation by Chemical and Protein Chaperones. Chem. Biol. 2013, 20, 1245–1254. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Ryu, S.Y.; Cho, M.H.; Lee, J. Ginkgolic Acids and Ginkgo Biloba Extract Inhibit Escherichia coli O157: H7 and Staphylococcus aureus Biofilm Formation. Int. J. Food Microbiol. 2014, 174, 47–55. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Ryu, S.Y.; Cho, M.H.; Lee, J. Resveratrol Oligomers Inhibit Biofilm Formation of Escherichia coli O157:H7 and Pseudomonas aeruginosa. J. Nat. Prod. 2014, 77, 168–172. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Cho, H.S.; Ryu, S.Y.; Cho, M.H.; Lee, J. Coumarins Reduce Biofilm Formation, and the Virulence of Escherichia coli O157:H7. Phytomedicine 2014, 21, 1037–1042. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lee, J.H.; Gwon, G.; Kim, S.I.; Park, J.G.; Lee, J. Essential Oils and Eugenols Inhibit Biofilm Formation and the Virulence of Escherichia coli O157:H7. Sci. Rep. 2016, 6, 36377. [Google Scholar] [CrossRef]
- Verstraeten, N.; Braeken, K.; Debkumari, B.; Fauvart, M.; Fransaer, J.; Vermant, J.; Michiels, J. Living on a Surface: Swarming and Biofilm Formation. Trends Microbiol. 2008, 16, 496–506. [Google Scholar] [CrossRef]
- Dakheel, M.M.; Alkandari, F.A.H.; Mueller-Harvey, I.; Woodward, M.J.; Rymer, C. Antimicrobial In Vitro Activities of Condensed Tannin Extracts on Avian Pathogenic Escherichia coli. Lett. Appl. Microbiol. 2020, 70, 165–172. [Google Scholar] [CrossRef]
- Priha, O.; Virkajärvi, V.; Juvonen, R.; Puupponen-Pimiä, R.; Nohynek, L.; Alakurtti, S.; Pirttimaa, M.; Storgårds, E. Quorum Sensing Signalling and Biofilm Formation of Brewery-derived Bacteria, and Inhibition of Signalling by Natural Compounds. Curr. Microbiol. 2014, 69, 617–627. [Google Scholar] [CrossRef]
- Borges, A.; Saavedra, M.J.; Simoes, M. The Activity of Ferulic and Gallic Acids in Biofilm Prevention and Control of Pathogenic bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef]
- Vikram, A.; Jayaprakasha, G.K.; Uckoo, R.M.; Patil, B.S. Inhibition of Escherichia coli O157:H7 Motility and Biofilm by β-Sitosterol Glucoside. Biochim. Biophys. Acta 2013, 1830, 5219–5228. [Google Scholar] [CrossRef]
- Cho, H.S.; Lee, J.H.; Ryu, S.Y.; Joo, S.W.; Cho, M.H.; Lee, J. Inhibition of Pseudomonas aeruginosa and Escherichia coli O157:H7 Biofilm Formation by Plant Metabolite ε-viniferin. J. Agric. Food Chem. 2013, 61, 7120–7126. [Google Scholar] [CrossRef]
