Abstract
The seeds of Zanthoxylum bungeanum seeds, a high-quality vegetable protein source, encounter application limitations due to their high molecular weight and anti-nutritional factors. This study focused on optimizing the fermentation process by investigating key parameters such as inoculation amount, inoculation ratio, material-to-liquid ratio, fermentation temperature, and fermentation time. Both single-factor experiments and response surface methodology were used to determine the optimal conditions. The effects of fermentation on particle size, surface morphology (scanning electron microscopy), water holding capacity, oil holding capacity, solubility, and emulsification properties of Zanthoxylum bungeanum seed protein were analyzed. In addition, acute toxicity was investigated at doses of 1.5 g/kg, 3 g/kg, 6 g/kg, and 12 g/kg. The results showed that the optimal fermentation conditions were an inoculum concentration of 10%, a ratio of Bacillus subtilis to Lactobacillus plantarum of 1:1, a material-to-liquid ratio of 0.8:1, a temperature of 35 °C, and a fermentation period of 4 days. Under these optimized conditions, the soluble protein content reached 153.1 mg/g. After fermentation, the functional properties of Zanthoxylum bungeanum seed protein improved significantly: the water holding capacity increased by 89%, the oil holding capacity by 68%, while the emulsifying activity and stability indices improved by 6% and 17%, respectively. The macromolecular proteins in the seeds of Zanthoxylum bungeanum were effectively broken down into smaller fragments during fermentation, resulting in a more folded and porous surface structure. In acute toxicity tests, all mice treated with fermented Zanthoxum seed protein survived for more than 7 days after injection, and there were no significant differences in body weight, organ index, and hematological tests between groups, but FZBSP of 1.5 g/kg~12 g/kg caused varying degrees of steatosis and inflammatory damage in the heart and liver. In conclusion, this study confirms that follow-up pilot studies using 1.5 g/kg FZBSP have the potential for further development and utilization.
1. Introduction
Zanthoxylum bungeanum seeds (ZBS) are one of the most important by-products of Zanthoxylum bungeanum, with a protein content of about 14–20% (based on Zanthoxylum bungeanum cultivars), which can reach more than 60% after defatting [1]. It is rich in amino acids and contains a variety of essential amino acids for the human body. In addition, it is easily absorbed and has a high nutritional value [2,3]. According to statistics, China produces about 1.1 million tons of ZBS every year [4]. In the food industry, ZBS are currently used as raw materials for the development of a variety of foods, including condiments [5], flavored snacks, and special beverages [6]. In medicine and health, ZBS have demonstrated antioxidant and anti-tumor activities [7] and cholesterol-lowering effects [8,9]. In agriculture, ZBS have been shown to improve the growth performance of livestock and poultry and at the same time reduce feed costs [10]. In industry, it can be used to produce insecticides and building materials with anti-corrosive properties [11,12]. These products are usually used as a primary processed product to replace some conventional feeds in the field of livestock and poultry breeding, thereby reducing the production costs of the breeding industry [13]. However, research and development of ZBS as highly processed products in the food and feed sectors is still limited. Studies have found that probiotics are dominant in fermented feed. Their metabolites can reduce feed pH [14], decrease harmful microorganisms [15,16], inhibit mycotoxin production, eliminate allergens and anti-nutritional factors [17], and enhance the crude protein content of feed [18]. There are significant quality differences among different fermentation processes. Currently, enhancing the nutritional value of feed through probiotic fermentation and the synergy between bacteria and enzymes has become a hot topic in new feed industry research [19,20].
Solid-state fermentation (SSF) is a common method for feed production, which is characterized by its low cost and mild reaction conditions. During the fermentation process, SSF can effectively remove harmful elements in feed, convert macromolecular proteins into low molecular weight proteins, and release free amino acids [21]. Common fermentative probiotics include Bacillus, lactic acid bacteria, and yeasts [22]. Among these Bacillus subtilis (B. subtilis) strains and Lactobacillus plantarum (L. plantarum) strains are common fermentation strains [23,24], which are characterized by rapid growth, relatively low nutrient requirements, rapid secretion of large amounts of proteins and metabolites, and no production of toxins [25]. The fermentation of functional hemp milk by B. subtilis can enhance its nutritional value and functional properties [26]. Fermentation by L. plantarum can improve the solubility, adsorption capacity, and paste viscosity of millet starch [26]. Co-fermentation feed with B. subtilis and L. plantarum can significantly improve the intestinal flora and strengthen the immunity of the animals [27].
It has been reported that probiotics capable of bioconverting alkylamides and alkaloids were isolated and identified from Zanthoxylum seed meal [28]. This research aimed to compare and analyze the effects of co-fermentation using B. subtilis and L. plantarum on the nutrient composition of ZBS. The objective is to explore multiple applications for ZBS in food, feed, environmental protection, agriculture, and other fields, thereby enhancing its comprehensive application value and economic benefits across various sectors [10,29]. The aim of this study was to characterize the changes in the fermented ZBS protein and to evaluate its physicochemical properties, including water holding capacity, oil holding capacity, and particle size distribution. We also investigated the safety of the fermented ZBS protein by performing acute toxicity tests in mice. The cumulative results of these studies pave the way for various potential applications of fermented ZBS protein in food, feed, environmental protection, and agriculture, thereby improving its utility and economic viability in various fields.
2. Materials and Methods
2.1. Materials and Reagents
The number of effective viable B. subtilis was ≥1.0 × 1010 CFU/g, and the number of effective viable L. plantarum was ≥1.0 × 1010 CFU/g. These were screened and stored by the Lanzhou Institute of Husbandry and Pharmaceutical Chinese Academy of Agricultural Sciences. The patent of B. subtilis and L. plantarum is No. ATCC6633 and No. CICC20871. The ZBS were collected in the planting base of the professional agricultural cooperative of Zanthoxylum bungeanum planting in Liangshui Town, Wudu District, Gansu Province, and the test ZBS were the grafted Zanthoxylum bungeanum of local “Dahongpao” Zanthoxylum bungeanum. ZBS refers to the ripe Zanthoxum seeds extracted from the peel of Zanthoxum after harvesting [30]. These are crushed (25,000 2 min) using a universal high-speed crusher (China solar ball TYQ-1000g, Guangzhou, China), passed through an 80-mesh sieve, and then stored in a refrigerator at −20 °C. The Bradford assay kit was purchased from Beijing Boxsun Technology Co., Ltd. (Beijing, China). The 2,2-diphenyl-1-picrylhydrazyl radical scavenging capacity (DPPH RSC) assay kit, the ABTS radical scavenging capacity (ABTS RSC) assay kit, and the hydroxyl radical scavenging capacity (HRSC) assay kit, respectively, following the manufacturer’s instructions. All assay kits were procured from Solarbio (Beijing, China). MRS medium and NB medium were purchased from Guangdong Huankai Biological Technology Co., Ltd. (Guangzhou, China). The complex protease (S10155, 120 U/g) was obtained from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). All other reagents used were of analytical grade.
