Advanced Extraction Techniques for Bioactive Compounds from Berry Fruits: Enhancing Functional Food Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
- Sea buckthorn (Hippophae rhamnoides L.)
- Chokeberry (Aronia melanocarpa (Michx.) Elliott)
- Black elderberry (Sambucus nigra L.)
- Rowan (Sorbus aucuparia L.)
- Wild blueberry (Vaccinium myrtillus L.)
- Wild rose (Rosa canina L.)
2.2. Extraction Methods
2.3. Determination of Dry Matter Content (DM)
2.4. Determination of Total Phenolic Content (TPC)
2.5. Determination of Total Carotenoids and β-Carotene Content
2.6. DPPH Method
2.7. Determination of Phenolic Compounds Using HPLC-ESI-MS/MS Analysis
2.8. MALDI-TOF-MS Analysis
3. Results and Discussion
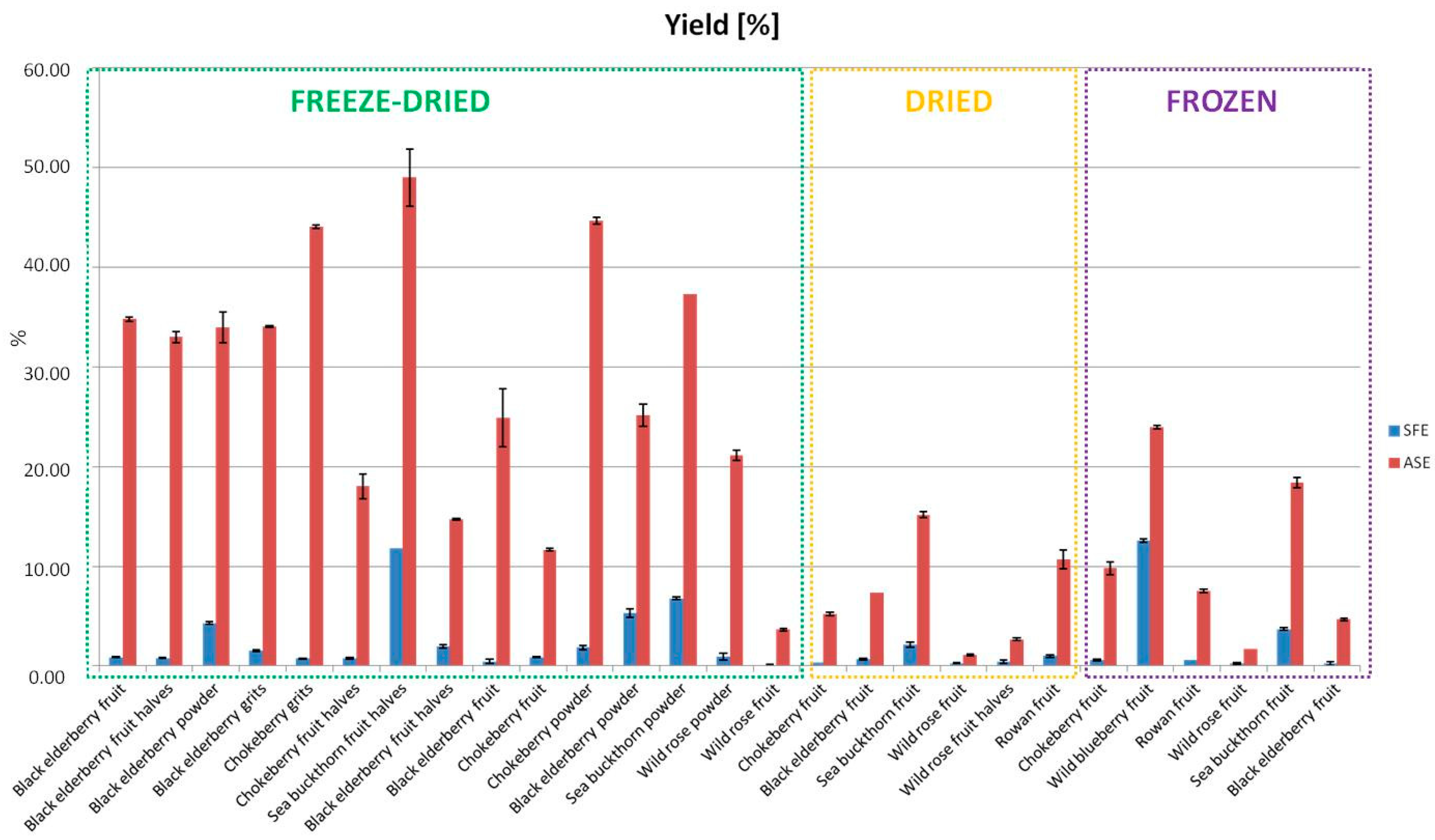
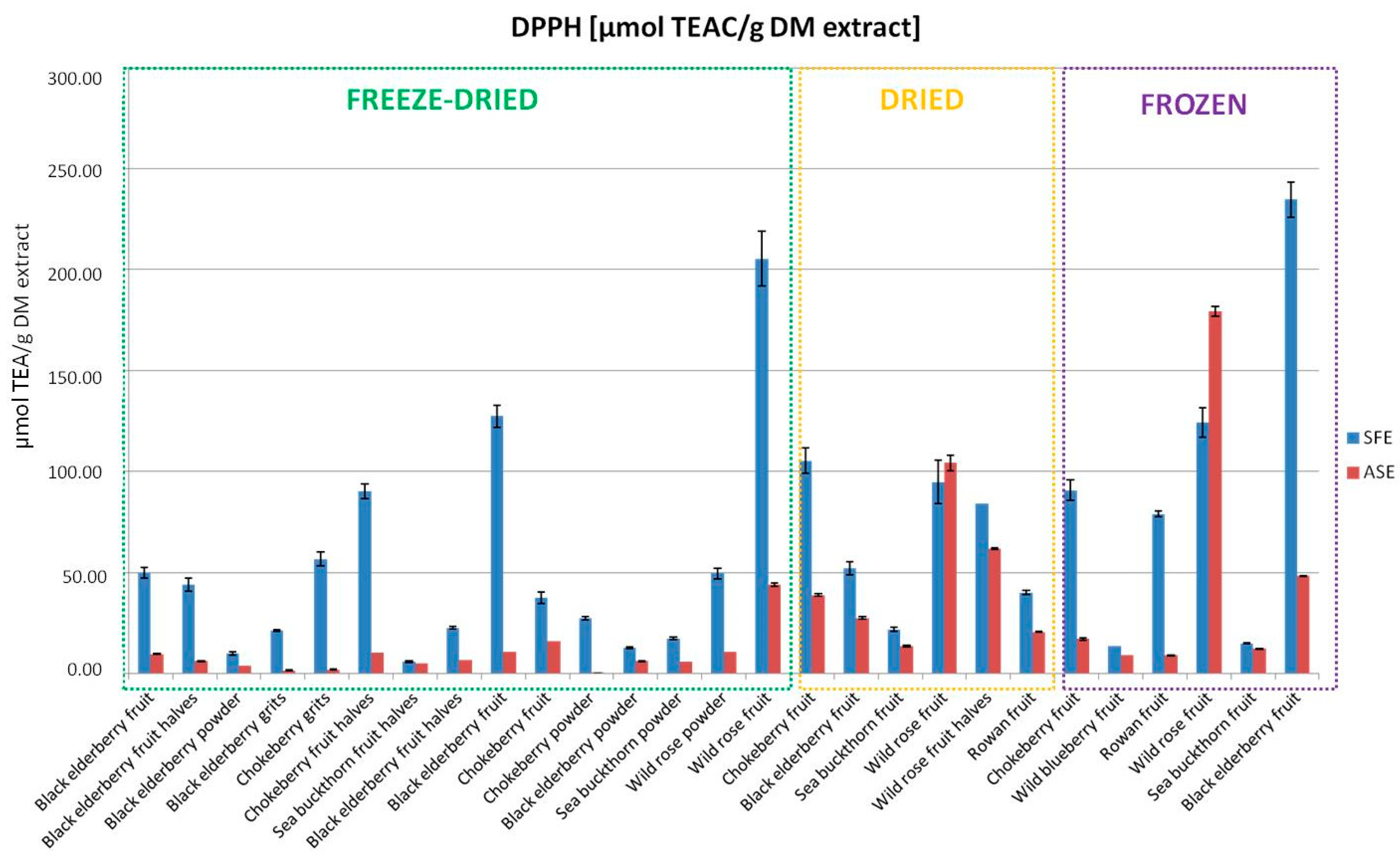
3.1. Comparison of Extraction Methods
- Yields of Extraction
- Total phenolic content
- Carotenoid Content
- Antioxidant Activity (DPPH)
3.2. Comparison of Different Methods of Processing Plant Material
- Freeze-drying
- Drying
- Frozen
3.3. Chemical Analysis of Obtained Extracts by HPLC-ESI-MS/MS
- Flavone
- Hesperidin
- Chlorogenic Acid
3.4. Selectivity of Extraction Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruxton, C.H.S.; Derbyshire, E.; Sievenpiper, I.J. Pure 100% fruit juices—More than just a source of free sugars? A review of the evidence of their effect on risk of cardiovascular disease, type 2 diabetes and obesity. Nutr. Bull. 