- Bai, Y.B.; Shi, M.Y.; Wang, W.W.; Wu, L.Y.; Bai, Y.T.; Li, B.; Zhou, X.Z.; Zhang, J.Y. Novel Quorum Sensing Inhibitor Echinatin as an Antibacterial Synergist Against Escherichia coli. Front. Microbiol. 2022, 13, 1003692. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, W.; Shi, M.; Wei, X.; Zhou, X.; Li, B.; Zhang, J. Novel Antibiofilm Inhibitor Ginkgetin as an Antibacterial Synergist against Escherichia coli. Int. J. Mol. Sci. 2022, 23, 8809. [Google Scholar] [CrossRef]
- Ivanov, M.; Novović, K.; Malešević, M.; Dinić, M.; Stojković, D.; Jovčić, B.; Soković, M. Polyphenols as Inhibitors of Antibiotic Resistant Bacteria-Mechanisms Underlying Rutin Interference with Bacterial Virulence. Pharmaceuticals 2022, 15, 385. [Google Scholar] [CrossRef]
- Kanamaru, K.; Kanamaru, K.; Tatsuno, I.; Tobe, T.; Sasakawa, C. SdiA, an Escherichia coli Homologue of Quorum-sensing Regulators, Controls the Expression of Virulence Factors in Enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 2000, 38, 805–816. [Google Scholar] [CrossRef]
- Zhu, L.; Liang, Z.; Xu, Y.; Chen, Z.; Wang, J.; Zhou, L. Ultrasensitive and Rapid Visual Detection of Escherichia coli O157:H7 Based on RAA-CRISPR/Cas12a System. Biosensors 2023, 13, 659. [Google Scholar] [CrossRef]
- Jiang, W.; He, C.; Bai, L.; Chen, Y.; Jia, J.; Pan, A.; Lv, B.; Tang, X.; Wu, X. A Rapid and Visual Method for Nucleic Acid Detection of Escherichia coli O157:H7 Based on CRISPR/Cas12a-PMNT. Foods 2023, 12, 236. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, W.; Shao, S.; Wang, Q. Gene Silencing through CRIPSR Interference in Bacteria: Current Advances and Future Prospects. Front. Microbiol. 2021, 12, 635227. [Google Scholar]
- Suvvari, T.K.; Kandula, V.D.K.; Kandi, V.; Thomas, V. Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-Cas13a: Future Hope to Tackle Anti-Microbial Resistance. Microbiol. Insights 2023, 16, 11786361231178623. [Google Scholar] [CrossRef]
- Hannu, T.; Mattila, L.; Rautelin, H.; Pelkonen, P.; Lahdenne, P.; Siitonen, A.; Leirisalo-Repo, M. Campylobacter-triggered Reactive Arthritis: A pPopulation-based Study. Rheumatology 2002, 41, 312–318. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef]
- Backert, S.; Tegtmeyer, N.; Cróinín, T.Ó.; Boehm, M.; Heimesaat, M.M. Chapter 1—Human Campylobacteriosis. In Campylobacter; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–25. [Google Scholar]
