Optimizing the Solvent Selection of the Ultrasound-Assisted Extraction of Sea Buckthorn (Hippophae rhamnoides L.) Pomace: Phenolic Profiles and Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. The Preparation of SBP Extracts
2.4. The Determination of Total Phenolic Content (TPC)
2.5. The Determination of Total Flavonoid Content (TFC)
2.6. DPPH Radical Scavenging Assay
2.7. ABTS Radical Scavenging Assay
2.8. Ferric Reducing Antioxidant Power (FRAP)
2.9. UPLC-MS/MS
2.10. Statistical Analysis
3. Results and Discussion
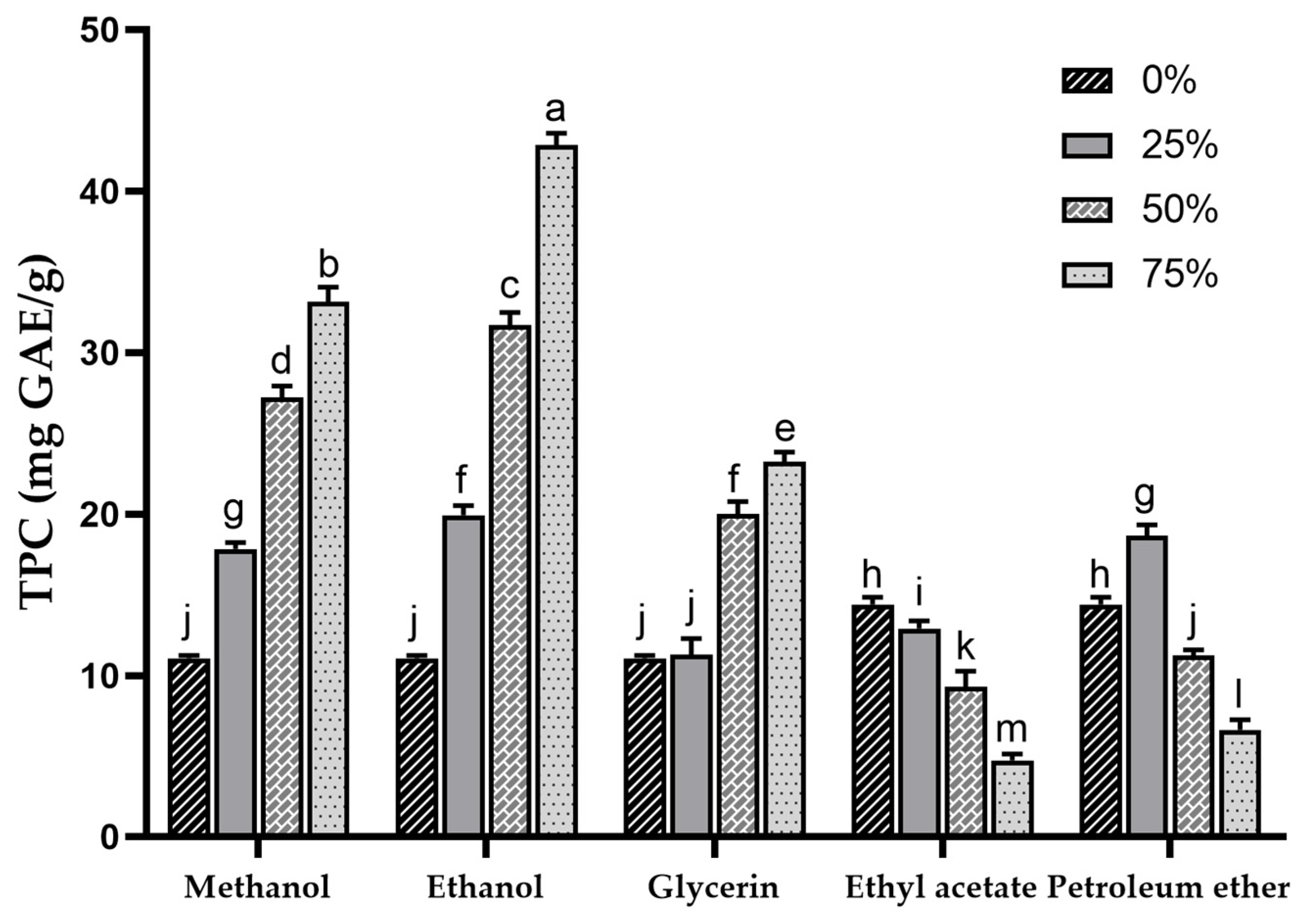
3.1. The Determination of Total Phenolic Content (TPC)
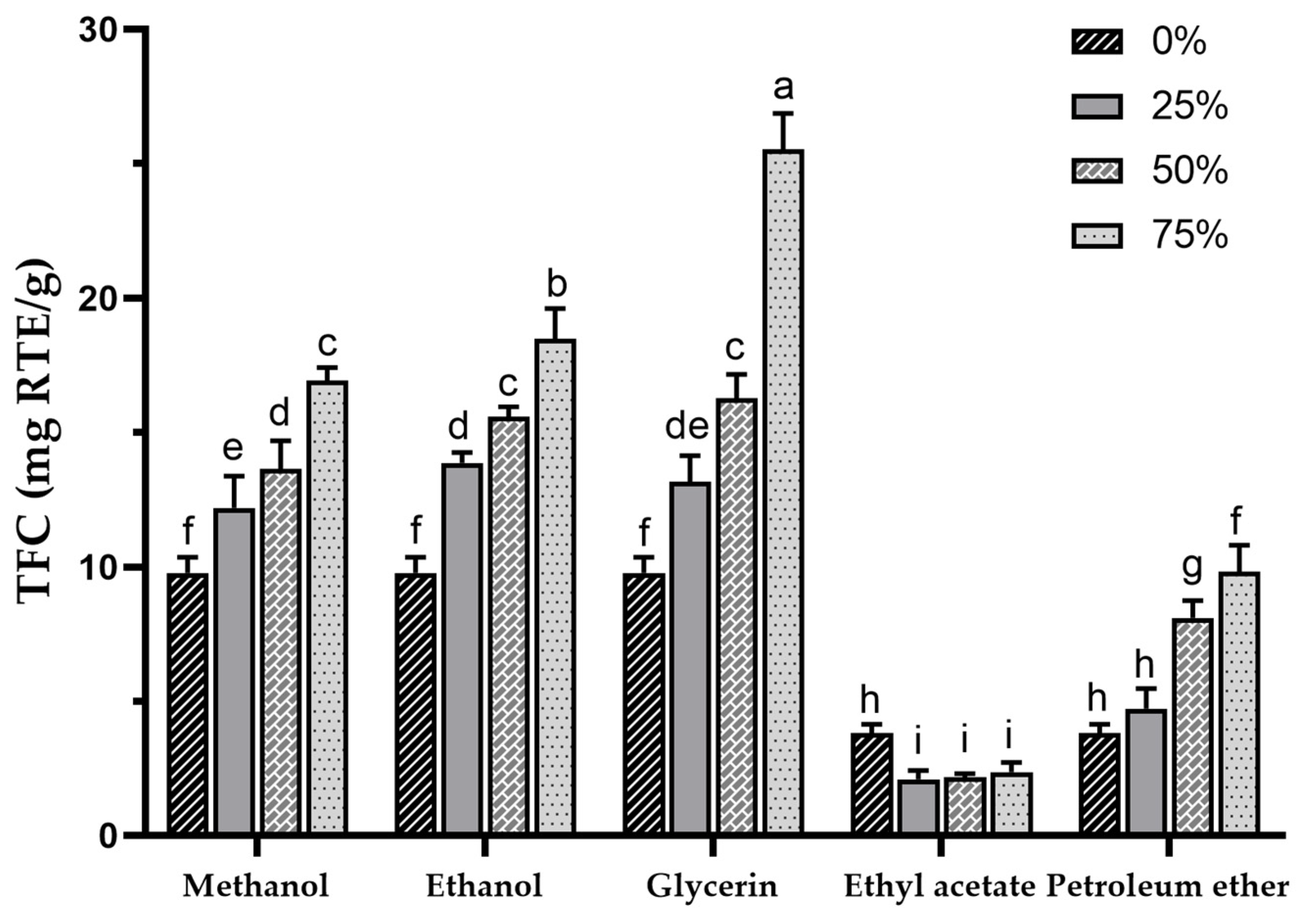
3.2. The Determination of Total Flavonoid Content (TFC)
3.3. Antioxidant Activity
3.3.1. DPPH Radical Scavenging Assay
3.3.2. ABTS Radical Scavenging Assay
3.3.3. Ferric-Reducing Antioxidant Power (FRAP)
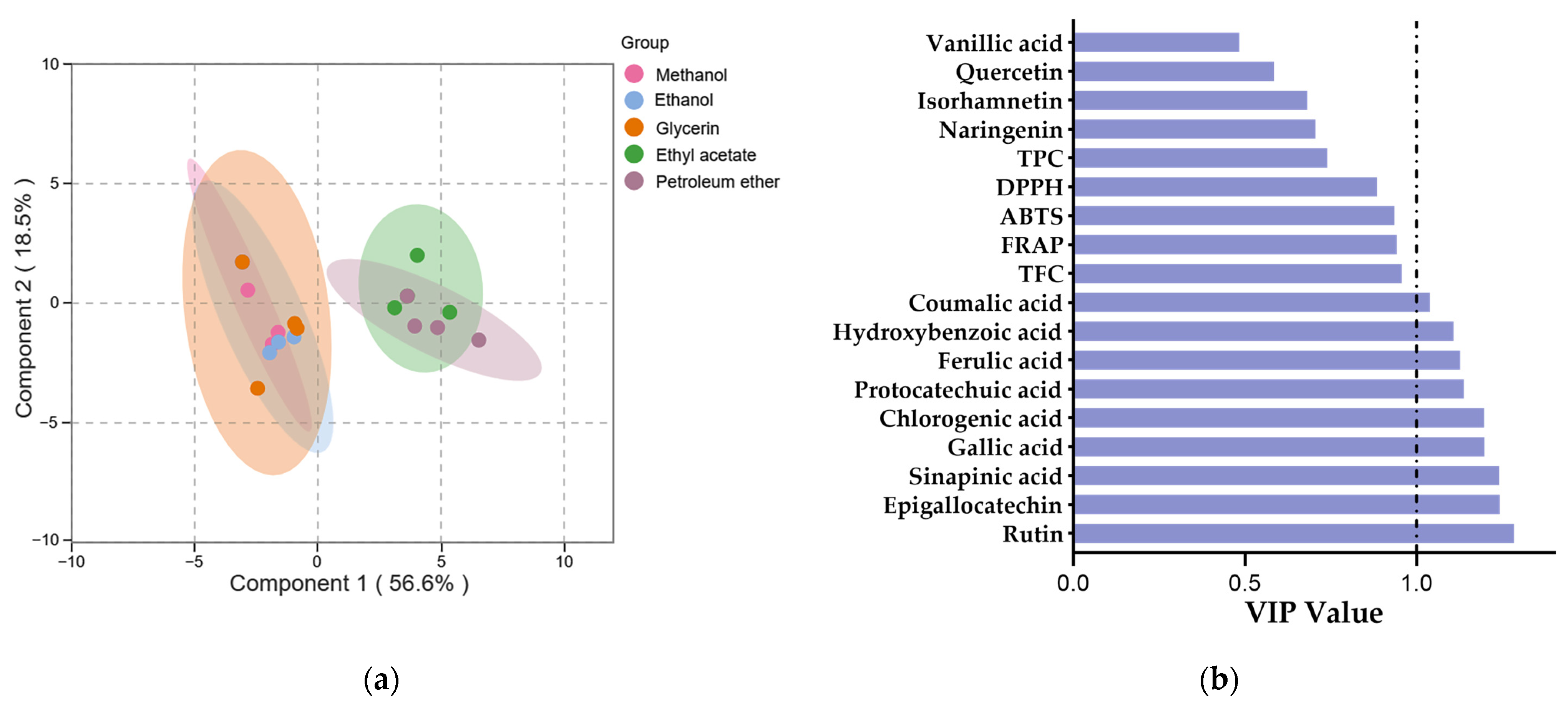
3.4. The Phenolic Profiles of Extracts
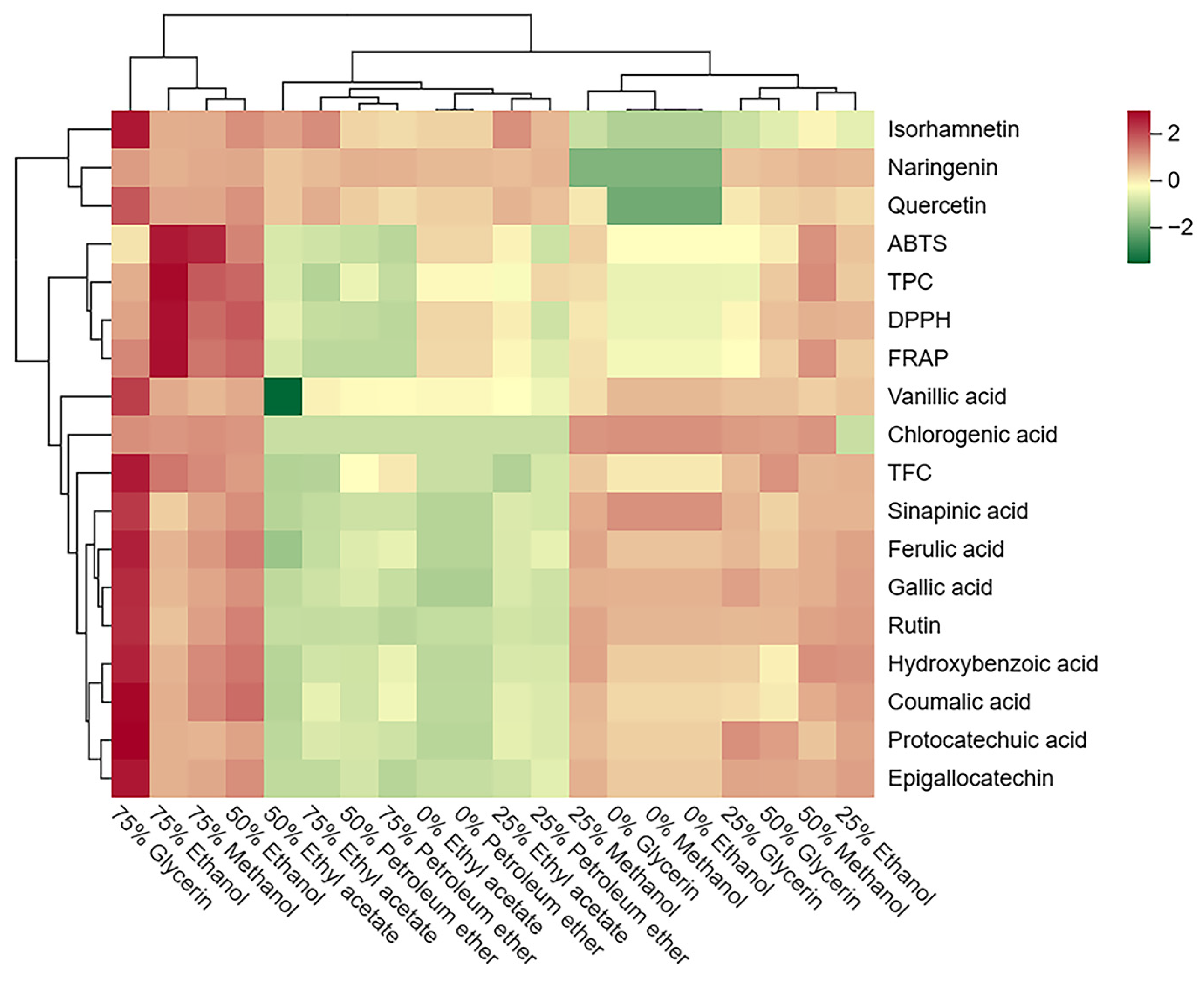
3.5. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gâtlan, A.-M.; Gutt, G. Sea Buckthorn in Plant Based Diets. An Analytical Approach of Sea Buckthorn Fruits Composition: Nutritional Value, Applications, and Health Benefits. Int. J. Environ. Res. Public Health 2021, 18, 8986. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Z.; Liao, X. Bioactive Compounds, Health Benefits and Functional Food Products of Sea Buckthorn: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6761–6782. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Bobak, Ł.; Nowicka, P. Anti-Oxidant and Anti-Enzymatic Activities of Sea Buckthorn (Hippophaë rhamnoides L.) Fruits Modulated by Chemical Components. Antioxidants 2019, 8, 618. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, G.; Li, X.; Cui, Q.; Gong, L.; Tian, Z. Non-Targeted Profiling of Sea Buckthorn Fruit Oil Fingerprints from 3 Regions and Study on Its Antioxidant Activity. Appl. Biochem. Biotechnol. 2023. Available online: https://link.springer.com/article/10.1007/s12010-023-04744-y (accessed on 7 January 2024). [CrossRef] [PubMed]
- Suryakumar, G.; Gupta, A. Medicinal and Therapeutic Potential of Sea Buckthorn (Hippophae rhamnoides L.). J. Ethnopharmacol. 2011, 138, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Zhao, P.; Qin, G.; Tang, S.; Li, B.; Zhang, J.; Peng, L. Genotoxicity and Teratogenicity of Seabuckthorn (Hippophae rhamnoides L.) Berry Oil. Drug Chem. Toxicol. 2020, 43, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Binosha Fernando, W.M.A.D.; Durham, R.; Stockmann, R.; Jayasena, V. Nutritional Value, Health-Promoting Benefits and Food Application of Sea Buckthorn. Food Rev. Int. 2023, 39, 2122–2137. [Google Scholar] [CrossRef]
- Zielińska, A.; Nowak, I. Abundance of Active Ingredients in Sea-Buckthorn Oil. Lipids Health Dis. 2017, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Ciesarová, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolková, B.; Koplík, R.; Belajová, E.; Kukurová, K.; Daško, Ľ.; Panovská, Z.; et al. Why Is Sea Buckthorn (Hippophae rhamnoides L.) so Exceptional? A Review. Food Res. Int. 2020, 133, 109170. [Google Scholar] [CrossRef] [PubMed]