2021, 46, 415–431. [Google Scholar] [CrossRef]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent Advances in Health Benefits of Bioactive Compounds from Food Wastes and By-Products: Biochemical Aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, M.; Pandey, P.; Martin, G.J.O.; Mishra, H.N.; Ashokkumar, M. Innovative Technologies for Extraction and Microencapsulation of Bioactives from Plant-Based Food Waste and their Applications in Functional Food Development. Foods 2021, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sanwal, N.; Bareen, M.A.; Barua, S.; Sharma, N.; Olatunji, O.J.; Nirmal, N.P.; Sahu, J.K. Trends in functional beverages: Functional ingredients, processing technologies, stability, health benefits, and consumer perspective. Food Res. Int. 2023, 170, 113046. [Google Scholar] [CrossRef]
- Herzyk, F.; Piłakowska-Pietras, D.; Korzeniowska, M. Supercritical Extraction Techniques for Obtaining Biologically Active Substances from a Variety of Plant Byproducts. Foods 2024, 13, 1713. [Google Scholar] [CrossRef]
- Krakowska, A.; Rafińska, K.; Walczak, J.; Buszewski, B. Enzyme-assisted optimized supercritical fluid extraction to improve Medicago sativa polyphenolics isolation. Ind. Crops Prod. 2018, 124, 931–940. [Google Scholar] [CrossRef]
