Schisandra chinensis Bee Pollen Ameliorates Colitis in Mice by Modulating Gut Microbiota and Regulating Treg/Th17 Balance
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of SCPE
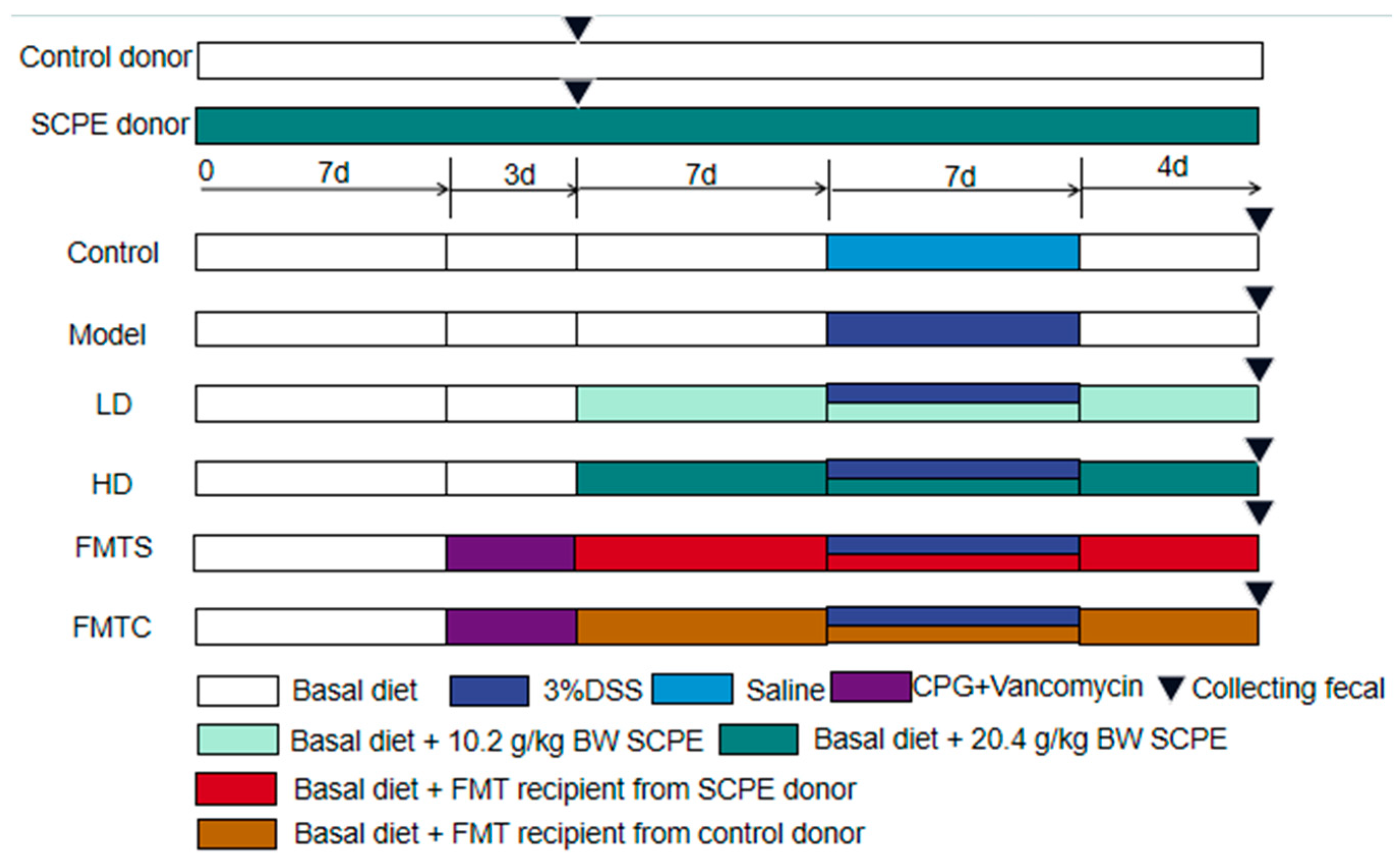
2.2. Animal Experiment
2.3. Fecal Microbiota Transplantation (FMT)
2.4. Histological Analysis
2.5. Measurement of Inflammatory Mediators
2.6. RNA Isolation and Quantitative Real-Time PCR
2.7. Flow Cytometry Analysis
2.8. Microbiota Analysis
2.9. Measurement of Short-Chain Fatty Acid
2.10. Statistical Analysis
3. Results
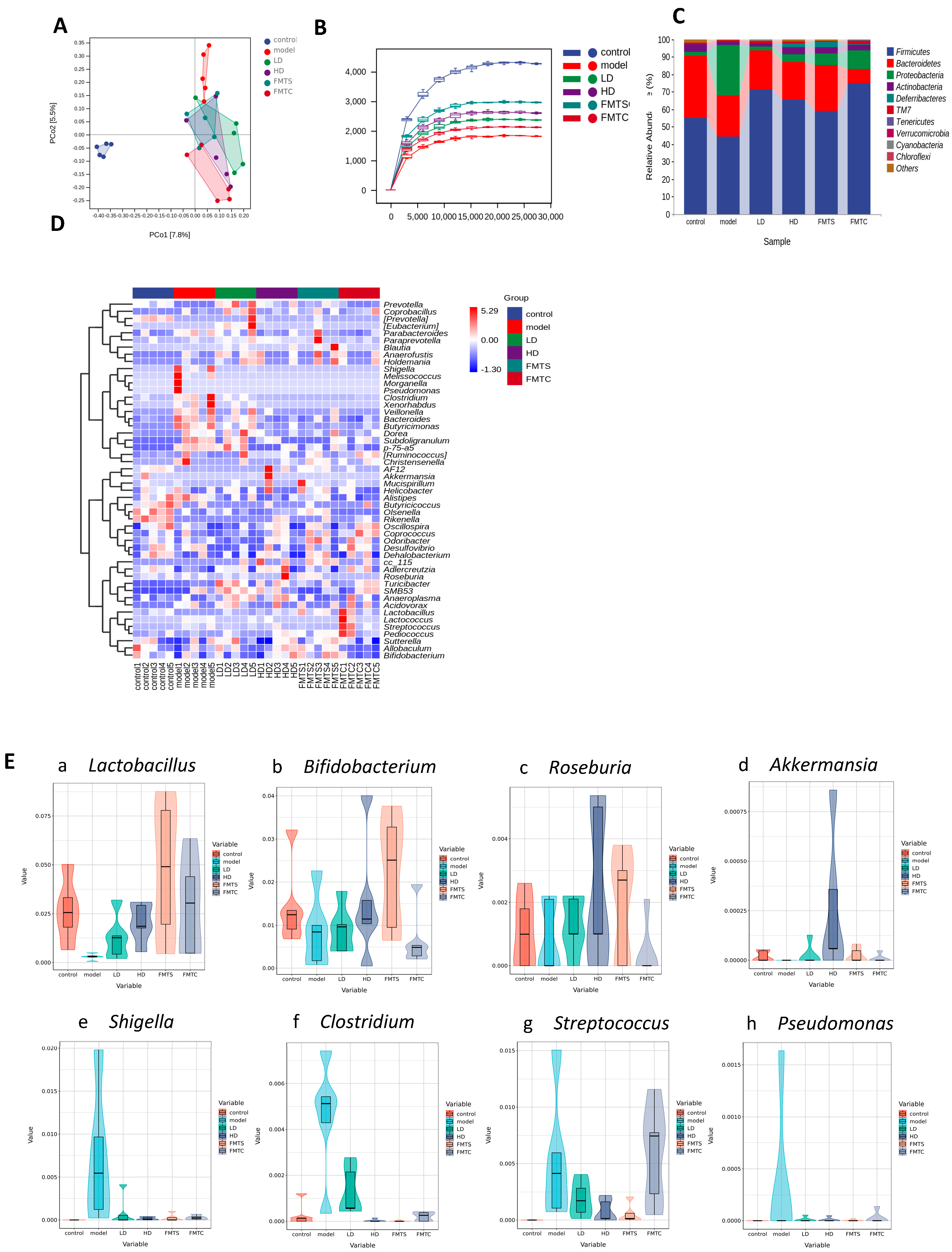
3.1. SCPE Altered Composition of Gut Microbiota
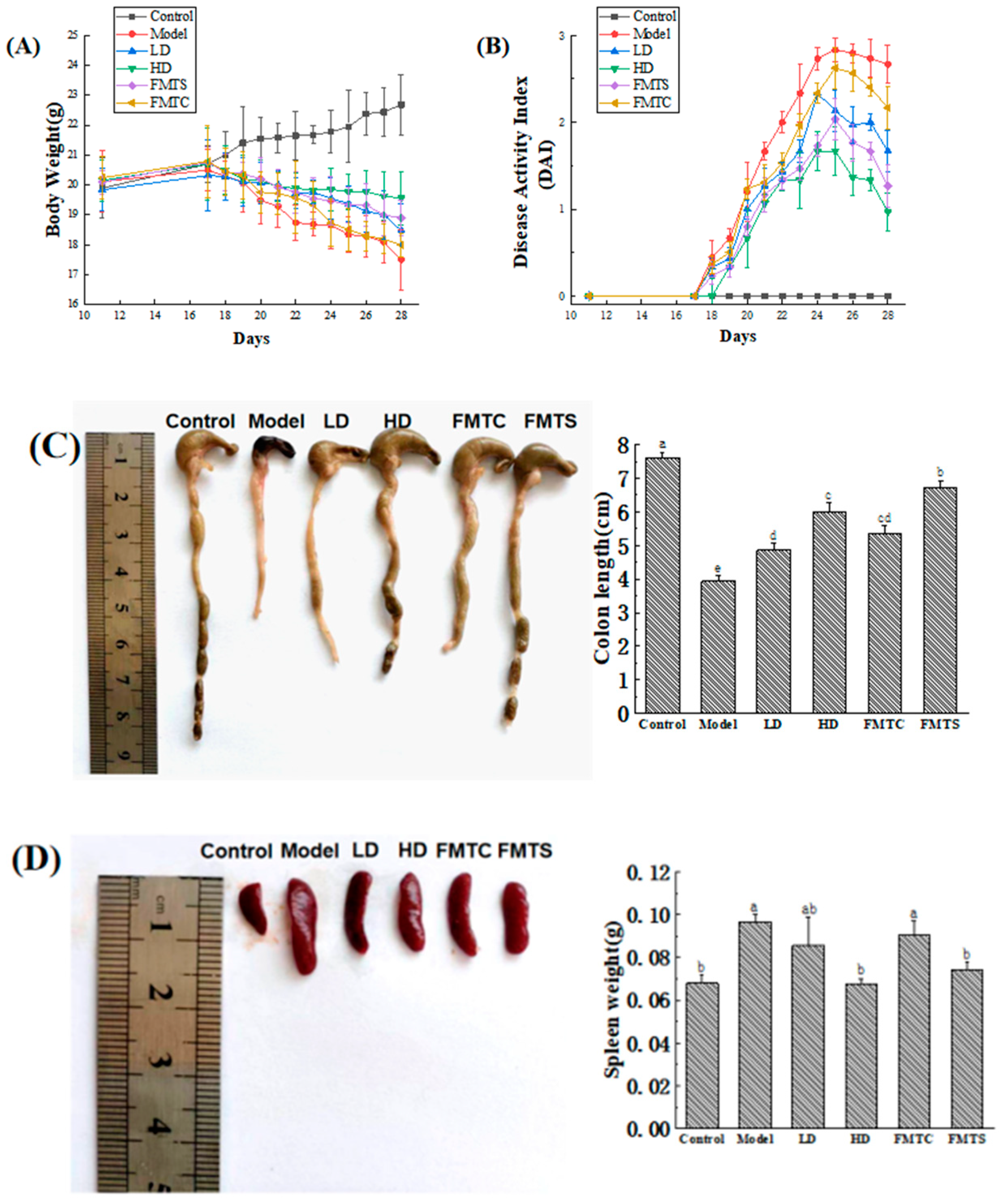
3.2. FMT from SCPE-Administration Mice Ameliorated DSS-Induced Colitis
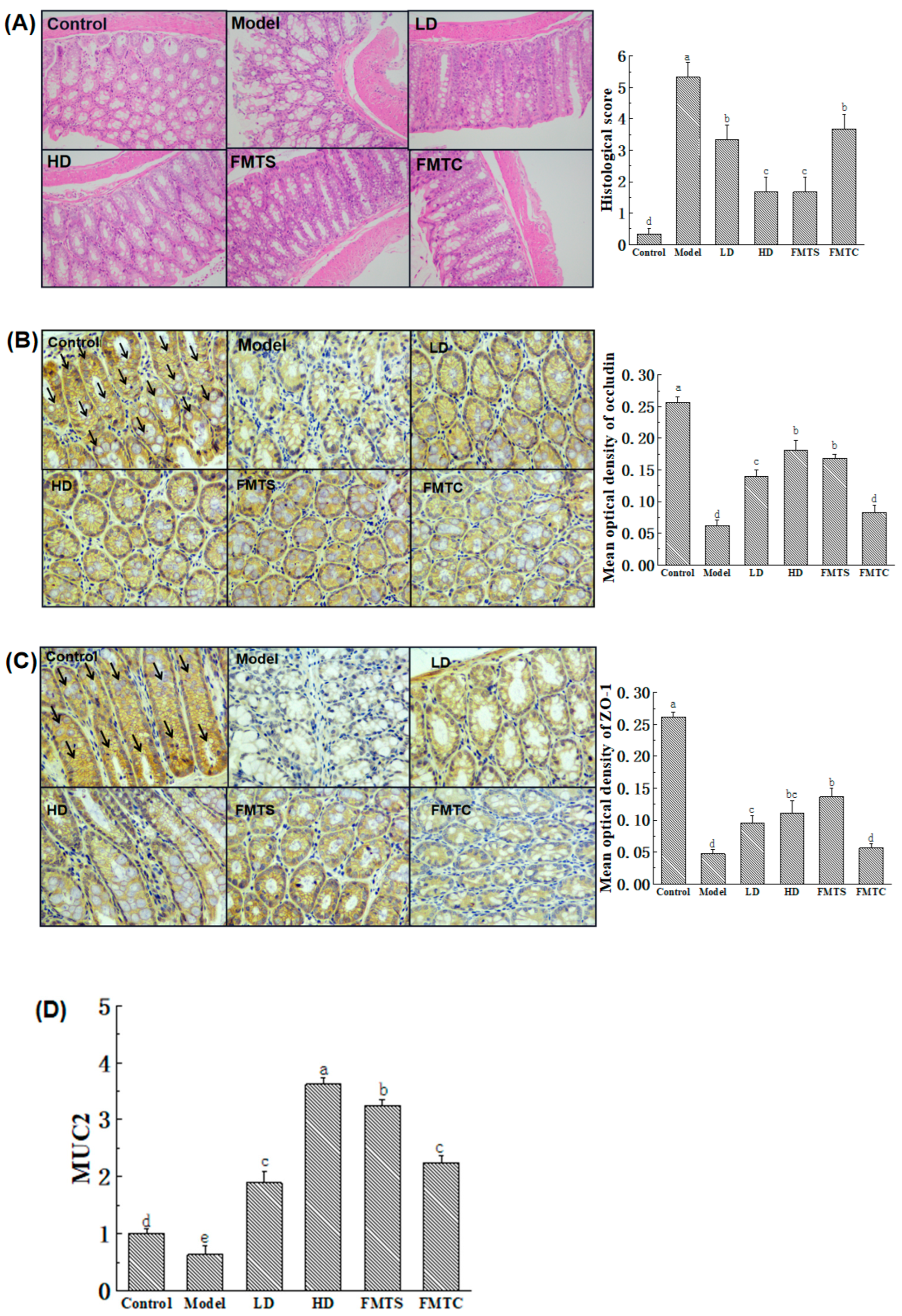
3.3. FMT from SCPE-Administration Mice Improved the Gut Barrier
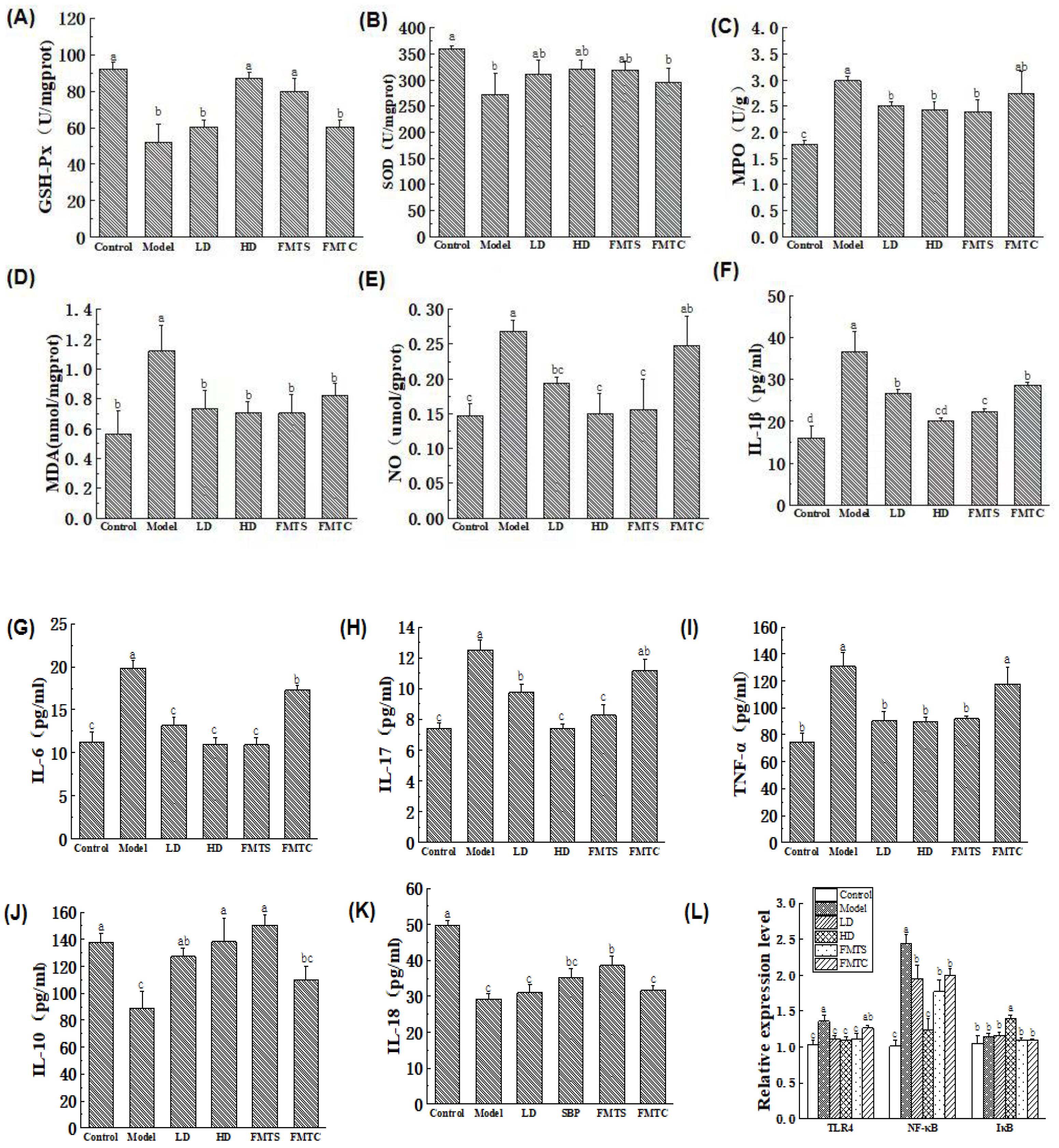
3.4. Effects of FMT from SCPE-Administration Mice on Inflammation and Oxidative Stress
3.5. FMT from SCPE-Administration Mice Modulated the Response of Immune Cells
3.6. FMT from SCPE-Administration Mice Improved Gut Microbial Structure and Function
3.7. FMT from SCPE-Administration Mice Promoted the Concentration of SCFAs in the Cecum
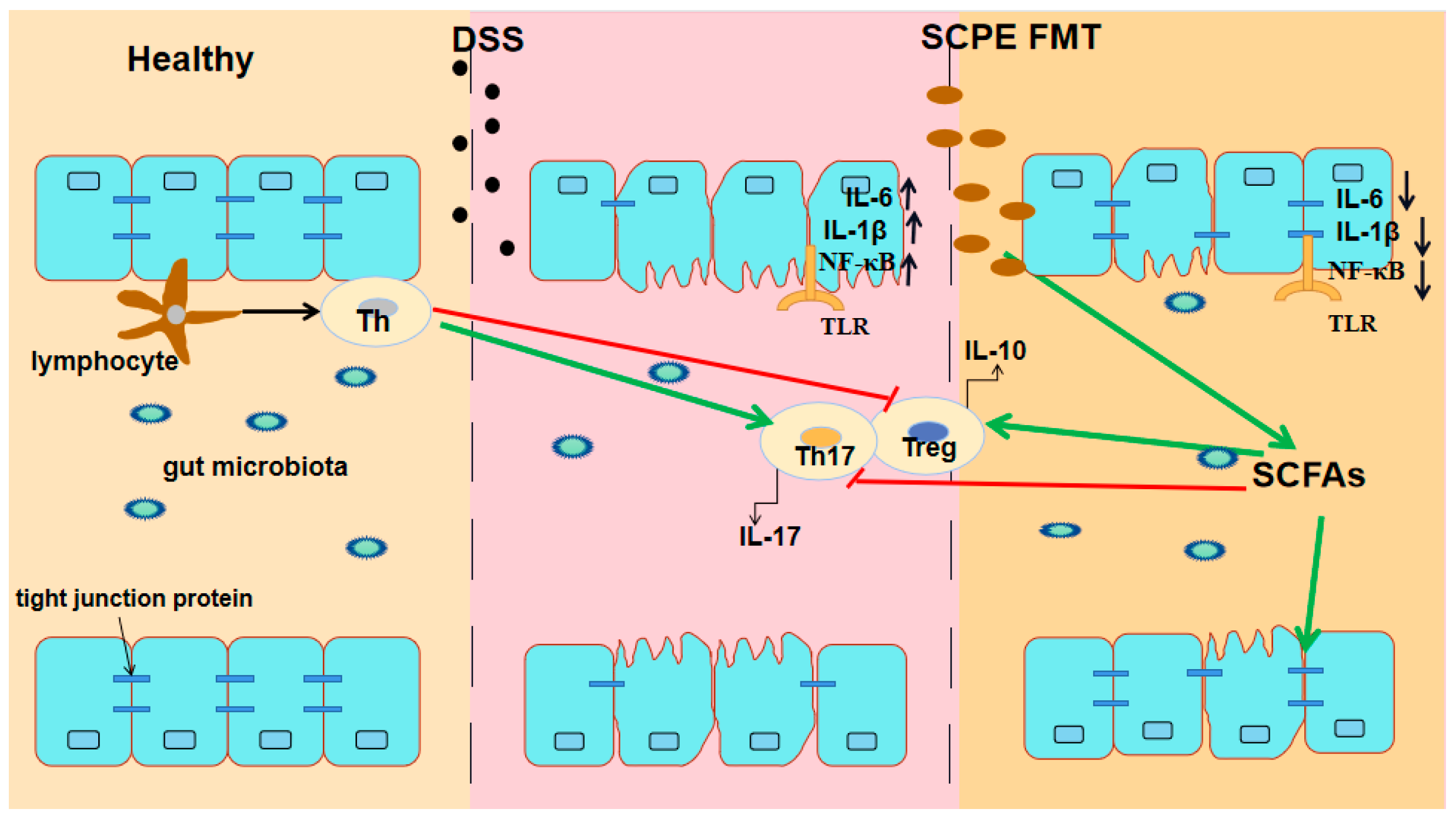
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martino, G.; Caputo, A.; Schwarz, P.; Bellone, F.; Fries, W.; Quattropani, M.C.; Vicario, C.M. Alexithymia and Inflammatory Bowel Disease: A Systematic Review. Front. Psychol. 2020, 11, 1763. