Abstract
Germination is an effective strategy to improve the nutritional and functional quality of Andean grains such as quinoa (Chenopodium quinoa Willd.); it helps reduce anti-nutritional components and enhance the digestibility and sensory aspects of the germinated. This work aimed to evaluate the effect of germination (0, 24, 48, and 72 h) on the physicochemical properties, content of bioactive compounds, and antioxidant capacity of three varieties of quinoa: white, red, and black high Andean from Peru. Color, nutritional composition, mineral content, phenolic compounds, flavonoids, and antioxidant activity were analyzed. Additionally, infrared spectra were obtained to elucidate structural changes during germination. The results showed color variations and significant increases (p < 0.05) in proteins, fiber, minerals, phenolic compounds, flavonoids, and antioxidant capacity after 72 h of germination, attributed to the activation of enzymatic pathways. In contrast, the infrared spectra showed a decrease in the intensity of functional groups –CH–, –CH2–, C–OH, –OH, and C–N. Correlation analysis showed that flavonoids mainly contributed to antioxidant activity (r = 0.612). Germination represents a promising alternative to develop functional ingredients from germinated quinoa flour with improved nutritional and functional attributes.
1. Introduction
Quinoa (Chenopodium quinoa Willd.), an Andean cereal, has positioned itself as a superfood of increasing popularity globally because of its excellent nutritional quality due to its balanced nutrient composition [1]; it contains significant amounts of protein (13.8 to 16.5%) of high biological value [2], with all essential amino acids such as phenylalanine (0.1–2.7 g/100 g), histidine (1.4–5.4 g/100 g), isoleucine (0.8–7.4 g/100 g), threonine (2.1–8.9 g/100 g), leucine (2.3–9.4 g/100 g), lysine (2.4–7.5 g/100 g), methionine (0.3–9.1 g/100 g), tryptophan (0.6–1.9 g/100 g) and valine (0.8–6.1 g/100 g) in ideal proportions for human nutrition [3,4]. Likewise, it is characterized by its high content of micronutrients such as iron, zinc, phosphorus, magnesium, manganese, B complex vitamins, vitamin E, and phenolic compounds [5,6,7,8,9,10].
Studies have reported important health benefits associated with quinoa consumption, including anti-inflammatory, antioxidant, anticancer, antihypertensive, hypo-cholesterolemic, prebiotic, and antidiabetic properties [11,12,13,14,15,16,17]. These benefits are attributed to bioactive compounds such as polyphenols, flavonoids, phytosterols, essential fatty acids, and dietary fiber [18,19,20,21]. In addition to its benefits as a food source, quinoa has significant potential as a functional ingredient in various products. However, some anti-nutritional factors in quinoa, such as saponins and trypsin inhibitors, could limit these applications [4,22]. An effective strategy to enhance the functional attributes of quinoa is the germination of the seeds.
Controlled germination is a biotechnological process that improves the nutritional value and quality of seeds and grains. During germination, various metabolic pathways are activated that mobilize reserve nutrients, synthesize new bioactive compounds, and reduce anti-nutritional factors [23,24,25,26,27]. Specifically in quinoa, germination increases the levels of phenolic compounds, unsaturated fatty acids, γ-aminobutyrate, carotenoids, and folates while decreasing the activity of trypsin inhibitors and the concentration of phytates [28,29,30]. Thus, germination enhances the quality and health benefits associated with quinoa consumption.
Although quinoa germination has been studied as a strategy to improve its nutritional and functional quality, there are still few works focused on high Andean varieties, and they still need to carry out a comprehensive characterization of the flour properties. This knowledge is critical to determining flour’s stability, texture, and suitability in food [1,23,24].
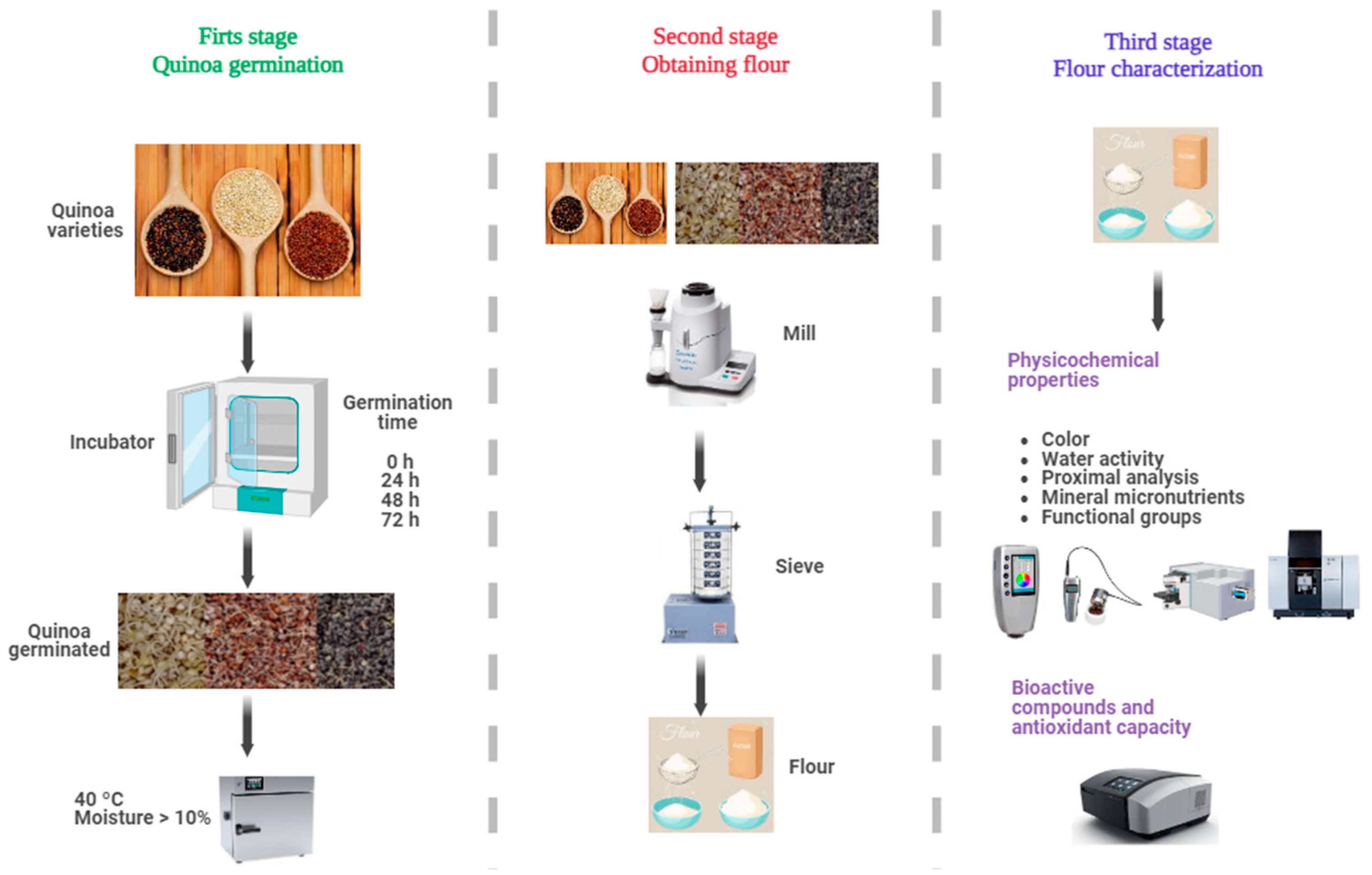
The main objective of the present study is to evaluate the effect of germination (0, 24, 48, and 72 h) on the physicochemical properties, content of bioactive compounds, and antioxidant capacity of three varieties of quinoa white, red, and black, grown in the high Andean area of Andahuaylas, Peru. Using a comprehensive approach, this research seeks to fill the knowledge gap to develop functional quinoa ingredients with improved quality attributes. The complete experimental flow chart is shown in Figure 1.
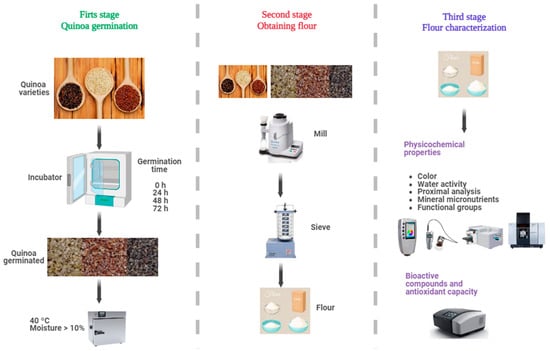
Figure 1.