- Rafińska, K.; Pomastowski, P.; Rudnicka, J.; Krakowska, A.; Maruśka, A.; Narkute, M.; Buszewski, B. Effect of solvent and extraction technique on composition and biological activity of Lepidium sativum extracts. Food Chem. 2019, 289, 16–25. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 11. [Google Scholar] [CrossRef]
- Alissa, E.M.; Ferns, G.A. Functional foods and nutraceuticals in the primary prevention of cardiovascular diseases. J. Nutr. Metab. 2012, 2012, 569486. [Google Scholar] [CrossRef]
- Klimczak, I.; Gliszczyńska-Świgło, A. The Influence of Wild Rose Extract on the Color of Cloudy Apple Juice. In Proceedings of the 10th International Conference on Research in Engineering, Science and Technology, Rome, Italy, 21–23 February 2020. [Google Scholar]
- Arvinte, O.M.; Senila, L.; Becze, A.; Amariei, S. Rowanberry—A Source of Bioactive Compounds and Their Biopharmaceutical Properties. Plants 2023, 12, 3225. [Google Scholar] [CrossRef]
- Dobros, N.; Zielińska, A.; Siudem, P.; Zawada, K.D.; Paradowska, K. Profile of Bioactive Components and Antioxidant Activity of Aronia melanocarpa Fruits at Various Stages of Their Growth, Using Chemometric Methods. Antioxidants 2024, 13, 462. [Google Scholar] [CrossRef] [PubMed]
- Mottaleb, M.A.; Sarker, S.D. Accelerated solvent extraction for natural products isolation. Methods Mol. Biol. 2012, 864, 75–87. [Google Scholar] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- PN-90/A-75101/12; Fruit and Vegetable Products. Determination of Total Carotenoids and β-Carotene. Polish Committee for Standardization: Warsaw, Poland, 1990.