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Fiocchi, C.; Panes, J. Drug development in IBD: From novel target identification to early clinical trials. Gut 2016, 65, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, R.; Gao, H.; Jung, S.; Gao, X.; Sun, R.; Liu, X.; Kim, Y.; Lee, H.S.; Kawai, Y.; et al. Genetic architecture of the inflammatory bowel diseases across East Asian and European ancestries. Nat. Genet. 2023, 55, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Knights, D.; Silverberg, M.S.; Weersma, R.K.; Gevers, D.; Dijkstra, G.; Huang, H.; Tyler, A.D.; van Sommeren, S.; Imhann, F.; Stempak, J.M.; et al. Complex host genetics influence the microbiome in inflammatory bowel disease. Genome Med. 2014, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hua, W.; Li, C.; Chang, H.; Liu, R.; Ni, Y.; Sun, H.; Li, Y.; Wang, X.; Hou, M.; et al. Protective Role of Fecal Microbiota Transplantation on Colitis and Colitis-Associated Colon Cancer in Mice Is Associated with Treg Cells. Front. Microbiol. 2019, 10, 2498. [Google Scholar] [CrossRef]
- Saez, A.; Herrero-Fernandez, B.; Gomez-Bris, R.; Sánchez-Martinez, H.; Gonzalez-Granado, J.M. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. Int. J. Mol. Sci. 2023, 24, 1526. [Google Scholar] [CrossRef]
- Ueno, A.; Jeffery, L.; Kobayashi, T.; Hibi, T.; Ghosh, S.; Jijon, H. Th17 plasticity and its relevance to inflammatory bowel disease. J. Autoimmun. 2018, 87, 38–49. [Google Scholar] [CrossRef]
- Jiang, P.; Zheng, C.; Xiang, Y.; Malik, S.; Su, D.; Xu, G.F.; Zhang, M.M. The involvement of TH17 cells in the pathogenesis of IBD. Cytokine Growth Factor Rev. 2023, 69, 28–42. [Google Scholar] [CrossRef]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Glendenning, L.M.; Cavanaugh, J.M.; McNeer, S.K.; Goodman, W.A.; Cobb, B.A. Intestinal Tr1 Cells Confer Protection against Colitis in the Absence of Foxp3+ Regulatory T Cell-Derived IL-10. Immunohorizons 2023, 7, 456–466. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Wang, D.D.; Yan, W.J. Treatment Effects of Natural Products on Inflammatory Bowel Disease In Vivo and Their Mechanisms: Based on Animal Experiments. Nutrients 2023, 15, 1031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qi, S.; Xue, X.; Al Naggar, Y.; Wu, L.; Wang, K. Understanding the Gastrointestinal Protective Effects of Polyphenols using Foodomics-Based Approaches. Front. Immunol. 2021, 12, 671150. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Deng, Y.; Long, S.Y.; Xu, H.Y.; Zeng, Y.T.; Peng, T.; Yang, C.M.; Du, J.; Zhang, X.Y. Metabolic flux and catabolic kinetics of prebiotic-like dietary polyphenol phlorizin in association with gut microbiota in vitro. Food Chem. 2024, 440, 138240. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhang, D.D.; Wu, J.; Liu, J.; Zhou, Y.C.; Tan, Y.Z.; Feng, W.W.; Peng, C. Interactions between gut microbiota and polyphenols: A mechanistic and metabolomic review. Phytomedicine 2023, 119, 154979. [Google Scholar] [CrossRef] [PubMed]
- Mascaraque, C.; Aranda, C.; Ocon, B.; Jesus Monte, M.; Dolores Suarez, M.; Zarzuelo, A.; Garcia Marin, J.J.; Martinez-Augustin, O.; Sanchez de Medina, F. Rutin has intestinal antiinflammatory effects in the CD4+ CD62L+ T cell transfer model of colitis. Pharmacol. Res. 2014, 90, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wei, C.; Wang, J.; Zhang, R.; Li, Y.; Wang, L. Effect of resveratrol on Treg/Th17 signaling and ulcerative colitis treatment in mice. Gastroenterol. WJG 2015, 21, 6572–6581. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, S.; Lv, S.Q.; Song, Y.; Guo, N.H. Resveratrol inhibits AhR/Notch axis and reverses Th17/Treg imbalance in purpura by activating Foxp3. Toxicol. Res. 2023, 12, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, S.W.; Das Neves, S.D.S.; Human, H.; Pirk, C.W.W. Digestibility and nutritional value of fresh and stored pollen for honey bees (Apis mellifera scutellata). J. Insect Physiol. 2018, 107, 302–308. [Google Scholar] [CrossRef]
- Cheng, N.; Chen, S.; Liu, X.; Zhao, H.; Cao, W. Impact of SchisandraChinensis Bee Pollen on Nonalcoholic Fatty Liver Disease and Gut Microbiota in HighFat Diet Induced Obese Mice. Nutrients 2019, 11, 346. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, H.; Cheng, N.; Cao, W. Rape bee pollen alleviates dextran sulfate sodium (DSS)-induced colitis by neutralizing IL-1β and regulating the gut microbiota in mice. Food Res. Int. 2019, 122, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Su, A.; Yang, W.; Zhao, L.; Pei, F.; Yuan, B.; Zhong, L.; Ma, G.; Hu, Q. Flammulina velutipes polysaccharides improve scopolamine-induced learning and memory impairment in mice by modulating gut microbiota composition. Food Funct. 2018, 9, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Pan, Q.; Shen, F.; Cao, H.; Ding, W.; Chen, Y.; Fan, J. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci. Rep. 2017, 7, 1529. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.J.; Stombaugh, K.; Bittinger, F.D.; Bushman, E.K.; Costello, N. QIIME allows analysis of high-throughputcommunity sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhong, X.; Wang, Y.; Xu, H. An approach to detecting species diversity of microfaunas in colonization surveys for marine bioassessment based on rarefaction curves. Mar. Pollut. Bull. 2014, 88, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 1993, 69, 238–249. [Google Scholar] [PubMed]