Experimental flow diagram.
2. Materials and Methods
2.1. Materials
The varieties of quinoa (Chenopodium quinoa Willd.) selected for this study (Figure 1) were Collana (black variety), Pasankalla (red variety), and an ecotype called Choclito (white variety). Collana is an original variety from the Altiplano, with small black grains, high saponin content, and a maturity cycle of 140 days. Pasankalla is a variety improved by the Instituto Nacional de Innovación Agraria—INIA, with large grains of intense red color, moderate saponin content, and a 144-day cycle. The Choclito ecotype is a traditional local variety from the department of Puno in Peru, white in color, medium grain, low saponin content, and a maturity cycle of 132 days [31]. The differences in days of maturity, stem shape, distinctive colors, and saponin content are typical attributes reported for each variety cultivated in the Andes. The samples were obtained from crops in the community of Chulcuisa, district of San Jerónimo, Province of Andahuaylas, at 3758 m of altitude; the seeds were cleaned and stored in polyethylene bags.
2.2. Germination
The germination process of quinoa grains was carried out following the methodology of Xing et al. [26] with some modifications. The quinoa grains were washed with plenty of distilled water and disinfected with a 1% sodium hypochlorite solution for 5 min. They were soaked in distilled water for 4 h at 20 °C until reaching a moisture content between 40 and 50% before germination.
The grains were placed in a humid chamber FOC 200 E (Velp ScientificaTM, Usmate Velate, Italy) at 25 °C and 95% relative humidity. After germination, the grains were collected at 24, 48, and 72 h, dried in a forced convection oven FED 115 (BINDER, Tuttlingen, Germany) at 40 °C to a moisture content below 10%, and finally ground at 150 rpm for 3 min in a cyclone mill Twister (Retsch, Haan, Alemania) and sieved into a 250 µm mesh, the germinated flour was stored in airtight glass containers for subsequent analysis.
2.3. Color
The color was measured using a CR-5 digital colorimeter (Konica Minolta, Tokyo, Japan); the results were obtained in CIE coordinates L* a* b*, using the standard illuminant D65 and a standard observer from 10°. L* indicates lightness from black (0) to white (100), a* refers to shades from green (−) to red (+) and b* from blue (−) to yellow (+). Readings were taken in reflectance modulus. The color difference (ΔE) between the germinated quinoa flour and the control (ungerminated) flour was calculated using Equation (1), while the whiteness index (WI) was calculated using Equation (2) [32,33].
2.4. Water Activity
Water activity was determined with a HygroPalm 23-AW meter (Rotronic brand, Bassersdorf, Switzerland). The instrument was calibrated with calibration standards, then 3 g of sample was taken and transferred to a disposable sample container, inserted into the chamber, and sample readings were taken [34,35].
2.5. Proximal Analysis
Proximate analyses of ungerminated and germinated quinoa samples were determined according to the standard methods of the Association of Official Analytical Chemists (A.O.A.C.) for moisture; the sample was dried to constant weight in a forced convection oven (AOAC 925.10). The protein was obtained using the Kjeldahl method through the destruction of organic matter with concentrated sulfuric acid (AOAC 955.04), the fat was extracted using petroleum ether in a Soxhlet extractor (AOAC 2003.05), the ash was determined by ignition at 600 °C of the sample in a muffle furnace (AOAC 942.05), and the fiber was determined by ignition of the dry residue after digestion of the sample with sulfuric acid and sodium hydroxide (AOAC 962.09). Carbohydrates were determined by difference according to Equation (3) [36].
2.6. Mineral Micronutrients
The content of mineral micronutrients was determined using an atomic absorption spectrometer model A6800 (Shimadzu, Kyoto, Japan). For this, 5 g of the sample was converted to ash by incineration at 550 °C for five hours. Subsequently, 0.1 g of each sample was digested with nitric acid in a microwave (SCP Science, Miniwave, Montreal, QC, Canada) oven. Finally, the elements were identified by their characteristic emission spectra compared to a standard curve [1].
2.7. Functional Groups
The functional groups were determined by Fourier transform infrared spectroscopy (FTIR). The Nicolet IS50 FTIR transmission module (ThermoFisher, Waltham, MA, USA) was used within a range of wave numbers between 4000 and 400 cm−1, 10 mg of sample was weighed, and 100 mg of potassium bromide to form the pellet in a press, the readings were taken with 32 scans and 8 cm−1 resolution [1,37].
2.8. Bioactive Compounds
The samples’ total phenolic compounds were estimated using Folin–Ciocalteu reagent. Extracts of germinated and ungerminated flour samples were prepared and mixed with 20% sodium carbonate, 0.25 N Folin–Ciocalteau reagent and deionized water. The samples were read at 755 nm on a spectrophotometer (Genesys 150, Thermo Fisher Scientific, Waltham, MA, USA). Gallic acid (GA) was used as a reference standard, and the results were expressed as mg equivalent of gallic acid/100 g of sample [38,39].
The total flavonoid content was determined using the method described by Suárez-Estrella et al. [6]. Extracts of germinated and ungerminated flour samples were mixed with methanol and aluminum chloride. The samples were read at 450 nm on a spectrophotometer (Genesys 150, Thermo Fisher Scientific, Waltham, MA, USA). The reference standard was Quercetin, and the results were expressed as mg of Quercetin equivalent/100 g of sample [40].
2.9. Antioxidant Capacity
The DPPH assay was performed using the stable radical 2,2-diphenyl-1-picrylhydrazyl. The samples were read at 515 nm on a spectrophotometer (Genesys 150, Thermo Fisher Scientific, Waltham, MA, USA). The sample extracts were mixed with the diluted DPPH solution, and the samples were read at 515 nm in a spectrophotometer. Trolox was used as a reference standard, and the results were expressed as mg Trolox equivalents/g sample [30,39,41,42].
2.10. Statistic Analysis
The results were analyzed through ANOVA, Tukey’s multiple comparison test, and Pearson correlation at 5% significance. Origin Pro 2023 software (Origin Lab Corporation, Northampton, MA, USA) was used for graphical representation and statistical tests.
3. Results and Discussion
3.1. Color
The color evaluation is shown in Table 1; germination decreased the brightness (L*) of the germinated quinoas, while redness (a*) and yellowness (b*) increased with germination time. These variations would be related to the hydrolysis of starch and proteins during germination [43]. Positive values of chroma a* and b* are associated with carotenoid pigments in foods [44]. In this study, the germination process significantly increased both chroma parameters, more evident at 72 h. These results suggest an accumulation of carotenoids, bioactive compounds essential for their provitamin and antioxidant activity [45,46]. Specifically, Darwish et al. [47] reported increases of 26.02% in total carotenes during quinoa germination, an effect that agrees with the trend observed in the present study. Taken together, the colorimetric analysis indicates that prolonged germination times could maximize the content of these nutraceuticals in quinoa with consequent nutritional and health benefits.

Table 1.
Lightness, chroma a* and b*, color variation, and whiteness index.
The color difference (Table 1) concerning the ungerminated control exceeded the visible threshold of three units (ΔE > 3) [48] in all varieties and times. Increasing the germination time to 72 h generated the greatest quantifiable color changes, with values of 6.61, 5.76, and 7.65 in white, red, and black quinoa, respectively. These results show that extending germination produces measurable color variations. Furthermore, the whiteness index progressively decreased as germination progressed. The low values suggest that the flour acquired a yellowish hue, possibly due to the formation of carotenoids. White quinoa, without colored pigments, easily reflects any change in color variation and whiteness index. In contrast, black and red quinoa have base pigments that partially mask the modifications. Antioxidant substances in red and black pigments could protect other compounds from oxidative degradation during germination, causing minor color alterations [49,50,51]. Likewise, the different content of phenolic compounds in each variety influences its susceptibility to changes during germination [52].