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef]
- Patra, A.; Abdullah, S.; Pradhan, R.C. Review on the extraction of bioactive compounds and characterization of fruit industry by-products. Bioresour. Bioprocess. 2022, 9, 14. [Google Scholar] [CrossRef]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Vafaei, N.; Rempel, C.B.; Scanlon, M.G.; Jones, P.J.H.; Eskin, M.N.A. Application of Supercritical Fluid Extraction (SFE) of Tocopherols and Carotenoids (Hydrophobic Antioxidants) Compared to Non-SFE Methods. AppliedChem 2022, 2, 68–92. [Google Scholar] [CrossRef]
- Mattea, F.; Gómez Martín, Á.; Cocero, M. Carotenoid processing with supercritical fluids. J. Food Eng. 2009, 93, 255–265. [Google Scholar] [CrossRef]
- Paes, J.; Dotta, R.; Barbero, G.F.; Martinez, J. Extraction of phenolic compounds from blueberry (Vaccinium myrtillus L.) residues using supercritical CO2 and pressurized water. In Proceedings of the III Iberoamerican Conference on Supercritical Fluids, Cartagena de Indias, Colombia, 1–5 April 2013. [Google Scholar]
- Shahid, M.; Yusuf, M.; Mohammad, F. Plant phenolics: A review on modern extraction techniques. In Analytical and Processing Techniques; Govil, J.N., Pathak, M., Eds.; Recent Progress in Medicinal Plants; Studium Press: New Delhi, India, 2016; Volume 41, pp. 1–23. [Google Scholar]
- Yaqoob, M.; Aggarwal, P.; Purandare, N. Extraction of Phenolic Compounds by Supercritical Fluid Extraction. In Advanced Nanotechnology and Application of Supercritical Fluids; Inamuddin Asiri, A.M., Ed.; Springer: Cham, Switzerland, 2020; pp. 125–139. [Google Scholar]
- Krakowska-Sieprawska, A.; Kiełbasa, A.; Rafińska, K.; Ligor, M.; Buszewski, B. Modern Methods of Pre-Treatment of Plant Material for the Extraction of Bioactive Compounds. Molecules 2022, 27, 730. [Google Scholar] [CrossRef]
- Ratti, C. Hot air and freeze-drying of high-value foods; a review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Turan, B.; Tekin-Cakmak, Z.H.; Kayacan Çakmakoglu, S.; Karasu, S.; Kasapoglu, M.Z.; Avci, E. Effect of Different Drying Techniques on Total Bioactive Compounds and Individual Phenolic Composition in Goji Berries. Processes 2023, 11, 754. [Google Scholar] [CrossRef]
- Ghafoor, K.; Ahmed, I.A.M.; Doğu, S.; Uslu, N.; Fadimu, G.J.; Al Juhaimi, F.; Babiker, E.E.; Özcan, M.M. The effect of heating temperature on total phenolic content, antioxidant activity, and phenolic compounds of plum and mahaleb fruits. Int. J. Food Eng. 2019, 15, 11–12. [Google Scholar] [CrossRef]
- Pavlovska, V.; Dukovska, E.; Knights, A.V.; Jankuloska, V. Influence of temperature and time of storage on amount of vitamin C in strawberries. J. Hyg. Eng. Des. 2015, 11, 15–19. [Google Scholar]