2.2. Experimental Animals
Healthy SPF mice (body weight, 20–22 g; age 4–6 weeks). All experimental animals used in this study were obtained from the Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences. The mice were adaptively fed for 5–7 days prior to the start of the experiment to minimize the effects of environmental stressors. The mice were then randomly assigned to different groups of six animals each, and the sawdust was replaced every 24 h. The mice had access to water AD libitum before and throughout the experiment. The animals were handled and euthanized according to internationally accepted guidelines. The research was licensed by the ethical approval of the Lanzhou Institute of Husbandry and Pharmaceutical Sciences of the Chinese Academy of Agricultural Science (Statement No. AEC-CAAS-20231124, Lanzhou, China).
2.3. Activation and Expansion of Strains
L. plantarum and B. subtilis stored at −80 °C were retrieved and dissolved in a water bath at 37 °C. L. plantarum was inoculated in MRS Broth, and B. subtilis in NB broth, then incubated at 37 °C with shaking at 120 rpm for 24 h to obtain the primary seed liquid. Using a 2% inoculum, the first-generation seed liquid was added to the corresponding broth medium for passage. The third-generation probiotics were then used as fermentation seed liquid for subsequent experiments.
2.4. Solid State Fermentation
Accurately weigh 100.0 g ZBS powder, 0.2 g ammonium sulfate, and 1 g glucose and add them into a 500 mL fermentation bottle to prepare SSF medium. A total of 8% of the amount of B. subtilis and L. plantarum inoculum liquids were added to the SSF medium, and the inoculum amount and ZBS were mixed thoroughly for fermentation.
2.5. Single Factor Experiment
According to the procedure described in 2.4, 100.0 g of Zanthoxylum seed powder was weighed and fermented at a fermentation temperature of 35 °C for 2 days, with an inoculation ratio of B. subtilis and L. plantarum of 1:1. The inoculations amount of B. subtilis and L. plantarum (6%, 8%, 10%, 12%, and 14%) were studied. Measured by substrate mass), inoculation proportion (1:1, 1:2, 2:1, 2:3, and 3:2), material–liquid ratio (0.6:1, 0.7:1, 0.8:1, 0.9:1, and 1:1), fermentation temperature (30 °C, 33 °C, 35 °C, 37 °C, and 40 °C), and fermentation time (0, 2, 4, 6, and 8 d). The specific experimental conditions of each factor are shown in Table 1. Only one variable was changed for each experiment, and the crude protein content at each factor level was used to determine the response surface test range. All tests were performed in triplicate according to LiW [31].

Table 1.
Single-factor test design.
2.6. Response Surface Optimization Experiment
Based on the results of the above single-factor experiments, response surface optimization tests were conducted. The inoculation amount (A), the material–liquid ratio (B), the fermentation temperature (C), and the fermentation time (D) were employed as independent variables, with the protein yield serving as the response value. Following the Box–Behnken experimental design, the fermented ZBS protein content was utilized as the response. The application’s response surface test can offer empirical relationships between the independent variables and the response variables based on parameter estimation [32], aiming to determine the optimal extraction process. The design of the response surface experimental factor level is presented in Table 2.

Table 2.
Factors and levels in the response surface analysis.
2.7. Protein Extraction from Fermented ZBS and ZBS
Based on the optimal fermentation conditions obtained by the above tests, the method of reference [33] was modified. Fermentation ZBS and ZBS (2 g), 5.43% complex protease (based on substrate mass), and phosphate buffer (2 g/50 mL) were placed in an Erlenmeyer flask and then extracted at 51.3 °C for 3 h. The supernatant was collected by centrifugation and precipitated with hydrochloric acid (1 mol/L). The precipitation was then centrifuged, collected, and dissolved with NaOH (1 mol/L), and the pH was adjusted to 7. The collected solution was freeze-dried to obtain fermented Zanthoxylum bungeanum seeds protein (FZBSP) or Zanthoxylum bungeanum seeds protein (ZBSP) or stored at −20 °C.
2.8. Structure Analysis
2.8.1. Particle Size Analysis
The ZBSP and FZBSP were dissolved in sterile water at a concentration of 1 mg/mL. This procedure was conducted following the method outlined by YILDIZ [34], with appropriate adjustments made to suit our specific needs. Subsequently, the samples were analyzed using a Mastersizer 3000 laser particle size analyzer to determine their particle size distribution.
2.8.2. Scanning Electron Microscope
Following the method described by DOU [35] and others, images of FZBSP and ZBSP structures were scanned. The FZBSP and ZBSP were placed on an aluminum plate coated with gold powder through sputtering. SEM images of the FZBSP and ZBSP were obtained using field emission SEM (JSM-840, JEOL, Tokyo, Japan).
2.9. Physicochemical Properties
2.9.1. Solubility
The method was adapted from MIR [36] and others with slight modifications. TFZBSP and ZBSP (100 mg) were stirred with distilled water (90 mL) at 25 °C for 1 h and then centrifuged (10,000 rpm, 30 min) to collect the supernatant. Mix the supernatant with 1 X Coomassie brilliant blue solution. Absorbance was measured at 595 nm using bovine serum albumin as the standard. The formula for calculating the solubility of a protein sample is as follows:
Type: S0 for solubility; Ns for the amount of protein in the supernatant; and Nt total is the amount of protein in the sample.
2.9.2. Water Holding Capacity
The methods reference Zhang [37] and others, with slight modifications. A 500 mg sample of FZBSP or ZBSP was weighed into a centrifuge tube, and 10 mL of distilled water was added to measure water holding capacity. The mixture was vortexed and shaken for 5 min, followed by centrifugation at 3050 g for 10 min. After centrifugation, the unbound water on the upper layer was poured off. The formula to calculate the water-holding capacity (WHC) of the protein is as follows:
where a is the weight of the centrifuge tube after dumping supernatant (g), b is the weight of the centrifuge tube and protein (g), and c is protein weight (g).
2.9.3. Oil Holding Capacity
Refer to the method described by Liu [38] et al., with a slight modification. A total of 500 mg of FZBSP or ZBSP samples were weighed into a 50 mL centrifuge tube, 10 g of soybean oil was added, vortexized for 30 s, placed at 37 °C for 24 h, and centrifuged at 4000 rpm for 20 min. Weighed after removing the upper oil, the protein oil holding capacity (OHC) is calculated as follows:
where a is the weight of the centrifuge tube after dumping supernatant (g), b is the weight of the centrifuge tube and protein (g), and c is protein weight (g).