3.2. Water Activity
Water activity, a parameter that quantifies water availability in food [28,40], presented its maximum values in white and red quinoa germinated for 72 and 48 h, respectively (Table 2). Although the levels obtained (water activity < 0.3) indicate that these germinated quinoa flours could be considered safe and stable ingredients for various food applications, some samples showed water activity lower than 0.2. According to Fontana [53], such low values of aqueous activity would be associated with a higher rate of lipid oxidation, a situation that could compromise the product’s useful life. Therefore, adequate control of drying kinetics is critical to obtaining a germinated quinoa ingredient with optimal levels of water availability.

Table 2.
Water activity quinoa varieties.
3.3. Proximal Analysis
The results of the proximal analysis (Table 3) showed a decrease in the carbohydrate content during the germination of the quinoa varieties, an effect attributed to hydrolysis processes mediated by endogenous amylolytic enzymes, which degrade starch, stored as amylose and amylopectin into sugars simple, that is, the reducing sugars glucose and maltose and, to a lesser extent, the non-reducing sugar sucrose [24,54]. On the contrary, germination markedly increased protein levels, especially at 72 h, reaching up to 10.65% for WQ, 11.35% for RQ, and 12.98% for BQ compared to non-germinated samples. During grain germination, storage proteins are hydrolyzed into peptides and amino acids by proteolytic enzymes after 2 to 3 days from imbibition, increasing nutrient bioavailability [23,54,55]. Likewise, the results showed increases in the total fiber content after the germination process for the three varieties. Previous studies attribute these changes to the fractionation of protein structures and greater solubilization of macromolecules during the biochemical process associated with seed germination [23]. Considering the current interest in plant-based ingredients rich in dietary fiber, germination would represent an effective strategy to enhance this attribute in quinoa for various food applications [38]. Other studies have reported decreases in fat content during quinoa germination, which are found in whole grains as triacylglycerols (TGA); their mobilization requires a coordinated metabolic activity that begins with germination, leading to the net conversion of fat into sugars [24,54,56]. Although in this work, the changes in fat were minimal, it is suggested that its variation be monitored due to the impact on the stability and shelf life of the ingredient. The ash content showed a slight but significant increase during the germination process of the three quinoa varieties analyzed. The germinated BQ variety presented the highest average percentage of ashes (2.58%) after 72 h. This effect could be due to a higher relative concentration of minerals remaining as inorganic residue after the ignition of the organic matter. Regarding moisture content, although the germinated RQ presented the highest average, the kinetics of variation between times and varieties did not show a uniform trend during germination. Previous studies attribute these differences in the final moisture percentage to factors such as the water absorption rate, respiratory activity, and efficiency of the drying process for each quinoa genotype [56].

Table 3.
Proximal analysis.
3.4. Mineral Micronutrients
The results showed significant increases (p-value < 0.05) in the content of several essential minerals, including calcium, phosphorus, iron, magnesium, and potassium, at different germination times for the three quinoa varieties studied (Table 4). These changes are attributed to the biochemical reactions and enzymatic activations typical of germination [47,57]. Furthermore, the concentration of the anti-nutrient phytate, which stores phosphorus in mature cereals, decreases during germination due to the action of phytases, thus increasing the bioavailability of phosphorus and other minerals [28,54,58]. The increases were greater than 72 h, agreeing with studies that report positive effects of prolonged germination times on the nutritional quality of quinoa. Controlled germination can increase the mineral content and nutritional functionality of different quinoa varieties.

Table 4.
Mineral micronutrients.
3.5. Functional Groups
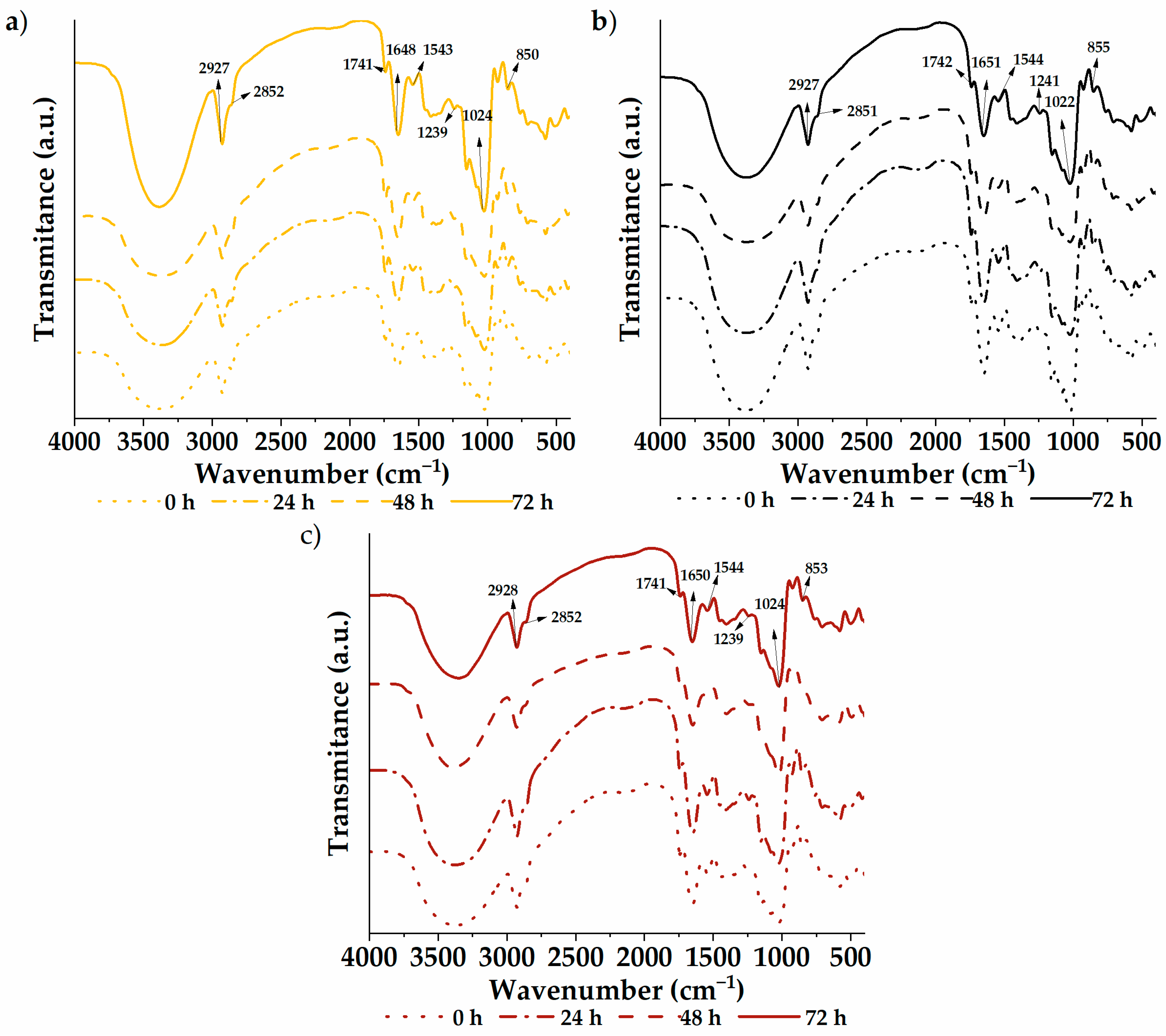
The infrared spectra of the germinated quinoa varieties are shown in Figure 2. A vibrational analysis was carried out through a transmission module to study the functional groups of the samples. The spectra were similar between varieties and germination times, with characteristic groups composed of –CH–, –CH2–, C−OH, and –OH found at wave numbers 2927, 2851, 1023, and 852 cm−1, respectively. Likewise, the C=O group was located at 1741 cm−1, possibly due to carboxylic acids or fats within the flour. The 1649 and 1543 cm−1 bands are attributed to amide I and II, respectively. These bands are associated with vibration modes of the amino groups of amino acids in protein structures. Therefore, they reflect modifications in the secondary protein structure [1]. In addition, the samples showed a signal at 1239 cm−1 (C–N vibrational mode) coming from amino acids. Analogous results were previously reported by García-Salcedo et al. [59] in quinoa, chia, and kiwicha flour and by Contreras-Jiménez et al. [1] in quinoa flour. Through analysis of the spectrograms, differences in intensities can be seen throughout the spectrum of the samples, especially between 500 and 2000 cm−1. The native sample exhibits higher intensities, with attenuation observed as germination time increases. This indicates increasing degradation with longer treatment time. However, no new functional group formation was evident from the germination process.