| Producer | Plant Material | Form of Plant Material Processing |
|---|---|---|
| LIOGAM Foryś, Kot, Prześlak sp.J Kielce Poland | Black elderberry fruit | Freeze-dried |
| Black elderberry fruit halves | Freeze-dried | |
| Black elderberry powder | Freeze-dried | |
| Black elderberry grits | Freeze-dried | |
| WPPH “ELENA”, Kalisz, Poland | Chokeberry grits | Freeze-dried |
| Chokeberry fruit halves | Freeze-dried | |
| Sea buckthorn fruit halves | Freeze-dried | |
| Black elderberry fruit halves | Freeze-dried | |
| PPHU “FROSTER”, Kielce, Poland | Black elderberry fruit | Freeze-dried |
| Chokeberry fruit | Freeze-dried | |
| Chokeberry powder | Freeze-dried | |
| Black elderberry powder | Freeze-dried | |
| Sea buckthorn powder | Freeze-dried | |
| Wild rose powder | Freeze-dried | |
| Wild rose fruit | Freeze-dried | |
| PPHU “AWB” Alina Becia Łańcut, Poland | Chokeberry fruit | Dried |
| Black elderberry fruit | Dried | |
| Sea buckthorn fruit | Dried | |
| Wild rose fruit | Dried | |
| Wild rose fruit halves | Dried | |
| Rowan fruit | Dried | |
| FUNGOPOL, Sp. z o.o Sp.k. Brusy, Poland | Chokeberry fruit | Frozen |
| Wild blueberry fruit | Frozen | |
| Rowan fruit | Frozen | |
| Wild rose fruit | Frozen | |
| Sea buckthorn fruit | Frozen | |
| Black elderberry fruit | Frozen |
| Total Carotenoids Content [mg/g DM Extract] | |||
|---|---|---|---|
| Plant Material | Form of Plant Material Processing | SFE | ASE |
| Black elderberry fruit | Freeze-dried | 21.09 ± 1.24 | 0.21 ± 0.02 |
| Black elderberry fruit halves | Freeze-dried | 23.25 ± 1.29 | 0.32 ± 0.01 |
| Black elderberry powder | Freeze-dried | 11.01 ± 0.42 | 0.28 ± 0.02 |
| Black elderberry grits | Freeze-dried | 20.64 ± 0.96 | 0.51 ± 0.04 |
| Chokeberry grits | Freeze-dried | 15.74 ± 1.78 | 0.33 ± 0.03 |
| Chokeberry fruit halves | Freeze-dried | 12.06 ± 0.73 | 0.23 ± 0.03 |
| Sea buckthorn fruit halves | Freeze-dried | 6.56 ± 0.93 | 0.15 ± 0.02 |
| Black elderberry fruit halves | Freeze-dried | 12.19 ± 1.20 | 0.57 ± 0.05 |
| Black elderberry fruit | Freeze-dried | 44.45 ± 3.03 | 0.37 ± 0.08 |
| Chokeberry fruit | Freeze-dried | 10.78 ± 0.10 | 0.31 ± 0.01 |
| Chokeberry powder | Freeze-dried | 5.95 ± 0.53 | 0.27 ± 0.05 |
| Black elderberry powder | Freeze-dried | 9.05 ± 1.01 | 0.26 ± 0.01 |
| Sea buckthorn powder | Freeze-dried | 15.55 ± 1.38 | 0.16 ± 0.01 |
| Wild rose powder | Freeze-dried | 15.05 ± 0.86 | 0.32 ± 0.01 |
| Wild rose fruit | Freeze-dried | 62.39 ± 4.13 | 0.56 ± 0.02 |
| Chokeberry fruit | Dried | 25.27 ± 1.32 | 0.23 ± 0.01 |
