Exploring the Impact of Different Saccharomyces cerevisiae Strains on the Flavor Profile of Greengage Alcoholic Beverage Using GC-E-Nose, HS-GC-IMS, and HS-SPME-GC-MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Chemicals
2.2. Fermentation of Greengage Alcoholic Beverage
2.3. Measurement of Physicochemical Properties
2.4. Sensory Analysis
2.5. GC-E-Nose Analysis
2.6. GC-IMS Analysis
2.7. HS-SPME-GC-MS Analysis
2.8. OAV Analysis
2.9. Statistical Analysis
3. Results
3.1. Analysis of the Quality Parameters of Greengage Alcoholic Beverage
3.2. GC-E-Nose Analysis of Aroma Differences in Greengage Alcoholic Beverage by Different S. cerevisiae
3.3. Identification of Volatile Organic Compounds by GC-IMS
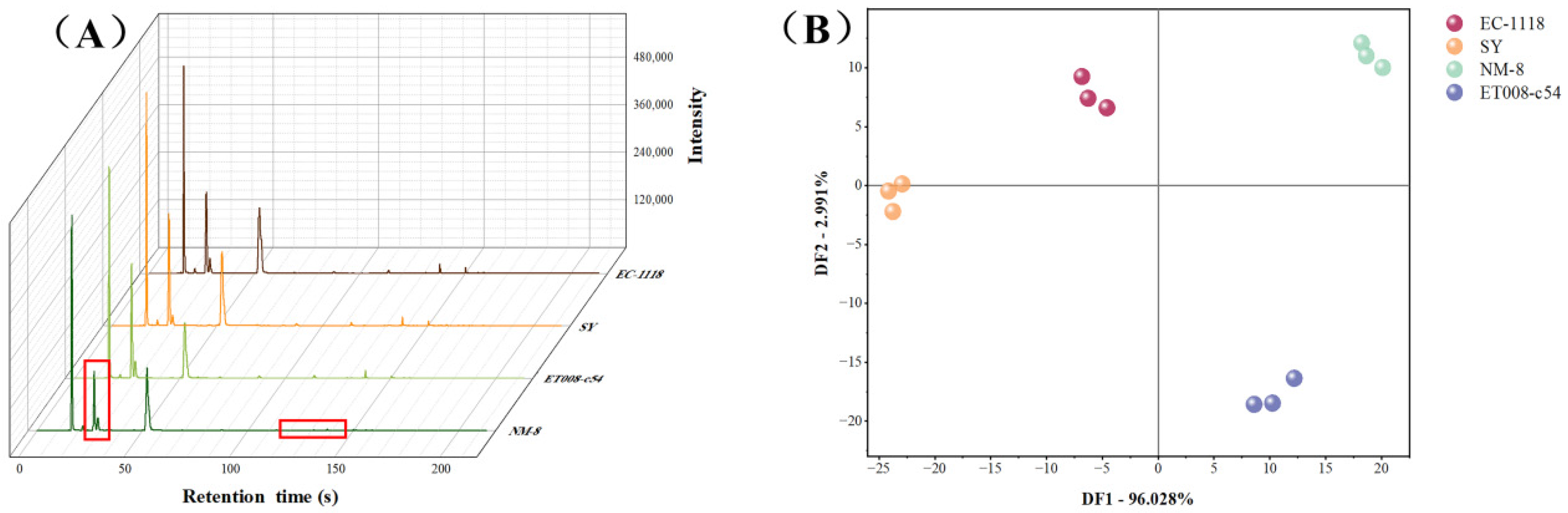
3.3.1. Comparison of GC-IMS Topographic Plots
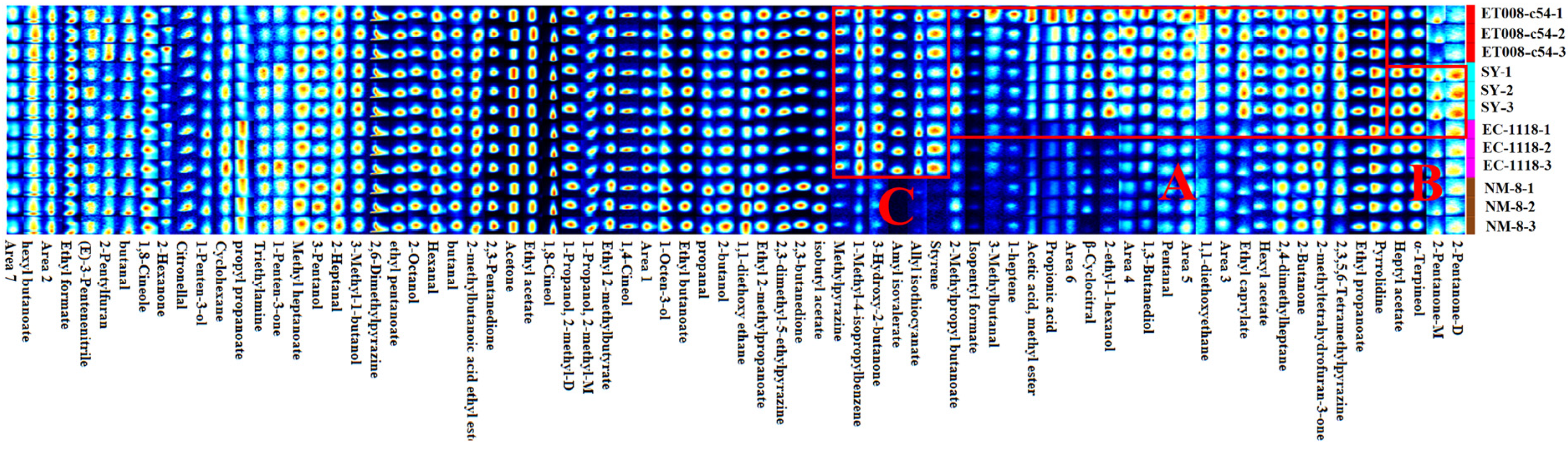
3.3.2. Analysis of GC-IMS Fingerprints
3.4. Identification of Volatile Organic Compounds by HS-SPME-GC–MS
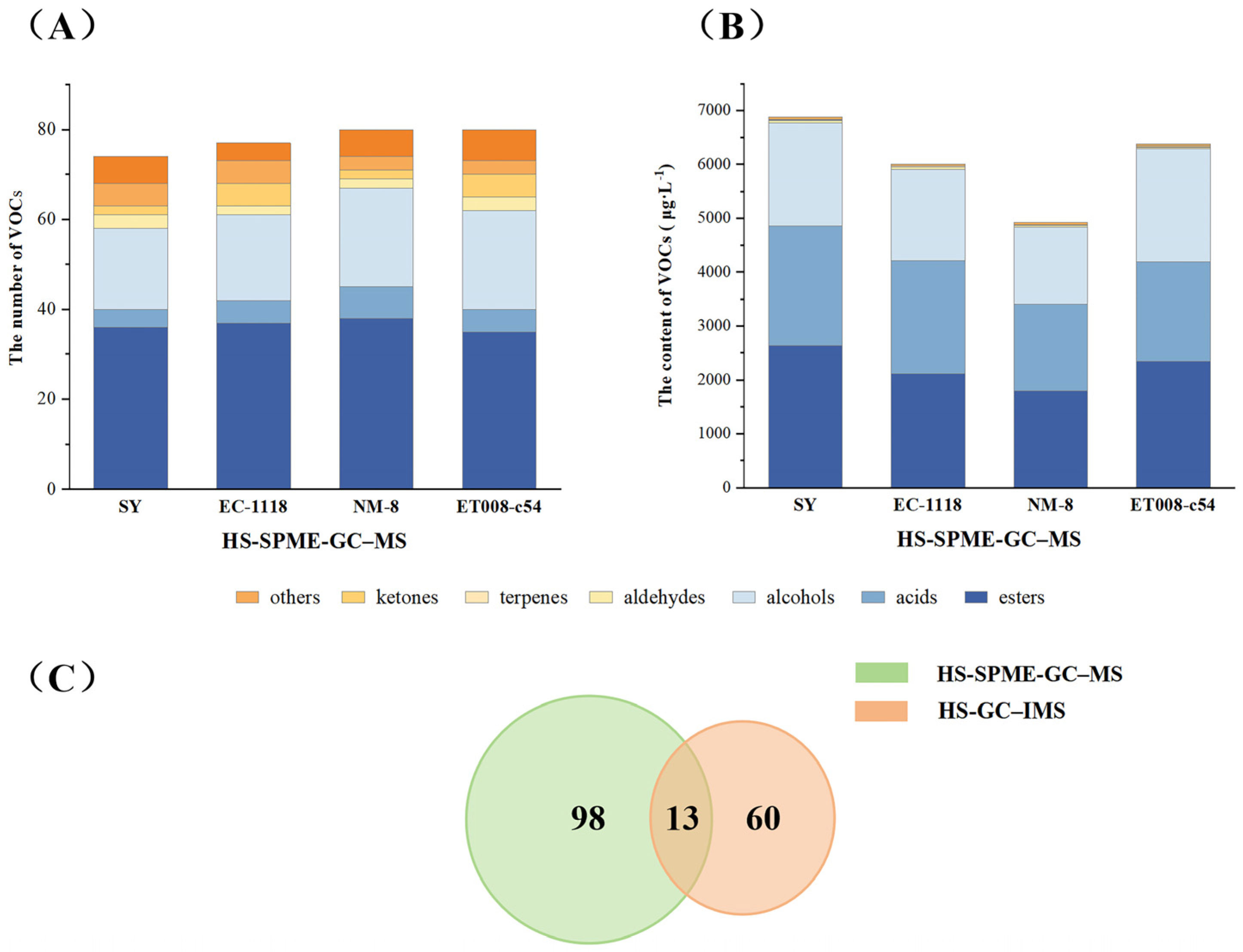
3.4.1. Characterization of VOCs in Different Fermentation Groups
3.4.2. Multivariate Statistical Analysis
3.4.3. Key Volatile Components of Greengage Alcoholic Beverage by OAV Analysis
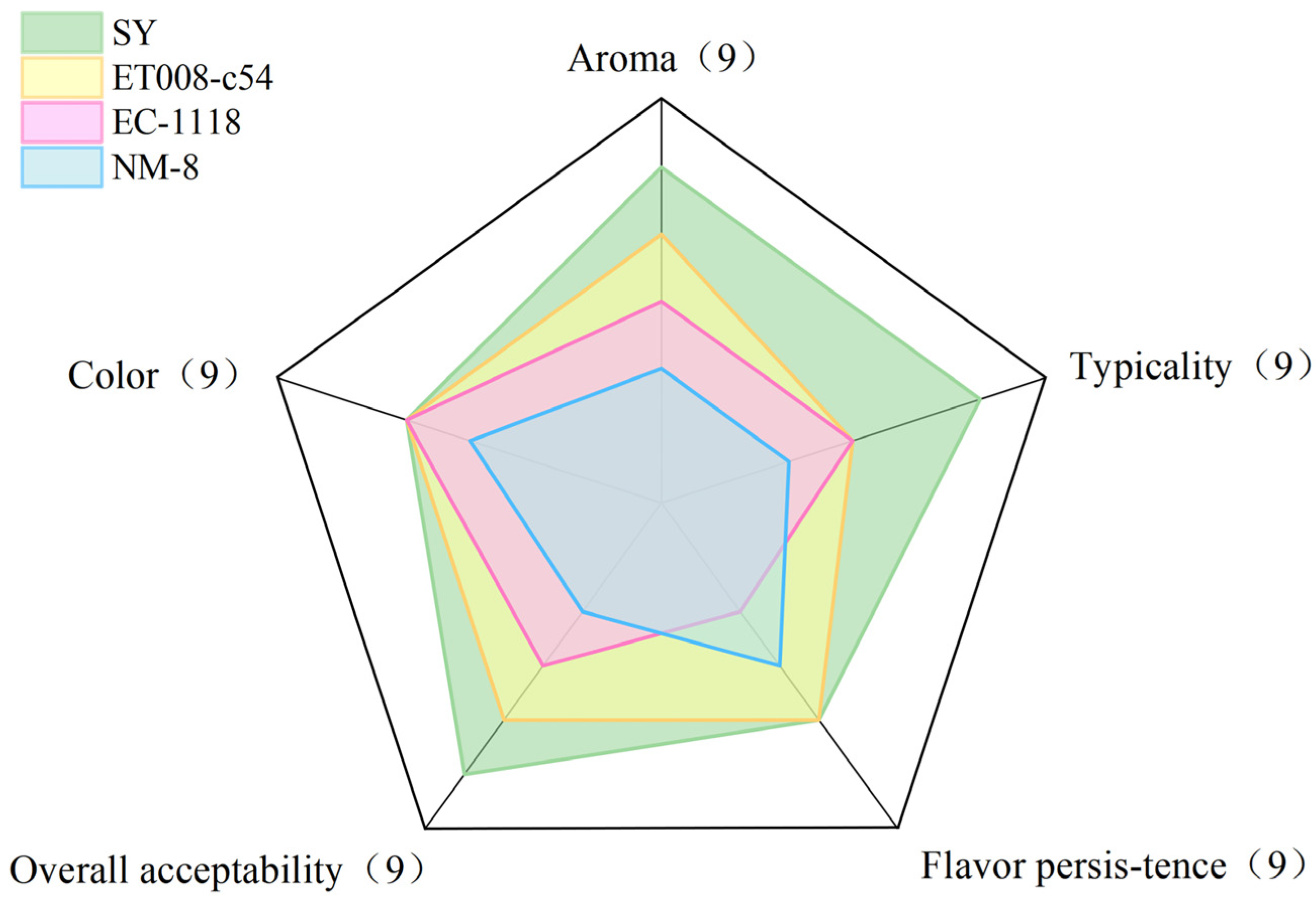
3.5. Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, T.T.; Cao, H.; Farag, M.A.; Fan, S.T.; Liu, L.X.; Yang, W.J.; Wang, Y.X.; Zou, L.; Cheng, K.W.; Wang, M.F.; et al. Current and potential trends in the bioactive properties and health benefits of Prunus mume Sieb. Et Zucc: A comprehensive review for value maximization. Crit. Rev. Food Sci. 2023, 63, 7091–7107. [Google Scholar] [CrossRef]
- Tian, T.T.; Yang, H.; Yang, F.; Li, B.W.; Sun, J.Y.; Wu, D.H.; Lu, J. Optimization of fermentation conditions and comparison of flavor compounds for three fermented greengage wines. LWT-Food Sci. Technol. 2018, 89, 542–550. [Google Scholar] [CrossRef]
- Cordente, A.G.; Curtin, C.D.; Varela, C.; Pretorius, I.S. Flavour-active wine yeasts. Appl. Microbiol. Biotechnol. 2012, 96, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Cordente, A.G.; Schmidt, S.; Beltran, G.; Torija, M.J.; Curtin, C.D. Harnessing yeast metabolism of aromatic amino acids for fermented beverage bioflavouring and bioproduction. Appl. Microbiol. Biotechnol. 2019, 103, 4325–4336. [Google Scholar] [CrossRef]
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascués, E.; Calderón, F.; Benito, A.; Rauhut, D.; Santos, A.; et al. Effects on varietal aromas during wine making: A review of the impact of varietal aromas on the flavor of wine. Appl. Microbiol. Biotechnol. 2019, 103, 7425–7450. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Huang, Y.M.; Górska-Horczyczak, E.; Wierzbicka, A.; Jelen, H.H. Rapid analysis of Baijiu volatile compounds fingerprint for their aroma and regional origin authenticity assessment. Food Chem. 2021, 337, 128002. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Ye, H.; Zou, Y.T.; He, Z.H.; Xu, B.T.; Wang, S.; Peng, C.N.; Zhou, X.R.; Zhang, Q.; Xiang, W.L.; et al. Flavor characteristics of navel orange wine fermented by Saccharomyces cerevisiae SC-125 and Angel yeast SY. Fermentation 2023, 9, 872. [Google Scholar] [CrossRef]