- Jaśniewska, A.; Diowksz, A. Wide Spectrum of Active Compounds in Sea Buckthorn (Hippophae Rhamnoides) for Disease Prevention and Food Production. Antioxidants 2021, 10, 1279. [Google Scholar] [CrossRef]
- Żuchowski, J. Phytochemistry and Pharmacology of Sea Buckthorn (Elaeagnus rhamnoides; Syn. Hippophae rhamnoides): Progress from 2010 to 2021. Phytochem. Rev. 2023, 22, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Dienaitė, L.; Baranauskienė, R.; Rimantas Venskutonis, P. Lipophilic Extracts Isolated from European Cranberry Bush (Viburnum opulus) and Sea Buckthorn (Hippophae rhamnoides) Berry sPomace by Supercritical CO2—Promising Bioactive Ingredients for Foods and Nutraceuticals. Food Chem. 2021, 348, 129047. [Google Scholar] [CrossRef] [PubMed]
- Jurevičiūtė, I.; Keršienė, M.; Bašinskienė, L.; Leskauskaitė, D.; Jasutienė, I. Characterization of Berry Pomace Powders as Dietary Fiber-Rich Food Ingredients with Functional Properties. Foods 2022, 11, 716. [Google Scholar] [CrossRef] [PubMed]
- Nour, V.; Panaite, T.D.; Corbu, A.R.; Ropota, M.; Turcu, R.P. Nutritional and Bioactive Compounds in Dried Sea-Buckthorn Pomace. Erwerbs-Obstbau 2021, 63, 91–98. [Google Scholar] [CrossRef]
- Chaouch, M.A.; Benvenuti, S. The Role of Fruit By-Products as Bioactive Compounds for Intestinal Health. Foods 2020, 9, 1716. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzińska, M. Seeds Recovered from Industry By-Products of Nine Fruit Species with a High Potential Utility as a Source of Unconventional Oil for Biodiesel and Cosmetic and Pharmaceutical Sectors. Ind. Crops Prod. 2016, 83, 329–338. [Google Scholar] [CrossRef]
- Wang, C.; Lei, M.; Zhang, H.; Zhao, L.; Mu, Z.; Wang, M.; Zhang, J.; Deng, Y.; Zhao, L. Comparison of Metabolites in Juice, Seed and Peel of Sea Buckthorn (Hippophae rhamnoides L. Subsp. Sinensis). J. Plant Biochem. Biotechnol. 2020, 29, 305–313. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef]
- Kitrytė, V.; Povilaitis, D.; Kraujalienė, V.; Šulniūtė, V.; Pukalskas, A.; Venskutonis, P.R. Fractionation of Sea Buckthorn Pomace and Seeds into Valuable Components by Using High Pressure and Enzyme-Assisted Extraction Methods. LWT—Food Sci. Technol. 2017, 85, 534–538. [Google Scholar] [CrossRef]
- Malenica, D.; Kass, M.; Bhat, R. Sustainable Management and Valorization of Agri-Food Industrial Wastes and By-Products as Animal Feed: For Ruminants, Non-Ruminants and as Poultry Feed. Sustainability 2023, 15, 117. [Google Scholar] [CrossRef]