| Black elderberry fruit | Dried | 11.11 ± 1.83 | 0.18 ± 0.01 |
| Sea buckthorn fruit | Dried | 3.71 ± 0.96 | 0.27 ± 0.03 |
| Wild rose fruit | Dried | 49.20 ± 3.32 | 1.08 ± 0.14 |
| Wild rose fruit halves | Dried | 33.88 ± 2.13 | 0.57 ± 0.04 |
| Rowan fruit | Dried | 7.19 ± 1.45 | 0.10 ± 0.01 |
| Chokeberry fruit | Frozen | 15.60 ± 0.24 | 0.21 ± 0.01 |
| Wild blueberry fruit | Frozen | 46.24 ± 3.06 | 0.34 ± 0.05 |
| Rowan fruit | Frozen | 15.47 ± 1.53 | 0.27 ± 0.01 |
| Wild rose fruit | Frozen | 34.23 ± 2.26 | 2.05 ± 0.15 |
| Sea buckthorn fruit | Frozen | 2.11 ± 0.07 | 0.25 ± 0.02 |
| Black elderberry fruit | Frozen | 64.76 ± 3.61 | 0.37 ± 0.01 |
| SFE | ASE | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant Material | Form of Plant Material Processing | Flavone [µg/100 g] | SD | Hesperidin [µg/100 g] | SD | Chlorogenic Acid [µg/100 g] | SD | Flavone [µg/100 g] | SD | Hesperidin [µg/100 g] | SD | Chlorogenic Acid [µg/100 g] | SD |
| Black elderberry fruit | Freeze-dried | 12.1 | 2.3 | 3.5 | 0.7 | 236.7 | 35.8 | 5.1 | 4.8 | 8.3 | 4.5 | 20.3 | 0.6 |
| Black elderberry fruit halves | Freeze-dried | 6.1 | 0.3 | 3.6 | 0.4 | 111.5 | 0.0 | 5 | 0.2 | 10.6 | 1.2 | 16.6 | 1.2 |
| Black elderberry powder | Freeze-dried | 2.4 | 0.3 | 1.2 | 0.1 | ND | ND | 3.8 | 0.3 | 6.3 | 0.2 | 5.5 | 0.2 |
| Black elderberry grits | Freeze-dried | 8.9 | 0.7 | 4.6 | 0.8 | 222.3 | 17.4 | 4.4 | 0.2 | 7.5 | 0.5 | 24.4 | 0.3 |
| Chokeberry grits | Freeze-dried | 7.8 | 0.3 | 7.4 | 0.7 | 249.0 | 35.4 | 3.2 | 0.0 | 13.6 | 0.1 | 33.1 | 0.4 |
| Chokeberry fruit halves | Freeze-dried | 22.5 | 2.1 | 5.1 | 0.1 | ND | ND | 6.2 | 0.1 | 29.9 | 0.3 | 41.4 | 0.0 |
| Sea buckthorn fruit halves | Freeze-dried | 23.6 | 1.1 | 11.0 | 0.2 | ND | ND | 4.1 | 0.8 | 9.3 | 0.2 | 0.1 | 0.0 |
| Black elderberry fruit halves | Freeze-dried | 13.2 | 3.8 | 3.4 | 0.1 | 202.4 | 1.0 | 8.8 | 0.2 | 16.7 | 0.4 | 48.7 | 0.0 |
| Black elderberry fruit | Freeze-dried | 32.7 | 1.1 | 3.4 | 0.5 | ND | ND | 8.4 | 0.3 | 14.3 | 0.7 | 15.6 | 0.0 |
| Chokeberry fruit | Freeze-dried | 25.1 | 0.3 | 6.6 | 0.1 | 98.0 | 4.0 | 14 | 0.7 | 18.9 | 1.6 | 84 | 0.8 |
| Chokeberry powder | Freeze-dried | 21.4 | 0.3 | 9.5 | 0.0 | 813.3 | 30.0 | 3.5 | 0.0 | 31.1 | 0.8 | 61.8 | 1.8 |
| Black elderberry powder | Freeze-dried | 15.7 | 0.0 | 1.9 | 0.0 | 63.8 | 7.6 | 3.6 | 0.3 | 77.1 | 0.0 | 11.6 | 0.8 |
| Sea buckthorn powder | Freeze-dried | 30.4 | 2.1 | 1 | 0.0 | 32.1 | 0.4 | 3.3 | 0.0 | 0.2 | 0.0 | 0.2 | 0.0 |
| Wild rose powder | Freeze-dried | 21.8 | 1.1 | ND | ND | 86.0 | 3.1 | 6.9 | 0.5 | 8.9 | 0.1 | 0.8 | 0.0 |