- Cai, W.; Tang, F.; Guo, Z.; Guo, X.; Zhang, Q.; Zhao, X.; Ning, M.; Shan, C. Effects of pretreatment methods and leaching methods on jujube wine quality detected by electronic senses and HS-SPME-GC-MS. Food Chem. 2020, 330, 127330. [Google Scholar] [CrossRef]
- Wang, S.Q.; Chen, H.T.; Sun, B.G. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Chi, X.L.; Zhang, Y.D.; Zheng, N.; Wang, J.Q.; Liu, H.M. HS-GC-IMS and HS-SPME/GC-MS coupled with E-nose and E-tongue reveal the flavors of raw milk from different regions of China. Curr. Res. Food Sci. 2024, 8, 100673. [Google Scholar] [CrossRef]
- Feng, X.Y.; Wang, H.W.; Wang, Z.R.; Huang, P.M.; Kan, J.Q. Discrimination and characterization of the volatile organic compounds in eight kinds of huajiao with geographical indication of China using electronic nose, HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2022, 375, 131671. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; Yu, Y.S.; Zou, B.; Yu, Y.Y.; Yang, J.G.; Xu, Y.J.; Chen, X.W.; Yang, F. Evaluation of dynamic changes and regularity of volatile flavor compounds for different Green Plum (Prunus mume sieb. et Zucc) varieties during the ripening process by HS-GC-IMS with PLS-DA. Foods 2023, 12, 551. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Chen, Z.; Huang, B.; Chen, X.; Liu, H.; Peng, Z.; Dong, P.; Lu, J.; Wu, D. Characterizing the key aroma compounds of barley malt from different origins using GC-E-Nose, HS-SPME-GC-MS, and HS-GC-IMS. Food Biosci. 2024, 58, 103707. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.; Ren, T.; Wang, J.; Niu, C.; Zheng, F.; Li, Q. Effect of Saccharomyces cerevisiae and non-Saccharomyces strains on alcoholic fermentation behavior and aroma profile of yellow-fleshed peach wine. LWT-Food Sci. Technol. 2022, 155, 112993. [Google Scholar] [CrossRef]
- Liu, Z.H.; Yang, Y.Z.; Sun, C.Z.; Wu, Y.; Li, X.B. Comparative phytochemical property and antioxidant activity of sixteen Mei (Prunus mume) fruit cultivars from six provinces in China. Int. J. Food Prop. 2022, 25, 617–633. [Google Scholar] [CrossRef]
- Casassa, L.F.; Mawdsley, P.F.W.; Stoffel, E.; Williams, P.; Peterson, J.C.D. Chemical and sensory effects of cofermentation and post-malolactic fermentation blending of Syrah with selected Rhone white cultivars. Aust. J. Grape Wine Res. 2020, 26, 41–52. [Google Scholar] [CrossRef]
- Olivero, R.E.; Aguas, M.Y.; Cury, R.K. Evaluación del efecto de diferentes cepas de levadura (Montrachet, K1-V1116, EC-1118, 71B-1122 y IVC-GRE®) y clarificantes sobre los atributos sensoriales del vino de naranja criolla (Citrus sinensis). Revista Colombiana de Biotecnología 2011, 13, 163–171. [Google Scholar]
- Wu, D.; Wu, Y.; Gu, Z.; Chen, X.; Liu, H.; Lu, J.; Xie, G. Multi-step screening of suitable Saccharomyces cerevisiae strain for lemon wine brewing. Food Biosci. 2023, 56, 103092. [Google Scholar] [CrossRef]
- Tian, T.T.; Wu, D.H.; Ng, C.T.; Yang, H.; Sun, J.Y.; Liu, J.M.; Lu, J. A multiple-step strategy for screening Saccharomyces cerevisiae strains with improved acid tolerance and aroma profiles. Appl. Microbiol. Biotechnol. 2020, 104, 3097–3107. [Google Scholar] [CrossRef]
