Quality Differences in Frozen Mackerel According to Thawing Method: Potential Classification via Hyperspectral Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Thawing Conditions
2.2. Total Viable Cell Counts
2.3. pH and Titratable Acidity
- VNaOH: volume of NaOH used (mL);
- NNaOH: normality of the NaOH solution (0.1 N);
- 0.090: the milliequivalent factor for lactic acid;
- Vsample: volume of the sample (mL).
2.4. Total Volatile Basic Nitrogen (TVB-N)
- a: volume (mL) of 0.01 N NaOH used for titrating the sample;
- b: volume (mL) of 0.01 N NaOH used for titrating the blank;
- f: factor of 0.01 N NaOH;
- 0.14: the milliequivalent of nitrogen;
- d: dilution factor;
- w: weight of the sample (g).
2.5. Texture Profile Analysis
2.6. Color Analysis
2.7. Hyperspectral Image Acquisition
2.8. Model Development and Performance Evaluation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Microbiological and Physicochemical Changes
3.2. Texture Profile Analysis by Thawing Method
3.3. Investigation of RGB Color Change
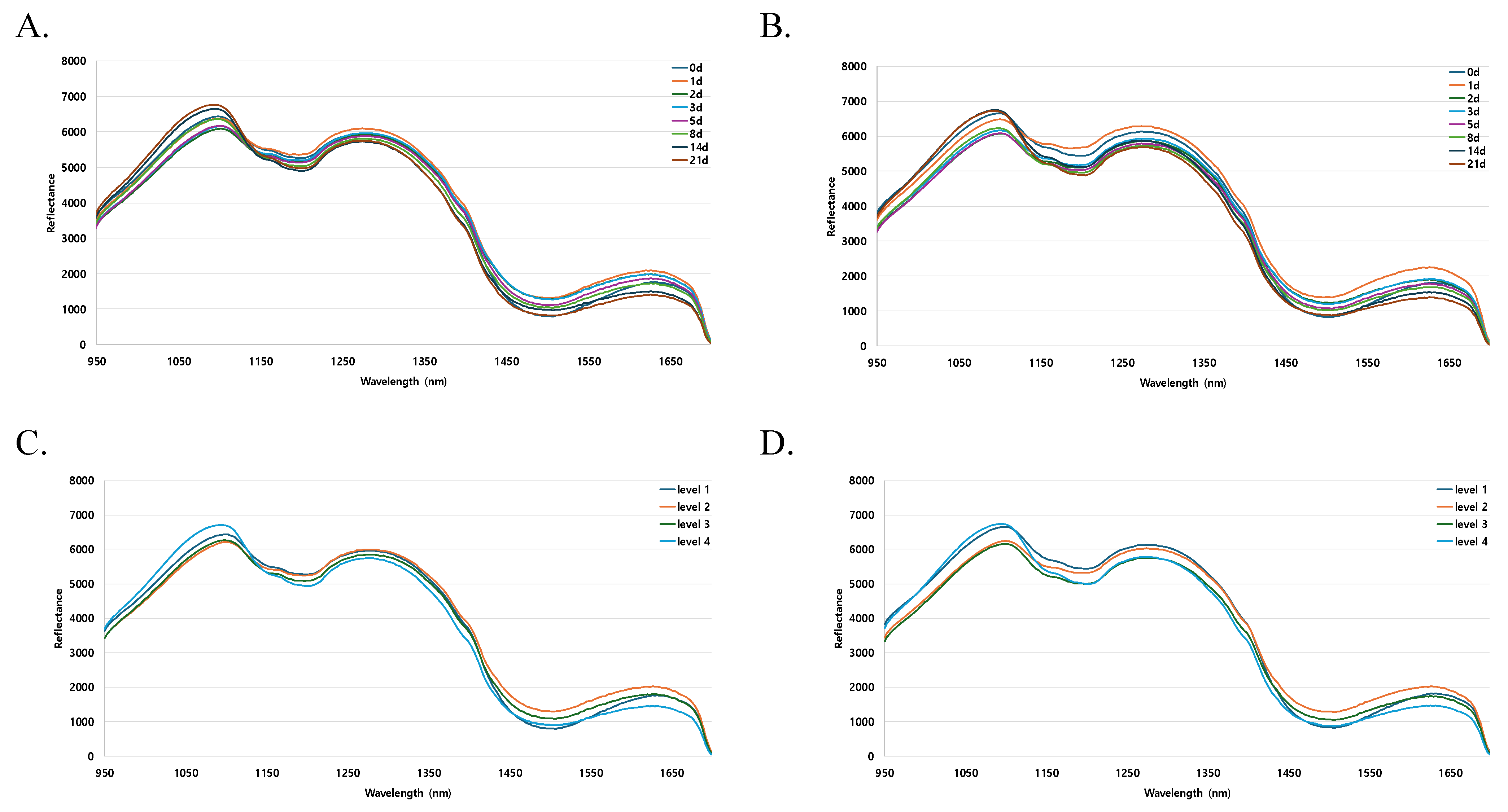
3.4. Characteristics of Hyperspectral Reflectance Spectra
3.5. Spectrum Classification According to the Thawing Method
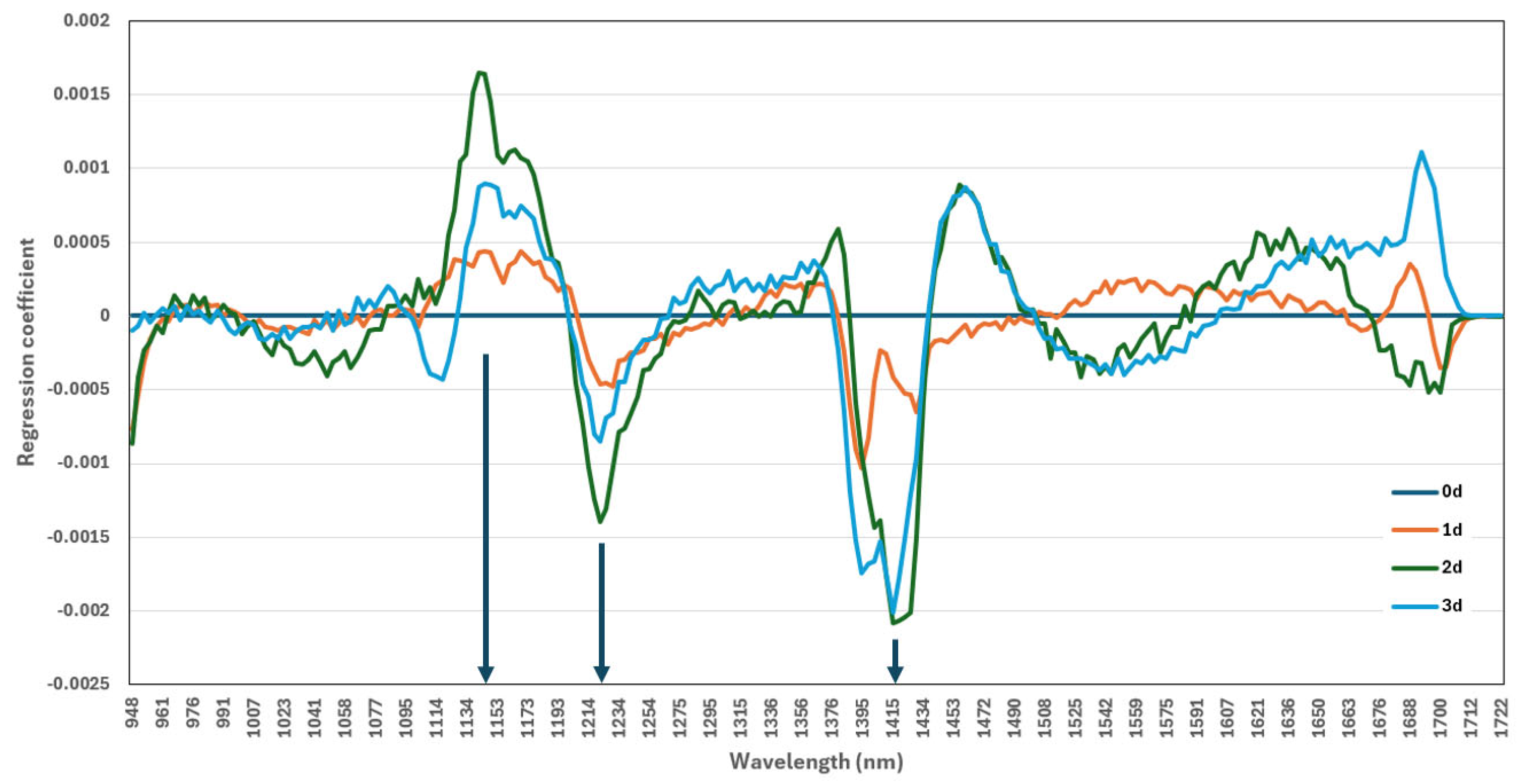
3.6. Identification of Key Wavelengths Using PLS-DA Beta Coefficient Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amit, S.K.; Uddin, M.M.; Rahman, R.; Islam, S.M.R.; Khan, M.S. A review on mechanisms and commercial aspects of food preservation and processing. Agric. Food Secur. 2017, 6, 51. [Google Scholar] [CrossRef]
- Floros, J.D.; Newsome, R.; Fisher, W.; Barbosa-Cánovas, G.V.; Chen, H.; Dunne, C.P.; German, J.B.; Hall, R.L.; Heldman, D.R.; Ziegler, G.R.; et al. Feeding the world today and tomorrow: The importance of food science and technology. Compr. Rev. Food Sci. Food Saf. 2010, 9, 572–599. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, G.G.; Evans, J.A. The use of supercooling for fresh foods: A review. J. Food Eng. 2015, 148, 74–79. [Google Scholar] [CrossRef]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Kaale, L.D.; Eikevik, T.M.; Rustad, T.; Kolsaker, K. Superchilling of food: A review. J. Food Eng. 2011, 107, 141–146. [Google Scholar] [CrossRef]
- Zhang, M.; Li, F.; Diao, X.; Kong, B.; Xia, X. Moisture migration, microstructure damage and protein structure changes in porcine longissimus muscle as influenced by multiple freeze-thaw cycles. Meat Sci. 2017, 133, 10–18. [Google Scholar] [CrossRef]
