Development of a Green, Quick, and Efficient Method Based on Ultrasound-Assisted Extraction Followed by HPLC-DAD for the Analysis of Bioactive Glycoalkaloids in Potato Peel Waste
Abstract
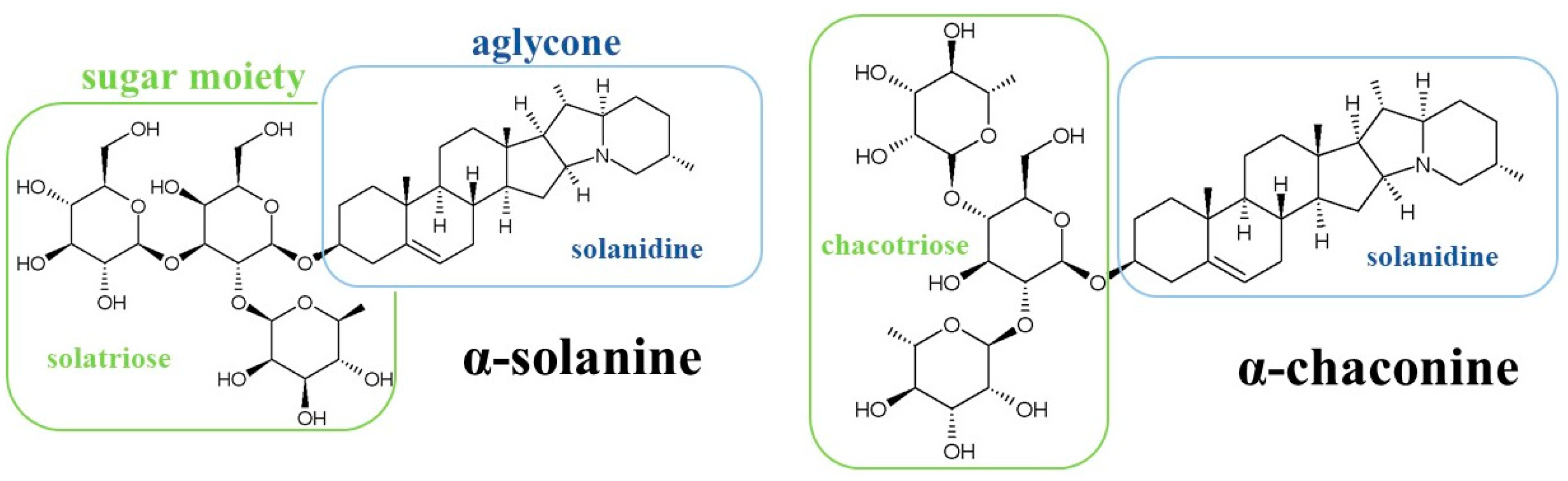
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Sample Collection and Moisture Determination
2.3. Optimization of the Sample Treatment Conditions and Statistical Analysis to Maximize the Extraction Yield of α-Solanine and α-Chaconine
2.3.1. Preliminary Studies
2.3.2. Design of Experiments to Reach the Optimal Extraction Conditions
2.3.3. Optimized Extraction Conditions
2.4. Optimal Chromatographic Conditions for Analysis
2.5. Method Validation
2.6. Evaluation of the Effect of the Drying Conditions on the α-Solanine and α-Chaconine Content in Potato Peels
3. Results and Discussion
3.1. Optimization of the Chromatographic Method for α-Solanine and α-Chaconine Determination
3.2. Optimization of the Extraction Methodology of α-Solanine and α-Chaconine from Potato Peels
3.2.1. Preliminary Studies
3.2.2. Experimental Design, Evaluation of the Variables Influencing the Extraction Efficiency, and Statistical Analysis
3.3. Method Validation
3.4. Greenness Evaluation of the Developed Method
3.5. Application to the Analysis of Different Potato Peels
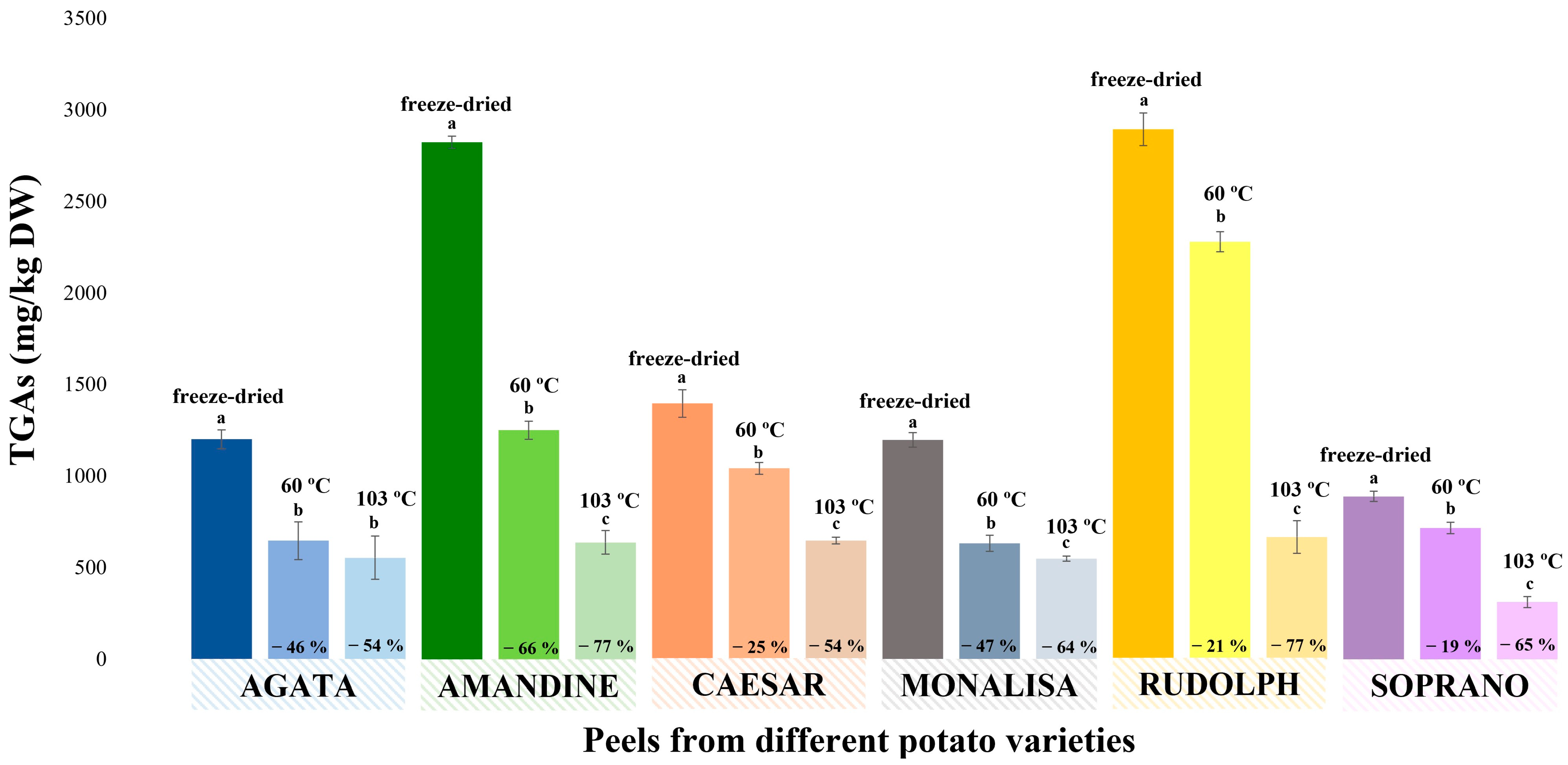
3.6. Evaluation of the Temperature Effect on the Concentration of α-Solanine and α-Chaconine during Drying
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Friedman, M. Potato glycoalkaloids and metabolites: Roles in the plant and in the diet. J. Agric. Food Chem. 2006, 54, 8655–8681. [Google Scholar] [CrossRef]
- Friedman, M. Analysis of biologically active compounds in potatoes (Solanum tuberosum), tomatoes (Lycopersicon esculentum), and jimson weed (Datura stramonium) seeds. J. Chromatogr. A 2004, 1054, 143–155. [Google Scholar] [CrossRef]
- Omayio, D.G.; Abong, G.O.; Okoth, M.W. A review of occurrence of glycoalkaloids in potato and potato products. Curr. Res. Nutr. Food Sci. 2016, 4, 195–202. [Google Scholar] [CrossRef]