- Tian, T.T. Screening of Saccharomyces cerevisiae for Greengage Wine and Its Mechanism of Acid Tolerance. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2019. [Google Scholar]
- GB/T 15038-2006; National Standards of the People’s Republic of China-Analytical Methods of Wine and Fruit Wine. Standardization Administration of China: Beijing, China, 2006.
- GB 5009.225-2023; National Standards of the People’s Republic of China-Determination of Ethanol Concentration in Wine and Edible Alcohol. Standardization Administration of China: Beijing, China, 2023.
- Qiu, S.; Chen, K.; Liu, C.; Wang, Y.; Chen, T.; Yan, G.; Li, J. Non-Saccharomyces yeasts highly contribute to characterisation of flavour profiles in greengage fermentation. Food Res. Int. 2022, 157, 111391. [Google Scholar] [CrossRef]
- NY/T 1508-2017; National standards of the People’s Republic of China-Green Food Fruit Wine. Standardization Administration of China: Beijing, China, 2017.
- Morata, A.; Loira, I.; Heras, J.M.; Callejo, M.J.; Tesfaye, W.; González, C.; Suárez-Lepe, J.A. Yeast influence on the formation of stable pigments in red wine making. Food Chem. 2016, 197, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.L.; Wang, L.L.; Deng, Y.L.; Yuan, H.B.; Zhu, J.Y.; Jiang, Y.W.; Yang, Y.Q. Characterization of the key odorants in floral aroma green tea based on GC-E-Nose, GC-IMS, GC-MS and aroma recombination and investigation of the dynamic changes and aroma formation during processing. Food Chem. 2023, 427, 136641. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, J.; Zhu, Z.; Lei, Y.; Huang, S.; Huang, M. Effect of ageing time on the flavour compounds in Nanjing water-boiled salted duck detected by HS-GC-IMS. LWT-Food Sci. Technol. 2022, 155, 112870. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.P.; Blank, I.; Li, F.; Li, C.; Liu, Y. GC x GC-ToF-MS and GC-IMS based volatile profile characterization of the Chinese dry-cured hams from different regions. Food Res. Int. 2021, 142, 110222. [Google Scholar] [CrossRef]
- Vararu, F.; Moreno-Garcia, J.; Niculaua, M.; Cotea, V.V.; Mayen, M.; Moreno, J. Fermentative volatilome modulation of Muscat Ottonel wines by using yeast starter cultures. LWT-Food Sci. Technol. 2020, 129, 109575. [Google Scholar] [CrossRef]
- Tian, T.; Sun, J.; Wu, D.; Xiao, J.; Lu, J. Objective measures of greengage wine quality: From taste-active compound and aroma-active compound to sensory profiles. Food Chem. 2021, 340, 128719. [Google Scholar] [CrossRef]
- Xi, B.N.; Zhang, J.J.; Xu, X.; Li, C.; Shu, Y.; Zhang, Y.; Shi, X.; Shen, Y. Characterization and metabolism pathway of volatile compounds in walnut oil obtained from various ripening stages via HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2024, 435, 137547. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhi, H.; Fang, Z.; Zhang, P. Genetic engineering of yeast, filamentous fungi and bacteria for terpene production and applications in food industry. Food Res. Int. 2021, 147, 110487. [Google Scholar] [CrossRef]