- Klančnik, A.; Šimunović, K.; Sterniša, M.; Ramič, D.; Možina, S.S.; Bucar, F. Anti-adhesion Activity of Phytochemicals to Prevent Campylobacter jejuni Biofilm Formation on Abiotic Surfaces. Phytochem. Rev. 2021, 20, 55–84. [Google Scholar] [CrossRef]
- Ica, T.; Caner, V.; Istanbullu, O.; Nguyen, H.D.; Ahmed, B.; Call, D.R.; Beyenal, H. Characterization of Mono- and Mixed-culture Campylobacter jejuni Biofilms. Appl. Environ. Microbiol. 2012, 78, 1033–1038. [Google Scholar] [CrossRef]
- Wagle, B.R.; Donoghue, A.M.; Jesudhasan, P.R. Select Phytochemicals Reduce Campylobacter jejuni in Postharvest Poultry and Modulate the Virulence Attributes of C. jejuni. Front. Microbiol. 2021, 12, 725087. [Google Scholar] [CrossRef]
- Wagle, B.R.; Upadhyay, A.; Upadhyaya, I.; Shrestha, S.; Arsi, K.; Liyanage, R.; Venkitanarayanan, K.; Donoghue, D.J.; Donoghue, A.M. Trans-cinnamaldehyde, Eugenol and Carvacrol Reduce Campylobacter jejuni Biofilms and Modulate Expression of Selected Genes and Proteins. Front. Microbiol. 2019, 10, 1837. [Google Scholar] [CrossRef]
- Wagle, B.R.; Donoghue, A.M.; Shrestha, S.; Upadhyaya, I.; Arsi, K.; Gupta, A.; Liyanage, R.; Rath, N.C.; Donoghue, D.J.; Upadhyay, A. Carvacrol Attenuates Campylobacter jejuni Colonization Factors and Proteome Critical for Persistence in the Chicken Gut. Poult. Sci. 2020, 99, 4566–4577. [Google Scholar] [CrossRef]
- Castillo, S.; Heredia, N.; García, S. 2(5H)-Furanone, Epigallocatechin Gallate, and a Citric-based Disinfectant Disturb Quorum-sensing Activity and Reduce Motility and Biofilm Formation of Campylobacter jejuni. Folia Microbiol. 2015, 60, 89–95. [Google Scholar] [CrossRef]
- Klančnik, A.; Gobin, I.; Vučković, D.; Možina, S.S.; Abram, M.; Jeršek, B. Reduced Contamination and Infection Via Inhibition of Adhesion of Foodborne Bacteria to Abiotic Polystyrene and Biotic Amoeba Surfaces. Int. J. Food Sci. Technol. 2018, 53, 1013–1020. [Google Scholar] [CrossRef]
- Klančnik, A.; Zorko, Š.; Toplak, N.; Kovač, M.; Bucar, F.; Jeršek, B.; Možina, S.S. Antiadhesion Activity of Juniper (Juniperus communis L.) Preparations Against Campylobacter jejuni Evaluated with PCR-based Methods. Phytother. Res. 2018, 32, 542–550. [Google Scholar] [CrossRef]