- Singh, B.; Dutt, S.; Raigond, P. Potato glycoalkaloids. In Potato: Nutrition and Food Security; Springer: Singapore, 2020; pp. 191–212. ISBN 9789811576621. [Google Scholar]
- Nema, P.K.; Ramayya, N.; Duncan, E.; Niranjan, K. Potato glycoalkaloids: Formation and strategies for mitigation. J. Sci. Food Agric. 2008, 88, 1869–1881. [Google Scholar] [CrossRef]
- EFSA-European Food Safety Authority. Risk assessment of glycoalkaloids in feed and food, in particular in potatoes and potato-derived products. ESFA J. 2020, 18, e06222. [Google Scholar]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; Nielsen, E.; et al. Commission Recommendation (EU) 2022/561 of 6 April 2022 on monitoring the presence of glycoalkaloids in potatoes and potato-derived products. Off. J. Eur. Union 2022, 18, 66–67. [Google Scholar]
- Ostreikova, T.O.; Kalinkina, O.V.; Bogomolov, N.G.; Chernykh, I.V. Glycoalkaloids of plants in the family Solanaceae (nightshade) as potential drugs. Pharm. Chem. J. 2022, 56, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Benkeblia, N. Potato glycoalkaloids: Occurrence, biological activities and extraction for biovalorisation—A review. Int. J. Food Sci. Technol. 2020, 55, 2305–2313. [Google Scholar] [CrossRef]
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and human health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef]
- Singh, B.; Raigond, P.; Dutt, S.; Manoj, K. Potatoes for food and nutritional security. In Potato: Nutrition and Food Security; Springer: Singapore, 2020; pp. 1–12. ISBN 9789811576621. [Google Scholar]
- Food and Agriculture Organisation (FAO). Role and Potential of Potato in Global Food Security Challenges of Global Food Security Contribution of Potato to the World Potential of Global Potato Production Strategies for Promoting Potato Development; FAO: Roma, Italy, 2022. [Google Scholar]