- Liu, D.; Liang, L.; Xia, W.; Regenstein, J.M.; Zhou, P. Biochemical and physical changes of grass carp (Ctenopharyngodon idella) fillets stored at −3 and 0 °C. Food Chem. 2013, 140, 105–114. [Google Scholar] [CrossRef]
- Sampels, S. The effects of processing technologies and preparation on the final quality of fish products. Trends Food Sci. Technol. 2015, 44, 131–146. [Google Scholar] [CrossRef]
- Sone, I.; Olsen, R.L.; Sivertsen, A.H.; Eilertsen, G.; Heia, K. Classification of fresh Atlantic salmon (Salmo salar L.) fillets stored under different atmospheres by hyperspectral imaging. J. Food Eng. 2019, 241, 97–105. [Google Scholar] [CrossRef]
- Nazemroaya, S.; Sahari, M.A.; Rezaei, M. Identification of fatty acid in mackerel (Scomberomorus commersoni) and shark (Carcharhinus dussumieri) fillets and their changes during six months of frozen storage at −18 °C. J. Agric. Sci. Technol. 2011, 13, 553–566. [Google Scholar]
- Roiha, I.S.; Jónsson, Á.; Backi, C.J.; Lunestad, B.T.; Karlsdóttir, M.G. A comparative study of quality and safety of Atlantic cod (Gadus morhua) fillets during cold storage, as affected by different thawing methods of pre-rigor frozen fish. J. Sci. Food Agric. 2018, 98, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, M.; Adhikari, B.; Sun, J. Effects of different thawing methods on the quality of protein-rich foods. Int. J. Food Sci. Technol. 2016, 51, 2379–2389. [Google Scholar]
- Zhou, G.; Xie, J. Recent advances in thawing methods for frozen food. Food Control 2021, 121, 107642. [Google Scholar]
- Cheng, J.H.; Sun, D.W.; Han, Z.; Zeng, X.A. Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Z.; Sun, D.W. Application of hyperspectral imaging in food safety inspection and control: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 1039–1058. [Google Scholar] [CrossRef]
- Yu, X.; Wang, J.; Wen, H.; Zhang, J.; Ding, Y. A comprehensive review of computer vision technology for meat freshness evaluation: From traditional methods to deep learning. Trends Food Sci. Technol. 2024, 143, 103594. [Google Scholar]
- Xie, A.; Sun, D.W.; Xu, Z.; Zhu, Z. Rapid detection of frozen pork quality without thawing by Vis-NIR hyperspectral imaging technique. Talanta 2015, 139, 208–215. [Google Scholar] [CrossRef]