- Sun, L.; Zhu, B.; Zhang, X.; Wang, H.; Yan, A.; Zhang, G.; Wang, X.; Xu, H. The accumulation profiles of terpene metabolites in three Muscat table grape cultivars through HS-SPME-GCMS. Sci. Data 2020, 7, 5. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, T.; Li, J.; Zhang, B.; Yu, Y.; Wang, Y.; Niu, H. Variations in main flavor compounds of freshly distilled brandy during the second distillation. Int. J. Food Eng. 2014, 10, 809–820. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, F.; Wu, W.; Wang, P.; Ye, N. Comparison of volatiles in different jasmine tea grade samples using electronic nose and automatic thermal desorption-gas chromatography-mass spectrometry followed by multivariate statistical analysis. Molecules 2020, 25, 380. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Ding, S.; Pan, Z.; Li, X.; Fu, F. Characteristic volatile fingerprints and odor activity values in different citrus-Tea by HS-GC-IMS and HS-SPME-GC-MS. Molecules 2020, 25, 6027. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Li, C.; Hao, J.; Hu, W. Study on the change of the main biochemical components, representative aromatic compounds as well as their correlation of green tea beverages during storage. J. Tea Sci. 2008, 28, 181–188. [Google Scholar]
- Noguerol-Pato, R.; Sieiro-Sampedro, T.; Gonzalez-Barreiro, C.; Cancho-Grande, B.; Simal-Gandara, J. Effect on the aroma profile of Graciano and Tempranillo red wines of the application of two antifungal treatments onto vines. Molecules 2014, 19, 17422–17423. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinionsand data contained inall publicationsare solely those of the individualauthor(s)and contributor(s)and not of MDPIand/or the editor(s). MDPIand/or the editor(s) disclaim responsibility forany injury to people or property resulting fromany ideas, methods, instructions or products referred to in the content. |



| Physicochemical Parameters | SY | ET008-c54 | EC-1118 | NM-8 |
|---|---|---|---|---|
| Reducing sugar (g·L−1) | 8.62 ± 0.59 a | 16.65 ± 1.12 b | 25.45 ± 1.11 c | 17.63 ± 0.63 b |
| Sugar utilization (%) | 0.87 ± 0.01 d | 0.83 ± 0.01 b | 0.78 ± 0 a | 0.77 ± 0.01 a |
| Total acid (g·L−1) | 10.04 ± 0.11 ab | 8.42 ± 1.95 a | 10.3 ± 0.23 ab | 10.56 ± 0.26 b |
| Alcohol degree (%v/v) | 11.78 ± 0.08 c | 11.17 ± 0.07 b | 10.53 ± 0.05 a | 10.45 ± 0.1 a |
| pH | 2.45 ± 0.02 b | 2.44 ± 0.01 ab | 2.41 ± 0.02 a | 2.45 ± 0.02 b |
| Color intensity | 0.61 ± 0.01 b | 0.67 ± 0.02 c | 0.56 ± 0.01 a | 0.57 ± 0.01 a |
| Color tonality | 2.43 ± 0.11 b | 2.97 ± 0.07 c | 1.93 ± 0.03 a | 1.97 ± 0.08 a |
| Compound | RI δ | Identification ε | Concentration (μg·L−1) η | Threshold (μg·L−1) θ | Aroma Description θ | |||
|---|---|---|---|---|---|---|---|---|
| SY | EC-1118 | NM-8 | ET008-c54 | |||||
| Ethyl Caprate | 1629 | MS, RI | 841.89 ± 8.95 a | 744.54 ± 88.57 a | 581.76 ± 14.49 b | 858.98 ± 15.46 a | 200 | brandy, fruit, grape |
| Ethyl Caprylate | 1426 | MS, RI | 529.24 ± 3.3 a | 412.26 ± 8.44 b | 348.37 ± 4.83 c | 426.17 ± 18.94 b | 5 | banana, fresh, fruit |
| Ethyl Laurate | 1831 | MS, RI | 425.85 ± 9.36 a | 259.86 ± 37.48 c | 231.07 ± 3.57c | 319.29 ± 0.68 b | 400 | cream, floral, green apple, |