- Wagle, B.R.; Arsi, K.; Upadhyay, A.; Shrestha, S.; Venkitanarayanan, K.; Donoghue, A.M.; Donoghue, D.J. β-Resorcylic Acid, a Phytophenolic Compound, Reduces Campylobacter jejuni in Postharvest Poultry. J. Food Prot. 2017, 80, 1243–1251. [Google Scholar] [CrossRef]
- Duarte, A.; Luís, A.; Oleastro, M.; Domingues, F.C. Antioxidant Properties of Coriander Essential Oil and Linalool and Their Potential to Control Camplyobacter spp. Food Control 2016, 61, 115–122. [Google Scholar] [CrossRef]
- Duarte, A.; Alves, A.C.; Ferreira, S.; Silva, F.; Domingues, F.C. Resveratrol Inclusion Complexes: Antibacterial and Anti-Biofilm Activity Against Campylobacter spp. and Arcobacter butzleri. Food Res. Int. 2015, 77, 244–250. [Google Scholar] [CrossRef]
- Klančnik, A.; Šikić, P.M.; Trošt, K.; Yušek, Z.M.; Vodopivec, M.B.; Smole, M.S. Anti-Campylobacter Activity of Resveratrol and an Extract from Waste. Pinot Noir Grape Skins and Seeds, and Resistance of Campylobacter jejuni Planktonic and Biofilm Cells, Mediated Via the CmeABC Efflux Pump. J. Appl. Microbiol. 2017, 122, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Pogačar, M.Š.; Klančnik, A.; Bucar, F.; Langerholc, T.; Možina, S.S. Alpinia katsumadai Extracts Inhibit Adhesion and Invasion of Campylobacter jejuni in Animal and Human Foetal Small Intestine Cell Lines. Phytother. Res. 2015, 29, 1585–1589. [Google Scholar] [CrossRef] [PubMed]
- Šikiæ, P.M.; Klanènik, A.; Bucar, F.; Langerholc, T.; Možina, S.S. Anti-adhesion activity of thyme (Thymus vulgaris L.) extract, thyme post-distillation waste, and olive (Olea europea L.) leaf extract against Campylobacter jejuni on polystyrene and intestine epithelial cells. J. Sci. Food Agric. 2016, 96, 2723–2730. [Google Scholar]
- Bensch, K.; Tiralongo, J.; Schmidt, K.; Matthias, A.; Bone, K.M.; Lehmann, R.; Tiralongo, E. Investigations Into the Antiadhesive Activity of Herbal Extracts Against Campylobacter jejuni. Phytother. Res. 2011, 25, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Šimunović, K.; Ramić, D.; Xu, C.; Možina, S.S. Modulation of Campylobacter jejuni Motility, Adhesion to Polystyrene Surfaces, and Invasion of INT407 Cells by Quorum-Sensing Inhibition. Microorganisms 2020, 8, 104. [Google Scholar] [CrossRef]