- Shahidi, S.-A. Effect of Solvent Type on Ultrasound-Assisted Extraction of Antioxidant Compounds from Ficaria kochii: Optimization by Response Surface Methodology. Food Chem. Toxicol. 2022, 163, 112981. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing by-Products: A Review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Leichtweis, M.G.; Pereira, C.; Prieto, M.A.; Barreiro, M.F.; Baraldi, I.J.; Barros, L.; Ferreira, I.C.F.R. Ultrasound as a Rapid and Low-Cost Extraction Procedure to Obtain Anthocyanin-Based Colorants from Prunus spinosa L. Fruit Epicarp: Comparative Study with Conventional Heat-Based Extraction. Molecules 2019, 24, 573. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in Ultrasound Assisted Extraction of Bioactive Compounds from Cash Crops—A Review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Umaña, M.; Eim, V.; Garau, C.; Rosselló, C.; Simal, S. Ultrasound-Assisted Extraction of Ergosterol and Antioxidant Components from Mushroom By-Products and the Attainment of a β-Glucan Rich Residue. Food Chem. 2020, 332, 127390. [Google Scholar] [CrossRef]
- Caldas, T.W.; Mazza, K.E.L.; Teles, A.S.C.; Mattos, G.N.; Brígida, A.I.S.; Conte-Junior, C.A.; Borguini, R.G.; Godoy, R.L.O.; Cabral, L.M.C.; Tonon, R.V. Phenolic Compounds Recovery from Grape Skin Using Conventional and Non-Conventional Extraction Methods. Ind. Crops Prod. 2018, 111, 86–91. [Google Scholar] [CrossRef]
- Chen, X.; Jia, X.; Yang, S.; Zhang, G.; Li, A.; Du, P.; Liu, L.; Li, C. Optimization of Ultrasonic-Assisted Extraction of Flavonoids, Polysaccharides, and Eleutherosides from Acanthopanax senticosus Using Response Surface Methodology in Development of Health Wine. LWT 2022, 165, 113725. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, H.; Cui, L.; Hussain, H.; Nadolnik, L.; Zhang, Z.; Zhao, Y.; Qin, X.; Li, J.; Park, J.H.; et al. Ultrasonic-Assisted Extraction of Flavonoids from Peanut Leave and Stem Using Deep Eutectic Solvents and Its Molecular Mechanism. Food Chem. 2024, 434, 137497. [Google Scholar] [CrossRef]
- Baite, T.N.; Mandal, B.; Purkait, M.K. Ultrasound Assisted Extraction of Gallic Acid from Ficus auriculata Leaves Using Green Solvent. Food Bioprod. Process. 2021, 128, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Wang, H.; Tong, M.; Gong, Y. Green and Enhanced Extraction of Coumarins from Cortex fraxini by Ultrasound-Assisted Deep Eutectic Solvent Extraction. J. Sep. Sci. 2020, 43, 3441–3448. [Google Scholar] [CrossRef]
- Savic, I.M.; Savic Gajic, I.M. Optimization of Ultrasound-Assisted Extraction of Polyphenols from Wheatgrass (Triticum aestivum L.). J. Food Sci. Technol. 2020, 57, 2809–2818. [Google Scholar] [CrossRef]
- Guo, Z.; Cheng, J.; Zheng, L.; Xu, W.; Xie, Y. Mechanochemical-Assisted Extraction and Hepatoprotective Activity Research of Flavonoids from Sea Buckthorn (Hippophaë rhamnoides L.) Pomaces. Molecules 2021, 26, 7615. [Google Scholar] [CrossRef]
- Ma, X.; Laaksonen, O.; Zheng, J.; Yang, W.; Trépanier, M.; Kallio, H.; Yang, B. Flavonol Glycosides in Berries of Two Major Subspecies of Sea Buckthorn (Hippophaë rhamnoides L.) and Influence of Growth Sites. Food Chem. 2016, 200, 189–198. [Google Scholar] [CrossRef]