| Ethyl Acetate | 850 | MS, RI | 226.37 ± 2.69 a | 145.13 ± 4.77 c | 221.09 ± 1.62 a | 205.25 ± 3.38 b | 750 | fruit, grape, pineapple |
| Ethyl Palmitate | 2235 | MS, RI | 125.85 ± 11.65 a | 122.6 ± 1.51 ab | 94.23 ± 18.54 b | 120.21 ± 6.48 ab | 2000 | fat, fruit, sweet |
| Ethyl Hexanoate | 1223 | MS, RI | 130.47 ± 3.87 a | 113.3 ± 8.39 b | 93.56 ± 0.43 c | 76.43 ± 3.62 d | 14 | apple, banana, cheese |
| Ethyl Benzoate | 1659 | MS, RI | 114.19 ± 1.81 a | 112.73 ± 8.01 a | 70.71 ± 4.25 c | 96.68 ± 2.8 b | 740 | fat, floral, flower |
| Ethyl 9-Hexadecenoate | 2262 | MS, RI | 26.6 ± 7.95 a | 25.36 ± 0.96 a | ND | 28.71 ± 0.05 a | NF | NF |
| Phenethyl Acetate | 1804 | MS, RI | 40.12 ± 1.52 b | 25.17 ± 1.97 c | 15.57 ± 0.46 d | 43.78 ± 0.21 a | 650 | floral, fruit, honey, rose |
| Ethyl Tetradecanoate | 2040 | MS, RI | 25.57 ± 0.14 a | 16.89 ± 0.49 b | 11.09 ± 1.04 c | 19.18 ± 0.98 d | 180 | cream, oil, pleasant |
| Isoamyl Acetate | 1110 | MS, RI | 29.05 ± 2.29 a | 19.36 ± 1.84 b | 28.23 ± 0.57 a | 27.86 ± 1.37 a | 30 | apple, banana, fruit |
| isoamyl Decanoate | 1850 | MS, RI | 20.09 ± 0.26 a | 18.8 ± 3.16 a | 8.03 ± 0.2 b | 19.14 ± 0.17 a | 5000 | wax |
| Ethyl Stearate | 2439 | MS, RI | 13 ± 1.08 b | 10.21 ± 0.69 c | 7.46 ± 1.32 d | 20.34 ± 0.18 a | 500 | wax |
| 2-Methylbutyl Laurate | 2059 | MS, RI | 9.94 ± 1.43 a | 5.55 ± 0.79 b | 2.91 ± 0.16 c | 6.8 ± 0 b | 450 | NF |
| Linoleic Acid Ethyl Ester | 2511 | MS, RI | 9.76 ± 0.61 a | 6.54 ± 0.35 b | 4.69 ± 0.13 c | 9.36 ± 0.04 a | 450 | fruit |
| 3-methylbutyl Octanoate | 1648 | MS, RI | 9.07 ± 0.09 a | 10.44 ± 1.16 a | 5.34 ± 0.14 b | 9.71 ± 0.06 a | 70 | baked apple |
| 2,2,4-Trimethyl-1,3-Pentanediol Diisobutyrate | 1864 | MS, RI | 2.9 ± 0.42 a | ND | 2.17 ± 0.02 b | ND | NF | NF |
| Ethyl Butanoate | 1022 | MS, RI | 7.59 ± 0.3 a | 5.59 ± 0.54 bc | 5.69 ± 0.1 b | 4.78 ± 0.19 c | 0.9 | apple, banana, fruit |
| Ethyl Phenylacetate | 1774 | MS, RI | 8.31 ± 0.29 a | 6.88 ± 0.44 c | 7.3 ± 0.27 bc | 7.89 ± 0.1 ab | 250 | honey, rose, sweet |
| R-γ-Decalactone | 2138 | MS, RI | 6.48 ± 0.15 a | 6.41 ± 0.14 a | 5.6 ± 0.5 b | 6.18 ± 0.11 ab | 70 | fruit, peach, pleasant |
| Isobutyl Decanoate | 1744 | MS, RI | ND | ND | ND | 5.16 ± 0.03 a | NF | baked apple |
| Ethyl 9-Decenoate | 1679 | MS, RI | ND | 8.83 ± 0.63 b | 4.05 ± 0.14c | 5.28 ± 0.28 d | NF | NF |
| Isopropyl Palmitate | 2221 | MS, RI | 4.93 ± 1.24 a | 5.16 ± 1.66 a | 4.33 ± 1.07 a | 5.13 ± 0.1 a | NF | NF |
| Benzylcarbinyl Caproate | 2157 | MS, RI | ND | 4.19 ± 0.02 a | ND | 3.74 ± 0.08 b | 94 | NF |
| Dibutyl Phthalate | 2684 | MS, RI | 5.33 ± 0.83 ab | 5.88 ± 0.94 ab | 7.81 ± 1.94 a | 4.62 ± 0.03 b | 260 | NF |
| 2-Phenylethyl Pentanoate | 2372 | MS, RI | 4.45 ± 0.02 a | ND | ND | ND | NF | NF |
| Ethyl Linolenate | 2578 | MS, RI | 2.92 ± 0.23 a | 1.88 ± 0.36 b | ND | 2.89 ± 0.09 a | NF | NF |
| Propanoic Acid, Ethyl Ester | 935 | MS, RI | 2.14 ± 0.14 a | 1.3 ± 0.1 c | 1.46 ± 0.01 c | 1.85 ± 0.02 b | 10 | apple, fruit, pineapple |
| Ethyl Isovalerate | 1051 | MS, RI | 1.08 ± 0.01 b | ND | 2.32 ± 0.3 a | ND | 3 | apple, citrus, fruit |