- Plummer, P.J. LuxS and Quorum-sensing in Campylobacter. Front. Cell. Infect. Microbiol. 2012, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Castillo, S.; Heredia, N.; Arechiga-Carvajal, E.; García, S. Citrus Extracts as Inhibitors of Quorum Sensing, Biofilm Formation and Motility of Campylobacter jejuni. Food Biotechnol. 2014, 28, 106–122. [Google Scholar] [CrossRef]
- Castillo, S.; Dávila-Aviña, J.; Heredia, N.; Garcia, S. Antioxidant Activity and Influence of Citrus by-product Extracts on Adherence and Invasion of Campylobacter jejuni and on the Relative Expression of cadF and ciaB. Food Sci. Biotechnol. 2017, 26, 453–459. [Google Scholar] [CrossRef]
- Bezek, K.; Kurinčič, M.; Knauder, E.; Klančnik, A.; Raspor, P.; Bucar, F.; Možina, S.S. Attenuation of Adhesion, Biofilm Formation and Quorum Sensing of Campylobacter jejuni by Euodia ruticarpa. Phytother. Res. 2016, 30, 1527–1532. [Google Scholar] [CrossRef]
- Elgamoudi, B.A.; Korolik, V. Campylobacter Biofilms: Potential of Natural Compounds to Disrupt Campylobacter jejuni Transmission. Int. J. Mol. Sci. 2021, 22, 12159. [Google Scholar] [CrossRef]
- Mohanraju, P.; Saha, C.; van Baarlen, P.; Louwens, R.; Staals, R.H.J.; van der Oost, J. Alternative functions of CRISPR—Cas systems in the evolutionary arms race. Nat. Rev. Microbiol. 2022, 20, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Püning, C.; Su, Y.; Lu, X.; Gölz, G. Molecular Mechanisms of Campylobacter Biofilm Formation and Quorum Sensing. Curr. Top. Microbiol. Immunol. 2021, 431, 293–319. [Google Scholar] [PubMed]
- Schauder, S.; Shokat, K.; Surette, M.G.; Bassler, B.L. The LuxS Family of Bacterial Autoinducers: Biosynthesis of a Novel Quorum-sensing Signal Molecule. Mol. Microbiol. 2001, 41, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chan, K.K.; Hua, M.Z.; Golz, G.; Lu, X. Inhibition of AI-2 Quorum Sensing and Biofilm Formation in Campylobacter jejuni by Decanoic and Lauric Acids. Front. Microbiol. 2022, 12, 811506. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Yu, Y.; Bai, X.; Jia, Y.; Tian, J.; Gu, K.; Zhao, M.; Zhou, C.; Zhang, X.; Wang, H.; et al. Pathogen-Specific Bactericidal Method Mediated by Conjugative Delivery of CRISPR-Cas13a Targeting Bacterial Endogenous Transcripts. Microbiol. Spectr. 2022, 10, e0130022. [Google Scholar] [CrossRef]