- Pu, H.; Sun, D.W.; Ma, J.; Cheng, J.H. Recent advances in intelligent hyperspectral methods for meat quality evaluation: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 296–322. [Google Scholar]
- Chen, Y.R.; Chao, K.; Kim, M.S. Machine vision technology for agricultural applications. Comput. Electron. Agric. 2012, 36, 173–191. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 22nd ed.; Association of Official Analytical Chemist: Rockville, MD, USA, 2020. [Google Scholar]
- Conway, E.J.; Byrne, A. An absorption apparatus for the micro-determination of certain volatile substances: The micro-determination of ammonia. Biochem. J. 1933, 27, 419–429. [Google Scholar]
- AOAC. Official Method 971.14. Trimethylamine Nitrogen in Seafood. In Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- Chaijan, M.; Benjakul, S.; Visessanguan, W.; Faustman, C. Changes of pigments and color in sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) muscle during iced storage. Food Chem. 2005, 93, 607–617. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Martinez, I.; Sánchez-Valencia, J.; Careche, M. Estimation of freezing storage time and quality changes in hake (Merluccius merluccius L.) by low field NMR. Food Chem. 2010, 135, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.H.; Sun, D.W. Rapid quantification analysis and visualization of Escherichia coli loads in grass crap fish flesh by hyperspectral imaging method. Food Bioprocess Tech. 2015, 8, 951–959. [Google Scholar] [CrossRef]
- Cho, J.S.; Choi, B.; Lim, J.H.; Choi, J.H.; Yun, D.Y.; Park, S.K.; Lee, G.; Park, K.-J.; Lee, J. Determination of freshness of mackerel (Scomber japonicus) using shortwave infrared hyperspectral imaging. Foods 2023, 12, 2305. [Google Scholar] [CrossRef]
- Ballabio, D.; Consonni, V. Classification tools in chemistry. Part 1: Linear models. PLS-DA. Anal. Methods 2013, 5, 3790–3798. [Google Scholar] [CrossRef]
- Martens, H.; Næs, T. Multivariate Calibration; John Wiley Sons: Hoboken, NJ, USA, 1992. [Google Scholar]
- Huss, H.H. Quality and Quality Changes in Fresh Fish; FAO Fisheries Technical Paper No. 348; Food and Agriculture Organization of the United Nations: Rome, Italy, 1995. [Google Scholar]