| Ethyl Isobutyrate | 944 | MS, RI | 1.69 ± 0.08 c | 0.67 ± 0.09 d | 2.46 ± 0.02 a | 2.08 ± 0.01 b | 0.1 | apple, floral, fruit, |
| Ethyl 2-Methylbutanoate | 1036 | MS, RI | 1.74 ± 0.01 a | 1.21 ± 0 b | 1.72 ± 0.04 a | 1.69 ± 0.04 a | 0.2 | apple, floral, fruit |
| Ethyl Margarate | 2337 | MS, RI | ND | 0.64 ± 0.01 a | ND | ND | NF | NF |
| Phenethyl Decanoate | 2589 | MS, RI | ND | ND | ND | 1.06 ± 0 a | NF | NF |
| Propyl Caprate | 1712 | MS, RI | 1.17 ± 0 ab | 1 ± 0.11 a | ND | 1.21 ± 0.08 b | NF | fruit |
| Hexyl Formate | 1339 | MS, RI | 0.73 ± 0.02 b | 0.97 ± 0.05 a | 1.01 ± 0.06 a | ND | NF | NF |
| Diethyl Bis(trimethylsilyl) Silicate | 1169 | MS, RI | 0.36 ± 0.04 a | 0.26 ± 0 b | 0.31 ± 0.02 ab | 0.33 ± 0.01 a | NF | NF |
| 2-Ethylhexyl Acetate | 1374 | MS, RI | ND | 0.15 ± 0.05 b | ND | 0.72 ± 0.03 a | 43 | NF |
| Octyl Formate | 1542 | MS, RI | ND | ND | 5.62 ± 0.55 a | ND | NF | NF |
| Phenethyl Nonanoate | 2157 | MS, RI | 4.45 ± 0.05 a | ND | ND | ND | NF | NF |
| Ethyl 3-Phenylpropanoate | 1873 | MS, RI | 2.44 ± 0.18 b | ND | 2.98 ± 0.12 a | 2.06 ± 0.07 c | 14 | floral, fruit, honey |
| Ethyl Valerate | 1122 | MS, RI | ND | 0.21 ± 0 b | 0.25 ± 0.01 a | ND | 1.5 | apple, herb, sweet |
| Phenethyl Butyrate | 1869 | MS, RI | ND | 3.03 ± 0.03 a | ND | ND | 961 | fruit |
| Isobutyl Acetate | 1000 | MS, RI | ND | ND | 0.69 ± 0 b | 0.72 ± 0 a | 25 | apple, floral, herb |
| Diisobutyl phthalate | 2529 | MS, RI | 3.27 ± 0.69 a | ND | 4.24 ± 0.74 a | ND | NF | NF |
| Benzyl Acetate | 1718 | MS, RI | ND | ND | 1.17 ± 0.03 a | ND | 364 | fresh, fruit, honey |
| Ethyl Hex-3-enoate | 1292 | MS, RI | ND | ND | 0.36 ± 0 a | ND | NF | NF |
| Ethyl (Z)-3-Hexenoate | 1291 | MS, RI | 0.35 ± 0 a | ND | ND | ND | 10 | fruit |
| 2-Phenylethyl Dodecanoate | 2588 | MS, RI | 1.55 ± 0.01 a | ND | ND | ND | NF | NF |
| Decyl Decanoate | 1744 | MS, RI | ND | 6.89 ± 0.7 a | 5.07 ± 0.01 b | ND | NF | NF |
| Octyl Octanoate | 1542 | MS, RI | ND | 5.36 ± 0.02 a | ND | ND | NF | NF |
| Ethyl Nonanoate | 1525 | MS, RI | ND | 4.96 ± 0.07 a | 5.38 ± 0 a | ND | 377 | banana, fruit, grape |
| Ethyl Heptanoate | 1322 | MS, RI | ND | ND | 0.43 ± 0.01 a | 0.41 ± 0 b | 18 | brandy, fruit, wine |
| 3-Methyl-1-Butanol | 1193 | MS, RI | 1109.8 ± 64.62 a | 946.3 ± 11.92 c | 890.19 ± 45.55 c | 1197.4 ± 12.09 a | 600 | banana, cheese, floral |
| Phenylethyl Alcohol | 1894 | MS, RI | 678.27 ± 40.71 ab | 617.67 ± 36.58 b | 367.15 ± 7.5 c | 714.4 ± 6.66 a | 564.23 | floral, fruit, honey, rose |
| Isobutanol | 1076 | MS, RI | 40.73 ± 2.57b | 49.65 ± 3.25 b | 67.85 ± 6.02 a | 61.82 ± 1.23 a | 25,000 | alcohol, apple |
| 2-Ethylhexanol | 1474 | MS, RI | 16.85 ± 0.52d | 23.16 ± 0.24 c | 24.92 ± 0.16b | 44.12 ± 0.92 a | 300 | citrus, green, oil, rose |
| Nerolidol | 2025 | MS, RI | 13.37 ± 0.96 a | 10.22 ± 1.24 b | 5.68 ± 0.12 c | 13.82 ± 0.28 a | 250 | oil, flower, wood |
| Benzyl Alcohol | 1860 | MS, RI | 12.44 ± 0.81b | 10.56 ± 0.71 c | 15.69 ± 0.33 a | 12.11 ± 0.27 b | 159,000 | floral, fruit |
| 1-Propanol | 1019 | MS, RI | 8.68 ± 0.67 a | 5.87 ± 0.38 b | 4.99 ± 0.53b | 7.62 ± 0.19 a | 25,000 | alcohol, candy, plastic |