- Costigan, R.; Stoakes, E.; Floto, R.A.; Parkhill, J.; Grant, A.J. Development and Validation of a CRISPR Interference System for Gene Regulation in Campylobacter jejuni. BMC Microbiol. 2022, 22, 238. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Tagliaferri, T.L.; Mendes, T.A. Potential of the endogenous and artificially inserted CRISPR-Cas system for controlling virulence and antimicrobial resistance of food pathogens. Food Chem. Adv. 2023, 2, 100229. [Google Scholar] [CrossRef]
- Zuberi, A.; Misba, L.; Khan, A.U. CRISPR Interference (CRISPRi) Inhibition of the luxS gene Expression in E. coli: An approach to inhibit biofilm. Front. Cell. Infect. Microbiol. 2017, 7, 214. [Google Scholar] [CrossRef]
- Alshammari, M.; Ahmad, A.; Alkhulaifi, M.; Farraj, D.A.; Alsudir, S.; Alawari, M.; Takashi, G.; Alyamani, E. Reduction of Biofilm Formation of Escherichia coli by Targeting Quorum Sensing and Adhesion Genes Using the CRIPR/Cas9-HDR Approach and its Clinical Application on Urinary Catheter. J. Infect. Public Health. 2023, 16, 1174–1183. [Google Scholar] [CrossRef]
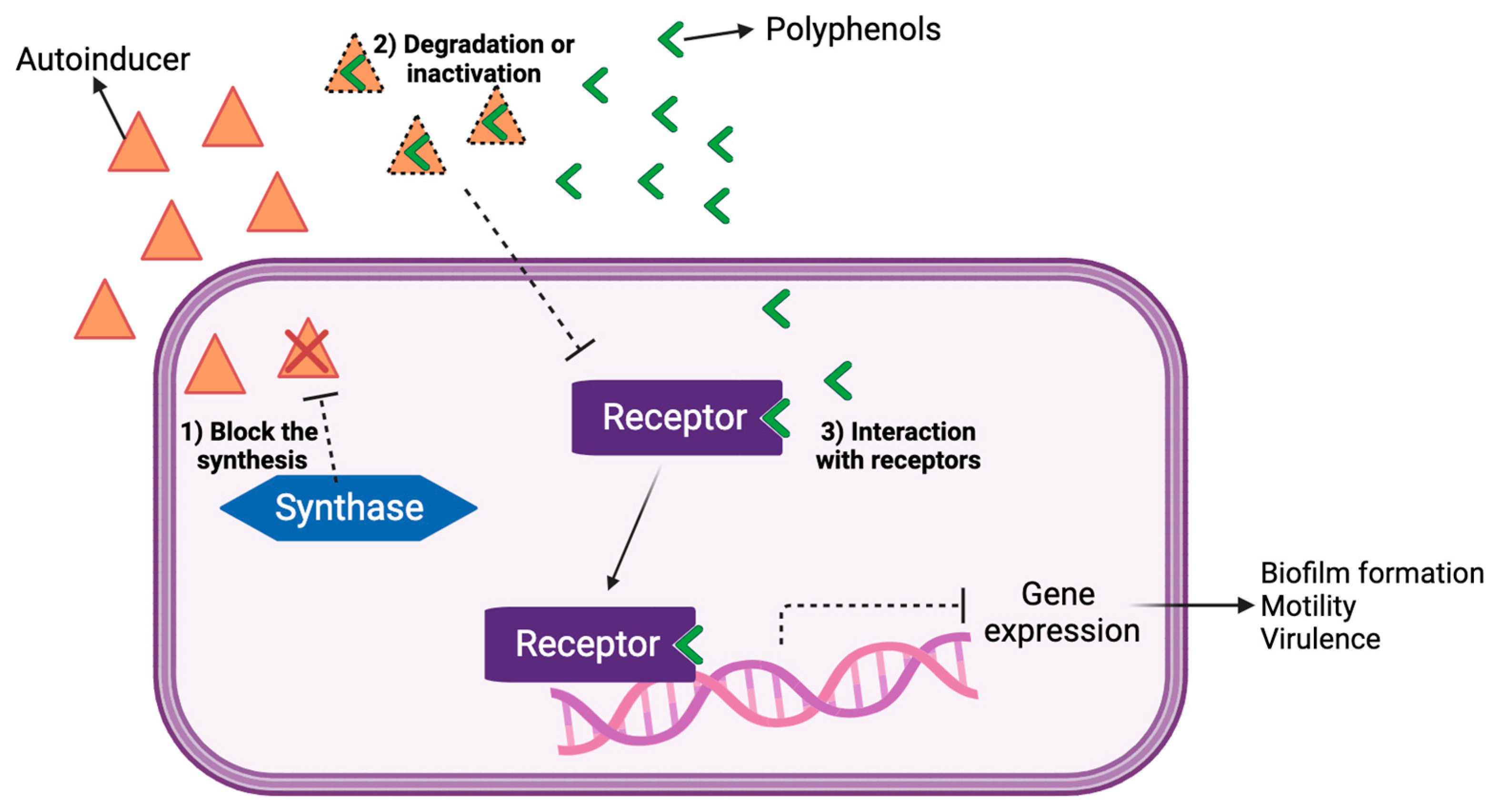
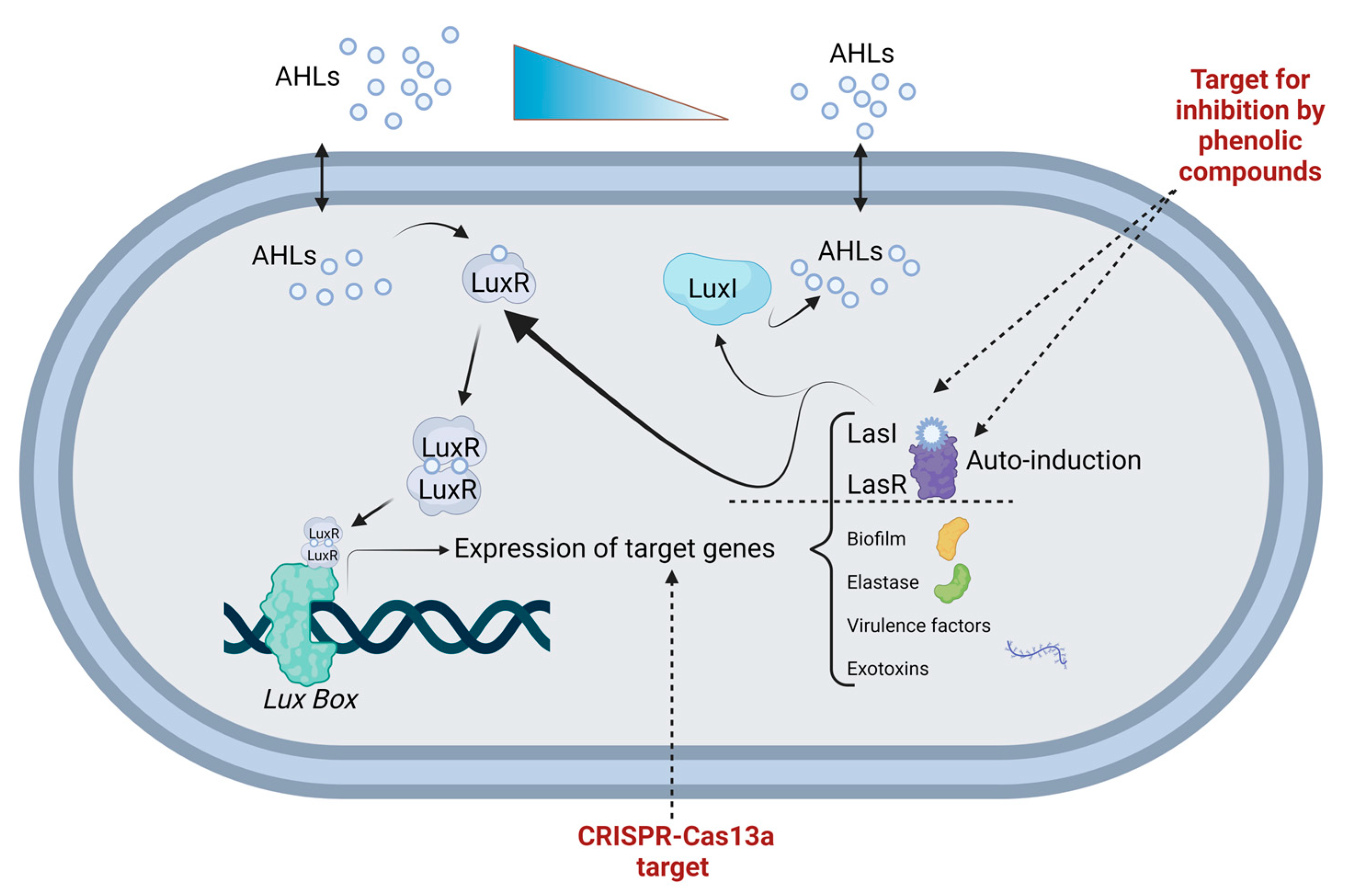
| Compound or Extract | Inhibitory Action | Reference |
|---|---|---|
| Coumarin | Not specified (Moderate) | [47] |
| Carvacrol Trans-cinnamaldehyde B-resorcylic acid Eugenol | Increased susceptibility to antibiotics | [48] |
| CD01374 RJF 004047 KM101117 | Inhibition of SDiA activity | [49] |
| Gallic acid Protocatechuic acid Vanillic Acid | Alteration of virulence genes and increased membrane permeability | [50] |
| Pyrogallol | Downregulation of virulence genes | [51] |
| Thymol | Biofilm inhibition, disruption of cell membrane and downregulation of virulence genes | [52] |
| Thymol | Lon protein degradation | [53] |
| Thymol and Pipperine | Synergistic effect with kanamycin and streptomycin | [54] |
| Compound or Extract | Inhibitory Action | Reference |
|---|---|---|
| 4-hydroxybenzoic, syringic, gallic, vanillic, cinnamic and p-coumaric acids, (+)-catechin, (−)-epicatechin, quercetin, polydatin and resveratrol. Bergamottin and dihydroxybergamottin Grapefruit seed extract | Inhibited the biofilm formation | [65,67,69] |
| Phloretin | Inhibited the biofilm formation by impaired autoinducer II expression and fimbriae expression. | [66] |
| Trans-resveratrol Naringenin, kaempferol, quercetin and apigenin. | Inhibited the biofilm formation by interfere of AI-2 signaling. | [27,68] |
| Grape seed extract | Suppresses QS with concomitant decrease in motility, flagella protein expression and Shiga toxin production. | [70] |