2.9.4. Emulsion Activity and Emulsion Stability
The method was adapted from Chen [39] et al., with slight modifications. To 1 mg/mL of FZBSP or ZBSP, 8 mL of solution mixed with 2 mL of soybean oil, homogeneous under an automatic homogenate machine (16,000 r/min, 1 min). Homogeneous in 0 min and 10 min after the completion of emulsion and 0.1% from the bottom of the drain, 100 mL + SDS solution diluted 50 times, 500 nm absorbance value, after blending with 0.1% SDS solution as the blank. The emulsifying activity index (EAI, m2/g) and emulsification stability (ESI, min) computation formulas are as follows:
Type: A0, 0 min absorbance value; A10, 10 min absorbance value; N, diluted times, 50; C, protein concentration, g/mL; and , oil phase volume fraction, 0.2.
2.10. Antioxidant Properties
To compare the antioxidant properties of FZBSP and ZBSP components, refer to Sittiruk’s [40] method, modified slightly. DPPH RSC, ABTS RSC, and HRSC were measured according to the instructions, and OD values were measured at 515 nm, 405 nm, and 536 nm, respectively. In the determination process, FZBSP was compared with ZBSP to prepare a 5 mg/mL solution, and all tests were performed 3 times.
2.11. Assessment of Acute Toxicity
Referring to Raskosha’s [41] method, sixty C57BL/6 mice were randomly divided into 5 groups with 12 mice in each group and were fed adaptively for 1 week in the control group and FZBSP group (1.5, 3, 6, 12 g/kg), respectively. The mice were fasted for 12 h before administration, given water and freedom, and the other groups were given the same dose of FZBSP except the blank group, which was given normal saline. Then, the appearance, signs, and adverse reactions of mice were observed for 7 consecutive days. At the end of the 7th day, blood was collected through the orbital vein, then the neck was removed and blood samples were collected for hematological and blood biochemical analysis. Then, the mice were surgically dissected, the lesions of the major internal organs were visually examined, the number of tissue organs and organ index were recorded, and the following organs were histologically analyzed: heart, liver, spleen, lung, and kidney.
3. Statistical Analysis
SPSS 26.0 was used to process the data. The response surface design was performed using Design Expert 11.0.4. Chart mapping was completed using GraphPad Prism 8. All experimental results are presented as “mean ± standard deviation”, with significant differences considered at p < 0.05 and no significant difference at p > 0.05.
4. Result and Analysis
4.1. Optimization of Fermentation Conditions
4.1.1. Single-Factor Analysis
To investigate the effects of different conditions on fermented ZBS, five sub-experiments were established for a single-factor experiment to determine the optimal conditions based on soluble protein content in the fermentation broth. As shown in Figure 1, the maximum soluble protein content in the fermentation broth was achieved at a strain inoculation amount of 10%. Similar trends were observed in the experiments in which the fermentation temperature, fermentation time, and material–liquid ratio were varied, and the maximum soluble protein content in the fermentation broth was obtained at an inoculation ratio of 1:1, a material–liquid ratio of 0.8:1, a fermentation temperature of 35 °C, and a fermentation time of 4 d.
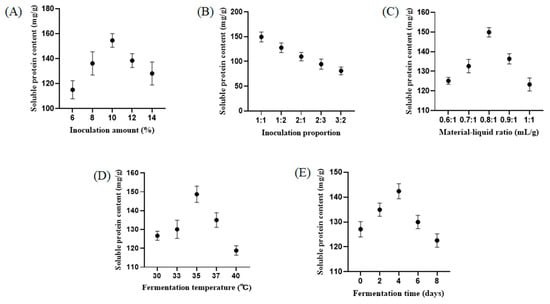
Figure 1.
Inoculation amount (A), inoculation proportion (B), material–liquid ratio (C), fermentation temperature (D), and fermentation time (E) on the yield of soluble protein content of FZBS. All tests were performed 3 times. Each data point represents the mean ± SD of triplicates.
4.1.2. Box–Behnken Design Results
The Box–Behnken design was implemented using Design-Expert 11.0.4., as shown in Table 2, Table 3 and Table 4. Fermented ZBS soluble protein was considered as the response value, with strain inoculation amount, material–liquid ratio, fermentation temperature, and fermentation time as the four factors for the experimental design. Quadratic polynomial regression analysis was performed on the experimental data, resulting in the equation

Table 3.
Experimental data of the four-factor, three-level Box–Behnken design for study response.

Table 4.
ANOVA with the response face quadratic model.
Y represents the soluble protein contents, while A, B, C, and D correspond to bacterial inoculum, material–liquid ratio, fermentation temperature, and fermentation time. This model’s coefficient of determination R2 is 0.9576, and the adjusted R2 (Adj. R2) is 0.9152. The model’s p-value was found to be less than 0.0001, indicating a strong correlation between the predicted and actual values. The results showed that B, D, AB, AC, CD, A2, B2, C2, and D2 have remarkable effects on the extraction yield of fermented ZBS soluble protein (p < 0.01). Furthermore, the p-values for AD, BC, and BD interactions were greater than 0.05, suggesting no significant interaction between these pairs of factors. This analysis demonstrates the effectiveness of the model in predicting and evaluating the influence of each factor on protein content.
As shown in Figure 2, the response surface curve and contour map were generated to analyze the impact of inoculum amount, material–liquid ratio, fermentation temperature, and fermentation time on soluble protein content. The optimal fermentation conditions predicted by the model were 11.5% inoculum volume, a 0.78:1 (g/mL) ratio of solid to liquid, a 35.13 °C fermentation temperature, and a 3.75 d fermentation time. According to the experimental conditions, the optimal fermentation conditions were determined as the inoculated amount of the strain of 10%, the solid–liquid ratio of 0.8:1 (g/mL), the fermentation temperature of 35 °C, and the fermentation time of 4 d. The soluble protein content could reach 153.1 (mg/g), which is in close agreement with the predicted value and shows that the model can well reflect the actual situation of optimizing the mixed fermentation process of B. subtilis and L. plantarum on ZBS.
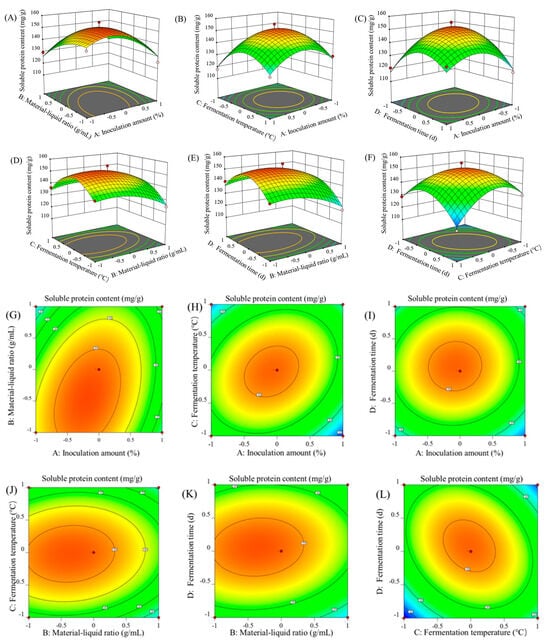
Figure 2.