- Khanal, S.; Karimi, K.; Majumdar, S.; Kumar, V.; Verma, R.; Bhatia, S.K.; Kuca, K.; Esteban, J.; Kumar, D. Sustainable utilization and valorization of potato waste: State of the art, challenges, and perspectives. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Pacifico, D.; Lanzanova, C.; Pagnotta, E.; Bassolino, L.; Mastrangelo, A.M.; Marone, D.; Matteo, R.; Lo Scalzo, R.; Balconi, C. Sustainable use of bioactive compounds from Solanum tuberosum and Brassicaceae wastes and by-products for crop protection—A review. Molecules 2021, 26, 2174. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 Principles of green analytical chemistry and the significance mnemonic of green analytical practices. Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Apel, C.; Lyng, J.G.; Papoutsis, K.; Harrison, S.M.; Brunton, N.P. Screening the effect of different extraction methods (ultrasound-assisted extraction and solid–liquid extraction) on the recovery of glycoalkaloids from potato peels: Optimisation of the extraction conditions using chemometric tools. Food Bioprod. Process. 2020, 119, 277–286. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A Comprehensive Review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochemistry 2023, 101, 106646. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Tiwari, B.K.; Gangopadhyay, N.; O’Donnell, C.P.; Brunton, N.P.; Rai, D.K. Ultrasonic extraction of steroidal alkaloids from potato peel waste. Ultrason. Sonochemistry 2014, 21, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.H.; Guo, H.C. An ultra-high-performance liquid chromatography-triple quadrupole mass spectrometry method for the detection of steroidal glycoalkaloids in potato samples. Anal. Methods 2017, 9, 6613–6621. [Google Scholar] [CrossRef]
- Tietje, C.; Brouder, E. Q2(R1) ICH Guidelines (Internation Council for Harmonisation, 2005). In Handbook of Transnational Economic Governance; Regimes: Brill, Nijhoff, 2010; pp. 1041–1053. [Google Scholar] [CrossRef]
- European Commission. SANTE/12682/2019 Guidance Document on Analytical Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed; European Commission: Brussels, Belgium, 2019.