| Ginkgolic acid | Repressed curli genes and prophage genes and influenced swarming and swimming motilities. | [75] |
| Vitisin B | Biofilm inhibition by fimbriae reduction. | [76] |
| Coumarin and umbelliferon | Repressed curli genes and motility genes. | [77] |
| Eugenol | Down-regulation of curly (csgABDFG) and type I fimbriae genes (fimCDH) and ler-controlled toxin genes (espD, escJ, escR, and tir), which are required for biofilm formation and the attachment and effacement phenotype. | [78] |
| Procyanidins and prodelphinidins | Showed antimicrobial activities against E. coli by affecting the growth biofilm formation and motility. | [80] |
| Gallic acid and ferulic acid | Inhibition of swarming in E. coli CECT 434 and thus reduced the biofilm mass considerably. | [82] |
| β-sitosterol glucoside | Inhibit the biofilm and motility through rssAB- and hns-mediated repression of flagellar master operon flhDC in E. coli O157:H7. | [83] |
| ε-viniferin | Downregulation of genes such as flhD, fimA, fimH and motB which are involved in motility regulation and adherence. | [68,82] |
| Hesperetin, hesperidin, naringenin, naringin, taxifolin, morin, chlorogenic acid, ferulic acid, p-coumaric acid, and gallic acid | Inhibit bacterial growth and biofilm formation of E. coli IBRS E003 and E. coli IMD989 | [87] |
| Compound or Extract | Inhibitory Action | Reference |
|---|---|---|
| Turmeric, curcumin, allyl sulfide, garlic oil, and ginger | Reduced the adhesion (except curcumin) and all the phytochemicals reduced quorum sensing. | [98] |
| Carvacrol | Inhibited the biofilm formation and adhesion of bacteria and decreasing motility, quorum sensing, and tolerance to stress in vitro. Downregulated bacterial cell mobility genes flaA, flaB, and flaG | [99] |
| Trans-cinnamaldehyde | Inhibited the biofilm formation and adhesion of bacteria. Downregulated bacterial cell mobility genes flaA, flaB, and flaG. | [99] |
| Epigallocatechin gallate | Disturbed quorum-sensing activity and reduced motility, adhesion, and biofilm formation. | [101,102] |
| Amentoflavone | Inhibited of adhesion. | [103] |
| β-resorcylic acid | Down-regulated expression of genes for motility (motA, motB) and attachment (cadF, ciaB) | [104] |
| Eugenol | Downregulated of genes (flaA, flaaG, flgA, waaF, cosR, and ahpC) critical for biofilm formation. | [99] |
| Linalool | Inhibited of adhesion and QS. | [105] |