| 1-Dodecanol | 1948 | MS, RI | 6.58 ± 1.79 a | 4.78 ± 1.36 a | 5.13 ± 0.35 a | 5.7 ± 0.57 a | 1000 | fat, wax |
| 1-Octanol | 1542 | MS, RI | 4.58 ± 0.06 a | ND | 5.27 ± 0.68 a | ND | 820 | citrus, fat, fruit, green |
| Dehydrolinalool | 1595 | MS, RI | 5.03 ± 0.26 b | 4.64 ± 0.11 b | 5.63 ± 0.12 a | 4.89 ± 0.09 b | NF | fresh, lemon, sweet |
| (R)-(+)-citronellol | 1748 | MS, RI | ND | ND | ND | 1.95 ± 0.49 a | 40 | floral |
| 1-Nonanol | 1643 | MS, RI | 1.85 ± 0.09 b | 2.05 ± 0.06 ab | 1.68 ± 0.14 b | 2.35 ± 0.31 a | 310 | fat, floral, green |
| Linalool | 1533 | MS, RI | 1.09 ± 0.24 b | 1.05 ± 0.12 b | ND | 2.23 ± 0.07 a | 6 | floral, flower, grape |
| 1-Hexadecanol | 2351 | MS, RI | ND | 2.97 ± 0.61 a | 3.98 ± 0.94 a | 3.93 ± 0.62 a | 1100 | flower, wax |
| Cis-Ocimenol | 1638 | MS, RI | 2.39 ± 0.05 b | 2.07 ± 0.07 c | ND | 3.15 ± 0.15 a | NF | NF |
| 1-Butanol | 1129 | MS, RI | 1.11 ± 0.08 ab | 1.07 ± 0.08 b | 1.29 ± 0.09 a | 0.73 ± 0.02 c | 459.2 | alcohol, fermented, fruit |
| 1-Octen-3-Ol | 1436 | MS, RI | 0.83 ± 0.01 a | ND | 0.77 ± 0.03 c | 0.64 ± 0 d | 20 | floral, green, herb |
| Farnesol | 2328 | MS, RI | 2.59 ± 0.03 a | 2.81 ± 0.76 a | 1.03 ± 0.03 b | 2.82 ± 0.1 a | 20 | floral, oil, weet |
| 1-Octadecanol | 2351 | MS, RI | 2.49 ± 0.01 a | ND | 1.06 ± 0.01 b | ND | NF | oil |
| (E)-2,6-Dimethyl-5,7-Octadien-2-Ol | 1638 | MS, RI | ND | ND | 2.78 ± 0.04 a | ND | NF | NF |
| Methionol | 1700 | MS, RI | ND | ND | 2.65 ± 0.01 b | 2.88 ± 0.14 a | 36 | cooked potato, earth |
| (Z)-Linalool Oxide (Furanoid) | 1431 | MS, RI | ND | ND | 1.81 ± 0.04 b | 1.88 ± 0.27 b | 100 | earth, flower, |
| 1-Hexanol | 1338 | MS, RI | ND | 0.9 ± 0.03 a | ND | 0.76 ± 0.06 b | 8000 | flower, fruit, green |
| (3S)-3,7-Dimethyloct-7-en-1-ol | 1748 | MS, RI | ND | 2.55 ± 0.03 a | ND | ND | NF | floral |
| 1-Decanol | 1745 | MS, RI | ND | ND | 5.5 ± 0.1 a | ND | 500 | fat, oil, plastic |
| α-Bisabolol | 2198 | MS, RI | ND | ND | ND | 3.4 ± 0.08 a | NF | flower, spice |
| Cis-3-Nonen-1-Ol | 1667 | MS, RI | ND | 0.64 ± 0.01 a | 0.43 ± 0.04 b | ND | NF | wax |
| α-Terpineol | 1684 | MS, RI | 6.05 ± 0.21 a | 5.67 ± 0.37 ab | 5.08 ± 0 b | 5.57 ± 0.27 ab | 250 | floral, fresh, oil |
| N-Decanoic Acid | 2247 | MS, RI | 1189.8 ± 74.33 a | 1145.04 ± 69.16 a | 803.28 ± 29.9 b | 1094.41 ± 20.65 a | 9400 | fat, grass, rancid |
| Octanoic Acid | 2046 | MS, RI | 901.38 ± 45.13 a | 865.57 ± 48.74 a | 707.06 ± 6.93 b | 659.3 ± 0 b | 500 | acid, cheese, fat |
| Dodecanoic Acid | 2470 | MS, RI | 106.43 ± 10.99 a | 69.26 ± 8.98 b | 57.27 ± 5.73 b | 71.49 ± 2.15 b | 7200 | fat, fruit, metal, wax |
| Acetic Acid | 1452 | MS, RI | 16.55 ± 2.85 c | 10.37 ± 0.87 d | 28.41 ± 0.05 a | 23.21 ± 1.31 b | 26,000 | acid, fruit, pungent |
| 9-Decenoic Acid | 2310 | MS, RI | ND | 6.98 ± 0.07 a | ND | ND | 4300 | soap |
| Benzoic Acid | 2448 | MS, RI | ND | ND | 3.14 ± 0.07 a | ND | 1000 | balsamic |
| Nonanoic Acid | 2144 | MS, RI | ND | ND | 11.19 ± 4.91 a | 4.15 ± 0.14 b | 1100 | cheese |
| 4-Hexyl-2,5-Dioxofuran-3-Acetic Acid | 2098 | MS, RI | ND | ND | 2.59 ± 0.3 a | ND | NF | NF |