- Varshneya, C.; Kant, V.; Mehta, M. Total Phenolic Contents and Free Radical Scavenging Activities of Different Extracts of Seabuckthorn (Hippophae rhamnoides) Pomace without Seeds. Int. J. Food Sci. Nutr. 2012, 63, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Zorig, A.; Sato, N.; Yanagihara, A.; Kanazawa, T.; Takasugi, M.; Arai, H. Effect of Polyphenols in Sea Buckthorn Berry on Chemical Mediator Release from Mast Cells. Prev. Nutr. Food Sci. 2023, 28, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Dienaitė, L.; Pukalskas, A.; Pukalskienė, M.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Phytochemical Composition, Antioxidant and Antiproliferative Activities of Defatted Sea Buckthorn (Hippophaë rhamnoides L.) Berry Pomace Fractions Consecutively Recovered by Pressurized Ethanol and Water. Antioxidants 2020, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Luntraru, C.M.; Apostol, L.; Oprea, O.B.; Neagu, M.; Popescu, A.F.; Tomescu, J.A.; Mulțescu, M.; Susman, I.E.; Gaceu, L. Reclaim and Valorization of Sea Buckthorn (Hippophae rhamnoides) By-Product: Antioxidant Activity and Chemical Characterization. Foods 2022, 11, 462. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Guo, X.; Li, T.; Fu, X.; Liu, R.H. Comparative Assessment of Phytochemical Profiles, Antioxidant and Antiproliferative Activities of Sea Buckthorn (Hippophaë rhamnoides L.) Berries. Food Chem. 2017, 221, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Visan, S.; Soritau, O.; Tatomir, C.; Baldasici, O.; Balacescu, L.; Balacescu, O.; Muntean, P.; Gherasim, C.; Pintea, A. The Bioactive Properties of Carotenoids from Lipophilic Sea Buckthorn Extract (Hippophae rhamnoides L.) in Breast Cancer Cell Lines. Molecules 2023, 28, 4486. [Google Scholar] [CrossRef] [PubMed]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Ferreres, F.; Moreno, D.A.; Nowicka, P. UPLC-PDA-Q/TOF-MS Profiling of Phenolic and Carotenoid Compounds and Their Influence on Anticholinergic Potential for AChE and BuChE Inhibition and on-Line Antioxidant Activity of Selected Hippophaë rhamnoides L. Cultivars. Food Chem. 2020, 309, 125766. [Google Scholar] [CrossRef]
- Li, Y.; Li, P.; Yang, K.; He, Q.; Wang, Y.; Sun, Y.; He, C.; Xiao, P. Impact of Drying Methods on Phenolic Components and Antioxidant Activity of Sea Buckthorn (Hippophae rhamnoides L.) Berries from Different Varieties in China. Molecules 2021, 26, 7189. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Saimaiti, A.; Luo, M.; Huang, S.-Y.; Xiong, R.-G.; Shang, A.; Gan, R.-Y.; Li, H.-B. Fermentation with Tea Residues Enhances Antioxidant Activities and Polyphenol Contents in Kombucha Beverages. Antioxidants 2022, 11, 155. [Google Scholar] [CrossRef]
- Wu, D.; Xia, Q.; Huang, H.; Tian, J.; Ye, X.; Wang, Y. Influence of Centrifugation and Transmembrane Treatment on Determination of Polyphenols and Antioxidant Ability for Sea Buckthorn Juice. Molecules 2023, 28, 2446. [Google Scholar] [CrossRef]
- NY/T3903-2021; Ministry of Agriculture and Rural Affairs of the People’s Republic of China, Determination of Flavonoids in Lycium barbarum L. China National Standard: Beijing, China, 2021.