| Wild rose fruit | Freeze-dried | 275.6 | 32.6 | ND | ND | ND | ND | 33.6 | 0.2 | 14.4 | 0.6 | 6.2 | 0.1 |
| Chokeberry fruit | Dried | 61.5 | 2.7 | 17.8 | 0.6 | ND | ND | 22.8 | 0.0 | 3.4 | 0.6 | 4.0 | 0.0 |
| Black elderberry fruit | Dried | 50.0 | 1.4 | 43.3 | 1.7 | ND | ND | 14.7 | 0.2 | 2.7 | 0.0 | ND | ND |
| Sea buckthorn fruit | Dried | 22.7 | 1.8 | 26.9 | 2.0 | 0.6 | 0.0 | 7.1 | 0.1 | 1.3 | 0.0 | 1.5 | 0.0 |
| Wild rose fruit | Dried | 38.6 | 0.4 | 19.3 | 0.5 | ND | ND | 129.1 | 13.0 | 22.8 | 0.8 | ND | ND |
| Wild rose fruit halves | Dried | 40.9 | 1.6 | 67.3 | 0.8 | ND | ND | 61.1 | 3.2 | 26.0 | 0.5 | ND | ND |
| Rowan fruit | Dried | 29.2 | 0.9 | 11.0 | 0.3 | 10.2 | 4.7 | 19.4 | 2.2 | 21.3 | 0.0 | 1.5 | 0.1 |
| Chokeberry fruit | Frozen | 29.4 | 4.7 | ND | ND | 596.0 | 35.7 | 10.3 | 1.2 | 2.1 | 0.0 | 23.5 | 1.1 |
| Wild blueberry fruit | Frozen | 0.9 | 0.0 | 14.7 | 2.0 | 30.1 | 1.0 | 5.2 | 0.0 | 62.1 | 4.6 | 138.4 | 0.5 |
| Rowan fruit | Frozen | 35.3 | 3.0 | 14.3 | 0.6 | ND | ND | 13.2 | 1.1 | 25.4 | 1.1 | ND | ND |
| Wild rose fruit | Frozen | 32.3 | 3.0 | 21.3 | 2.6 | 6.6 | 0.1 | 117.1 | 9.8 | 199.7 | 15.1 | 149.8 | 5.3 |
| Sea buckthorn fruit | Frozen | 22.7 | 1.1 | 21.4 | 1.1 | ND | ND | 6.2 | 0.4 | 2.8 | 0.1 | 1.0 | 0.1 |
| Black elderberry fruit | Frozen | 81.1 | 7.9 | 65.1 | 5.2 | ND | ND | 24.6 | 1.5 | 12.4 | 1.4 | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krakowska-Sieprawska, A.; Walczak-Skierska, J.; Pomastowski, P.; Sobolewska, R.; Głogowski, J.; Bernat, C.; Rafińska, K. Advanced Extraction Techniques for Bioactive Compounds from Berry Fruits: Enhancing Functional Food Applications. Foods 2024, 13, 4115. https://doi.org/10.3390/foods13244115
Krakowska-Sieprawska A, Walczak-Skierska J, Pomastowski P, Sobolewska R, Głogowski J, Bernat C, Rafińska K. Advanced Extraction Techniques for Bioactive Compounds from Berry Fruits: Enhancing Functional Food Applications. Foods. 2024; 13(24):4115. https://doi.org/10.3390/foods13244115
Chicago/Turabian StyleKrakowska-Sieprawska, Aneta, Justyna Walczak-Skierska, Paweł Pomastowski, Róża Sobolewska, Jarosław Głogowski, Cezary Bernat, and Katarzyna Rafińska. 2024. "Advanced Extraction Techniques for Bioactive Compounds from Berry Fruits: Enhancing Functional Food Applications" Foods 13, no. 24: 4115. https://doi.org/10.3390/foods13244115
APA StyleKrakowska-Sieprawska, A., Walczak-Skierska, J., Pomastowski, P., Sobolewska, R., Głogowski, J., Bernat, C., & Rafińska, K. (2024). Advanced Extraction Techniques for Bioactive Compounds from Berry Fruits: Enhancing Functional Food Applications. Foods, 13(24), 4115. https://doi.org/10.3390/foods13244115