| Resveratrol | Inhibited the biofilm formation and adhesion. | [106,107] |
| Alpinia katsumadai extracts Grape extract Thyme extract Herbal extracts Lavender, juniper, rosemary, cloves, thyme (essential oil) | Inhibited adhesion. | [107,108,109,110,111] |
| Coriander | Inhibited of adhesion and QS. | [105] |
| Citrus extracts | Reduce motility, biofilm formation, invasion, and adhesion of epithelial cells and virulence of C. jejuni by modulation of QS. | [113,114] |
| Euodia ruticarpa | Inhibited of QS. | [115] |
| Cas system | Organism | Application | Reference |
|---|---|---|---|
| CRISPR-Cas13 | Salmonella Typhimurium | Reduction of S. Typhimurium colonization in the intestinal tract. | [121] |
| RAA-CRISPR/12a | Escherichia coli O157:H7 | Highly efficient diagnostic test development. | [122] |
| CRISPR-dCas9 | Campylobacter jejuni | Verified the variation in the reduction of mobility that exists in flagellum phenotypes | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higuera-Ciapara, I.; Benitez-Vindiola, M.; Figueroa-Yañez, L.J.; Martínez-Benavidez, E. Polyphenols and CRISPR as Quorum Quenching Agents in Antibiotic-Resistant Foodborne Human Pathogens (Salmonella Typhimurium, Campylobacter jejuni and Escherichia coli 0157:H7). Foods 2024, 13, 584. https://doi.org/10.3390/foods13040584
Higuera-Ciapara I, Benitez-Vindiola M, Figueroa-Yañez LJ, Martínez-Benavidez E. Polyphenols and CRISPR as Quorum Quenching Agents in Antibiotic-Resistant Foodborne Human Pathogens (Salmonella Typhimurium, Campylobacter jejuni and Escherichia coli 0157:H7). Foods. 2024; 13(4):584. https://doi.org/10.3390/foods13040584
Chicago/Turabian StyleHiguera-Ciapara, Inocencio, Marieva Benitez-Vindiola, Luis J. Figueroa-Yañez, and Evelin Martínez-Benavidez. 2024. "Polyphenols and CRISPR as Quorum Quenching Agents in Antibiotic-Resistant Foodborne Human Pathogens (Salmonella Typhimurium, Campylobacter jejuni and Escherichia coli 0157:H7)" Foods 13, no. 4: 584. https://doi.org/10.3390/foods13040584
APA StyleHiguera-Ciapara, I., Benitez-Vindiola, M., Figueroa-Yañez, L. J., & Martínez-Benavidez, E. (2024). Polyphenols and CRISPR as Quorum Quenching Agents in Antibiotic-Resistant Foodborne Human Pathogens (Salmonella Typhimurium, Campylobacter jejuni and Escherichia coli 0157:H7). Foods, 13(4), 584. https://doi.org/10.3390/foods13040584