Response surface plots (A–F) and the corresponding contour plots (G–L) showing inoculation amount and material–liquid ratio (A,G); inoculation amount and fermentation temperature (B,H); inoculation amount and fermentation time (C,I); material–liquid ratio and fermentation temperature (D,J); material–liquid ratio and fermentation time (E,K); and fermentation temperature and fermentation time (F,L).
4.2. The Structural Characteristics of ZBSP and FZBSP
4.2.1. Particle Size Distribution
As shown in Figure 3, the alterations in particle size and distribution of FZBSP and ZBSP were illustrated. The particle size of ZBSP was about 275.22 μm, while the particle number of FZBSP was significantly reduced to about 216.33 μm. After fermentation, the particle size of FZBSP was reduced by 27.2% compared with that of ZBSP.
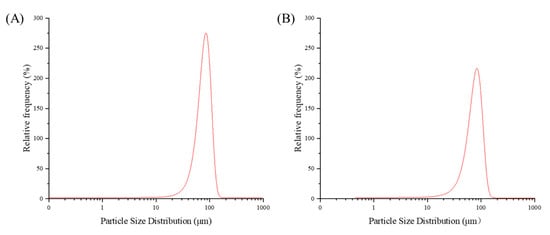
Figure 3.
The particle size distribution of ZBSP (A) and FZBSP (B). All tests were performed 3 times.
4.2.2. Scanning Electron Microscopy
As Figure 4 shows, ZBSP exhibited a flat layered structure on the surface under the electron microscope, with the larger particles having smooth edges. In contrast, FZBSP exhibited a more fragmented and irregular layered structure, and the surface of the particles became wrinkled and porous due to the co-fermentation of B. subtilis and L. plantarum. After fermentation, the protein structure was looser, indicating that the mixed fermentation of B. subtilis and L. plantarum could completely decompose the protein material, which was more conducive to digestion and absorption and also improved the nutritional properties of ZBSP.
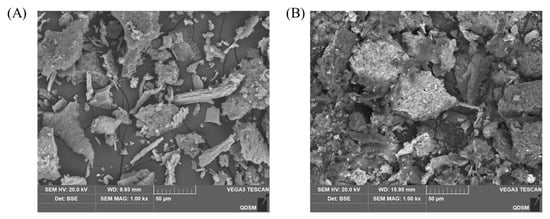
Figure 4.
Scanning Electron Microscopy of ZBSP (A) and FZBSP (B). All tests were performed 3 times.
4.3. The Physicochemical Properties of ZBSP and FZBSP
As shown in Figure 5, the solubility of ZBSP before and after fermentation was low at pH 3, which is close to the isoelectric point, but showed an upward trend with the increase of pH from 5 to 9. At pH 5 and pH 7, the solubility of peppercorn seed protein after fermentation was 45.34% and 21.72% higher than before fermentation, respectively. At pH 9, the solubility of ZBSP and FZBSP was 52.28% and 60.85%, respectively. According to Table 5, the WHC and OHC of FZBSP were 3.87 ± 0.11 (g/g) and 6.16 ± 0.19 (g/g), respectively, and those of ZBSP were 1.99 ± 0.10 (g/g) and 3.07 ± 0.01 (g/g), respectively. EAI and ESI of FZBSP were 16.22 ± 0.17 (m2/g) and 54.61 ± 2.41 (min), respectively, and those of ZBSP were 15.26 ± 0.29 (m2/g) and 46.82 ± 1.64 (m2/g), respectively. After fermentation, WHC, OHC, EAI, and ESI of FZBSP were significantly increased (p < 0.01).
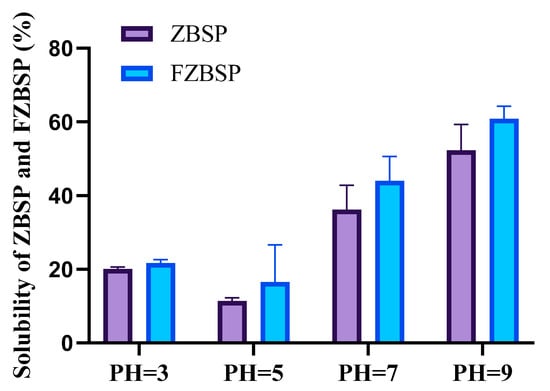
Figure 5.
When pH = 3, pH = 5, pH = 7, pH = 39, the solubility of ZBSP and FZBSP. Results are expressed as mean ± SD (n = 3). ZBSP means Zanthoxylum bungeanum seeds protein, and FZBSP means fermented Zanthoxylum bungeanum seeds protein.

Table 5.
Physicochemical properties of ZBSP and FZBSP.
4.4. Antioxidant Activity
According to Table 6, the antioxidant activity of ZBSP was significantly improved after fermentation with probiotics. The DPPH RSC, ABTS RSC, and HRSC in FZBSP were 63.85%, 95.42%, and 65.39%, respectively, which were higher than those of ZBSP.

Table 6.
Antioxidant activity of FZBSP and ZBSP (%).
4.5. Acute Toxicity Test
4.5.1. Effect on General Condition
After intragastric administration, all mice in each dose group survived and showed normal behavior with food and water intake, good mental status, no signs of irritability or excessive startle response, and other abnormalities.
4.5.2. Effect on Body Weight
As shown in Figure 6, the mice in the 1.5 g/kg, 3 g/kg, 6 g/kg, and 12 g/kg dose groups showed no significant changes in body weight after 7 days of feeding compared to the blank group (p > 0.05), but a steady increase in body weight was observed throughout the study period. The organ indices of the 1.5 g/kg, 3 g/kg, 6 g/kg, and 12 g/kg dose groups were compared with those of the CON group. As shown in Table 7, there was no significant difference in the organ index between the groups (p > 0.05).
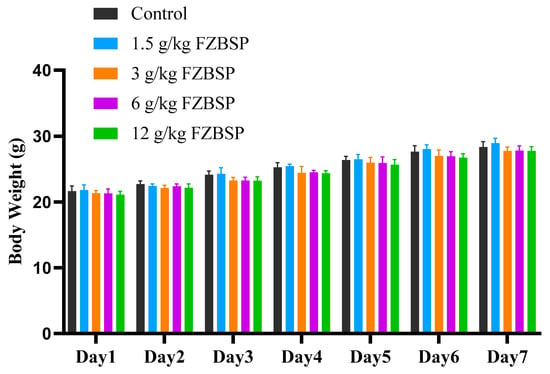
Figure 6.
Impact on body weight of mice in FZBSP toxicity. Six mice were randomly selected in each group.

Table 7.
Effect of FZBSP on organ index in mice.
4.5.3. Routine Blood Tests
As shown in Table 8, the blood parameters (WBC, LYM, MON, NEU, RBC, HGB, and MCV) in the 1.5 g/kg, 3 g/kg, 6 g/kg, and 12 g/kg dose groups compared with the CON group all showed abnormal changes attributable to drug administration. No significant difference (p > 0.05) was observed.