- Ocaño-Higuera, V.M.; Marquez-Ríos, E.; Canizales-Dávila, M.; Castillo-Yáñez, F.J.; Pacheco-Aguilar, R.; Lugo-Sánchez, M.E.; García-Orozco, K.D.; Graciano-Verdugo, A.Z. Postmortem changes in cazon fish muscle stored on ice. Food Chem. 2011, 126, 1603–1608. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, M.J.; Kim, G.U.; Choi, H.D.; Park, S.Y.; Kim, M.J.; Kim, K.-B.-W.-R.; Kim, Y.-M.; Nam, T.-K.; Hong, C.W.; et al. Assessment of quality changes in mackerel Scomber Japonicus during refrigerated storage: Development of a freshness indicator. Korean J. Fish. Aquat. Sci. 2016, 49, 731–736. [Google Scholar] [CrossRef]
- Boonsumrej, S.; Chaiwanichsiri, S.; Tantratian, S.; Suzuki, T.; Takai, R. Effects of freezing and thawing on the quality changes of tiger shrimp (Penaeus monodon) frozen by air-blast and cryogenic freezing. J. Food Eng. 2007, 80, 292–299. [Google Scholar] [CrossRef]
- Ersoy, B.; Aksan, E.; Özeren, A. The effect of thawing methods on the quality of eels (Anguilla anguilla). Food Chem. 2008, 111, 377–380. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Z.; Zhou, L.; Niu, Y.; Yuan, C.; Tian, Y.; Takaki, K. Ultrastructural and biochemical changes of sarcoplasmic reticulum in Spotted Mackerel (Scomber australasicus Cuvier, 1832) muscle during cold storage at 5 °C. Int. J. Food Sci. Technol. 2023, 58, 3810–3818. [Google Scholar] [CrossRef]
- Careche, M.; Herrero, A.M.; Rodríguez-Casado, A.; Del Mazo, M.L.; Carmona, P. Structural changes of hake (Merluccius merluccius L.) fillets: Effects of freezing and frozen storage. J. Agric. Food Chem. 1999, 47, 952–959. [Google Scholar] [CrossRef] [PubMed]
- HSIEH, Y.L.; REGENSTEIN, J.M. Texture changes of frozen stored cod and ocean perch minces. J. Food Sci. 1989, 54, 824–826. [Google Scholar] [CrossRef]
- Sharifian, S.; Alizadeh, E.; Mortazavi, M.S.; Shahriari Moghadam, M. Effects of refrigerated storage on the microstructure and quality of Grouper (Epinephelus coioides) fillets. J. Food Sci. Technol. 2014, 51, 929–935. [Google Scholar] [CrossRef]
- Suárez, M.D.; Martínez, T.F.; Saez, M.I.; Alferez, B.; García-Gallego, M. Changes in muscle properties during postmortem storage of farmed sea bream (Sparus aurata). J. Food Process Eng. 2011, 34, 922–946. [Google Scholar] [CrossRef]
- Genç, İ.Y. Prediction of storage time in different seafood based on color values with artificial neural network modeling. J. Food Sci. Technol. 2022, 59, 2501–2509. [Google Scholar] [CrossRef]