- Xie, S.; Liu, B.; Ye, H.; Li, Q.; Pan, L.; Zha, X.; Liu, J.; Duan, J.; Luo, J. Dendrobium huoshanense polysaccharide regionally regulates intestinal mucosal barriers function and intestinal microbiota in mice. Carbohydr. Polym. 2019, 206, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and prebiotics:mechanisms and healthbenefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Kmieć, Z.; Cyman, M.; Ślebioda, T.J. Cells of the innate and adaptive immunity and their interactions in inflammatory bowel disease. Adv. Med. Sci. 2017, 62, 1–16. [Google Scholar] [CrossRef]
- Chang, C.; Lin, C.; Lu, C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.; Chen, Y.; Young, J.D.; et al. Corrigendum: Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 16130. [Google Scholar] [CrossRef]
- Amaro, M.I.; Rocha, J.; Vila-Real, H.; Eduardo-Figueira, M.; Mota-Filipe, H.; Sepodes, B.; Ribeiro, M.H. Anti-inflammatory activity of naringin and the biosynthesised naringenin by naringinase immobilized in microstructured materials in a model of DSS-induced colitis in mice. Food Res. Int. 2009, 42, 1010–1017. [Google Scholar] [CrossRef]
- Chaen, Y.; Yamamoto, Y.; Suzuki, T. Naringenin promotes recovery from colonic damage through suppression of epithelial tumor necrosis factor-α production and induction of M2-type macrophages in colitic mice. Nutr. Res. 2019, 64, 82–92. [Google Scholar] [CrossRef]
- Song, H.Y.; Kim, W.S.; Kim, J.M.; Bak, D.H.; Han, J.M.; Lim, S.T.; Byun, E.B. A hydroxyethyl derivative of chrysin exhibits anti-inflammatory activity in dendritic cells and protective effects against dextran sodium salt-induced colitis in mice. Int. Immunopharmacol. 