Table 8.
Hematological data of the toxicity study of the FZBSP.
4.5.4. Serum Biochemical Tests
According to Table 9, compared with the CON group, no significant changes were observed in ALP, ALT, BUN, GLU, and TP levels when fed FZBSP at 1.5 g/kg, 3 g/kg, 6 g/kg, and 12 g/kg compared with the CON group, indicating no obvious toxic effects on liver function and renal function.

Table 9.
Effect on biochemical indicators of the FZBSP.
4.5.5. Pathological Section
Figure 7 shows the pathological staining sections of heart, liver, spleen, lung, and kidney tissues of mice from the CON group and the 1.5 g/kg~12 g/kg FZBSP dose groups. The study shows that the structural integrity of spleen, kidney, and lung tissue sections in the control group and all drug-treated groups is well preserved with clear structures; no significant inflammatory cell infiltration was observed. Consistent with the findings in the CON group. Steatosis and inflammatory infiltration were observed in the heart and liver in the FZBSP dose groups compared to the CON group.
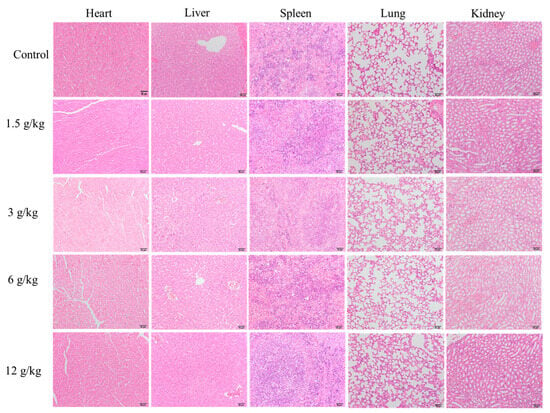
Figure 7.
Impact on histopathological examination of mice in FZBSP toxicity.
5. Discussion
In recent years, probiotic fermentation has become increasingly important as a means of supporting the extraction of active plant ingredients [42]. At the same time, solid-state fermentation is being used in many industries to improve the nutritional quality and functional properties of agricultural by-products [43]. It has been found that the co-fermentation of Lactobacillus plantarum and Bacillus subtilis can effectively increase the solubility of TCA protein content in rice bran [44], promote direct absorption by the gastrointestinal tract, and induce antioxidant and immunoregulatory functions [45]. Fermented soybean meal can increase the crude protein content and lactate content of soybean meal by 50.1% and 94.9%, respectively [46]. The physical and chemical properties of roasted soybean flour can be changed [47]. In this study, the effects of inoculation amount, inoculation ratio, solid–liquid ratio, fermentation temperature, and fermentation time on FZBSP during mixed fermentation of Bacillus subtilis and Lactobacillus plantarum were evaluated. The results showed that as the inoculation amount, fermentation temperature, and time were increased, the FZBSP content first increased and then decreased. The optimum fermentation conditions for the extraction of FZBSP from ZBS were an inoculation amount of 10%, an inoculation ratio of 1:1, a solid–liquid ratio of 0.8:1, a fermentation temperature of 35 °C, and a fermentation time of 4 d. The technological fermentation technology obtained in this study provides a theoretical basis for the further development and utilization of ZBS.
The combination of scanning electron microscopy and particle size analysis showed that FZBSP has a rough surface and consists of small particles. This observation can be related to the protein-degrading enzymes produced during the fermentation process [48]. Furthermore, the reduction in particle size of FZBSP suggests that FZBSP undergoes more thorough hydrolysis, resulting in the formation of protein structures with smaller molecules. Compared to ZBSP, the protein properties of FZBSP are significantly improved. In particular, the water holding capacity is increased by 89%, the oil holding capacity by 68%, and the EAI and ESI are increased by 6% and 17%, respectively. These improvements may be attributed to increased protein charge and intermolecular electrostatic repulsion, increased protein surface area, reduced size, and inhibition of protein aggregation facilitated by fermentation [49]. Protein solubility is considered the most practical indicator of the functional properties of proteins. Adequate solubility is a fundamental prerequisite for effective protein expression and emulsification. Consequently, improving protein solubility can improve the overall performance of protein emulsification [50,51,52]. Oil-holding capacity indicates the ability of a protein to bind with free fatty acids. A higher oil-holding capacity indicates a stronger ability of the protein to bind oil, thus minimizing oil loss during production and improving food flavor [53]. Due to their amphiphilic properties, proteins can act as emulsifiers and increase the stability of emulsions by inhibiting processes such as emulsification, coalescence, precipitation, and flocculation of droplets [54]. Antioxidant defense serves as a universal mechanism in the body [55,56], capable of repairing and preventing oxidative damage [57]. During the fermentation process, compounds present in ZBSP undergo biotransformation, resulting in the production of compounds with varied antioxidant properties [58]. Following fermentation, there is an increase in DPPH RSC clearance by 8%, ABTS RSC clearance by 7%, and HRSC clearance by 22.5%. These increases indicate that fermentation enhances antioxidant performance, consistent with the findings of Wu et al. in their study [59].
In this study, no deaths or abnormal reactions were observed in mice within 7 days after a high dose (12 g/kg) of FZBSP. According to the World Health Organization standards for acute toxicity of exogenous chemicals, the FZBSP used in this study is considered safe, non-toxic, and well tolerated. Blood plays a crucial role in maintaining the homeostasis of the animal’s internal environment, so relevant serum indicators can objectively reflect the health status of the body [60,61]. Compared with the control group, there were no significant changes in the serum levels of ALP, ALT, BUN, GLU, and TP in each dose group of FZBSP. However, FZBSP caused steatosis and inflammatory injury of the heart and liver and had no adverse effects on the spleen, kidney function, or lungs. In addition, we found no statistically significant changes in the mice’s organ weight or organ-to-body ratio. Therefore, combined with the results of blood analysis and pathological sections, the FZBSP (1.5 g/kg) dose is recommended for subsequent experimental studies.
6. Conclusions
The optimal fermentation conditions for ZBS using a mixed culture of B. subtilis and L. plantarum were beneficial for increasing the FZBSP content. Compared to ZBSP, particle size analysis and SEM images show that FZBSP has a more porous structure on the surface, with protein particles degrading on the surface. In terms of protein properties, FZBSP exhibited increased solubility, water binding, oil binding, and emulsifiability, favoring digestion and absorption. The acute toxicity test of FZBSP on mice showed that the growth performance, blood analysis, and pathological sections of the spleen, lung, and kidney of mice had no significant changes at the dose of FZBSP, but the heart and liver showed varying degrees of steatosis and inflammatory damage. Considering the changes in protein structure and properties and the results of the acute toxicity test in mice, we can summarize that the nutritional quality of ZBSP after solid-phase fermentation with B. subtilis and L. plantarum is optimized, its nutritional value is enhanced, and a dose of 1.5 g/kg is recommended for subsequent relevant experimental studies. This study paves the way for a re-evaluation of FZBSP as a potential functional food ingredient.