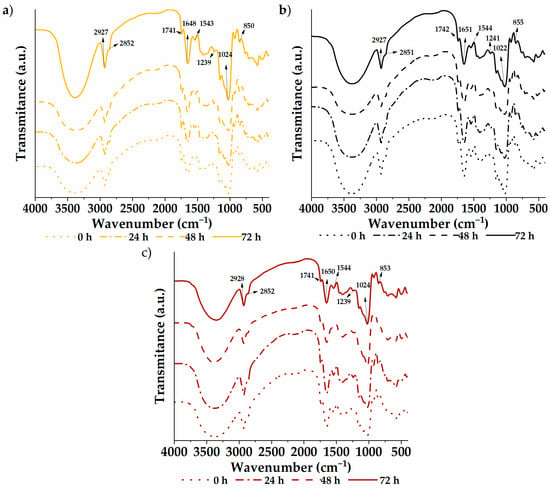
Figure 2.
Functional groups: (a) white quinoa, (b) black quinoa, and (c) red quinoa.
3.6. Bioactive Compounds
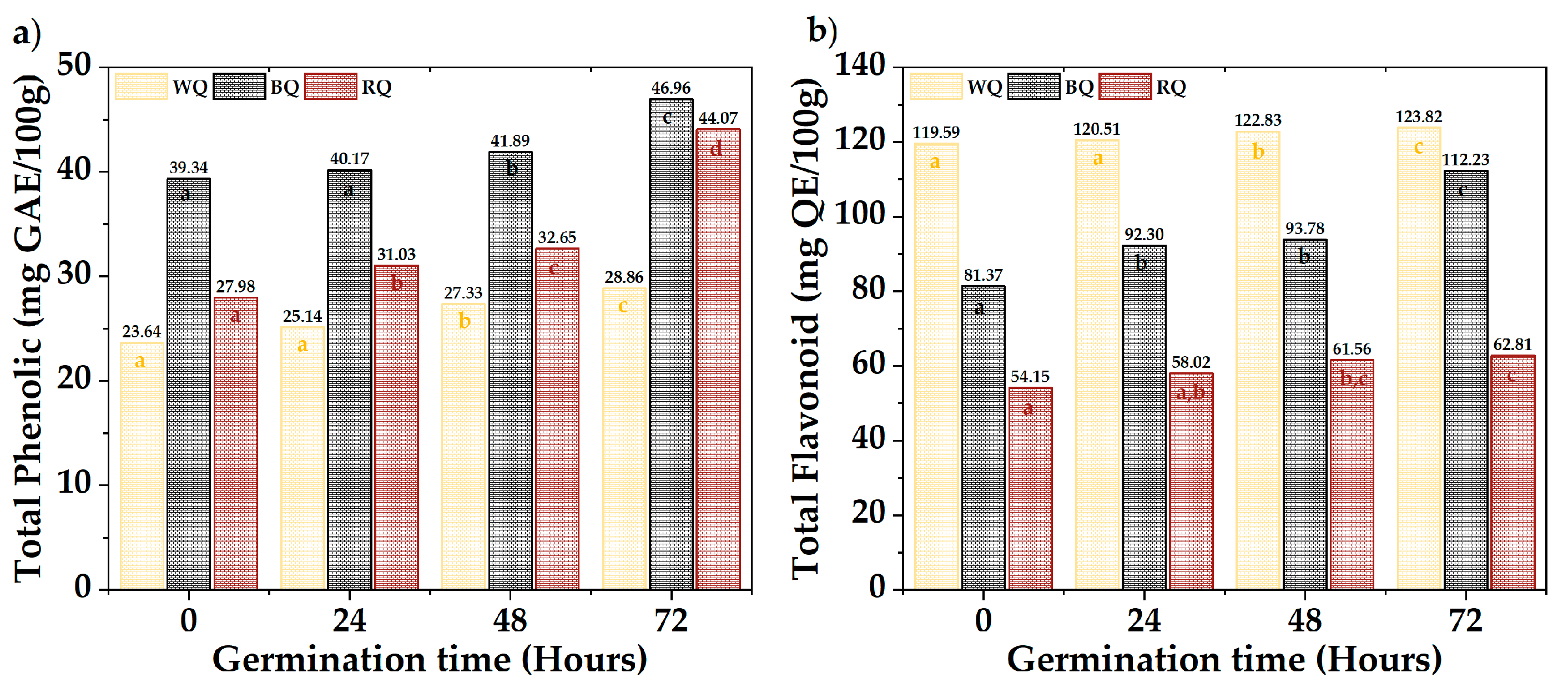
Figure 3 shows the results of total phenolics and flavonoids. The QR (44.07 mg AGE/100 g) and QN (46.96 mg AGE/100 g) presented the highest phenolic contents, while the QB (123.82 mg QE/100 g) had high levels of flavonoids. A significant difference was observed between varieties and times (p-value < 0.05). Total phenols increased with a prolonged germination time, except in QB, whose increase occurred after 48 h. Flavonoids fluctuated in QB and QR, but there was a substantial increase during germination in QN. The different biosynthetic routes (Shikimate and phenylpropanoids) and metabolite synthesis rates could explain the differences between varieties [51,52,60,61,62,63]. This occurs as a protective response when seeds break dormancy during germination [64,65,66,67,68].
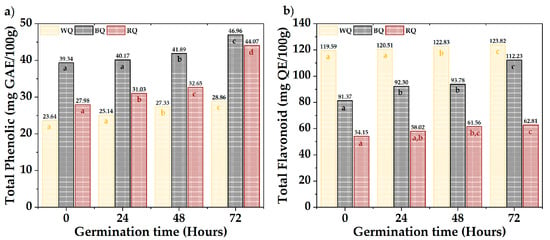
Figure 3.
Bioactive compounds, (a) total phenolic, and (b) total flavonoids. Equal letters mean that there is no significant difference, evaluated through the Tukey test, with α = 5%.