- European Commission (EC). Commission Regulation 401/2006 of 23 February 2006 Laying Down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Food Stuffs; European Commission: Brussels, Belgium, 2006.
- Bagheri, M.; Bushehri, A.A.S.; Hassandokht, M.R.; Naghavi, M.R. Evaluation of solasonine content and expression patterns of sgt1 gene in different tissues of two iranian eggplant (Solanum melongena L.) Genotypes. Food Technol. Biotechnol. 2017, 55, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Mennella, G.; Lo Scalzo, R.; Fibiani, M.; D’Alessandro, A.; Francese, G.; Toppino, L.; Acciarri, N.; De Almeida, A.E.; Rotino, G.L. Chemical and bioactive quality traits during fruit ripening in eggplant (S. Melongena L.) and allied species. J. Agric. Food Chem. 2012, 60, 11821–11831. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, G.; Cahill, M.G.; Vittori, S.; James, K.J. Liquid chromatography-hybrid linear ion trap-high-resolution mass spectrometry (LTQ-Orbitrap) method for the determination of glycoalkaloids and their aglycons in potato samples. Food Anal. Methods 2014, 7, 1367–1372. [Google Scholar] [CrossRef]
- Dzakovich, M.P.; Hartman, J.L.; Cooperstone, J.L. A high-throughput extraction and analysis method for steroidal glycoalkaloids in tomato. Front. Plant Sci. 2020, 11, 767. [Google Scholar] [CrossRef]
- Rasool, S.; Cárdenas, P.D.; Pattison, D.I.; Jensen, B.; Meyling, N. V Isolate-specific effect of entomopathogenic endophytic fungi on population growth of two-spotted spider mite (Tetranychus Urticae Koch) and levels of steroidal glycoalkaloids in tomato. J. Chem. Ecol. 2021, 47, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Martínez, E.; Adalid-Martínez, A.M.; García-Martínez, M.D.; Mangino, G.; Raigón, M.D.; Plazas, M.; Gramazio, P.; Prohens, J.; Vilanova, S. Fruit composition of eggplant lines with introgressions from the wild relative S. Incanum: Interest for breeding and safety for consumption. Agronomy 2022, 12, 266. [Google Scholar] [CrossRef]
- Wojnowski, W.; Tobiszewski, M.; Pena-Pereira, F.; Psillakis, E. AGREEprep—Analytical Greenness Metric for Sample Preparation. Trends Anal. Chem. 2022, 149, 116553. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The ten principles of green sample preparation. Trends Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]
- Musita, C.N.; Omayio, D.G.; Abong, G.O.; Okoth, M.W. Glycoalkaloids in commercial potato varieties traded in Nairobi, Kenya. F1000Research 2020, 9, 423. [Google Scholar] [CrossRef]