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Kuhkheil, A.; Badi, H.; Mehrafarin, A.; Abdossi, V. Chemical Constituents of Sea Buckthorn (Hippophae rhamnoides L.) Fruit in Populations of Central Alborz Mountains in Iran. Res. J. Pharmacogn. 2017, 4, 1–12. [Google Scholar]
- Rashid, R.; Mohd Wani, S.; Manzoor, S.; Masoodi, F.A.; Masarat Dar, M. Green Extraction of Bioactive Compounds from Apple Pomace by Ultrasound Assisted Natural Deep Eutectic Solvent Extraction: Optimisation, Comparison and Bioactivity. Food Chem. 2023, 398, 133871. [Google Scholar] [CrossRef] [PubMed]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of Extraction Solvent on Total Phenol Content, Total Flavonoid Content, and Antioxidant Activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-L.; Sun, P.; Feng, J.; Yuan, J.; Wang, Y.; Shang, Y.-F.; Niu, X.-L.; Yang, S.-H.; Wei, Z.-J. Solvent Effect on Phenolics and Antioxidant Activity of Huangshan Gongju (Dendranthema morifolium (Ramat) Tzvel. Cv. Gongju) Extract. Food Chem. Toxicol. 2021, 147, 111875. [Google Scholar] [CrossRef] [PubMed]
- Chaabani, E.; Abert Vian, M.; Bettaieb Rebey, I.; Bourgou, S.; Zar Kalai, F.; Chemat, F.; Ksouri, R. Ethanol–Water Binary Solvent Affects Phenolic Composition and Antioxidant Ability of Pistacia lentiscus L. Fruit Extracts: A Theoretical versus Experimental Solubility Study. J. Food Meas. Charact. 2023, 17, 4705–4714. [Google Scholar] [CrossRef]
- Afshari, K.; Javanmard Dakheli, M.; Ramezan, Y.; Bassiri, A.; Ahmadi Chenarbon, H. Physicochemical and Control Releasing Properties of Date Pit (Phoenix dactylifera L.) Phenolic Compounds Microencapsulated through Fluidized-Bed Method. Food Sci. Nutr. 2023, 11, 1367–1382. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ou, X.; Zhang, X.; Zhou, Z.; Ma, L. Effect of Different Solvents on the Measurement of Phenolics and the Antioxidant Activity of Mulberry (Morus atropurpurea Roxb.) with Accelerated Solvent Extraction. J. Food Sci. 2017, 82, 605–612. [Google Scholar] [CrossRef]
- Guido, L.F.; Moreira, M.M. Techniques for Extraction of Brewer’s Spent Grain Polyphenols: A Review. Food Bioprocess Technol. 2017, 10, 1192–1209. [Google Scholar] [CrossRef]
- Cavalcanti, V.P.; Aazza, S.; Bertolucci, S.K.V.; Rocha, J.P.M.; Coelho, A.D.; Oliveira, A.J.M.; Mendes, L.C.; Pereira, M.M.A.; Morais, L.C.; Forim, M.R.; et al. Solvent Mixture Optimization in the Extraction of Bioactive Compounds and Antioxidant Activities from Garlic (Allium sativum L.). Molecules 2021, 26, 6026. [Google Scholar] [CrossRef] [PubMed]
- Rösch, D.; Bergmann, M.; Knorr, D.; Kroh, L.W. Structure−Antioxidant Efficiency Relationships of Phenolic Compounds and Their Contribution to the Antioxidant Activity of Sea Buckthorn Juice. J. Agric. Food Chem. 2003, 51, 4233–4239. [Google Scholar] [CrossRef] [PubMed]
- Kreps, F.; Tobolková, B.; Ciesarová, Z.; Potočňáková, M.; Janotková, L.; Schubertová, S.; Ház, A.; Schmidt, Š.; Jablonský, M. Total Content of Polyphenols, Flavonoids, Rutin, and Antioxidant Activity of Sea Buckthorn Juice. BioResources 2021, 16, 4743–4751. [Google Scholar] [CrossRef]
- Xiao, P.; Liu, S.; Kuang, Y.; Jiang, Z.; Lin, Y.; Xie, Z.; Liu, E.-H. Network Pharmacology Analysis and Experimental Validation to Explore the Mechanism of Sea Buckthorn Flavonoids on Hyperlipidemia. J. Ethnopharmacol. 2021, 264, 113380. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Zhu, C.; Gao, X.; Chu, W. Evaluation of Antioxidative and Neuroprotective Activities of Total Flavonoids From Sea Buckthorn (Hippophae rhamnoides L.). Front. Nutr. 2022, 9, 861097. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, X.; Liu, F.; Zhang, J.; Zhang, X.; Liu, J.; Li, S.; Wang, D.; Guan, H.; Hou, D. Total Flavonoids of Sea Buckthorn (Hippophae rhamnoides L.) Improve MC903-Induced Atopic Dermatitis-like Lesions. J. Ethnopharmacol. 2022, 292, 115195. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, G.; Chen, N.; Zhang, J.; He, C. Metabolomic and Transcriptomic Analyses Provide Insights into the Flavonoid Biosynthesis in Sea Buckthorn (Hippophae rhamnoides L.). LWT 2023, 187, 115276. [Google Scholar] [CrossRef]
- Geng, Z.; Wang, J.; Zhu, L.; Yu, X.; Zhang, Q.; Li, M.; Hu, B.; Yang, X. Metabolomics Provide a Novel Interpretation of the Changes in Flavonoids during Sea Buckthorn (Hippophae rhamnoides L.) Drying. Food Chem. 2023, 413, 135598. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, J.; Su, T.; Li, S.; Sheng, W.; Feng, L.; Bi, Y. Flavonoid Extract from Seed Residues of Hippophae rhamnoides ssp. Sinensis Protects against Alcohol-Induced Intestinal Barrier Dysfunction by Regulating the Nrf2 Pathway. Antioxidants 2023, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Ding, J.; Lu, S.; Wen, X.; Hu, J.; Ruan, C. Identification of the Key Flavonoid and Lipid Synthesis Proteins in the Pulp of Two Sea Buckthorn Cultivars at Different Developmental Stages. BMC Plant Biol. 2022, 22, 299. [Google Scholar] [CrossRef]