3.7. Antioxidant Capacity
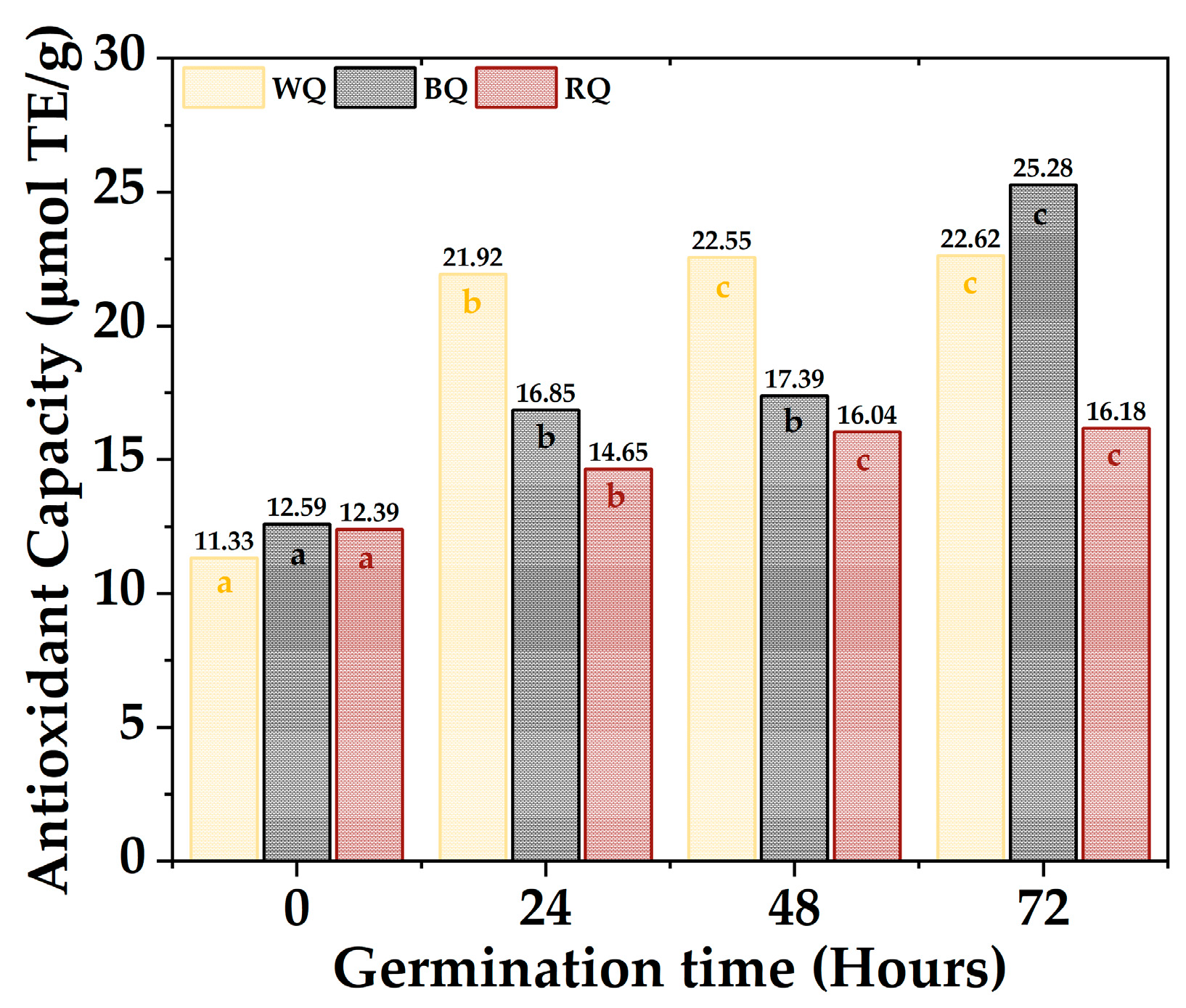
The antioxidant activity capacity equivalent to Trolox of the quinoa varieties is shown in Figure 4; the values ranged between 11.33 µmol TE/g to 22.62 µmol TE/g for white quinoa, between 12.59 µmol TE/g to 25.28 µmol TE/g for black quinoa, and between 12.39 µmol TE/g to 16.18 µmol TE/g. Furthermore, it was found that most of the samples present significant differences (p-value < 0.05), with the antioxidant activity being higher during 72 h, especially for BQ and WQ. The polyphenolic compounds responsible for antioxidant capacity are secondary metabolites present in plants, which are formed during their development and under stress conditions; these include simple phenols, phenolic acids, coumarins, flavonoids, stilbenes, hydrolyzable and condensed tannins, lignans, and lignins [69,70]. Furthermore, these polyphenols could be altered during germination, increasing their content and antioxidant capacity [71].
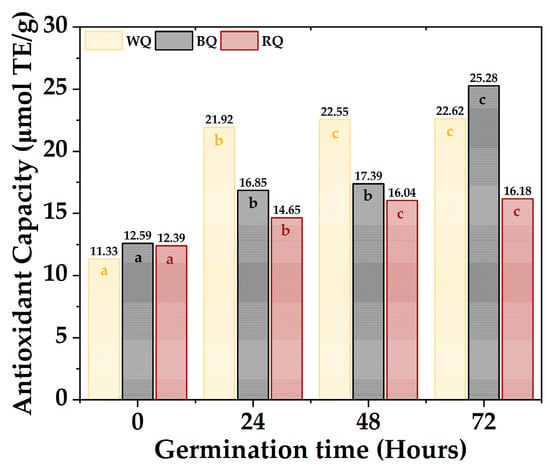
Figure 4.
Antioxidant capacity quinoa varieties. Equal letters mean that there is no significant difference, evaluated through the Tukey test, with α = 5%.
3.8. Correlation of Physicochemical Parameters, Bioactive Compounds, and Antioxidant Activity
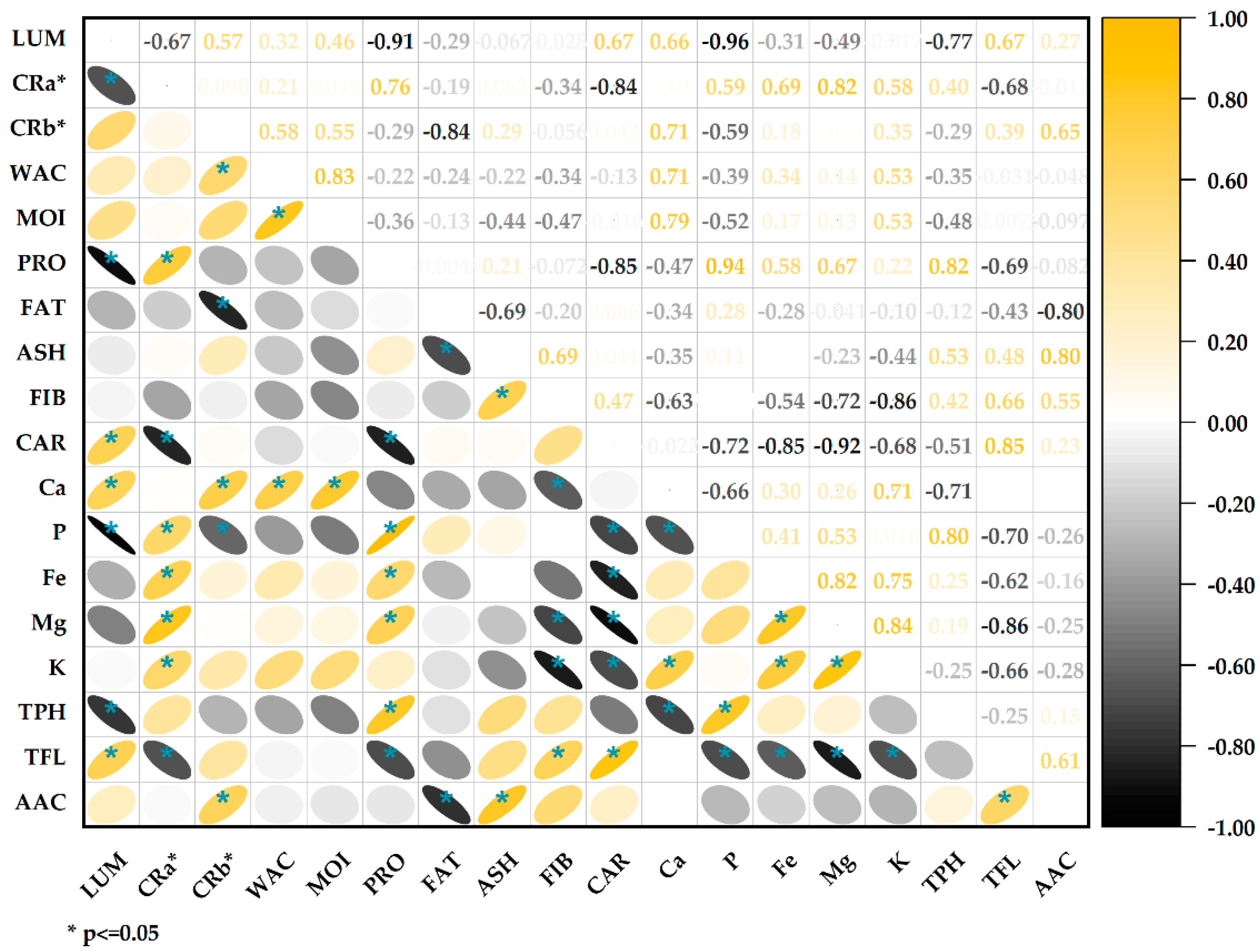
The correlation analysis (Figure 5) showed a significant negative association between protein content and lightness values (r = −0.91); this inverse relationship agrees with previous studies that report lower lightness in flours with higher protein content [72], the increase in proteins during germination increases the opacity of the flours by providing more excellent light dispersion, thus reducing lightness values [73]. The chrome a* (red/green) was positively correlated with proteins (r = 0.76) and negatively with carbohydrates (r = −0.84). This is consistent with the previous point, indicating that the higher the protein content, the higher the reddish tone. The water activity and moisture are strongly correlated (r = 0.83), which makes sense since both parameters are related to the aqueous content [40]. The fat content negatively correlates with the ashes (r = −0.69). This may indicate a dilution effect; the higher the fat content, the lower the ash content. There is a positive correlation between total flavonoids and antioxidant capacity (r = 0.61); flavonoids are a type of phenolic compound with particular chemical structures that confer a high antioxidant capacity [69,74]. The positive correlation indicates that flavonoids are the primary metabolites responsible for the antioxidant capacity in these samples of quinoa. Likewise, the high correlation between proteins and phenolic compounds (0.82) could be seen, associated with stress during germination that increases the levels in the germinated grains.