- Khoshnoudi-Nia, S.; Moosavi-Nasab, M. Prediction of various freshness indicators in fish fillets by one multispectral imaging system. Sci. Rep. 2019, 9, 14704. [Google Scholar] [CrossRef]
- Choudhry, P. High-throughput method for automated colony and cell counting by digital image analysis based on edge detection. PLoS ONE 2016, 11, e0148469. [Google Scholar] [CrossRef]
- Xiong, Z.; Sun, D.W.; Pu, H.; Xie, A.; Han, Z.; Luo, M. Non-destructive prediction of thiobarbituricacid reactive substances (TBARS) value for freshness evaluation of chicken meat using hyperspectral imaging. Food Chem. 2015, 179, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Riccioli, C.; Sun, D.W. Efficient integration of particle analysis in hyperspectral imaging for rapid assessment of oxidative degradation in salmon fillet. J. Food Eng. 2016, 169, 259–271. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, L.; Tian, X.; Zheng, M.; Su, Z.; Zhong, N. Rapid non-destructive evaluation of texture properties changes in crispy tilapia during crispiness using hyperspectral imaging and data fusion. Food Control 2024, 162, 110446. [Google Scholar] [CrossRef]
- Li, P.; Tang, S.; Chen, S.; Tian, X.; Zhong, N. Hyperspectral imaging combined with convolutional neural network for accurately detecting adulteration in Atlantic salmon. Food Control 2023, 147, 109573. [Google Scholar] [CrossRef]
- Wilson, R.H.; Nadeau, K.P.; Jaworski, F.B.; Tromberg, B.J.; Durkin, A.J. Review of short-wave infrared spectroscopy and imaging methods for biological tissue characterization. J. Biomed. Opt. 2015, 20, 030901. [Google Scholar] [CrossRef] [PubMed]
- Arena, F.; La Cava, F.; Faletto, D.; Roberto, M.; Crivellin, F.; Stummo, F.; Adamo, A.; Boccalon, M.; Napolitano, R.; Reitano, E.; et al. Short-wave infrared fluorescence imaging of near-infrared dyes with robust end-tail emission using a small-animal imaging device. PNAS Nexus 2023, 2, pgad250. [Google Scholar] [CrossRef] [PubMed]
- Rossel, R.V.; Behrens, T.; Ben-Dor, E.; Brown, D.J.; Demattê, J.A.M.; Shepherd, K.D.; Shi, Z.; Stenberg, B.; Stevens, A.; Ji, W.; et al. A global spectral library to characterize the world’s soil. Earth-Sci. Rev. 2016, 155, 198–230. [Google Scholar] [CrossRef]
- Currà, A.; Gasbarrone, R.; Cardillo, A.; Trompetto, C.; Fattapposta, F.; Pierelli, F.; Missori, P.; Bonifazi, G.; Serranti, S. Near-infrared spectroscopy as a tool for in vivo analysis of human muscles. Sci. Rep. 2019, 9, 8623. [Google Scholar] [CrossRef]