Author Contributions
Conceptualization, G.X. and C.L.; formal analysis, H.E.; funding acquisition, J.L. (Jianxi Li); investigation, B.M., X.T., X.W. (Xiaoya Wang) and H.C.; methodology, L.W.; project administration, J.Z.; software, R.M. and M.Z.; supervision, X.W. (Xuezhi Wang); validation, K.Z. and J.L. (Jiahui Liu); writing—original draft, X.G. All authors have read and agreed to the published version of the manuscript.
Funding
Supported by the National Key Research and Development Program (2022YFD1602204) and the Chinese Veterinary Medicine and Clinical Innovation Team of the Chinese Academy of Agricultural Sciences (CAAS-ASITP-2015-LIHPS-06).
Institutional Review Board Statement
The animal study protocol was approved by the Lanzhou Institute of Husbandry and Pharmaceutical sciences of CAAS (protocol code 2023-31 and 2023-11-24).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hou, X.; Li, S.; Luo, Q.; Shen, G.; Wu, H.; Li, M.; Liu, X.; Chen, A.; Ye, M.; Zhang, Z. Discovery and Identification of Antimicrobial Peptides in Sichuan Pepper (Zanthoxylum bungeanum Maxim) Seeds by Peptidomics and Bioinformatics. Appl. Microbiol. Biotechnol. 2019, 103, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Sun, X. Optimization of preparation technology and composition analysis of seed protein of Zanthoxylum liyingqiu. China Condiments 2024, 49, 101–108. [Google Scholar]
- Tang, W.; Xie, Q.; Guan, J.; Jin, S.; Zhao, Y. Phytochemical Profiles and Biological Activity Evaluation of Zanthoxylum bungeanum Maxim Seed against Asthma in Murine Models. J. Ethnopharmacol. 2014, 152, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, J.; Pan, X. Analysis of volatile components of Li Yanmei Yuqing Zanthoxylum essential oil and its effect on differentiation and apoptosis of HEL cells in erythroleukemia. J. Food Saf. Qual. Insp. 2024, 15, 317–326. [Google Scholar]
- Liu, Y.; Li, J.; Wang, G. Comparative analysis of flavor and comprehensive quality of seed oil of Sun Guohao and Zanthoxylum zanthoxylum oil. Food Sci. 2021, 42, 195–201. [Google Scholar]
- Yang, X.; Zhang, X.; Xu, H.; Wang, X.; Liu, Y.; Xie, N.; Guo, X. Research progress on nutrients, extraction technology and application of Zanthoxylum hanxiao seed. Chin. Oils Fats 2024, 1–12. [Google Scholar] [CrossRef]
- Wang, W.; Pang, W.; Yan, S.; Zheng, X.; Han, Q.; Yao, Y.; Jin, L.; Zhang, C. Zanthoxylum bungeanum Seed Oil Inhibits Tumorigenesis of Human Melanoma A375 by Regulating CDC25A/CyclinB1/CDK1 Signaling Pathways in Vitro and in Vivo. Front. Pharmacol. 2023, 14, 1165584. [Google Scholar] [CrossRef]
- Li, D.; Yang, H.; Li, Q.; Ma, K.; Wang, H.; Wang, C.; Li, T.; Ma, Y. Prickly Ash Seeds Improve Immunity of Hu Sheep by Changing the Diversity and Structure of Gut Microbiota. Front. Microbiol. 2023, 14, 1273714. [Google Scholar] [CrossRef]
- Hou, J.; Wang, J.; Meng, J.; Zhang, X.; Niu, Y.; Gao, J.; Bai, Y.; Zhou, J. Zanthoxylum bungeanum Seed Oil Attenuates LPS-Induced BEAS-2B Cell Activation and Inflammation by Inhibiting the TLR4/MyD88/NF-kB Signaling Pathway. Evid. Based Complement. Altern. Med. 2021, 2021, 2073296. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Zheng, A.; Wang, Z.; Wei, X.; Li, S.; Purba, A.; Chen, Z.; Liu, G. Dietary Replacement of Soybean Meal with Zanthoxylum bungeanum Seed Meal on Growth Performance, Blood Parameters, and Nutrient Utilization in Broiler Chickens. Animals 2024, 14, 1420. [Google Scholar] [CrossRef]
- Duan, Y. Development and utilization of Zanthoxylum seed oil in. West. Leather 2020, 42, 84. [Google Scholar]
- Zhang, J.; Jiang, L. Acid-Catalyzed Esterification of Zanthoxylum bungeanum Seed Oil with High Free Fatty Acids for Biodiesel Production. Bioresour. Technol. 2008, 99, 8995–8998. [Google Scholar] [CrossRef] [PubMed]
- Song, C.H.; Oh, S.M.; Lee, S.; Choi, Y.; Kim, J.D.; Jang, A.; Kim, J. The Ratio of Dietary N-3 Polyunsaturated Fatty Acids Influences the Fat Composition and Lipogenic Enzyme Activity in Adipose Tissue of Growing Pigs. Food Sci. Anim. Resour. 2020, 40, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, Z.; Wu, G.; Xu, F.; Zhang, J.; Luo, X.; Ma, Y.; Pang, H.; Duan, Y.; Chen, J.; et al. Effects of Probiotic-Fermented Feed on the Growth Profile, Immune Functions, and Intestinal Microbiota of Bamei Piglets. Animals 2024, 14, 647. [Google Scholar] [CrossRef]
- Zhu, X.; Tao, L.; Liu, H.; Yang, G. Effects of Fermented Feed on Growth Performance, Immune Organ Indices, Serum Biochemical Parameters, Cecal Odorous Compound Production, and the Microbiota Community in Broilers. Poult. Sci. 2023, 102, 102629. [Google Scholar] [CrossRef]
- Xu, B.; Zhu, L.; Fu, J.; Li, Z.; Wang, Y.; Jin, M. Overall Assessment of Fermented Feed for Pigs: A Series of Meta-Analyses. J. Anim. Sci. 2019, 97, 4810–4821. [Google Scholar] [CrossRef]
- Yang, A.; Zuo, L.; Cheng, Y.; Wu, Z.; Li, X.; Tong, P.; Chen, H. Degradation of Major Allergens and Allergenicity Reduction of Soybean Meal through Solid-State Fermentation with Microorganisms. Food Funct. 2018, 9, 1899–1909. [Google Scholar] [CrossRef]
- Huang, J.; Dai, Y.; Zhang, Y.; Liu, G.; Peng, F.; Xie, M.; Xiong, T. Dynamics of Bacterial Community, Metabolites Profile and Physicochemical Characteristics during Solid-State Fermentation of Soybean Meal and Corn Mixed Substrates Inoculated with Bacillus Pumilus and Limosilactobacillus Fermentum. J. Sci. Food Agric. 2023, 103, 5588–5599. [Google Scholar] [CrossRef]