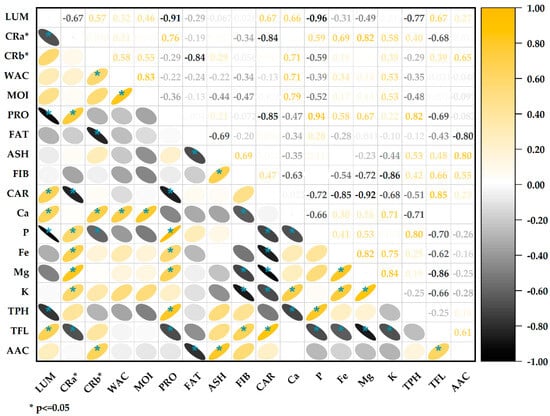
Figure 5.
Correlation of physicochemical parameters, bioactive compounds, and antioxidant capacity.
4. Conclusions
Germination significantly increased the content of protein, dietary fiber, mineral micronutrients, total phenolics, flavonoids, and antioxidant capacity in white, red, and black quinoa varieties. The most excellent bioactive compounds and antioxidant capacity increases were achieved after 72 h of germination. Flavonoids showed the most significant correlation with antioxidant capacity, suggesting they are mainly responsible.
Carrying out these studies would provide solid support for the potential use of germinated quinoa flours as a functional ingredient in developing new foods or dietary supplements. Germination is an effective strategy to improve quinoa’s nutritional and functional profile. Therefore, further research in this area is warranted to determine the feasibility of applying this process at an industrial level and harnessing its benefits in developing healthier food products.
In conclusion, the results of this study demonstrate that germination substantially improves quinoa’s nutritional composition and antioxidant capacity. Further studies on bioactive compounds’ stability, bioavailability, and bioaccessibility are required to support the potential use of germinated quinoa flours as functional ingredients in developing foods or dietary supplements. Research in this area would promote using quinoa germination’s benefits at an industrial level.
Author Contributions
Conceptualization, B.S.R.-P. and D.C.-Q.; methodology, C.A.L.-S., B.S.R.-P. and E.M.-M.; software, B.S.R.-P.; validation, D.C.-Q., C.A.L.-S. and A.M.S.-R.; formal analysis, C.A.L.-S., D.C.-Q., E.M.-M., A.M.S.-R. and D.E.P.-G.; investigation, B.S.R.-P., C.A.L.-S., D.C.-Q., E.M.-M., H.P.-R., Y.C.-Q. and Á.S.A.-P.; resources, B.S.R.-P.; data curation, D.C.-Q., Y.C.-Q. and C.A.L.-S.; writing—original draft preparation, B.S.R.-P. and D.C.-Q.; writing—review and editing, B.S.R.-P., D.C.-Q., E.M.-M., H.P.-R., D.E.P.-G. and Y.C.-Q.; visualization, C.A.L.-S. and A.M.S.-R.; supervision, B.S.R.-P., D.C.-Q. and C.A.L.-S.; project administration, H.P.-R. and D.E.P.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Vicerrectorado de Investigación of the Universidad Nacional José María Arguedas, Andahuaylas, Apurímac, and Universidad Nacional de San Antonio Abad del Cusco.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors thank the Viccerrectorado de Investigación of the Universidad Nacional José María Arguedas, Andahuaylas, Apurímac, the Yachayninchis Wiñarinanpaq program of the Universidad Nacional de San Antonio Abad del Cusco, the Food Nanotechnology Research Laboratory, the Materials Research Laboratory for Water Treatment and Food and the Nutraceuticals, and Biomaterials Research Group of the UNAJMA for their support of the project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Contreras-Jiménez, B.; Torres-Vargas, O.L.; Rodríguez-García, M.E. Physicochemical characterization of quinoa (Chenopodium quinoa) flour and isolated starch. Food Chem. 2019, 298, 124982. [Google Scholar] [CrossRef]
- Agarwal, A.; Rizwana; Tripathi, A.D.; Kumar, T.; Sharma, K.P.; Patel, S.K. Nutritional and Functional New Perspectives and Potential Health Benefits of Quinoa and Chia Seeds. Antioxidants 2023, 12, 1413. [Google Scholar] [CrossRef] [PubMed]
- Campos-Rodriguez, J.; Acosta-Coral, K.; Paucar-Menacho, L.M. Quinua (Chenopodium quinoa): Composición nutricional y Componentes bioactivos del grano y la hoja, e impacto del tratamiento térmico y de la germinación. Sci. Agropecu. 2022, 13, 209–220. [Google Scholar] [CrossRef]
- Pathan, S.; Siddiqui, R.A. Nutritional Composition and Bioactive Components in Quinoa (Chenopodium quinoa Willd.) Greens: A Review. Nutrients 2022, 14, 558. [Google Scholar] [CrossRef]
- Alandia, G.; Rodriguez, J.P.; Jacobsen, S.E.; Bazile, D.; Condori, B. Global expansion of quinoa and challenges for the Andean region. Glob. Food Secur. 2020, 26, 100429. [Google Scholar] [CrossRef]
- Suárez-Estrella, D.; Borgonovo, G.; Buratti, S.; Ferranti, P.; Accardo, F.; Pagani, M.A.; Marti, A. Sprouting of quinoa (Chenopodium quinoa Willd.): Effect on saponin content and relation to the taste and astringency assessed by electronic tongue. LWT 2021, 144, 111234. [Google Scholar] [CrossRef]
- Anchico-Jojoa, W.; Peixoto, J.R.; Júnior, A.A.d.O. Physicochemical characterization and antioxidant capacity of quinoa progenies from Colombia, Brazil and Ecuador produced in the Brazilian Savanna. Rev. Colomb. Cienc. Hortícolas 2023, 17, 1–11. [Google Scholar] [CrossRef]
- Craine, E.B.; Murphy, K.M. Seed Composition and Amino Acid Profiles for Quinoa Grown in Washington State. Front. Nutr. 2020, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz-Arango, J. La quinua en el Perú: Pseudocereal andino, alimento de generaciones presentes y futuras. J. Selva Andin. Biosph. 2023, 11, 1–3. [Google Scholar] [CrossRef]
- Angeli, V.; Miguel Silva, P.; Crispim Massuela, D.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium quinoa Willd.): An Overview of the Potentials of the “Golden Grain” and Socio-Economic and Environmental Aspects of Its Cultivation and Marketization. Foods 2020, 9, 216. [Google Scholar] [CrossRef]
- Tang, Y.; Tsao, R. Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects: A review. Mol. Nutr. Food Res. 2017, 61, 1600767. [Google Scholar] [CrossRef]
- El Makawy, A.I.; Abdel-Aziem, S.H.; Mohammed, S.E.; Ibrahim, F.M.; Abd El-Kader, H.A.; Sharaf, H.A.; Youssef, D.A.; Mabrouk, D.M. Exploration of tumor growth regression of quinoa and chia oil nanocapsules via the control of PIK3CA and MYC expression, anti-inflammation and cell proliferation inhibition, and their hepatorenal safety in rat breast cancer model. Bull. Natl. Res. Cent. 2024, 48, 7. [Google Scholar] [CrossRef]
- Jan, N.; Hussain, S.Z.; Naseer, B.; Bhat, T.A. Amaranth and quinoa as potential nutraceuticals: A review of anti-nutritional factors, health benefits and their applications in food, medicinal and cosmetic sectors. Food Chem. X 2023, 18, 100687. [Google Scholar] [CrossRef]
- Kwon, C.; Kim, H.R.; Moon, T.W.; Lee, S.H.; Lee, C.J. Structural and Physicochemical Characteristics of Granular Malic Acid-Treated Sweet Potato Starch Containing Heat-Stable Resistant Starch. J. Chem. 2019, 2019, 2903252. [Google Scholar] [CrossRef]
- Ranjan, S.; Sow, S.; Ghosh, M.; Padhan, S.R.; Kumar, S.; Gitari, H.; Mirriam, A.; Nath, D. Nutraceutical properties and secondary metabolites of quinoa (Chenopodium quinoa Willd.): A review. Int. J. Food Prop. 2023, 26, 3477–3491. [Google Scholar] [CrossRef]