2019, 77, 105958. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Zhang, J.J.; Zhang, E.Y.; Sun, A.N.; Ding, L.L.; Chou, G.X.; Wang, Z.T.; Mani, S. Chrysin Ameliorates Chemically Induced Colitis in the Mouse through Modulation of a PXR/NF-κB Signaling Pathway. J. Pharmacol. Exp. Ther. 2013, 345, 473–482. [Google Scholar] [CrossRef]
- Mascaraque, C.; López-Posadas, R.; Monte, M.J.; Romero-Calvo, I.; Daddaoua, A.; González, M.; Martínez-Plata, E.; Suárez, M.D.; González, R.; Marín, J.J.G.; et al. The small intestinal mucosa acts as a rutin reservoir to extend flavonoid anti-inflammatory activity in experimental ileitis and colitis. J. Funct. Foods 2015, 13, 117–125. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Farup, P.G.; Rudi, K.; Hestad, K. Faecal short-chain fatty acids—A diagnostic biomarker for irritable bowel syndrome? BMC Gastroenterol. 2016, 16, 51. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, W.; Lan, P.; Mou, X. The microbiome in inflammatory bowel diseases: From pathogenesis to therapy. Protein Cell 2021, 12, 331–345. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Fachi, J.L.; Felipe, J.S.; Pral, L.P.; da Silva, B.K.; Corrêa, R.O.; de Andrade, M.C.P.; da Fonseca, D.M.; Basso, P.J.; Câmara, N.O.S.; de Sales e Souza, É.L.; et al. Butyrate Protects Mice from Clostridium difficile-Induced Colitis through an HIF-1-Dependent Mechanism. Cell Rep. 2019, 27, 750–761. [Google Scholar] [CrossRef]
- Norman, J.M.; Handley, S.A.; Baldridge, M.T.; Droit, L.; Liu, C.; Keller, B.C.; Kambal, A.; Monaco, C.L.; Zhao, G.; Fleshner, P.; et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 2015, 160, 447–460. [Google Scholar] [CrossRef]