- Huang, B.; Jia, H.; Han, X.; Gou, J.; Huang, C.; Wang, J.; Wei, J.; Wang, J.; Zhang, C. Effects of Biocontrol Bacillus and Fermentation Bacteria Additions on the Microbial Community, Functions and Antibiotic Resistance Genes of Prickly Ash Seed Oil Meal-Biochar Compost. Bioresour. Technol. 2021, 340, 125668. [Google Scholar] [CrossRef]
- Mukherjee, R.; Chakraborty, R.; Dutta, A. Role of Fermentation in Improving Nutritional Quality of Soybean Meal—A Review. Asian-Australas. J. Anim. Sci. 2016, 29, 1523–1529. [Google Scholar] [CrossRef]
- Dai, C.; Hou, Y.; Xu, H.; Huang, L.; Dabbour, M.; Mintah, B.K.; He, R.; Ma, H. Effect of Solid-State Fermentation by Three Different Bacillus Species on Composition and Protein Structure of Soybean Meal. J. Sci. Food Agric. 2022, 102, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Yasar, S.; Tosun, R.; Sonmez, Z. Fungal Fermentation Inducing Improved Nutritional Qualities Associated with Altered Secondary Protein Structure of Soybean Meal Determined by FTIR Spectroscopy. Measurement 2020, 161, 107895. [Google Scholar] [CrossRef]
- Sungatullina, A.; Petrova, T.; Nikitina, E. Investigation on Fermented Milk Quality after the Addition of Flaxseed Mucilage and the Use of Lactobacillus delbrueckii subsp. bulgaricus and Lactiplantibacillus plantarum AG9. Front. Biosci. Elite 2024, 16, 11. [Google Scholar] [CrossRef]
- Prakash, T.J.; Kharnaior, P.; Pariyar, P. Whole Genome Sequencing of the Poly-γ-Glutamic Acid-Producing Novel Bacillus Subtilis Tamang Strain, Isolated from Spontaneously Fermented Kinema. Food Res. Int. 2024, 190, 114655. [Google Scholar] [CrossRef]
- Miao, X.; Niu, H.; Sun, M.; Li, D.; Hua, M.; Wang, J.; Su, Y. Structural Characterization and Properties of Modified Soybean Meal Protein via Solid-State Fermentation by Bacillus subtilis. Molecules 2023, 28, 8015. [Google Scholar] [CrossRef]
- Karabulut, G.; Nemzer, B.V.; Feng, H. γ-Aminobutyric Acid (GABA)-Enriched Hemp Milk by Solid-State Co-Fermentation and Germination Bioprocesses. Plant Foods Hum. Nutr. 2024, 79, 322–329. [Google Scholar] [CrossRef]
- Chen, J.; Mou, L.; Wang, L.; Wu, G.; Dai, X.; Chen, Q.; Zhang, J.; Luo, X.; Xu, F.; Zhang, M.; et al. Mixed Bacillus subtilis and Lactiplantibacillus plantarum-Fermented Feed Improves Gut Microbiota and Immunity of Bamei Piglet. Front. Microbiol. 2024, 15, 1442373. [Google Scholar] [CrossRef]
- Li, C.; Kong, Q.; Mou, H.; Jiang, Y.; Du, Y.; Zhang, F. Biotransformation of Alkylamides and Alkaloids by Lactic Acid Bacteria Strains Isolated from Zanthoxylum Bungeanum Meal. Bioresour. Technol. 2021, 330, 124944. [Google Scholar] [CrossRef]
- Diao, W.R.; Hu, Q.P.; Feng, S.S.; Li, W.Q.; Xu, J.G. Chemical Composition and Antibacterial Activity of the Essential Oil from Green Huajiao (Zanthoxylum schinifolium) against Selected Foodborne Pathogens. J. Agric. Food Chem. 2013, 61, 6044–6049. [Google Scholar] [CrossRef]
- Chruma, J.J.; Cullen, D.J.; Bowman, L.; Toy, P.H. Polyunsaturated Fatty Acid Amides from the Zanthoxylum Genus-from Culinary Curiosities to Probes for Chemical Biology. Nat. Prod. Rep. 2018, 35, 54–74. [Google Scholar] [CrossRef]
- Li, W.; Wang, T. Effect of Solid-State Fermentation with Bacillus Subtilis Lwo on the Proteolysis and the Antioxidative Properties of Chickpeas. Int. J. Food Microbiol. 2021, 338, 108988. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zheng, H.; Lu, J.; Li, S.; Anchen, W.; Huang, M.; Fu, Y.; Zhuang, L. Extraction, Identification, Mechanism of Action, and Application of Hu Zhang (Polygonum cuspidatum) Extracts against Non-O1/O139 Vibrio cholerae in Prawn Storage. LWT Food Sci. Technol. 2023, 185, 115135. [Google Scholar] [CrossRef]
- Jiang, X.; Gao, F.; Ma, Y.; Huo, N.; Guo, Y.; Yu, Y. Protein from Tiger Nut Meal Extracted by Deep Eutectic Solvent and Alkali-Soluble Acid Precipitation: A Comparative Study on Structure, Function, and Nutrition. Food Chem. 2024, 452, 139608. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, G. Effect of pH-Shifting Method on Solubility and Emulsifying Properties of Soy Protein Concentrate. Harran Tarim. Ve Gida Bilim. Derg. Harran J. Agric. Food Sci. 2019, 23, 159–166. [Google Scholar] [CrossRef]
- Dou, Z.; Chen, C.; Fu, X. The Effect of Ultrasound Irradiation on the Physicochemical Properties and α-Glucosidase Inhibitory Effect of Blackberry Fruit Polysaccharide. Food Hydrocoll. 2019, 96, 568–576. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Effect of pH and Holding Time on the Characteristics of Protein Isolates from Chenopodium Seeds and Study of Their Amino Acid Profile and Scoring. Food Chem. 2019, 272, 165–173. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Sun, X.; Chen, F.; Wu, Q. Effects of AOT Reverse Micelle Extraction on Structure and Emulsifying Properties of Soybean Protein. J. Am. Oil Chem. Soc. 2021, 98, 923–932. [Google Scholar] [CrossRef]
- Liu, X.; Suo, K.; Wang, P.; Li, X.; Hao, L.; Zhu, J.; Yi, J.; Kang, Q.; Huang, J.; Lu, J. Modification of Wheat Bran Insoluble and Soluble Dietary Fibers with Snail Enzyme. Food Sci. Hum. Wellness 2021, 10, 356–361. [Google Scholar] [CrossRef]
- Chen, W.; Wang, W.; Ma, X.; Lv, R.; Balaso, W.R.; Ding, T.; Ye, X.; Liu, D. Effect of pH-Shifting Treatment on Structural and Functional Properties of Whey Protein Isolate and Its Interaction with (-)-Epigallocatechin-3-Gallate. Food Chem. 2019, 274, 234–241. [Google Scholar] [CrossRef]
- Roytrakul, S.; Charoenlappanit, S.; Kittisenachai, S.; Siangpro, N.; Sichaem, J.; Chuakrut, S.; Sarin, S.; Jutakanoke, R. Antimicrobial and Antioxidant Activities of Peptide Derived from Turmeric Plant (Curcuma loznga L.). PLoS ONE 2024, 19, e0314482. [Google Scholar] [CrossRef]