| Traits | Thawing Condition | Storage Days (Day) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 5 | 8 | 14 | 21 | ||
| pH | WT * | 6.38 ± 0.07 a** | 6.47 ± 0.11 ab | 6.49 ± 0.09 ab | 6.53 ± 0.03 ab | 6.55 ± 0.04 ab | 6.64 ± 0.07 b | 6.85 ± 0.22 c | 6.90 ± 0.29 c |
| RT * | 6.38 ± 0.07 a | 6.42 ± 0.04 a | 6.38 ± 0.07 a | 6.45 ± 0.05 ab | 6.41 ± 0.08 a | 6.61 ± 0.05 b | 6.84 ± 0.18 c | 6.88 ± 0.29 c | |
| Titratable Acidity (%) | WT | 1.79 ± 0.16 b | 1.74 ± 0.23 b | 1.76 ± 0.23 b | 1.78 ± 0.02 b | 1.67 ± 0.07 b | 1.62 ± 0.11 b | 1.31 ± 0.24 a | 1.32 ± 0.28 a |
| RT | 1.79 ± 0.16 bc | 2.03 ± 0.07 d | 1.92 ± 0.07 cd | 1.87 ± 0.12 cd | 1.81 ± 0.08 bcd | 1.60 ± 0.15 ab | 1.38 ± 0.14 a | 1.52 ± 0.33 a | |
| TVB-N (mg/100 g) | WT | 12.27 ± 1.54 a | 11.78 ± 1.60 a | 12.98 ± 0.70 ab | 16.27 ± 0.77 bc | 18.52 ± 1.17 cd | 20.20 ± 1.25 d | 44.33 ± 2.91 e | 64.81 ± 6.90 f |
| RT | 12.27 ± 1.54 a | 11.78 ± 0.77 a | 12.91 ± 1.17 a | 16.55 ± 1.17 b | 18.80 ± 0.77 bc | 19.92 ± 1.17 c | 43.21 ± 3.04 d | 69.30 ± 4.82 e | |
| Total Viable Cell Count (Log CFU/g) | WT | 3.71 ± 0.30 d | 2.20 ± 0.15 a | 2.74 ± 0.30 b | 3.15 ± 0.22 bc | 3.56 ± 0.23 cd | 4.34 ± 0.46 e | 4.90 ± 0.52 f | 5.56 ± 0.21 g |
| RT | 3.71 ± 0.30 b | 2.56 ± 0.70 a | 3.02 ± 0.34 a | 2.87 ± 0.19 a | 3.86 ± 0.42 bc | 4.30 ± 0.12 c | 5.24 ± 0.45 d | 5.20 ± 0.11 d | |
| Traits | Thawing Condition | Storage Days (Day) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 5 | 8 | 14 | 21 | ||
| Hardness | WT * | 1311.71 ± 234.62 d* | 1062.78 ± 109.68 bc | 848.04 ± 131.55 a | 854.83 ± 191.14 a | 1012.76 ± 85.33 ab | 1040.85 ± 75.64 bc | 1184.86 ± 123.12 bcd | 1210.83 ± 69.11 cd |
| RT* | 1311.71 ± 234.62 cd | 1081.12 ± 78.35 b | 1011.82 ± 175.00 b | 647.10 ± 238.67 a | 1036.57 ± 157.63 b | 1054.22 ± 156.20 b | 1156.11 ± 95.32 bc | 1480.51 ± 87.74 d | |
| Cohesiveness | WT | 0.27 ± 0.01 bc | 0.27 ± 0.02 c | 0.24 ± 0.03 ab | 0.24 ± 0.02 a | 0.30 ± 0.03 d | 0.28 ± 0.02 cd | 0.28 ± 0.02 cd | 0.28 ± 0.02 cd |
| RT | 0.27 ± 0.01 bc | 0.24 ± 0.01 a | 0.27 ± 0.01 cd | 0.25 ± 0.02 ab | 0.28 ± 0.02 cd | 0.28 ± 0.02 cd | 0.26 ± 0.01 bc | 0.29 ± 0.01 d | |
| Springiness | WT | 0.29 ± 0.01 a | 0.34 ± 0.04 bc | 0.32 ± 0.01 ab | 0.31 ± 0.01 ab | 0.35 ± 0.03 cd | 0.36 ± 0.03 cd | 0.32 ± 0.01 ab | 0.38 ± 0.02 d |