- Ren, G.; Teng, C.; Fan, X.; Guo, S.; Zhao, G.; Zhang, L.; Liang, Z.; Qin, P. Nutrient composition, functional activity and industrial applications of quinoa (Chenopodium quinoa Willd.). Food Chem. 2023, 410, 135290. [Google Scholar] [CrossRef] [PubMed]
- Romero-Benavides, J.C.; Guaraca-Pino, E.; Duarte-Casar, R.; Rojas-Le-Fort, M.; Bailon-Moscoso, N. Chenopodium quinoa Willd. and Amaranthus hybridus L.: Ancestral Andean Food Security and Modern Anticancer and Antimicrobial Activity. Pharmaceuticals 2023, 16, 1728. [Google Scholar] [CrossRef] [PubMed]
- Graf, B.L.; Rojas-Silva, P.; Rojo, L.E.; Delatorre-Herrera, J.; Baldeón, M.E.; Raskin, I. Innovations in Health Value and Functional Food Development of Quinoa (Chenopodium quinoa Willd.). Compr. Rev. Food Sci. Food Saf. 2015, 14, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, X.; Zhang, B.; Chen, P.X.; Liu, R.; Tsao, R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 166, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Hernandez, M.; Zhang, H.; Marcone, M.F.; Liu, R.; Tsao, R. Characterisation of fatty acid, carotenoid, tocopherol/tocotrienol compositions and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 174, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Liu, R.; Hernandez, M.; Draves, J.; Marcone, M.F.; Tsao, R. Assessing the Fatty Acid, Carotenoid, and Tocopherol Compositions of Amaranth and Quinoa Seeds Grown in Ontario and Their Overall Contribution to Nutritional Quality. J. Agric. Food Chem. 2016, 64, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Abugoch James, L.E. Quinoa (Chenopodium quinoa Willd.): Composition, chemistry, nutritional, and functional properties. Adv. Food Nutr. Res. 2009, 58, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Saxena, D.C.; Singh, S. Comparative study of raw and germinated Chenopodium (Chenopodium album) flour on the basis of thermal, rheological, minerals, fatty acid profile and phytocomponents. Food Chem. 2018, 269, 173–180. [Google Scholar] [CrossRef]
- Jimenez, M.D.; Lobo, M.; Sammán, N. 12th IFDC 2017 Special Issue–Influence of germination of quinoa (Chenopodium quinoa) and amaranth (Amaranthus) grains on nutritional and techno-functional properties of their flours. J. Food Compos. Anal. 2019, 84, 103290. [Google Scholar] [CrossRef]
- Pilco-Quesada, S.; Tian, Y.; Yang, B.; Repo-Carrasco-Valencia, R.; Suomela, J.-P. Effects of germination and kilning on the phenolic compounds and nutritional properties of quinoa (Chenopodium quinoa) and kiwicha (Amaranthus caudatus). J. Cereal Sci. 2020, 94, 102996. [Google Scholar] [CrossRef]
- Xing, B.; Teng, C.; Sun, M.; Zhang, Q.; Zhou, B.; Cui, H.; Ren, G.; Yang, X.; Qin, P. Effect of germination treatment on the structural and physicochemical properties of quinoa starch. Food Hydrocoll. 2021, 115, 106604. [Google Scholar] [CrossRef]
- Bhinder, S.; Kumari, S.; Singh, B.; Kaur, A.; Singh, N. Impact of germination on phenolic composition, antioxidant properties, antinutritional factors, mineral content and Maillard reaction products of malted quinoa flour. Food Chem. 2021, 346, 128915. [Google Scholar] [CrossRef] [PubMed]
- Guardianelli, L.M.; Salinas, M.V.; Brites, C.; Puppo, M.C. Germination of White and Red Quinoa Seeds: Improvement of Nutritional and Functional Quality of Flours. Foods 2022, 11, 3272. [Google Scholar] [CrossRef]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Lê, K.-A.; Van den Broeck, H.C.; Brouns, F.J.P.H.; De Brier, N.; et al. Impact of Cereal Seed Sprouting on Its Nutritional and Technological Properties: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Cao, B.; Wei, X.; Shen, Z.; Su, N. Assessment and comparison of nutritional qualities of thirty quinoa (Chenopodium quinoa Willd.) seed varieties. Food Chem. X 2023, 19, 100808. [Google Scholar] [CrossRef]
- Vilca, S.M.; Espinoza, P.; Vidal, A.P. Multiplicación de semilla de variedades y ecotipos de quinua en valle de majes-Arequipa. Rev. Investig. Altoandinas 2015, 17, 2. [Google Scholar]
- Ligarda-Samanez, C.A.; Moscoso-Moscoso, E.; Choque-Quispe, D.; Ramos-Pacheco, B.S.; Arévalo-Quijano, J.C.; Cruz, G.D.; Huamán-Carrión, M.L.; Quispe-Quezada, U.R.; Gutiérrez-Gómez, E.; Cabel-Moscoso, D.J.; et al. Native Potato Starch and Tara Gum as Polymeric Matrices to Obtain Iron-Loaded Microcapsules from Ovine and Bovine Erythrocytes. Polymers 2023, 15, 3985. [Google Scholar] [CrossRef]
- Choque-Quispe, D.; Choque-Quispe, Y.; Ligarda-Samanez, C.A.; Peralta-Guevara, D.E.; Solano-Reynoso, A.M.; Ramos-Pacheco, B.S.; Taipe-Pardo, F.; Martínez-Huamán, E.L.; Aguirre Landa, J.P.; Agreda Cerna, H.W.; et al. Effect of the Addition of Corn Husk Cellulose Nanocrystals in the Development of a Novel Edible Film. Nanomaterials 2022, 12, 3421. [Google Scholar] [CrossRef] [PubMed]
- Fasoyiro, S.; Hovingh, R.; Gourama, H.; Cutter, C. Change in Water Activity and Fungal Counts of Maize-pigeon Pea Flour During Storage Utilizing Various Packaging Materials. Procedia Eng. 2016, 159, 72–76. [Google Scholar] [CrossRef]
- Ligarda-Samanez, C.A.; Choque-Quispe, D.; Moscoso-Moscoso, E.; Huamán-Carrión, M.L.; Ramos-Pacheco, B.S.; Peralta-Guevara, D.E.; De la Cruz, G.; Martínez-Huamán, E.L.; Arévalo-Quijano, J.C.; Muñoz-Saenz, J.C.J.F. Obtaining and characterizing andean multi-floral propolis nanoencapsulates in polymeric matrices. Foods 2022, 11, 3153. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W. Official Methods of Analysis of AOAC International. In Volume I, Agriculture Chemicasl, Contaminants, Drugs; AOAC International: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Choque-Quispe, D.; Mojo-Quisani, A.; Ligarda-Samanez, C.A.; Calla-Florez, M.; Ramos-Pacheco, B.S.; Zamalloa-Puma, L.M.; Peralta-Guevara, D.E.; Solano-Reynoso, A.M.; Choque-Quispe, Y.; Zamalloa-Puma, A.; et al. Preliminary Characterization of a Spray-Dried Hydrocolloid from a High Andean Algae (Nostoc sphaericum). Foods 2022, 11, 1640. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Lucas-Gonzales, R.; Ricci, A.; Fontecha, J.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, fatty acid, polyphenolic profile, techno-functional and antioxidant properties of flours obtained from quinoa (Chenopodium quinoa Willd) seeds. Ind. Crops Prod. 2018, 111, 38–46. [Google Scholar] [CrossRef]
- Ligarda-Samanez, C.A.; Choque-Quispe, D.; Moscoso-Moscoso, E.; Pozo, L.M.F.; Ramos-Pacheco, B.S.; Palomino-Rincón, H.; Gutiérrez, R.J.G.; Peralta-Guevara, D.E. Effect of Inlet Air Temperature and Quinoa Starch/Gum Arabic Ratio on Nanoencapsulation of Bioactive Compounds from Andean Potato Cultivars by Spray-Drying. Molecules 2023, 28, 7875. [Google Scholar] [CrossRef] [PubMed]