- Merga, Y.; Campbell, B.J.; Rhodes, J.M. Mucosal barrier, bacteria and inflammatory bowel disease: Possibilities for therapy. Dig. Dis. 2014, 32, 475–483. [Google Scholar] [CrossRef]
- Rescigno, M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011, 32, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Funderburg, N.T.; Stubblefield, P.S.R.; Sung, H.C.; Hardy, G.; Clagett, B.; Ignatz-Hoover, J.; Harding, C.V.; Fu, P.; Katz, J.A.; Lederman, M.M.; et al. Circulating CD4+ and CD8+ T cells are activated in inflammatory bowel disease and are associated with plasma markers of inflammation. Immunology 2013, 140, 87–97. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeong, J.J.; Le, T.H.; Eun, S.H.; Nguyen, M.D.; Park, J.H.; Kim, D.H. Ocotillol, a majonoside R2 metabolite, ameliorates 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice by restoring the balance of Th17/Treg cells. J. Agric. Food Chem. 2015, 63, 7024–7031. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Yousaf, W.; Giannella, R.; Shata, M.T. Th17 cells: Interactions with predisposing factors in the immunopathogenesis of inflammatory bowel disease. Expert Rev. Clin. Immunol. 2012, 8, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Round, J.L.; Kasper, D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 2008, 453, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Ansaldo, E.; Slayden, L.C.; Ching, K.L.; Koch, M.A.; Wolf, N.K.; Plichta, D.R.; Brown, E.M.; Graham, D.B.; Xavier, R.J.; Moon, J.J.; et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science 2019, 364, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ren, D.; Zhao, Y.; Liu, L.; Yang, X. Fuzhuan Brick Tea Polysaccharide Improved Ulcerative Colitis in Association with Gut Microbiota-Derived Tryptophan Metabolism. J. Agric. Food Chem. 2021, 69, 8448–8459. [Google Scholar] [CrossRef] [PubMed]