| RT | 0.29 ± 0.01a | 0.32 ± 0.04 ab | 0.30 ± 0.02 a | 0.35 ± 0.03 bcd | 0.35 ± 0.02 bcd | 0.36 ± 0.02 cd | 0.34 ± 0.03 bc | 0.38 ± 0.04 d | |
| Gumminess | WT | 345.76 ± 51.11 b | 293.28 ± 44.54 b | 215.06 ± 46.57 a | 204.78 ± 51.16 a | 313.23 ± 31.02 b | 293.14 ± 31.34 b | 329.50 ± 22.21 b | 328.65 ± 40.37 b |
| RT | 345.76 ± 51.11 c | 240.17 ± 18.45 b | 273.05 ± 38.73 b | 160.23 ± 87.17 a | 281.68 ± 28.41 b | 289.48 ± 40.30 b | 298.77 ± 32.50 bc | 409.76 ± 31.82 d | |
| Chewiness | WT | 100.59 ± 9.96 b | 98.40 ± 13.66 b | 64.62 ± 14.76 a | 64.98 ± 13.93 a | 112.70 ± 13.99 bc | 109.97 ± 19.34 bc | 110.24 ± 14.42 bc | 119.86 ± 9.19 c |
| RT | 100.59 ± 9.96 bc | 80.31 ± 17.17 b | 83.66 ± 19.05 b | 54.09 ± 24.99 a | 100.01 ± 16.16 bc | 111.36 ± 29.65 c | 105.29 ± 16.37 bc | 158.10 ± 24.40 d | |
| Resilience | WT | 0.10 ± 0.00 a | 0.09 ± 0.01 a | 0.10 ± 0.01 a | 0.10 ± 0.01 a | 0.09 ± 0.01 a | 0.09 ± 0.00 a | 0.09 ± 0.00 a | 0.09 ± 0.01 a |
| RT | 0.10 ± 0.00 d | 0.09 ± 0.01 cd | 0.09 ± 0.00 bcd | 0.09 ± 0.01 ab | 0.09 ± 0.01 abcd | 0.09 ± 0.01 abc | 0.08 ± 0.01 a | 0.10 ± 0.01 d | |
| Traits | Thawing Condition | Storage Days (Day) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 5 | 8 | 14 | 21 | ||
| Red (R) | WTS * | 139.39 ± 7.80 c** | 154.12 ± 5.13 d | 141.03 ± 12.69 c | 132.66 ± 10.05 bc | 123.43 ± 12.36 ab | 123.59 ± 5.95 ab | 125.25 ± 10.71 ab | 113.53 ± 7.08 a |
| RTS * | 139.39 ± 7.80 cd | 126.97 ± 9.34 b | 142.13 ± 7.60 d | 132.45 ± 16.87 cd | 140.10 ± 13.50 cd | 126.94 ± 8.68 b | 131.26 ± 7.99 cd | 111.04 ± 5.05 a | |
| WTW * | 133.44 ± 10.73 c | 123.07 ± 6.65 bc | 112.53 ± 5.81 ab | 106.83 ± 8.01 a | 106.38 ± 5.73 a | 108.44 ± 4.17 a | 101.20 ± 5.17 a | 86.21 ± 4.13 a | |
| RTW * | 132.95 ± 10.88 c | 116.03 ± 4.55 b | 111.41 ± 7.13 ab | 103.05 ± 0.87 a | 110.33 ± 5.20 ab | 103.56 ± 3.38 a | 100.51 ± 2.33 a | 84.67 ± 2.84 a | |
| Green (G) | WTS | 97.22 ± 7.62 bc | 101.59 ± 4.16 d | 99.51 ± 10.03 d | 93.49 ± 7.93 bc | 88.14 ± 7.95 ab | 89.65 ± 3.31 ab | 90.13 ± 7.31 ab | 83.79 ± 3.63 a |
| RTS | 97.22 ± 7.62 bc | 89.79 ± 6.04 ab | 98.74 ± 5.07 bc | 94.16 ± 12.53 bc | 100.71 ± 9.98 c | 90.69 ± 6.40 bc | 94.58 ± 6.10 bc | 80.67 ± 4.21 a | |
| WTW | 133.08 ± 10.92 c | 125.84 ± 6.64 c | 113.68 ± 7.25 b | 108.39 ± 6.84 b | 104.99 ± 4.91 ab | 109.16 ± 4.29 b | 95.83 ± 5.37 a | 80.10 ± 4.51 a | |