- Ligarda-Samanez, C.A.; Choque-Quispe, D.; Moscoso-Moscoso, E.; Palomino-Rincón, H.; Taipe-Pardo, F.; Aguirre Landa, J.P.; Arévalo-Quijano, J.C.; Muñoz-Saenz, J.C.; Quispe-Quezada, U.R.; Huamán-Carrión, M.L.; et al. Nanoencapsulation of Phenolic Extracts from Native Potato Clones (Solanum tuberosum spp. andigena) by Spray Drying. Molecules 2023, 28, 4961. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Rico, D.; Peñas, E.; García, M.D.; Martínez-Villaluenga, C.; Rai, D.K.; Birsan, R.I.; Frias, J.; Martín-Diana, A.B. Sprouted Barley Flour as a Nutritious and Functional Ingredient. Foods 2020, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Garbanzo, C.; Pérez, A.M.; Vaillant, F.; Pineda-Castro, M.L. Physicochemical and antioxidant composition of fresh peach palm (Bactris gasipaes Kunth) fruits in Costa Rica. Braz. J. Food Technol. 2016, 19, 1–9. [Google Scholar] [CrossRef]
- Amengual, J. Bioactive Properties of Carotenoids in Human Health. Nutrients 2019, 11, 2388. [Google Scholar] [CrossRef] [PubMed]
- González-Peña, M.A.; Ortega-Regules, A.E.; Anaya de Parrodi, C.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids-A Review. Plants 2023, 12, 313. [Google Scholar] [CrossRef]
- Darwish, A.M.G.; Al-Jumayi, H.A.O.; Elhendy, H.A. Effect of germination on the nutritional profile of quinoa (Cheopodium quinoa Willd.) seeds and its anti-anemic potential in Sprague–Dawley male albino rats. Cereal Chem. 2021, 98, 315–327. [Google Scholar] [CrossRef]
- Adekunte, A.O.; Tiwari, B.K.; Cullen, P.J.; Scannell, A.G.M.; O’Donnell, C.P. Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chem. 2010, 122, 500–507. [Google Scholar] [CrossRef]
- Pedrali, D.; Giupponi, L.; De la Peña-Armada, R.; Villanueva-Suárez, M.J.; Mateos-Aparicio, I. The quinoa variety influences the nutritional and antioxidant profile rather than the geographic factors. Food Chem. 2023, 402, 133531. [Google Scholar] [CrossRef]
- Peng, M.; Yin, L.; Dong, J.; Shen, R.; Zhu, Y. Physicochemical characteristics and in vitro digestibility of starches from colored quinoa (Chenopodium quinoa) varieties. J. Food Sci. 2022, 87, 2147–2158. [Google Scholar] [CrossRef]
- Qian, G.; Li, X.; Zhang, H.; Zhang, H.; Zhou, J.; Ma, X.; Sun, W.; Yang, W.; He, R.; Wahab, A.-t.; et al. Metabolomics analysis reveals the accumulation patterns of flavonoids and phenolic acids in quinoa (Chenopodium quinoa Willd.) grains of different colors. Food Chem. X 2023, 17, 100594. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cao, D.; Yao, Y.; Wang, J.; Li, Z.; Liu, B. Understanding the chemical foundation and genetic mechanism of the black grain trait in quinoa by integrating metabolome and transcriptome analyses. Biotechnol. Biotechnol. Equip. 2020, 34, 1095–1103. [Google Scholar] [CrossRef]
- Fontana, A.J. Understanding the importance of water activity in food. Cereal Foods World 2000, 45, 7–10. [Google Scholar]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.; Hilhorst, H. Seeds: Physiology of Development, Germination and Dormancy; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Aguilar, J.; Miano, A.C.; Obregón, J.; Soriano-Colchado, J.; Barraza-Jáuregui, G. Malting process as an alternative to obtain high nutritional quality quinoa flour. J. Cereal Sci. 2019, 90, 102858. [Google Scholar] [CrossRef]
- Demir, B.; Bilgiçli, N. Changes in chemical and anti-nutritional properties of pasta enriched with raw and germinated quinoa (Chenopodium quinoa Willd.) flours. J. Food Sci. Technol. 2020, 57, 3884–3892. [Google Scholar] [CrossRef]
- Maldonado-Alvarado, P.; Pavón-Vargas, D.J.; Abarca-Robles, J.; Valencia-Chamorro, S.; Haros, C.M. Effect of Germination on the Nutritional Properties, Phytic Acid Content, and Phytase Activity of Quinoa (Chenopodium quinoa Willd). Foods 2023, 12, 389. [Google Scholar] [CrossRef] [PubMed]
- García-Salcedo, Á.J.; Torres-Vargas, O.L.; Ariza-Calderón, H. Physical-chemical characterization of quinoa (Chenopodium quinoa Willd.), amaranth (Amaranthus caudatus L.), and chia (Salvia hispanica L.) flours and seeds. Acta Agronómica 2018, 67, 215–222. [Google Scholar] [CrossRef]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.d.P.; Abrahão, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Valanciene, E.; Jonuskiene, I.; Syrpas, M.; Augustiniene, E.; Matulis, P.; Simonavicius, A.; Malys, N. Advances and Prospects of Phenolic Acids Production, Biorefinery and Analysis. Biomolecules 2020, 10, 874. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, L.; Li, Q.; Xie, H.; Guo, Y.; Huang, T.; Zhang, X.; Liu, J.; Zhang, P.; Li, L.; et al. Integrative Analysis of the Metabolome and Transcriptome Provides Insights into the Mechanisms of Flavonoid Biosynthesis in Quinoa Seeds at Different Developmental Stages. Metabolites 2022, 12, 887. [Google Scholar] [CrossRef]
- Norma Francenia, S.-S.; Raúl, S.-C.; Beatriz, H.-C.; Claudia, V.-C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; Marcos, S.-H., Rosario, G.-M., Mariana, P.-T., Eds.; IntechOpen: Rijeka, Croatia, 2019; Volume 1, pp. 1–15. [Google Scholar]
- Balakrishnan, G.; Schneider, R.G. Quinoa flavonoids and their bioaccessibility during in vitro gastrointestinal digestion. J. Cereal Sci. 2020, 95, 103070. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Y.; Qin, L. The cause of germination increases the phenolic compound contents of Tartary buckwheat (Fagopyrum tataricum). J. Future Foods 2022, 2, 372–379. [Google Scholar] [CrossRef]
- Gharachorloo, M.; Ghiassi Tarzi, B.; Baharinia, M. The Effect of Germination on Phenolic Compounds and Antioxidant Activity of Pulses. J. Am. Oil Chem. Soc. 2013, 90, 407–411. [Google Scholar] [CrossRef]
- Khang, D.T.; Dung, T.N.; Elzaawely, A.A.; Xuan, T.D. Phenolic Profiles and Antioxidant Activity of Germinated Legumes. Foods 2016, 5, 27. [Google Scholar] [CrossRef]
- Xiang, J.; Yuan, Y.; Du, L.; Zhang, Y.; Li, C.; Beta, T. Modification on phenolic profiles and enhancement of antioxidant activity of proso millets during germination. Food Chem. X 2023, 18, 100628. [Google Scholar] [CrossRef]
- Van Hung, P. Phenolic Compounds of Cereals and Their Antioxidant Capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Enciso-Roca, E.C.; Aguilar-Felices, E.J.; Tinco-Jayo, J.A.; Arroyo-Acevedo, J.L.; Herrera-Calderon, O. Biomolecules with Antioxidant Capacity from the Seeds and Sprouts of 20 Varieties of Chenopodium quinoa Willd. (Quinoa). Plants 2021, 10, 2417. [Google Scholar] [CrossRef] [PubMed]
- Fenn, D.; Lukow, O.M.; Humphreys, G.; Fields, P.G.; Boye, J.I. Wheat-Legume Composite Flour Quality. Int. J. Food Prop. 2010, 13, 381–393. [Google Scholar] [CrossRef]
- Mikus, M.; Galus, S.; Ciurzyńska, A.; Janowicz, M. Development and Characterization of Novel Composite Films Based on Soy Protein Isolate and Oilseed Flours. Molecules 2021, 26, 3738. [Google Scholar] [CrossRef]
- Sarker, U.; Hossain, M.M.; Oba, S. Nutritional and antioxidant components and antioxidant capacity in green morph Amaranthus leafy vegetable. Sci. Rep. 2020, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).