- Liu, R.; Yang, Y.; Zhao, M.; Wang, Y.; Meng, X.; Yan, T.; Ho, C.-T. Effect of Heat-Treating Methods on Components, Instrumental Evaluation of Color and Taste, and Antioxidant Properties of Sea Buckthorn Pulp Flavonoids. J. Food Sci. 2022, 87, 5442–5454. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, G.; Baj, T.; Kowalski, R.; Szymańska, J. Optimization of Glycerol–Water Extraction of Selected Bioactive Compounds from Peppermint and Common Nettle. Antioxidants 2021, 10, 817. [Google Scholar] [CrossRef] [PubMed]
- Anis, N.; Ahmed, D. Modelling and Optimization of Polyphenol and Antioxidant Extraction from Rumex hastatus by Green Glycerol-Water Solvent According to Response Surface Methodology. Heliyon 2022, 8, e11992. [Google Scholar] [CrossRef]
- Nastasi, J.R.; Daygon, V.D.; Kontogiorgos, V.; Fitzgerald, M.A. Qualitative Analysis of Polyphenols in Glycerol Plant Extracts Using Untargeted Metabolomics. Metabolites 2023, 13, 566. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P.; Lalas, S. Glycerol and Glycerol-Based Deep Eutectic Mixtures as Emerging Green Solvents for Polyphenol Extraction: The Evidence So Far. Molecules 2020, 25, 5842. [Google Scholar] [CrossRef]
- Zhang, X.; Su, J.; Chu, X.; Wang, X. A Green Method of Extracting and Recovering Flavonoids from Acanthopanax senticosus Using Deep Eutectic Solvents. Molecules 2022, 27, 923. [Google Scholar] [CrossRef]
- Yangthong, M.; Hutadilok-Towatana, N.; Phromkunthong, W. Antioxidant Activities of Four Edible Seaweeds from the Southern Coast of Thailand. Plant Foods Hum. Nutr. 2009, 64, 218–223. [Google Scholar] [CrossRef]
- Sharma, U.K.; Sharma, K.; Sharma, N.; Sharma, A.; Singh, H.P.; Sinha, A.K. Microwave-Assisted Efficient Extraction of Different Parts of Hippophae rhamnoides for the Comparative Evaluation of Antioxidant Activity and Quantification of Its Phenolic Constituents by Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC). J. Agric. Food Chem. 2008, 56, 374–379. [Google Scholar] [CrossRef]
- Saleem, M.; Durani, A.I.; Asari, A.; Ahmed, M.; Ahmad, M.; Yousaf, N.; Muddassar, M. Investigation of Antioxidant and Antibacterial Effects of Citrus Fruits Peels Extracts Using Different Extracting Agents: Phytochemical Analysis with in Silico Studies. Heliyon 2023, 9, e15433. [Google Scholar] [CrossRef] [PubMed]
- Kocak, M.S.; Uren, M.C.; Calapoglu, M.; Tepe, A.S.; Mocan, A.; Rengasamy, K.R.R.; Sarikurkcu, C. Phenolic Profile, Antioxidant and Enzyme Inhibitory Activities of Stachys annua subsp. Annua Var. Annua. S. Afr. J. Bot. 2017, 113, 128–132. [Google Scholar] [CrossRef]
- Wakeel, A.; Jan, S.A.; Ullah, I.; Shinwari, Z.K.; Xu, M. Solvent Polarity Mediates Phytochemical Yield and Antioxidant Capacity of Isatis tinctoria. PeerJ 2019, 7, e7857. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Li, B.; Ji, B.; Yang, J.; Zhang, G.; Chen, Y.; Luo, Y. Antioxidant and Antimicrobial Activities of Consecutive Extracts from Galla chinensis: The Polarity Affects the Bioactivities. Food Chem. 2009, 113, 173–179. [Google Scholar] [CrossRef]
- Liu, S.; Xiao, P.; Kuang, Y.; Hao, J.; Huang, T.; Liu, E. Flavonoids from Sea Buckthorn: A Review on Phytochemistry, Pharmacokinetics and Role in Metabolic Diseases. J. Food Biochem. 2021, 45, e13724. [Google Scholar] [CrossRef] [PubMed]
- Pop, R.M.; Socaciu, C.; Pintea, A.; Buzoianu, A.D.; Sanders, M.G.; Gruppen, H.; Vincken, J.-P. UHPLC/PDA-ESI/MS Analysis of the Main Berry and Leaf Flavonol Glycosides from Different Carpathian Hippophaë rhamnoides L. Varieties. Phytochem. Anal. PCA 2013, 24, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Teleszko, M.; Wojdyło, A.; Rudzińska, M.; Oszmiański, J.; Golis, T. Analysis of Lipophilic and Hydrophilic Bioactive Compounds Content in Sea Buckthorn (Hippophaë rhamnoides L.) Berries. J. Agric. Food Chem. 2015, 63, 4120–4129. [Google Scholar] [CrossRef]
- Ghafoor, K.; Al Juhaimi, F.; Özcan, M.M.; Uslu, N.; Babiker, E.E.; Mohamed Ahmed, I.A. Total Phenolics, Total Carotenoids, Individual Phenolics and Antioxidant Activity of Ginger (Zingiber officinale) Rhizome as Affected by Drying Methods. LWT 2020, 126, 109354. [Google Scholar] [CrossRef]