- Oksana, R.; Anatoly, K.; Anastasia, E.; Lyudmila, B.; Yana, P.; Natalia, S.; Irina, B.; Elena, R.; Ludmila, K. Evaluation of Safety and Biomedical Potential of Water-Soluble Oat Lignin Avena sativa L. Int. J. Biol. Macromol. 2024, 283, 137609. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.B.; Baiseitova, A.; Zahoor, M.; Ahmad, I.; Ikram, M.; Bakhsh, A.; Shah, M.A.; Ali, I.; Idress, M.; Ullah, R.; et al. Probiotic Significance of Lactobacillus Strains: A Comprehensive Review on Health Impacts, Research Gaps, and Future Prospects. Gut Microbes 2024, 16, 2431643. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Jiang, Z.; Wang, C.; Zhang, Y.; Gong, T.; Wang, F.; Jin, M.; Wang, Y.; Lu, Z. Co-Fermented Defatted Rice Bran Alters Gut Microbiota and Improves Growth Performance, Antioxidant Capacity, Immune Status and Intestinal Permeability of Finishing Pigs. Anim. Nutr. 2022, 11, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Jiang, Z.; Wang, C.; Xu, B.; Lu, Z.; Wang, F.; Zong, X.; Jin, M.; Wang, Y. Dynamics of Defatted Rice Bran in Physicochemical Characteristics, Microbiota and Metabolic Functions during Two-Stage Co-Fermentation. Int. J. Food Microbiol. 2022, 362, 109489. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.R.; Wong, E.A.; Webb, K.J. Board-Invited Review: Peptide Absorption and Utilization: Implications for Animal Nutrition and Health. J. Anim. Sci. 2008, 86, 2135–2155. [Google Scholar] [CrossRef]
- Effect of Bacillus Subtilis and Lactobacillus plantarum on Solid-State Fermentation of Soybean Meal—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/37140367/ (accessed on 27 November 2024).
- Park, M.-J.; General, T.; Lee, S.-P. Physicochemical Properties of Roasted Soybean Flour Bioconverted by Solid-State Fermentation Using Bacillus subtilis and Lactobacillus plantarum. Prev. Nutr. Food Sci. 2012, 17, 36–45. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Zhao, Z.; Yu, W.; Zheng, L.; Li, L.; Gu, W.; Xu, H.; Wei, B.; Yan, X. Nutritional Quality Improvement of Soybean Meal by Bacillus velezensis and Lactobacillus plantarum during Two-Stage Solid-State Fermentation. AMB Express 2021, 11, 23. [Google Scholar] [CrossRef]
- Li, M.; Yang, R.; Feng, X.; Fan, X.; Liu, Y.; Xu, X.; Zhou, G.; Zhu, B.; Ullah, N.; Chen, L. Effects of Low-Frequency and High-Intensity Ultrasonic Treatment Combined with Curdlan Gels on the Thermal Gelling Properties and Structural Properties of Soy Protein Isolate. Food Hydrocoll. 2022, 127, 107506. [Google Scholar] [CrossRef]
- Gouvea, L.D.P.; Caldeira, R.; Azevedo, T.D.L.; Galdeano, M.C.; Felberg, I.; Lima, J.R.; Mellinger, C.G. Physical and Techno-Functional Properties of a Common Bean Protein Concentrate Compared to Commercial Legume Ingredients for the Plant-Based Market. Food Hydrocoll. 2023, 137, 108351. [Google Scholar] [CrossRef]
- Arzeni, C.; Martinez, K.; Zema, P.; Arias, A.; Perez, O.E.; Pilosof, A.M.R. Comparative Study of High Intensity Ultrasound Effects on Food Proteins Functionality. J. Food Eng. 2012, 108, 463–472. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse Proteins: Processing, Characterization, Functional Properties and Applications in Food and Feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Tarahi, M.; Abdolalizadeh, L.; Hedayati, S. Mung Bean Protein Isolate: Extraction, Structure, Physicochemical Properties, Modifications, and Food Applications. Food Chem. 2024, 444, 138626. [Google Scholar] [CrossRef] [PubMed]
- Brishti, F.H.; Chay, S.Y.; Muhammad, K.; Ismail-Fitry, M.R.; Zarei, M.; Karthikeyan, S.; Saari, N. Effects of Drying Techniques on the Physicochemical, Functional, Thermal, Structural and Rheological Properties of Mung Bean (Vigna radiata) Protein Isolate Powder. Food Res. Int. 2020, 138, 109783. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Li, X.; Zhang, Z.; Chen, A.; Li, S.; Shen, G.; Li, M.; Liu, X.; Yin, X.; Cheng, L.; et al. Extraction of Zanthoxylum Seed Protein and Identification of Its Simulated Digestion Products. LWT Food Sci. Technol. 2022, 161. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding Oxidants and Antioxidants: Classical Team with New Players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef]
- Cömert, E.D.; Gökmen, V. Antioxidants Bound to an Insoluble Food Matrix: Their Analysis, Regeneration Behavior, and Physiological Importance. Compr. Rev. Food Sci. Food Saf. 2017, 16, 382–399. [Google Scholar] [CrossRef]
- Liang, S.; Yu, S.; Qin, Y.; Yu, H.; Zhao, Z.; Xu, Y.; Zhang, G.; Li, C.; Liu, L.; Peng, D.; et al. Blue Honeysuckle Fermentation with Lacticaseibacillus rhamnosus L08 Improves Its Biological Activity, Sensory and Flavor Characteristics, and Storage Stability. Food Chem. X 2024, 23, 101659. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Tao, Y.; Li, D.; Han, Y.; Show, P.L.; Wen, G.; Zhou, J. Fermentation of Blueberry and Blackberry Juices Using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of Probiotics, Metabolism of Phenolics, Antioxidant Capacity in Vitro and Sensory Evaluation. Food Chem. 2021, 348, 129083. [Google Scholar] [CrossRef]
- Li, C.; Zhao, P.; Shao, Q.; Chen, W.; Huang, S.; Wang, X.; Zhang, C.; He, L. Effects of Dietary Glycyrrhiza polysaccharide on Growth Performance, Blood Parameters and Immunity in Weaned Piglets. J. Anim. Physiol. Anim. Nutr. 2023, 107, 136–146. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Zheng, N.; Guo, L.; Song, X.; Zhao, S.; Wang, J. Biological System Responses of Dairy Cows to Aflatoxin B1 Exposure Revealed with Metabolomic Changes in Multiple Biofluids. Toxins 2019, 11, 77. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).