| Factor Type | Symbol | Factor Levels | ||
|---|---|---|---|---|
| Categorical | Type 1 | Type 2 | Type 3 | |
| Extraction type | A | VA-SLE 1 min | MgS-SLE 5 min | UAE 5 min |
| Solvent type | B | MeOH | EtOH | H2O |
| Numerical | Low (−1) | Medium (0) | High (+1) | |
| Sample/solvent ratio (w/v (g/mL)) | C | 1:10 | 1:20 | 1:40 |
| RUN | Factor * | Responses (Mean Concentration, mg/kg DW ± SD) | ||||
|---|---|---|---|---|---|---|
| A | B | C | α-Solanine (Y1) | α-Chaconine (Y2) | TGAs (Y3) | |
| 1 | 1 | 1 | −1 | 748 ± 13 | 818 ± 38 | 1566 ± 41 |
| 2 | 1 | 1 | 0 | 658 ± 44 | 876 ± 54 | 1584 ± 88 |
| 3 | 1 | 1 | 1 | 482 ± 12 | 726 ± 58 | 1208 ± 47 |
| 4 | 1 | 2 | −1 | 238 ± 28 | 179 ± 13 | 417 ± 41 |
| 5 | 1 | 2 | 0 | 283 ± 46 | 424 ± 54 | 696 ± 104 |
| 6 | 1 | 2 | 1 | 421 ± 89 | 471 ± 50 | 892 ± 85 |
| 7 | 1 | 3 | −1 | 58 ± 22 | 63 ± 18 | 121 ± 27 |
| 8 | 1 | 3 | 0 | 111 ± 18 | 51 ± 5 | 162 ± 20 |
| 9 | 1 | 3 | 1 | 94 ± 7 | 39 ± 3 | 132 ± 9 |
| 10 | 2 | 1 | −1 | 528 ± 21 | 546 ± 25 | 1074 ± 43 |
| 11 | 2 | 1 | 0 | 456 ± 30 | 768 ± 33 | 1225 ± 37 |
| 12 | 2 | 1 | 1 | 917 ± 78 | 646 ± 58 | 1564 ± 127 |
| 13 | 2 | 2 | −1 | 447 ± 49 | 423 ± 13 | 870 ± 62 |
| 14 | 2 | 2 | 0 | 346 ± 67 | 523 ± 23 | 869 ± 32 |
| 15 | 2 | 2 | 1 | 729 ± 40 | 291 ± 23 | 1020 ± 61 |
| 16 | 2 | 3 | −1 | 32 ± 9 | 25 ± 0.6 | 57 ± 9 |
| 17 | 2 | 3 | 0 | 62 ± 3 | 98 ± 14 | 160 ± 14 |
| 18 | 2 | 3 | 1 | 94 ± 5 | 60 ± 2 | 154 ± 4 |
| 19 | 3 | 1 | −1 | 735 ± 52 | 887 ± 151 | 1622 ± 157 |
| 20 | 3 | 1 | 0 | 671 ± 13 | 915 ± 42 | 1586 ± 57 |
| 21 | 3 | 1 | 1 | 617 ± 3 | 898 ± 29 | 1515 ± 32 |
| 22 | 3 | 2 | −1 | 465 ± 47 | 496 ± 118 | 961± 128 |
| 23 | 3 | 2 | 0 | 454 ± 46 | 654 ± 59 | 1108 ± 102 |
| 24 | 3 | 2 | 1 | 543 ± 16 | 732 ± 6 | 1275 ± 22 |
| 25 | 3 | 3 | −1 | 24 ± 15 | 24 ± 7 | 48 ± 22 |
| 26 | 3 | 3 | 0 | 16 ± 3 | 63 ± 11 | 79 ± 13 |
| 27 | 3 | 3 | 1 | 15 ± 6 | 77 ± 11 | 92 ± 14 |
| Dependent Variable | Prediction | Lower Limit 95% | Upper Limit 95% | Experimental Value |
|---|---|---|---|---|
| α-solanine * | 774 | 557 | 990 | 735 |
| α-chaconine * | 784 | 687 | 881 | 829 |
| TGAs * | 1559 | 1330 | 1788 | 1565 |
| Linearity | Method Accuracy d | Method Precision d | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Linear Range (mg/L) | Calibration Line (R2) a | LOD (mg/L) b | LOQ (mg/L) c | Recovery (% ± SD) | Mean Recovery (% ± SD) | Intra-Day (%RSD) | Inter-Day (%RSD) | |||
| α-solanine | 1–100 | y = 50.765x − 98.094 (0.9906) | 0.30 | 1 | Low | 104 ± 5 | 103 ± 5 | Low | 5 | 4 |