| β-Lonone | 1930 | MS, RI | 7.75 ± 0.29 a | 5.78 ± 0.01 c | 6.61 ± 0.11 b | 6.88 ± 0.18 b | 0.007 | floral, raspberry, seaweed |
| 2-Octanone | 1278 | MS, RI | ND | 2.06 ± 0.25 a | ND | 2.08 ± 0.07 a | 50.2 | fat, fragant, green |
| 2-Nonanone | 1382 | MS, RI | ND | 0.68 ± 0.02 a | ND | 0.5 ± 0.07 b | 10.9 | fragant, fruit, green |
| 2,6-Di-Tert-Butyl-4-Hydroxy-4-Methylcyclohexa-2,5-Dien-1-One | 2082 | MS, RI | 1.54 ± 0.44 a | 1.21 ± 0.01 a | 1.69 ± 0.02 a | 1.75 ± 0.13 a | NF | NF |
| Irisone | 1929 | MS, RI | ND | 6.73 ± 0.01 a | ND | 2.68 ± 0.15 b | 0.45 | floral, sweet, violet |
| 3,4-Dimethylbenzaldehyde | 1814 | MS, RI | 32.4 ± 1.04 a | 28.5 ± 2.77 ab | 26.2 ± 1.58 b | ND | NF | NF |
| Benzaldehyde | 1523 | MS, RI | 9.79 ± 1.25 a | 10.62 ± 0.5 a | 9.32 ± 0.32 a | 11.2 ± 0.65 a | 350 | almond, berry, bitter |
| Isovaleraldehyde | 896 | MS, RI | 0.33 ± 0.01 b | ND | ND | 0.43 ± 0.04 a | 4.6 | chocolate, corn flakes |
| 2,5-Dimethylbenzaldehyde | 1814 | MS, RI | ND | ND | ND | 12.79 ± 1.08 a | 200 | NF |
| 2,4-Di-Tert-Butylphenol | 2282 | MS, RI | 20.88 ± 0.38 a | 19.22 ± 1.92 ab | 18.05 ± 1 ab | 16.55 ± 1.98 b | 500 | NF |
| Eugenol | 2152 | MS, RI | 9.2 ± 0.57 a | ND | 9.68 ± 0.15 a | 8.56 ± 0.86 a | 10 | burnt, clove, smoke |
| 4-tert-Amylphenol | 2389 | MS, RI | 2.77 ± 0.06 a | ND | 2.22 ± 0.07 b | 2.25 ± 0.1 b | 800 | NF |
| 3-Ethoxy-1-propanol | 1362 | MS, RI | 5.07 ± 0.17 a | 4.5 ± 0.18 b | ND | 4.68 ± 0.32 ab | 100 | fruit |
| Nerol Oxide | 1462 | MS, RI | 3.54 ± 0.01 a | 2.77 ± 0.01 b | 3.35 ± 0.2 a | 3.56 ± 0.14 a | 80 | flower, oil |
| Geranic Oxide | 1095 | MS, RI | 1.9 ± 0.06 b | 1.84 ± 0.29 b | 2.26 ± 0.08 ab | 2.41 ± 0.12 a | NF | NF |
| Ocimene Quintoxide | 1233 | MS, RI | ND | ND | 1.1 ± 0.06 a | 0.91 ± 0.08 b | NF | Citrus, wood |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Li, W.; Chen, Z.; Peng, Z.; Ma, M.; Zhang, J.; Wu, D.; Xie, G.; Lu, J. Exploring the Impact of Different Saccharomyces cerevisiae Strains on the Flavor Profile of Greengage Alcoholic Beverage Using GC-E-Nose, HS-GC-IMS, and HS-SPME-GC-MS. Foods 2024, 13, 3984. https://doi.org/10.3390/foods13243984
Shi Z, Li W, Chen Z, Peng Z, Ma M, Zhang J, Wu D, Xie G, Lu J. Exploring the Impact of Different Saccharomyces cerevisiae Strains on the Flavor Profile of Greengage Alcoholic Beverage Using GC-E-Nose, HS-GC-IMS, and HS-SPME-GC-MS. Foods. 2024; 13(24):3984. https://doi.org/10.3390/foods13243984
Chicago/Turabian StyleShi, Zhenbao, Wenzhe Li, Ziqiang Chen, Zhengcong Peng, Mingtao Ma, Jinglong Zhang, Dianhui Wu, Guangfa Xie, and Jian Lu. 2024. "Exploring the Impact of Different Saccharomyces cerevisiae Strains on the Flavor Profile of Greengage Alcoholic Beverage Using GC-E-Nose, HS-GC-IMS, and HS-SPME-GC-MS" Foods 13, no. 24: 3984. https://doi.org/10.3390/foods13243984
APA StyleShi, Z., Li, W., Chen, Z., Peng, Z., Ma, M., Zhang, J., Wu, D., Xie, G., & Lu, J. (2024). Exploring the Impact of Different Saccharomyces cerevisiae Strains on the Flavor Profile of Greengage Alcoholic Beverage Using GC-E-Nose, HS-GC-IMS, and HS-SPME-GC-MS. Foods, 13(24), 3984. https://doi.org/10.3390/foods13243984