- Wu, S.; Shen, D.; Wang, R.; Li, Q.; Mo, R.; Zheng, Y.; Zhou, Y.; Liu, Y. Phenolic Profiles and Antioxidant Activities of Free, Esterified and Bound Phenolic Compounds in Walnut Kernel. Food Chem. 2021, 350, 129217. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, Y.; Zhang, M.; Qi, J.; Zhao, W.; Gu, J.; Guo, W.; Li, Y. Screening of Specific Quantitative Peptides of Beef by LC–MS/MS Coupled with OPLS-DA. Food Chem. 2022, 387, 132932. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Chen, W.; Lei, Y.; Li, F.; Li, H.; Deng, W.; Jiang, G. Discrimination of Authenticity of Fritillariae cirrhosae Bulbus Based on Terahertz Spectroscopy and Chemometric Analysis. Microchem. J. 2021, 168, 106440. [Google Scholar] [CrossRef]
| Solvent of Extracts | DPPH (mg TE/g) | ABTS (mg TE/g) | FRAP (mg FE/g) | |
|---|---|---|---|---|
| Methanol | 0% | 2.26 ± 0.39 g | 19.99 ± 1.18 i | 18.54 ± 0.58 j |
| 25% | 4.33 ± 0.63 ef | 32.83 ± 0.91 f | 31.81 ± 1.22 g | |
| 50% | 6.76 ± 0.66 cd | 50.04 ± 1.63 d | 55.88 ± 1.19 e | |
| 75% | 9.92 ± 0.24 b | 80.25 ± 1.28 b | 65.04 ± 1.08 c | |
| Ethanol | 0% | 2.26 ± 0.39 g | 19.99 ± 1.18 i | 18.54 ± 0.58 j |
| 25% | 6.53 ± 0.16 cd | 36.35 ± 0.89 e | 38.32 ± 1.26 f | |
| 50% | 10.7 ± 0.72 b | 53.96 ± 2.14 c | 70.13 ± 1.5 b | |
| 75% | 13.93 ± 0.41 a | 84.22 ± 1.31 a | 96.81 ± 1.33 a | |
| Glycerin | 0% | 2.26 ± 0.39 g | 19.99 ± 1.18 i | 18.54 ± 0.58 j |
| 25% | 3.62 ± 0.11 f | 20.08 ± 0.32 i | 22.51 ± 0.77 i | |
| 50% | 6.06 ± 0.14 d | 25.41 ± 0.89 gh | 37.29 ± 1.43 f | |
| 75% | 7.39 ± 0.12 c | 27.44 ± 1.35 g | 59.64 ± 1.92 d | |
| Ethyl acetate | 0% | 5.1 ± 0.12 e | 31.32 ± 1.10 f | 34.31 ± 1.35 g |
| 25% | 4.01 ± 0.13 f | 23.43 ± 0.50 h | 25.14 ± 1.60 h | |
| 50% | 1.95 ± 0.03 g | 8.69 ± 0.67 j | 10.09 ± 1.29 k | |
| 75% | 0.63 ± 0.19 hi | 6.66 ± 0.54 jk | 1.62 ± 1.44 l | |
| Petroleum ether | 0% | 5.1 ± 0.12 e | 31.32 ± 1.10 f | 34.31 ± 1.35 g |
| 25% | 1.04 ± 0.19 h | 5.54 ± 0.66 kl | 11.92 ± 1.13 k | |
| 50% | 0.49 ± 0.06 hi | 4.14 ± 0.36 l | 1.76 ± 0.53 l | |
| 75% | 0.14 ± 0.03 i | 0.55 ± 0.66 m | 1.58 ± 0.56 l | |
| Solvent of Extracts | Gallic Acid | Ferulic Acid | Naringenin | Quercetin | Chlorogenic Acid | Rutin | Protocatechuic Acid | |
|---|---|---|---|---|---|---|---|---|
| mg/kg | ||||||||
| Methanol | 0% | 7.05 ± 0.14 de | 2.38 ± 0.08 c | ND | ND | 1.52 ± 0.07 ab | 100.67 ± 4.64 de | 14.47 ± 1.55 d |
| 25% | 7.10 ± 0.09 de | 2.47 ± 0.04 bc | ND | 2.85 ± 0.15 h | 1.48 ± 0.01 bc | 112.04 ± 5.18 cd | 15.73 ± 1.55 bcd | |
| 50% | 7.17 ± 0.08 d | 2.44 ± 0.02 bc | 1.81 ± 0.16 bcde | 3.33 ± 0.12 efg | 1.48 ± 0.04 bc | 113.77 ± 13.25 c | 15.20 ± 2.45 cd | |
| 75% | 7.42 ± 0.10 c | 2.51 ± 0.10 bc | 1.90 ± 0.02 bc | 3.99 ± 0.15 c | 1.53 ± 0.10 ab | 115.87 ± 12.07 c | 16.20 ± 1.00 bcd | |
| Ethanol | 0% | 7.05 ± 0.14 de | 2.38 ± 0.08 c | ND | ND | 1.52 ± 0.07 ab | 100.67 ± 4.64 de | 14.47 ± 1.55 d |
| 25% | 7.63 ± 0.09 c | 2.48 ± 0.09 bc | 1.78 ± 0.03 bcdef | 3.10 ± 0.16 fgh | ND | 118.25 ± 11.66 c | 17.17 ± 2.13 bcd | |
| 50% | 8.08 ± 0.06 b | 2.59 ± 0.07 b | 1.92 ± 0.10 ab | 4.32 ± 0.21 b | 1.47 ± 0.04 bc | 135.90 ± 10.08 b | 17.41 ± 1.99 bc | |
| 75% | 6.88 ± 0.10 e | 2.42 ± 0.08 c | 1.84 ± 0.05 bcd | 4.00 ± 0.13 c | 1.47 ± 0.05 bc | 93.22 ± 8.76 e | 16.41 ± 0.94 bcd | |
| Glycerin | 0% | 7.05 ± 0.14 de | 2.38 ± 0.08 c | ND | ND | 1.52 ± 0.07 ab | 100.67 ± 4.64 de | 14.47 ± 1.55 d |
| 25% | 7.61 ± 0.21 c | 2.41 ± 0.08 c | 1.67 ± 0.02 ef | 2.83 ± 0.10 h | 1.43 ± 0.03 cd | 98.59 ± 8.63 de | 18.56 ± 0.76 b | |
| 50% | 6.96 ± 0.15 de | 2.35 ± 0.04 c | 1.73 ± 0.06 def | 3.21 ± 0.04 efg | 1.39 ± 0.04 d | 99.25 ± 8.54 de | 17.67 ± 1.68 bc | |
| 75% | 11.25 ± 0.06 a | 2.88 ± 0.12 a | 2.04 ± 0.16 a | 5.33 ± 0.07 a | 1.55 ± 0.05 a | 192.21 ± 8.19 a | 27.90 ± 2.38 a | |
| Ethyl acetate | 0% | 2.13 ± 0.10 j | 1.97 ± 0.15 de | 1.76 ± 0.08 cdef | 3.26 ± 0.07 efg | ND | 11.61 ± 2.73 fg | 6.79 ± 1.56 e |
| 25% | 3.49 ± 0.09 f | 2.08 ± 0.10 d | 1.73 ± 0.14 def | 3.73 ± 0.06 cd | ND | 20.64 ± 3.00 f | 9.57 ± 0.68 e | |
| 50% | 2.71 ± 0.11 i | 1.89 ± 0.09 e | 1.66 ± 0.06 f | 3.41 ± 0.08 ef | ND | 13.15 ± 2.23 fg | 7.08 ± 0.60 e | |
| 75% | 3.14 ± 0.06 g | 2.01 ± 0.19 de | 1.75 ± 0.03 cdef | 3.85 ± 0.16 c | ND | 12.01 ± 1.28 fg | 8.93 ± 1.13 e | |
| Petroleum ether | 0% | 2.13 ± 0.10 j | 1.97 ± 0.15 de | 1.76 ± 0.08 cdef | 3.26 ± 0.07 efg | ND | 11.61 ± 2.73 fg | 6.79 ± 1.56 e |
| 25% | 3.18 ± 0.06 g | 2.12 ± 0.07 d | 1.81 ± 0.04 bcdef | 3.52 ± 0.46 de | ND | 18.27 ± 3.44 fg | 9.00 ± 1.56 e | |
| 50% | 3.51 ± 0.11 f | 2.09 ± 0.06 d | 1.84 ± 0.02 bcd | 3.31 ± 0.22 efg | ND | 12.94 ± 4.11 fg | 8.60 ± 0.69 e | |
| 75% | 2.94 ± 0.25 h | 2.12 ± 0.03 d | 1.83 ± 0.07 bcd | 3.05 ± 0.10 gh | ND | 4.70 ± 2.03 g | 8.19 ± 0.83 e | |
| Solvent of Extracts | Vanillic Acid | Hydroxybenzoic Acid | Coumalic Acid | Sinapinic Acid | Isorhamnetin | Epigallocatechin | Total Amount | |