| Group | SCFAs Concentrations (mg/g) | |||
|---|---|---|---|---|
| AA | PA | BA | VA | |
| Control | 1.59 ± 0.12 a | 0.63 ± 0.09 a | 1.35 ± 0.53 ab | 0.12 ± 0.04 |
| Model | 0.62 ± 0.30 b | 0.26 ± 0.11 b | 0.37 ± 0.06 c | 0.08 ± 0.05 |
| LD | 1.05 ± 0.33 a | 0.27 ± 0.14 b | 1.04 ± 0.58 ab | 0.09 ± 0.01 |
| HD | 1.40 ± 0.31 a | 0.37 ± 0.11 ab | 1.29 ± 0.41 ab | 0.10 ± 0.03 |
| FMTS | 1.40 ± 0.63 a | 0.42 ± 0.15 ab | 1.65 ± 0.51 a | 0.12 ± 0.04 |
| FMTC | 1.00 ± 0.46 a | 0.29 ± 0.08 b | 0.49 ± 0.33 c | 0.08 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, N.; Wang, X.; Zhou, Y.; Zhao, X.; Chen, M.; Zhao, H.; Cao, W. Schisandra chinensis Bee Pollen Ameliorates Colitis in Mice by Modulating Gut Microbiota and Regulating Treg/Th17 Balance. Foods 2024, 13, 585. https://doi.org/10.3390/foods13040585
Cheng N, Wang X, Zhou Y, Zhao X, Chen M, Zhao H, Cao W. Schisandra chinensis Bee Pollen Ameliorates Colitis in Mice by Modulating Gut Microbiota and Regulating Treg/Th17 Balance. Foods. 2024; 13(4):585. https://doi.org/10.3390/foods13040585
Chicago/Turabian StyleCheng, Ni, Xiaochao Wang, Yaoyao Zhou, Xuanxuan Zhao, Minghao Chen, Haoan Zhao, and Wei Cao. 2024. "Schisandra chinensis Bee Pollen Ameliorates Colitis in Mice by Modulating Gut Microbiota and Regulating Treg/Th17 Balance" Foods 13, no. 4: 585. https://doi.org/10.3390/foods13040585
APA StyleCheng, N., Wang, X., Zhou, Y., Zhao, X., Chen, M., Zhao, H., & Cao, W. (2024). Schisandra chinensis Bee Pollen Ameliorates Colitis in Mice by Modulating Gut Microbiota and Regulating Treg/Th17 Balance. Foods, 13(4), 585. https://doi.org/10.3390/foods13040585