| High | 101 ± 6 | High | 6 | 13 | ||||||
| α-chaconine | 1–100 | y = 51.854x − 15.205 (0.9910) | 0.30 | 1 | Low | 100 ± 4 | 100 ± 4 | Low | 4 | 5 |
| High | 100 ± 4 | High | 4 | 5 | ||||||
| Variety | α-Solanine (mg/kg DW) | α-Solanine (%) | α-Chaconine (mg/kg DW) | α-Chaconine (%) | TGAs (mg/kg DW) |
|---|---|---|---|---|---|
| Agata | 307 ± 21 | 26 | 894 ± 35 | 74 | 1201 ± 51 |
| Agria | 234 ± 32 | 48 | 251 ± 41 | 52 | 485 ± 72 |
| Amandine | 1081 ± 27 | 39 | 1742 ± 8 | 61 | 2823 ± 33 |
| Amaris | 239 ± 26 | 42 | 327 ± 38 | 58 | 566 ± 20 |
| Caesar | 594 ± 23 | 43 | 803 ± 67 | 57 | 1397 ± 75 |
| Colomba | 217 ± 5 | 31 | 471 ± 41 | 69 | 681 ± 40 |
| Evolution | 262 ± 20 | 34 | 502 ± 52 | 66 | 764 ± 40 |
| Frisia | 247 ± 11 | 24 | 797 ± 70 | 76 | 1044 ± 68 |
| Memphis | 198 ± 6 | 23 | 680 ± 27 | 77 | 878 ± 28 |
| Monalisa | 306 ± 19 | 25 | 892 ± 29 | 75 | 1198 ± 40 |
| Lady Amarilla | 235 ± 12 | 37 | 393 ± 25 | 63 | 629 ± 31 |
| Rudolph | 1266 ± 78 | 44 | 1629 ± 44 | 56 | 2895 ± 89 |
| Soprano | 343 ± 12 | 39 | 546 ± 17 | 61 | 889 ± 28 |
| Universa | 193 ± 19 | 38 | 316 ± 16 | 62 | 509 ± 24 |
| Vivaldi | 143 ± 9 | 55 | 117 ± 9 | 45 | 260 ± 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-García, I.; Gaona-Scheytt, C.; Morante-Zarcero, S.; Sierra, I. Development of a Green, Quick, and Efficient Method Based on Ultrasound-Assisted Extraction Followed by HPLC-DAD for the Analysis of Bioactive Glycoalkaloids in Potato Peel Waste. Foods 2024, 13, 651. https://doi.org/10.3390/foods13050651
Martínez-García I, Gaona-Scheytt C, Morante-Zarcero S, Sierra I. Development of a Green, Quick, and Efficient Method Based on Ultrasound-Assisted Extraction Followed by HPLC-DAD for the Analysis of Bioactive Glycoalkaloids in Potato Peel Waste. Foods. 2024; 13(5):651. https://doi.org/10.3390/foods13050651
Chicago/Turabian StyleMartínez-García, Isabel, Carlos Gaona-Scheytt, Sonia Morante-Zarcero, and Isabel Sierra. 2024. "Development of a Green, Quick, and Efficient Method Based on Ultrasound-Assisted Extraction Followed by HPLC-DAD for the Analysis of Bioactive Glycoalkaloids in Potato Peel Waste" Foods 13, no. 5: 651. https://doi.org/10.3390/foods13050651
APA StyleMartínez-García, I., Gaona-Scheytt, C., Morante-Zarcero, S., & Sierra, I. (2024). Development of a Green, Quick, and Efficient Method Based on Ultrasound-Assisted Extraction Followed by HPLC-DAD for the Analysis of Bioactive Glycoalkaloids in Potato Peel Waste. Foods, 13(5), 651. https://doi.org/10.3390/foods13050651