| mg/kg | ||||||||
| Methanol | 0% | 4.29 ± 0.09 b | 6.49 ± 0.24 e | 7.29 ± 0.20 e | 7.31 ± 0.27 b | 2.19 ± 0.33 j | 45.81 ± 0.49 f | 199.46 ± 3.06 f |
| 25% | 3.80 ± 0.40 bc | 6.95 ± 0.15 cd | 7.75 ± 0.26 d | 7.06 ± 0.12 c | 3.58 ± 0.26 i | 53.74 ± 0.46 e | 224.56 ± 3.10 de | |
| 50% | 4.01 ± 0.30 bc | 7.19 ± 0.16 bc | 7.92 ± 0.20 d | 6.97 ± 0.08 c | 7.37 ± 0.17 g | 54.92 ± 0.94 e | 233.57 ± 13.43 cd | |
| 75% | 4.3 ± 0.55 b | 7.25 ± 0.03 bc | 8.46 ± 0.36 bc | 7.12 ± 0.13 bc | 11.43 ± 0.56 d | 56.68 ± 0.79 d | 244.66 ± 14.85 c | |
| Ethanol | 0% | 4.29 ± 0.09 b | 6.49 ± 0.24 e | 7.29 ± 0.20 e | 7.31 ± 0.27 b | 2.19 ± 0.33 j | 45.81 ± 0.49 f | 199.46 ± 3.06 f |
| 25% | 4.15 ± 0.41 bc | 7.14 ± 0.12 c | 8.14 ± 0.14 cd | 6.98 ± 0.16 c | 5.20 ± 0.29 h | 59.55 ± 1.60 c | 241.57 ± 12.16 c | |
| 50% | 4.49 ± 0.40 b | 7.48 ± 0.20 b | 8.86 ± 0.17 b | 7.34 ± 0.19 b | 13.08 ± 0.36 b | 65.37 ± 1.69 b | 278.30 ± 11.11 b | |
| 75% | 4.49 ± 0.11 b | 6.77 ± 0.06 de | 7.87 ± 0.29 d | 6.74 ± 0.11 d | 11.36 ± 0.08 d | 53.70 ± 2.32 e | 217.17 ± 12.06 e | |
| Glycerin | 0% | 4.29 ± 0.09 b | 6.49 ± 0.24 e | 7.29 ± 0.20 e | 7.31 ± 0.27 b | 2.19 ± 0.33 j | 45.81 ± 0.49 f | 199.46 ± 3.06 f |
| 25% | 4.18 ± 0.94 bc | 6.45 ± 0.44 e | 7.20 ± 0.45 e | 6.98 ± 0.16 c | 3.77 ± 0.29 i | 57.64 ± 0.46 d | 219.34 ± 7.10 de | |
| 50% | 4.18 ± 0.36 bc | 6.08 ± 0.13 f | 7.02 ± 0.24 e | 6.70 ± 0.13 d | 4.99 ± 0.16 h | 57.35 ± 0.95 d | 218.88 ± 8.25 de | |
| 75% | 5.91 ± 0.67 a | 8.45 ± 0.34 a | 10.37 ± 0.23 a | 8.14 ± 0.10 a | 20.01 ± 0.63 a | 105.49 ± 0.69 a | 401.53 ± 11.81 a | |
| Ethyl acetate | 0% | 3.46 ± 0.35 bc | 5.12 ± 0.12 hi | 5.71 ± 0.21 h | 5.58 ± 0.16 f | 9.32 ± 0.37 f | 8.68 ± 0.44 i | 65.40 ± 2.33 ij |
| 25% | 3.36 ± 0.62 bc | 5.45 ± 0.17 gh | 6.27 ± 0.23 fg | 5.93 ± 0.09 e | 13.13 ± 0.32 b | 11.18 ± 0.40 h | 86.56 ± 3.29 gh | |
| 50% | ND | 5.09 ± 0.13 i | 5.62 ± 0.14 h | 5.59 ± 0.02 f | 12.10 ± 0.32 c | 6.54 ± 0.28 j | 64.83 ± 2.66 ij | |
| 75% | 3.55 ± 0.45 bc | 5.38 ± 0.05 ghi | 6.34 ± 0.21 fg | 5.70 ± 0.18 ef | 13.25 ± 0.27 b | 6.54 ± 0.14 j | 72.44 ± 1.51 hi | |
| Petroleum ether | 0% | 3.46 ± 0.35 bc | 5.12 ± 0.12 hi | 5.71 ± 0.21 h | 5.58 ± 0.16 f | 9.32 ± 0.37 f | 8.68 ± 0.44 i | 65.40 ± 2.33 ij |
| 25% | 3.12 ± 1.09 c | 5.44 ± 0.05 gh | 6.16 ± 0.08 fg | 5.87 ± 0.06 e | 10.77 ± 0.39 e | 18.43 ± 0.05 g | 87.68 ± 4.68 g | |
| 50% | 3.43 ± 0.90 bc | 5.35 ± 0.05 ghi | 5.99 ± 0.09 gh | 5.80 ± 0.03 ef | 9.21 ± 0.40 f | 12.44 ± 0.28 h | 74.51 ± 5.18 ghi | |
| 75% | 3.41 ± 0.89 bc | 5.65 ± 0.16 g | 6.46 ± 0.29 f | 5.80 ± 0.06 ef | 8.77 ± 0.07 f | 3.80 ± 0.45 k | 56.72 ± 3.25 j | |
| DPPH | ABTS | FRAP | |
|---|---|---|---|
| TPC | 0.937 ** | 0.916 ** | 0.951 ** |
| TFC | 0.688 ** | 0.575 * | 0.724 ** |
| Gallic acid | 0.618 ** | 0.517 * | 0.653 ** |
| Ferulic acid | 0.635 ** | 0.542 * | 0.680 ** |
| Naringenin | 0.227 | 0.110 | 0.200 |
| Quercetin | 0.405 | 0.263 | 0.406 |
| Chlorogenic acid | 0.088 | 0.208 | 0.199 |
| Rutin | 0.658 ** | 0.564 * | 0.694 ** |
| Protocatechuic acid | 0.588 * | 0.446 | 0.625 ** |
| Vanillic acid | 0.602 * | 0.440 | 0.643 ** |
| Hydroxybenzoic acid | 0.667 ** | 0.597 * | 0.714 ** |
| Coumalic acid | 0.669 ** | 0.572 * | 0.713 ** |
| Sinapinic acid | 0.583 * | 0.506 * | 0.627 ** |
| Isorhamnetin | 0.211 | 0.084 | 0.239 |
| Epigallocatechin | 0.653 ** | 0.531 * | 0.691 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Yang, Z.; Li, J.; Huang, H.; Xia, Q.; Ye, X.; Liu, D. Optimizing the Solvent Selection of the Ultrasound-Assisted Extraction of Sea Buckthorn (Hippophae rhamnoides L.) Pomace: Phenolic Profiles and Antioxidant Activity. Foods 2024, 13, 482. https://doi.org/10.3390/foods13030482
Wu D, Yang Z, Li J, Huang H, Xia Q, Ye X, Liu D. Optimizing the Solvent Selection of the Ultrasound-Assisted Extraction of Sea Buckthorn (Hippophae rhamnoides L.) Pomace: Phenolic Profiles and Antioxidant Activity. Foods. 2024; 13(3):482. https://doi.org/10.3390/foods13030482
Chicago/Turabian StyleWu, Dan, Zhihao Yang, Jiong Li, Huilin Huang, Qile Xia, Xingqian Ye, and Donghong Liu. 2024. "Optimizing the Solvent Selection of the Ultrasound-Assisted Extraction of Sea Buckthorn (Hippophae rhamnoides L.) Pomace: Phenolic Profiles and Antioxidant Activity" Foods 13, no. 3: 482. https://doi.org/10.3390/foods13030482
APA StyleWu, D., Yang, Z., Li, J., Huang, H., Xia, Q., Ye, X., & Liu, D. (2024). Optimizing the Solvent Selection of the Ultrasound-Assisted Extraction of Sea Buckthorn (Hippophae rhamnoides L.) Pomace: Phenolic Profiles and Antioxidant Activity. Foods, 13(3), 482. https://doi.org/10.3390/foods13030482