| RTW | 132.03 ± 10.75 d | 116.43 ± 1.59 c | 111.68 ± 8.00 bc | 104.18 ± 0.87 b | 111.74 ± 5.84 bc | 101.23 ± 4.14 b | 76.28 ± 2.71 a | 77.50 ± 3.39 a | |
| Blue (B) | WTS | 87.63 ± 5.70 cd | 90.13 ± 2.59 d | 87.14 ± 6.59 cd | 82.21 ± 5.93 bc | 77.58 ± 5.47 ab | 77.91 ± 2.27 ab | 78.37 ± 4.72 ab | 73.26 ± 2.39 a |
| RTS | 87.63 ± 5.70 d | 80.51 ± 4.38 b | 86.47 ± 3.53 bc | 83.02 ± 8.46 bc | 85.81 ± 6.51 bc | 80.42 ± 4.43 b | 80.98 ± 3.34 bc | 72.02 ± 2.06 a | |
| WTW | 114.98 ± 9.87 c | 113.60 ± 5.86 c | 103.10 ± 8.43 b | 98.87 ± 3.93 b | 93.30 ± 4.22 b | 94.06 ± 3.16 b | 78.93 ± 4.07 a | 64.11 ± 3.57 a | |
| RTW | 113.41 ± 9.04 d | 106.02 ± 1.50 cd | 99.78 ± 6.91 bc | 94.38 ± 2.60 b | 100.07 ± 6.35 bc | 83.83 ± 3.37 a | 94.13 ± 2.80 b | 62.40 ± 2.93 | |
| Storage Days | Model Performance * (Train) | Classification Accuracy (Test) | |||
|---|---|---|---|---|---|
| n | RC2 | RMSEC | n | Accuracy (%) | |
| 0 | 70 | 0.6734 | 0.4813 | 30 | 80.00 |
| 1 | 70 | 0.9547 | 0.1064 | 30 | 100.00 |
| 2 | 70 | 0.7683 | 0.2407 | 30 | 96.67 |
| 3 | 70 | 0.8168 | 0.2140 | 30 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-K.; Cho, J.-S.; Won, D.-H.; Kim, S.S.; Lim, J.-H.; Choi, J.H.; Yun, D.-Y.; Park, K.-J.; Lee, G. Quality Differences in Frozen Mackerel According to Thawing Method: Potential Classification via Hyperspectral Imaging. Foods 2024, 13, 4005. https://doi.org/10.3390/foods13244005
Park S-K, Cho J-S, Won D-H, Kim SS, Lim J-H, Choi JH, Yun D-Y, Park K-J, Lee G. Quality Differences in Frozen Mackerel According to Thawing Method: Potential Classification via Hyperspectral Imaging. Foods. 2024; 13(24):4005. https://doi.org/10.3390/foods13244005
Chicago/Turabian StylePark, Seul-Ki, Jeong-Seok Cho, Dong-Hoon Won, Sang Seop Kim, Jeong-Ho Lim, Jeong Hee Choi, Dae-Yong Yun, Kee-Jai Park, and Gyuseok Lee. 2024. "Quality Differences in Frozen Mackerel According to Thawing Method: Potential Classification via Hyperspectral Imaging" Foods 13, no. 24: 4005. https://doi.org/10.3390/foods13244005
APA StylePark, S.-K., Cho, J.-S., Won, D.-H., Kim, S. S., Lim, J.-H., Choi, J. H., Yun, D.-Y., Park, K.-J., & Lee, G. (2024). Quality Differences in Frozen Mackerel According to Thawing Method: Potential Classification via Hyperspectral Imaging. Foods, 13(24), 4005. https://doi.org/10.3390/foods13244005






